Weekly oritavancin plus ampicillin continuous infusion combination therapy was used to successfully treat a deep spine vancomycin-resistant Enterococcus faecium infection associated with hardware. Checkerboard and time-kill assays confirmed synergy between these two antibiotics.
KEYWORDS: ampicillin, oritavancin, vancomycin-resistant Enterococcus faecium
ABSTRACT
Weekly oritavancin plus ampicillin continuous infusion combination therapy was used to successfully treat a deep spine vancomycin-resistant Enterococcus faecium infection associated with hardware. Checkerboard and time-kill assays confirmed synergy between these two antibiotics. Further synergies of oritavancin and ampicillin with rifampin or the endogenous human antimicrobial peptide cathelicidin LL-37 were demonstrated.
CASE PRESENTATION
A 59-year-old man (118 kg, 36.3 kg/m2 body mass index) with type 2 diabetes mellitus, peripheral vascular disease, and prior toe amputations due to osteomyelitis was admitted with back pain and methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (vancomycin MIC, 1 mg/liter, tested by microdilution). A transthoracic and subsequent transesophageal echocardiogram did not demonstrate valvular vegetations or perivalvular abscesses. Magnetic resonance imaging (MRI) of the thoracic and lumbar spine showed a possible arachnoid cyst versus early epidural abscess in the thoracic spine, erector spinae myositis from L2 to S1, and perifacet inflammation of L5 to S1 without discitis, osteomyelitis, or abscess. Erythrocyte sedimentation rate (ESR) was 130 mm/h (normal range, 0 to 25 mm/h) and C-reactive protein (CRP) was 210 mg/liter (normal range, 0 to 7.5 mg/liter). Bacteremia cleared in 48 h, and intravenous vancomycin, dosed to achieve a serum trough of 15 to 20 mg/liter, was prescribed for 6 weeks. Unfortunately, the development of acute kidney injury 14 days into therapy prompted a switch to intravenous daptomycin 8 mg/kg to complete the course. Two weeks later, the patient developed some patchy lung infiltration as seen on chest x-ray, along with some pulmonary symptoms. Daptomycin was switched to clindamycin for the final 10 days because of concerns for possible eosinophilic pneumonitis, related to use of daptomycin, and primary medical team insistence. At completion of the intravenous (i.v.) antibiotic course, ESR was 61 mm/h, and CRP was 243 mg/liter.
Two months after therapy was completed, the patient presented with worsening low-back pain and MRSA bacteremia (vancomycin MIC, ≤0.5 mg/liter). The patient was started on i.v. vancomycin, and bacteremia cleared within 72 h. The patient’s ESR was 61 mm/h, and CRP was 193 mg/liter. Noncontrast thoracic and lumbar spine MRI showed resolution of the prior epidural collection and a new 1.4- by 1.6- by 4.5-cm lumbar epidural abscess causing marked narrowing of the thecal sac and probable impingement of the cauda equina. The patient underwent posterior spinal decompression from L3 to S1 with irrigation and debridement of the epidural abscess and posterior spinal instrumentation and fusion from L4 to S1. Postoperatively, the patient continued on vancomycin and was discharged to a subacute nursing facility to complete 10 weeks of therapy with close monitoring.
Before completion of the prescribed vancomycin course and before any additional fluid aspirations, a repeat lumbar spine MRI showed a large (12.2- by 6.9- by 9.5-cm) fluid collection surrounding the posterior rods and screws from L4 to S1 (Fig. 1A). ESR was 41 mm/h, and CRP was 35 mg/liter. Interventional radiology-guided aspiration with drain placement yielded purulent fluid that grew vancomycin-resistant Enterococcus faecium with the following MICs (mg/liter): vancomycin, >32; daptomycin, 16; ampicillin, >32; linezolid, 2; tedizolid, 0.25; tigecycline, 0.25; quinupristin-dalfopristin, 0.5; oritavancin (ORI), 0.06; streptomycin, synergy resistant; and gentamicin, synergy susceptible. Tigecycline was initiated but discontinued after just 2 days because of intolerable nausea. Quinupristin-dalfopristin was attempted next but caused severe intolerable myalgias. After only 4 days, the posterior spinal drainage catheter accidentally became dislodged and was not replaced.
FIG 1.
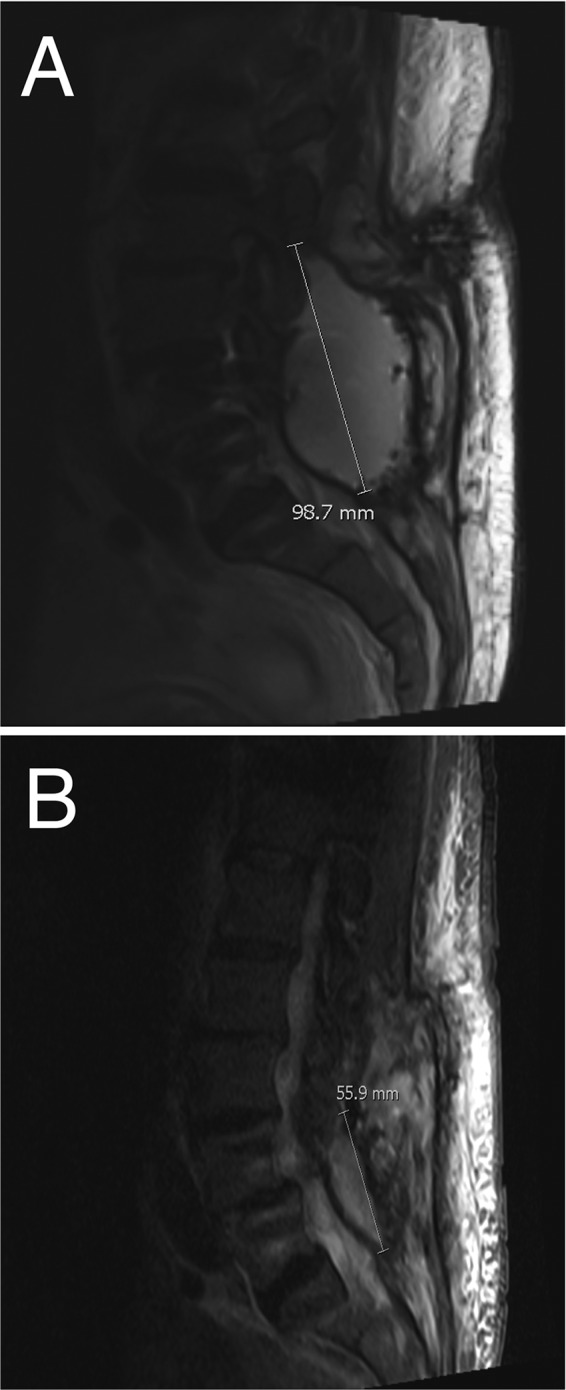
Representative MRI images of lumbosacral fluid collection before (A) and after (B) treatment with ORI plus ampicillin after VRE was isolated. The volume of the fluid collection was reduced by >90% on this therapy.
CHALLENGE QUESTION
Given the above susceptibility report and the patient’s antibiotic intolerance history, which antimicrobial regimen would you consider next?
A. Continue quinupristin-dalfopristin plus tigecycline at half of approved doses
B. Tedizolid
C. Oritavancin
D. Oritavancin plus ampicillin
E. Telavancin plus ampicillin
TREATMENT AND OUTCOME
ORI demonstrates excellent in vitro activity against vancomycin-resistant E. faecium (VRE) (1–3). Based on a previous case report utilizing prolonged ORI in the treatment of VRE endocarditis (4) and demonstrated synergy between ORI and β-lactams in vitro against VRE (5), ORI plus ampicillin combination therapy was used. ORI 1.2 g i.v. weekly for 2 weeks then decreased to 800 mg i.v. weekly for 8 weeks with concomitant ampicillin 12 g/day was given as a continuous infusion. This dose was selected based on previous case series utilizing multiple-dose ORI (6). In total, the patient received a full 10 weeks of i.v. ORI-ampicillin. Oxazolidinone therapy with linezolid or tedizolid was reserved as a “mop-up” regimen after the parenteral regimen was completed. Given that pharmacokinetic and preclinical laboratory science predicts a lower risk of neuropathic (7) and hematological (8) side effects with tedizolid, this may have been the preferred agent for long-term use (e.g., 6 months) after completion of i.v. therapy, although clinical data are lacking to support this decision. After the 10 weeks of combination i.v. therapy, ESR was 44 mm/h, and CRP was 26 mg/liter. A subsequent MRI just before the completion of therapy showed a considerable decrease in size (>90% volume) of the fluid collection to 2.5 by 4.4 by 5.6 cm (Fig. 1B).
MIC testing and synergy tests were performed by checkerboard assay in triplicate, as described previously (9), using an inoculum of 1 × 106 CFU/ml in brain heart infusion (BHI) broth supplemented with 0.002% polysorbate-80. BHI was utilized because the fastidious strain did not reliably grow well in Mueller-Hinton broth (MHB), which potentially would have compromised testing, but may have accounted for the much higher ORI MIC (1 mg/liter) observed in the research laboratory than seen in the clinical microbiology laboratory (0.06 mg/liter). These results confirmed the anticipated synergy between ORI and ampicillin (Table 1). The combination of ORI and rifampin was studied by checkerboard assay for possible future consideration. Time-kill assays were performed to examine the activities of ORI (0.06 mg/liter), ampicillin (4 mg/liter), and rifampin (2 mg/liter) alone or in combination against the clinical VRE isolate (10). Each assay was performed in triplicate in 96-well plates using a starting inoculum of 1 × 108 CFU/ml in cation-adjusted MHB and repeated three times. These concentrations were chosen to simulate values below MICs that would be readily achieved in vivo with standard human dosing under the more rigorous high-inoculum conditions presumably present in this serious infection (2, 3). Viable bacteria were quantified at 4, 6, and 24 h (37°C, without shaking) using 25-μl aliquots for serial dilution in phosphate-buffered saline and plating on Todd-Hewitt agar plates (Hardy Diagnostics, Santa Maria, CA). The results are shown in Fig. 2. Despite being at concentrations well below MICs, thereby allowing for growth under single-drug conditions, the combination of two and three drugs exerted much more potent effects than single drugs (Fig. 2B). Although we did not use rifampin in the case described, the in vitro studies suggested an additional benefit of rifampin over ORI and ampicillin in achieving complete stasis at very low drug concentrations.
TABLE 1.
Susceptibilities and synergy assessment by checkerboard assays against the clinical VRE isolate
| Drug or combination | MIC (mg/liter) | FICIa |
|---|---|---|
| Oritavancin | 1 | |
| Ampicillin | >128 | |
| Rifampin | 8 | |
| ORI-ampicillin | 0.140 (synergy) | |
| ORI-rifampin | 0.375 (synergy) |
FICI, fractional inhibitory concentration index.
FIG 2.
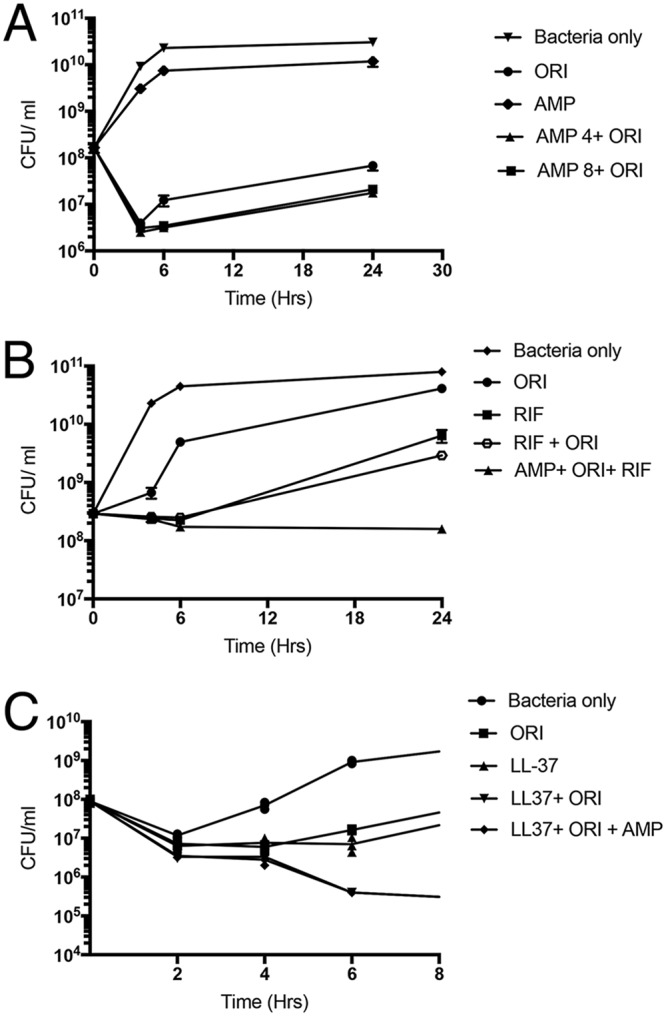
Time-kill curves evaluating ORI (0.06 mg/liter, 1/16 MIC) and ampicillin (4 and 8 mg/liter) alone or in various combinations (A) or oritavancin with rifampin (2 mg/liter, 1/4 MIC) and oritavancin, rifampin, and ampicillin (4 mg/liter) alone or in various combinations (B), as shown against the clinical VRE isolate. Data plotted are means ± SEM and represent the mean of triplicates. ORI, oritavancin; RIF, rifampin; AMP, ampicillin. (C) Relative activities over 8 h of ORI (0.12 mg/liter, 1/8 MIC) and ampicillin (4 mg/liter) with or without cathelicidin LL-37 (2 μM). Each experiment was done in triplicate and independently repeated three times.
The use of ORI-ampicillin, although not providing killing in our assays, appeared to eradicate the organism and result in significant radiographic and clinical improvement. We hypothesized that the activity of ORI-ampicillin may be augmented in vivo by various arms of the innate host response (e.g., cationic antimicrobial peptides produced by neutrophils and epithelial cells) (11, 12). We performed additional killing assays utilizing ORI and ampicillin alone or in combination in the presence or absence of 2 μM cathelicidin LL-37, a concentration selected based on the pathogen’s MIC and relevance to concentrations present in vivo (12, 13). The results shown in Fig. 2C demonstrate that addition of LL-37 to ORI or ORI plus ampicillin led to considerably greater killing than LL-37 or the exogenously administered antibiotics alone, supporting the possible additional antibacterial effect with host factors.
Repeat diagnostic aspiration was performed 11 days before completion of the 10-week planned course of ORI and ampicillin. The aspiration yielded 5 ml of sanguineous fluid, consistent with hematoma, and grew a few colonies of methicillin-resistant Staphylococcus epidermidis (vancomycin MIC, 1 mg/liter). Although of unclear significance, given the proximity of the fluid collection to adjacent spinal hardware, the patient was treated as though he had a different possible hardware infection with S. epidermidis. The treatment chosen was daptomycin 8 mg/kg (800 mg i.v. every 48 h) in combination with ceftaroline 600 mg i.v. every 12 h. Daptomycin was retried in this patient, given that the initial concern for eosinophilic pneumonitis was felt to be unsubstantiated; the patchy lung infiltration started to resolve before discontinuation of daptomycin, there was no documentation of eosinophils in sputum, and there was no sign of peripheral eosinophilia. During this treatment, the patient developed complications related to his comorbidities, including bradycardia, heart failure, and acute kidney injury, and opted for comfort care only. He expired 1 day after stopping all antibiotics.
COMMENTARY
VRE is one of the most difficult-to-treat pathogens, and daptomycin is often considered the drug of choice for the treatment of serious VRE infections. However, daptomycin is also for several other indications, and prior exposure to daptomycin for any reason can lead to subsequent daptomycin-resistant VRE (14). This case highlights this key point, wherein brief exposure to daptomycin for a complicated MRSA bacteremia likely contributed to the development of a hardware-associated spinal infection caused by daptomycin-resistant VRE. Unfortunately, treatments for daptomycin-resistant VRE are limited. ORI is a synthetic derivative of the naturally occurring glycopeptide chloroeremomycin, which, because of a lipophilic 4′-chlorobiphenyl moiety, retains activity against VRE harboring the vanA gene, including daptomycin-resistant strains. Thus, ORI is an attractive option to treat these increasingly common infections.
The authors approached this case in a reasonable manner and first attempted to treat the patient with both tigecycline and quinupristin-dalfopristin, only to have toxicity preclude the continued use of either agent. They then elected to treat with a combination of ORI (1,200 mg i.v. weekly for 2 weeks, followed by 800 mg i.v. weekly) and ampicillin (12 g i.v. daily, as a continuous infusion) under the hypothesis that the combination would be synergistic. Treatment appeared to be successful after 8 weeks of therapy, with a significant decrease in the size of the infected fluid collection and failure to regrow the Enterococcus. However, the same repeat culture did grow S. epidermidis, and it is unclear whether this was a breakthrough infection that developed on therapy or a contaminant. Nevertheless, antibiotics were changed to daptomycin and ceftaroline, but the patient died shortly thereafter.
To verify that the combination of ORI and ampicillin was synergistic, the investigators performed a series of in vitro tests. In a checkerboard assay, ORI and ampicillin were highly synergistic. In time-kill studies, however, the picture became less clear. ORI alone and in combination with ampicillin led to an initial bacterial kill, but regrowth occurred with both regimens by 24 h. The lack of sustained kill at 24 h with the ORI-ampicillin combination is in line with the findings of Smith et al. (5), who evaluated ORI-β-lactam (i.e., ampicillin, ceftaroline, or ertapenem) combinations against four VRE strains in an in vitro time-kill model and found that synergy was both strain and β-lactam dependent. However, these authors further demonstrated that the endogenous antimicrobial peptide LL-37 restored ORI-ampicillin synergy in the same time-kill model. Thus, the answer to the question of whether ampicillin-ORI is synergistic against VRE is, at best, unclear. The authors showed that the addition of rifampin to ORI-ampicillin also restored synergy, whereas others have shown synergy between ORI and gentamicin (15). Therefore, either rifampin-ORI-ampicillin or ORI-gentamicin may have been reasonable treatment options in this patient.
Because this patient was treated with ampicillin and ORI for the duration of therapy, it is impossible to know whether the combination was any more or less effective than ORI alone. Nevertheless, this case adds to the limited literature regarding the use of ORI for infections caused by VRE. The authors chose their dose based on a small case series, which included three cases of daptomycin-resistant VRE (6). A separate report of ORI for daptomycin-resistant VRE endocarditis used a dose of 1,200 mg i.v. every 48 h for three doses, followed by 1,200 mg i.v. weekly (4). In vitro pharmacokinetic-pharmacodynamic modeling showed regrowth of daptomycin-resistant VRE after 48 h following a single dose of ORI 1,200 mg, and a phase 2 clinical trial indicated that daily dosing is safe; thus, a front-loaded strategy as employed by Johnson et al. (4) and others may be preferred (2, 16). However, clinical trials and more robust clinical data are needed to verify the optimal treatment approach.
Although the emergence of bacteria resistant to ORI has not been reported in the clinical setting, the S. epidermidis isolated from aspirated fluid after 10 weeks of combination therapy may have been resistant to ORI. To date, the emergence of ORI resistance during therapy has not been reported, and potential mechanisms are poorly described. However, as long as ORI is used for difficult-to-treat infections, as in this case, treatment-emergent resistance is unlikely to be far behind.
The authors chose to use ORI and ampicillin in this situation based on their expertise and clinical experience, and that choice appears to have been the right one for this patient. Many questions remain, however, regarding the optimal approach to treating infections caused by daptomycin-resistant VRE.
ACKNOWLEDGMENTS
G.S. has received speaking honoraria from Allergan, Sunovion, and Melinta and consulting fees from Allergan and Paratek Pharmaceuticals. V.N. has received consulting fees from Cidara Therapeutics. S.L.A. is on the advisory board for Merck, Sharpe, & Dohme and has received research support from Melinta Therapeutics and Merck, Sharpe, & Dohme.
This journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Two expert clinicians then provide a commentary on the case.
REFERENCES
- 1.Mendes RE, Woosley LN, Farrell DJ, Sader HS, Jones RN. 2012. Oritavancin activity against vancomycin-susceptible and vancomycin-resistant enterococci with molecularly characterized glycopeptide resistance genes recovered from bacteremic patients, 2009-2010. Antimicrob Agents Chemother 56:1639–1642. doi: 10.1128/AAC.06067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belley A, Arhin FF, Moeck G. 2018. Evaluation of oritavancin dosing strategies against vancomycin-resistant Enterococcus faecium isolates with or without reduced susceptibility to daptomycin in an in vitro pharmacokinetic/pharmacodynamics model. Antimicrob Agents Chemother 62:e01873-17. doi: 10.1128/AAC.01873-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belley A, Lalode-Seguin D, Arhin FF, Moeck G. 2017. Comparative pharmacokinetics of single-dose oritavancin and daily high-dose daptomycin regimens against vancomycin-resistant Enterococcus faecium in an in vitro pharmacokinetic/pharmacodynamics model of infection. Antimicrob Agents Chemother 61:e01265-17. doi: 10.1128/AAC.01265-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JA, Feeney ER, Kubiak DW, Gorey GR. 2015. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis 2:ofv156. doi: 10.1093/ofid/ofv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JR, Yim J, Raut A, Rybak MJ. 2016. Oritavancin combinations with beta-lactams against multidrug-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Antimicrob Agents Chemother 60:2352–2358. doi: 10.1128/AAC.03006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz LT, Dworkin E, Dela-Pena J, Rose WE. 2018. Multiple-dose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy 38:152–159. doi: 10.1002/phar.2057. [DOI] [PubMed] [Google Scholar]
- 7.Schlosser MJ, Hosako H, Radovsky A, Butt MT, Draganov D, Vija J, Oleson F. 2015. Lack of neuropathological changes in rats administered tedizolid phosphate for nine months. Antimicrob Agents Chemother 59:475–481. doi: 10.1128/AAC.03950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikamo H, Takesue Y, Iwamoto Y, Tanigawa T, Kato M, Tanimura Y, Kohno S. 2018. Efficacy, safety, and pharmacokinetics of tedizolid versus linezolid in patients with skin and soft tissue infections in Japan-results of a randomized, multicenter phase 3 study. J Infect Chemother 24:434–442. doi: 10.1016/j.jiac.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Orhan G, Bayram A, Zer Y, Balci I. 2005. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol 43:140–143. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40:1914–1918. doi: 10.1128/AAC.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakoulas G, Okumura CY, Thienphrapa W, Olson J, Nonejuie P, Dam Q, Dhand A, Pogliano J, Yeaman MR, Hensler ME, Bayer AS, Nizet V. 2014. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med 92:139–149. doi: 10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byfield FJ, Wen Q, Leszczyńska K, Kułakowska A, Namiot Z, Janmey PA, Bucki R. 2011. Cathelicidin LL-37 peptide regulates endothelial cell stiffness and endothelial barrier permeability. Am J Physiol Cell Physiol 300:C105–C112. doi: 10.1152/ajpcell.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPippo AJ, Tverdek FP, Tarrand JJ, Munita JM, Tran TT, Arias CA, Shelburne SA, Aitken SL. 2017. Daptomycin non-susceptible Enterococcus faecium in leukemia patients: role of prior daptomycin exposure. J Infect 74:243–247. doi: 10.1016/j.jinf.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu T, Meyer K, Harrington AT, Danziger LH, Wenzler E. 2019. In vitro activity of oritavancin alone or in combination against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. doi: 10.1093/jac/dkz010. [DOI] [PubMed] [Google Scholar]
- 16.Bhavnani SM, Passarell JA, Owen JS, Loutit JS, Porter SB, Ambrose PG. 2006. Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50:994–1000. doi: 10.1128/AAC.50.3.994-1000.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


