There is a great need for efficacious therapies against Gram-negative bacteria. Double β-lactam combination(s) (DBL) are relatively safe, and preclinical data are promising; however, their clinical role has not been well defined.
KEYWORDS: double beta-lactam, Gram-negative bacteria, beta-lactamase inhibitor, combination therapy, meta-analysis, randomized controlled clinical trial
ABSTRACT
There is a great need for efficacious therapies against Gram-negative bacteria. Double β-lactam combination(s) (DBL) are relatively safe, and preclinical data are promising; however, their clinical role has not been well defined. We conducted a metaanalysis of the clinical and microbiological efficacy of DBL compared to β-lactam plus aminoglycoside combinations (BLAG). PubMed, Embase, ISI Web of Knowledge, and Cochrane Controlled Trials Register database were searched through July 2018. We included randomized controlled clinical trials that compared DBL with BLAG combinations. Clinical response was used as the primary outcome and microbiological response in Gram-negative bacteria as the secondary outcome; sensitivity analyses were performed for Pseudomonas aeruginosa, Klebsiella spp., and Escherichia coli. Heterogeneity and risk of bias were assessed. Safety results were classified by systems and organs. Thirteen studies evaluated 2,771 cases for clinical response and 665 cases for microbiological response in various Gram-negative species. DBL achieved slightly, but not significantly, better clinical response (risk ratio, 1.05; 95% confidence interval [CI], 0.99 to 1.11) and microbiological response in Gram-negatives (risk ratio, 1.11; 95% CI, 0.99 to 1.25) compared with BLAG. Sensitivity analyses by pathogen showed the same trend. No significant heterogeneity across studies was found. DBL was significantly safer than BLAG regarding renal toxicity (6.6% versus 8.8%, P = 0.0338) and ototoxicity (0.7 versus 3.1%, P = 0.0137). Other adverse events were largely comparable. Overall, empirically designed DBL showed comparable clinical and microbiological responses across different Gram-negative species, and were significantly safer than BLAG. Therefore, DBL should be rationally optimized via the latest translational approaches, leveraging mechanistic insights and newer β-lactams for future evaluation in clinical trials.
INTRODUCTION
Antimicrobial resistance is causing a global public health crisis with increasing mortality, morbidity, and medical cost due to serious bacterial infections with resistant strains (1). This situation is more severe for infections with multidrug-resistant Gram-negative pathogens (MDRGN), considering the epidemiology and shortage of efficacious antibiotics (2, 3). Combination therapy is widely used to treat serious infections by MDRGN (4) and usually includes antibiotics from different classes to achieve synergistic bacterial killing. However, to target this global health crisis, innovative therapies and new antibiotics are urgently needed. β-Lactam antibiotics are likely to be an indispensable part of these combinations and are safe in patients of all ages. Indeed, against carbapenemase-producing Klebsiella pneumoniae strains, better clinical outcomes were observed for carbapenem-containing combinations compared with those for combinations that lacked a carbapenem (5). Presently, extensive efforts are being made to identify novel and efficacious combinations to combat infections with MDRGN (6).
All β-lactams are known to covalently bind to and thereby inactivate one or multiple penicillin-binding proteins (PBPs); however, β-lactams differ greatly in their binding patterns for various PBPs. Combining two β-lactams enables inactivation of multiple PBPs to achieve synergistic bacterial killing and minimize resistance. Such combinations have been widely investigated in preclinical and clinical studies from the 1970s to 1990s (7–10) to target both Gram-negative and Gram-positive pathogens. However, the advantages in spectrum have diminished with the availability of newer broad-spectrum antibiotics, including carbapenems and fluoroquinolones. Unfortunately, this has led to the global emergence of drastic resistance to fluoroquinolone. Since then, the clinical interest in double β-lactam combination(s) (DBL) has declined, and only a small number of clinical case studies have been published.
A series of novel molecular insights and translational approaches now enable us to design and rationally optimize DBL. Comprehensive PBP receptor binding data were recently published for K. pneumoniae and Acinetobacter baumannii (11–13), and such binding data are available over a series of papers on Pseudomonas aeruginosa and Escherichia coli (7, 10, 14–17). Some outer membrane permeability data are available for β-lactams in P. aeruginosa (18), and novel and efficient permeability assays for β-lactams and β-lactamase inhibitors in MDRGN have been recently developed (19). Addressing the key gaps in our understanding of β-lactam antibiotic action and resistance (13) enables the rational design of mechanistically optimized DBL with or without a β-lactamase inhibitor (11, 20). β-Lactams present the largest antibiotic class with abundant clinical pharmacokinetic (PK) and safety data. This presents a substantial advantage for translating these DBL to patients.
Inspired by these novel mechanistic advances, we performed a systematic review and meta-analysis of the clinical performance of DBL. We aimed to compare clinical and microbiological responses for key Gram-negative pathogens between DBL and β-lactam plus aminoglycoside combinations (BLAG) based on all published randomized controlled clinical trials. The majority of these trials were in patients with febrile neutropenia. The present analysis includes more clinical trials, as well as a meta-analysis that has not been performed in prior reviews (7, 8). The insights gained from these large, early clinical trials add considerable value and a clinical perspective to the future design, optimization, and implementation of innovative DBL that can successfully combat infections by MDRGN.
(Part of this work was presented as an ePoster presentation at the European Congress of Clinical Microbiology and Infectious Diseases [ECCMID] 2017 in Vienna, Austria.)
RESULTS
Study selection.
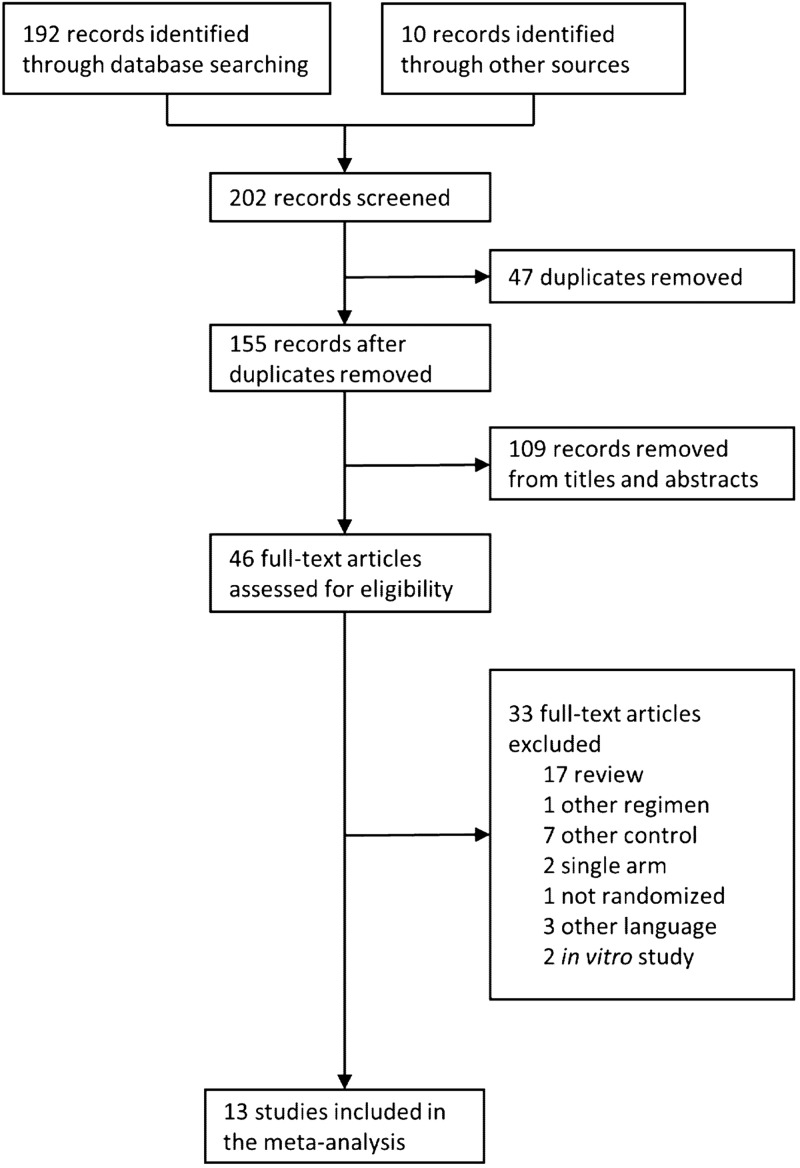
A total of 202 publications were identified during the database searches and by evaluating the references within the identified papers (Fig. 1). Forty-seven duplicates from different databases were removed, and 109 records (e.g., animal and in vitro studies) were excluded based on titles and abstracts. Thirty-three records were further removed for other reasons; these were nonclinical studies (n = 2), studies reported in another language (n = 3), review-only publications (n = 17), and trials with designs that did not meet the inclusion criteria (such as trials lacking a comparator group; n = 11). The final data set included 13 randomized controlled clinical trials reported between 1972 and 1993 (Fig. 1) (21–33).
FIG 1.
Flow of information in the systematic review.
Four of these thirteen trials were multicenter studies (Table 1), and eleven of these trials assessed febrile neutropenic patients. The latter accounted for 94% of the whole patient population (i.e., 3,257 cases of febrile neutropenia out of 3,476 overall cases). Age, sex, and other demographic variables were distributed evenly between both groups, with the exception of two studies that did not balance sex (22, 31).
TABLE 1.
Main characteristics of the trials included in the meta-analysisk
| Author (yr) (reference) | Trial typed | Type of infection | Treatment, dosage frequencyb |
Avg treatment duration (days) | Overall no. of casese (no. of patients) | Demographic characteristic |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Double β-lactam | β-Lactam plus aminoglycoside (daily doses) | Mean age (yrs) |
Men (%) |
|||||||
| DBL | BLAG | DBL | BLAG | |||||||
| Middleman (1972) (21) | Multicenter | Febrile neutropenia | CAR, 5 g q4h | CAR, 5 g q4h | 5–10 | 179 (151) | 30.5f | 36f | NAc | NA |
| CEF, 3 g q6h | KAN, 200 mg/m2 q8h (14.8 mg/kg/day) | |||||||||
| Klastersky (1975) (22) | Single center | Severe Infection | TIC, 10 g t.i.d. | CEF, 3 g t.i.d. | 6.3–7.8 | 186g (186) | 52.7 | 67.7 | 12.5 | 40 |
| CEF, 3 g t.i.d. | TOB, 4.5 mg/kg in 3 doses (4.5 mg/kg/day) | |||||||||
| TIC, 10 g t.i.d. | 50.7 | 25 | ||||||||
| TOB, 4.5 mg/kg in 3 doses (4.5 mg/kg/day) | ||||||||||
| Schimpff (1976) (23) | Single center | Febrile neutropenia | TIC, 5 g q6h | TIC, 5 g q6h | NA | 127 (NA) | 40 | 41 | 61.3 | 56.9 |
| CEF, 3 g q6h | GEN, 180 mg/m2/day in 4 doses (4.45 mg/kg/day) | |||||||||
| EORTCa (1978) (24) | Multicenter | Febrile neutropenia | TIC, 5 g q6h | TIC, 5 g q6h | NA | 625 (NA) | NA | NA | NA | NA |
| CEF, 3 g q6h | GEN, 180 mg/m2/day in 4 doses (4.45 mg/kg/day) | |||||||||
| CAR, 10 g q6h | CAR, 10 g q6h (CEF, 3 g q6h) | 43h | 43h | 54h | 54h | |||||
| CEF, 3 g q6h | GEN, 180 mg/m2/day in 4 doses (4.45 mg/kg/day) | |||||||||
| CEF, 3 g q6h | 43h | 54h | ||||||||
| GEN, 180 mg/m2/day in 4 doses (4.45 mg/kg/day) | ||||||||||
| Bodey (1979) (25) | Single center | Febrile neutropenia | CAR, 5 g q4h | CAR, 5 g q4h | 6–7f | 490i (NA) | NA | NA | 54 | 60 |
| FAM, 3 g loading, followed by 12 g over 24 h | TOB, 90 mg/m2 loading dose followed by 360/m2/day over 24 h (11.1 mg/kg/day on day 1; then 8.9 mg/kg/day) | |||||||||
| CAR, 5 g q4h | NA | 59 | ||||||||
| FAM, 3 g q6h | ||||||||||
| Wiston (1984) (26) | Single center | Febrile neutropenia | MOX, 57.5 mg/kg q12h (40 mg/kg, q8h) | MOX, 57.5 mg/kg q12h (or 40 mg/kg q8h) | 12 | 295 (NA) | 42f | 35f | 58.8 | 53.4 |
| PIP, 75 mg/kg q6h | AMK, 3.75 mg/kg q6h (15 mg/kg/day) | |||||||||
| Fainstein (1984) (27) | Single center | Febrile neutropenia | MOX, 2 g q4h | MOX, 2 g q4h | 8.0 | 495 (270) | 42 | 40 | 61.8 | 55.0 |
| TIC, 4 g q4h | TOB, 90 mg/m2 loading dose followed by 360/m2/day over 24 h (11.1 mg/kg/day on day 1; then 8.9 mg/kg/day) | |||||||||
| Feld (1985) (28) | Multicenter | Febrile neutropenia | TIC, 3 g q4h | TIC, 3 g q4h | 10 | 277 (244) | 50 | 53 | 45.3 | 53.4 |
| MOX, 2 g q4h | TOB, 1.25 mg/kg q6h (5 mg/kg/day) | |||||||||
| De Jongh (1986) (29) | Single center | Febrile neutropenia | MOX, 4 g q12h | MOX, 4 g q12h | 6 | 302 (220) | 48f | 48f | NA | NA |
| PIP, 5 g q6h | AMK, 250 mg q6h (14.3 mg/kg/day) | |||||||||
| Rostein (1988) (30) | Multicenter | Febrile neutropenia | CFP, 4 g q12h | MEZ, 3 g q4h | 11 | 60 (54) | 46 | 47 | 63.3 | 43.3 |
| PIP, 3 g q4h | TOB, 2 mg/kg loading dose, 1.5 mg/kg,j (3.5 mg/kg/day on day 1; then 1.5 mg/kg/day) | |||||||||
| Torres (1989) (31) | Single center | Severe nosocomial pneumonia | CTX, 2 g q6h | CTX, 2 g q6h | NA | 33g (33) | 64 | 53 | 84.6 | 100.0 |
| ATM, 1 g q8h | AMK, 500 mg q12h (14.3 mg/kg/day) | |||||||||
| Kibbler (1989) (32) | Single center | Febrile neutropenia | PIP, 4 g q.i.d. | PIP, 4 g q.i.d. | 7–10 | 202 (130) | 28.4 | 33.2 | 70.3 | 65.7 |
| CAZ, 2 g t.i.d. | NET, 3.5 mg/kg b.i.d. (7 mg/kg/day) | |||||||||
| AZL, 5 g t.i.d. | AZL, 5 g t.i.d. | 27.9 | 32.8 | 52.7 | 64.3 | |||||
| CAZ, 2 g t.i.d. | NET, 3.5 mg/kg b.i.d. (7 mg/kg/day) | |||||||||
| Joshi (1993) (33) | Single center | Febrile neutropenia | CAZ, 1.5 g q6h | CAZ, 1.5 g q6h | 7 | 205 (159) | 58 | 50 | NA | NA |
| PIP, 5 g q6h | TOB, 5 mg/kg/day in 3 doses (5 mg/kg/day) | |||||||||
EORTC, European Organization for Research on Treatment of Cancer.
AZL, azlocillin; ATM, aztreonam; CAR, carbenicillin; CFP, cefoperazone; CTX, cefotaxime; CEF, cephalothin (cefalotin); CAZ, ceftazidime; FAM, cefamandole; MEZ, mezlocillin; MOX, moxalactam; TIC, ticarcillin; PIP, piperacillin; AMK, amikacin; GEN, gentamicin; KAN, kanamycin; NET, netilmicin; TOB, tobramycin; q4h, q6h, q8h, q12h, every 4, 6, 8, or 12 hours, respectively; b.i.d., twice a day; t.i.d., three times a day; q.i.d., four times a day.
NA, not applicable.
All studies were randomized, controlled trials.
Cases included episodes or patient-trials. The number of cases was larger than the number of patients, since some patients had multiple episodes of febrile neutropenia. The number of evaluable cases was used to calculate clinical response.
Median.
The original study only provided the number of subjects but not the number of episodes; the number of subjects was used as the number of cases, since there was usually only one episode of a severe infection per patient in these two studies.
Only overall demographic data were available.
Only 450 episodes were evaluable. Of these, 234 episodes represented fever with unknown origin, and 216 episodes were caused by clinically or microbiologically documented infections.
Dosing interval adjusted by renal function.
Please note: the clinical evaluation time has not been precisely reported in these studies; therefore, it is not presented in this table.
A variety of β-lactams and aminoglycosides, as well as their dosage regimens, were investigated by different studies (Table 1). Given the time period in which these trials were performed, the employed β-lactams did not include carbapenems. Definitions for infection and criteria for clinical or microbiological cure were obtained as specified by the respective trial (Tables S1 and S2). The median duration of treatment ranged from 5 to 12 days (Table 1).
Clinical and microbiological response.
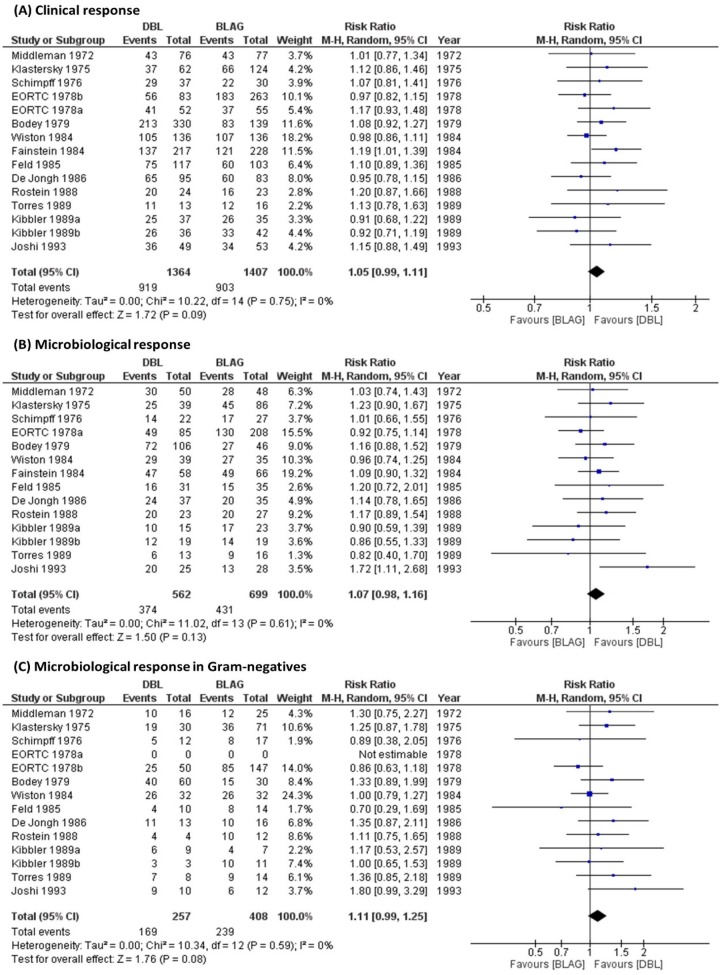
All pooled risk ratios for the different outcomes were above 1.00 and therefore slightly favored DBL compared to BLAG therapy. The therapeutic advantage of DBL over BLAG was not statistically significant, since all 95% confidence intervals (CIs) included 1.00 (Table 2, Fig. 2). Meta-analysis results from a fixed-effect model (not shown) were nearly identical to those reported in Table 2. Sensitivity analyses of the microbiological responses in the different Gram-negative pathogens (P. aeruginosa, Klebsiella spp., and E. coli) all showed the same trend (Table 2, Fig. S1).
TABLE 2.
Summary of clinical and microbiological responses comparing double β-lactam with β-lactam plus aminoglycoside therapya
| Outcome | Double β-lactam (% [no. of responses/total]) | β-Lactam plus aminoglycoside (% [no. of responses/total]) | Risk ratio (95% confidence interval)c | No. of evaluable casesb |
|---|---|---|---|---|
| Clinical response | 67.4 (919/1,364) | 64.2 (903/1,407) | 1.05 (0.99, 1.11) | 2,771 |
| Microbiological response | ||||
| Overall | 66.5 (374/562) | 61.7 (431/699) | 1.07 (0.98, 1.16) | 1,261 |
| Gram-negative species | 65.8 (169/257) | 58.6 (239/408) | 1.11 (0.99, 1.25) | 665 |
| Pseudomonas aeruginosa | 58.5 (38/65) | 60.6 (60/99) | 1.02 (0.81, 1.27) | 164 |
| Klebsiella spp. | 60.8 (31/51) | 50.5 (52/103) | 1.16 (0.89, 1.51) | 154 |
| Escherichia coli | 72.3 (60/83) | 65.2 (86/132) | 1.08 (0.90, 1.28) | 215 |
From the meta-analysis based on thirteen randomized, controlled clinical trials.
Evaluable cases included evaluable episodes or evaluable patient-trials. The overall number of cases could be equal to or larger than the number of evaluable cases.
Risk ratios were calculated using a random-effects model. There were minor numerical differences between the RevMan and R meta package results, but these did not change any conclusions. This applied especially for comparison of outcomes with relatively small sample size (i.e., microbiological response in the Gram-negative species, P. aeruginosa, Klebsiella spp., and E. coli). Results for the fixed-effect model were identical in both software packages.
FIG 2.
Forest plots for double β-lactam combinations compared with β-lactam plus aminoglycoside combinations for (A) clinical response, (B) microbiological response, and (C) microbiological response in various Gram-negative species. A random-effects model was used. Dots represent the summary measure and 95% confidence interval. The left column shows the numeric values for each study and summary measure.
No significant heterogeneity (P > 0.1) was detected across studies. For all analyses, the degree of heterogeneity (I2) was below 25% (Fig. 2, Fig. S1). A sensitivity analysis was performed for clinical response by excluding the two trials which did not assess febrile neutropenic patients; the risk ratio (95% CI) was 1.04 (0.99 to 1.10) in agreement with the analysis of all thirteen trials (Table 2, Fig. S3). The risk ratio (95% CI) was 1.35 (0.75 to 2.42) in the two trials with severe infections (Fig. S4) (22, 31).
A partial least-squares analysis identified the presence of tobramycin in BLAG to yield favorable results for DBL regarding microbiological response in all bacteria and microbiological response in Gram-negative bacteria. The pooled risk ratio (95% confidence interval) for the six trials with tobramycin was 1.18 (1.08, 1.32) for microbiological response in all bacteria and 1.25 (1.02, 1.52) for microbiological response in Gram-negative bacteria (Fig. S5). Thus, DBL yielded significantly better microbiological responses compared to BLAG regimens that included tobramycin. Likewise, when piperacillin was used in DBL but not in BLAG, the pooled risk ratio (95% confidence interval) for these four studies was 1.17 (0.94, 1.44) for microbiological response in all bacteria and 1.17 (0.93, 1.47) for microbiological response in Gram-negative bacteria (Fig. S6).
Assessment of bias.
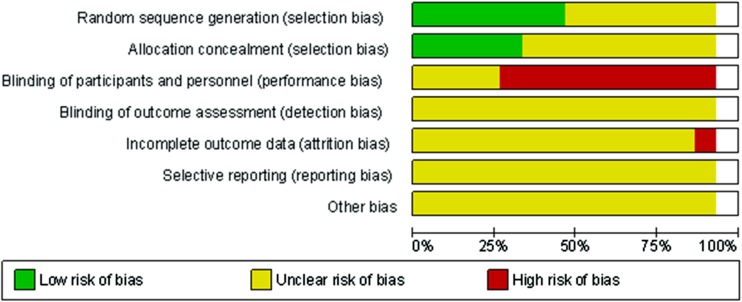
For selection bias regarding to random sequence generation and allocation concealment, slightly less than half of the studies were at low risk and the other studies at unclear risk of bias (Fig. 3). For performance bias regarding whether participants and personnel were blinded to study conditions, the majority of studies were at high risk of bias, since the dosing schedules differed. For other types of bias, such as blinding of outcome assessment, incomplete outcome data, or selective reporting, the risk of bias was unclear (Fig. 3).
FIG 3.
Risk of bias for assessment of the included studies.
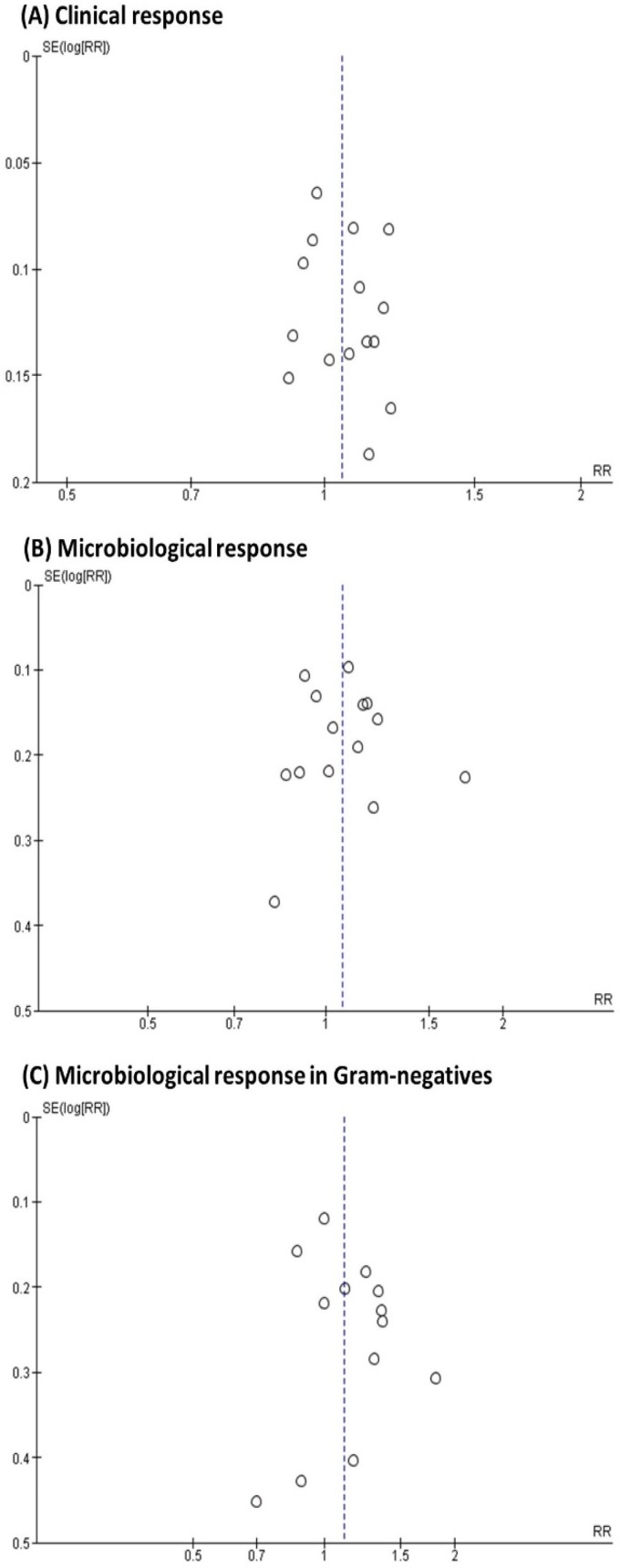
Specifically, for publication bias, symmetric patterns (i.e., visually suggesting no bias) were observed in the funnel plots for clinical response, overall microbiological response, and microbiological response in P. aeruginosa and E. coli (Fig. 4, Fig. S2). The arcsine-Thompson test showed no significant statistical publication bias for all studied outcomes, including sensitivity analyses in Gram-negative species (P = 0.246) and in Klebsiella spp. (P = 0.174). Due to zero cases for some of the groups, a continuity correction was applied by adding a value of 0.5 to the corresponding observations.
FIG 4.
Funnel plots of included trials comparing double β-lactam combinations with β-lactam plus aminoglycoside combinations for (A) clinical response, (B) microbiological response, and (C) microbiological response in specific Gram-negative species. Pooled risk ratios are indicated by the dotted line.
Safety.
Significantly lower nephrotoxicity (including renal dysfunction, serum creatinine elevation, and azotemia; P = 0.0338, n = 2,626) was observed for the DBL (6.6%) than for the BLAG group (8.8%, Table 3). Likewise, ototoxicity was significantly lower (P = 0.0137) for the DBL (0.7%) than for the BLAG group (3.1%; Table 3), as expected. Renal toxicity ranged from 2.1% to 7.4% in the four studies with cephalothin in DBL, while this risk was 16.3% to 20.8% in the two studies that combined cephalothin with an aminoglycoside (Table S3). In contrast, when ticarcillin was used in BLAG with gentamicin or tobramycin, nephrotoxicity occurred in 8 of 319 cases (2.5%); these were significantly fewer cases (Fisher’s exact test; P < 0.0001) than the 117 of 1,110 cases (10.6%) for BLAG therapy without ticarcillin. For BLAG combinations with ticarcillin or carbenicillin, nephrotoxicity occurred in 34 of 540 cases (6.2%) compared to the significantly higher risk (P = 0.009) with 91 of 879 cases (10.4%) for BLAG that contained other β-lactams.
TABLE 3.
Safety of double β-lactam compared with β-lactam plus aminoglycoside combinations in 13 published clinical trialsa
| System | Adverse drug events | Treatment |
Overall no. of cases | P valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Double β-lactam |
β-Lactam plus aminoglycoside |
||||||||
| n | Total | % | n | Total | % | ||||
| Renal | Renal dysfunction/toxicity | 19 | 292 | 7.0 | 43 | 515 | 8.3 | 807 | |
| Serum creatinine elevation | 2 | 62 | 3.2 | 0 | 65 | 0.0 | 127 | ||
| Azotemia | 66 | 739 | 9.0 | 63 | 588 | 10.7 | 1,327 | ||
| All renal events | 79 | 1,207 | 6.6 | 125 | 1,419 | 8.8 | 2,626 | 0.0338 | |
| Hearing | Ototoxicity | 3 | 411 | 0.7 | 14 | 455 | 3.1 | 866 | 0.0137 |
| Infection | Superinfection | 122 | 821 | 14.9 | 118 | 802 | 14.7 | 1,623 | |
| Colonization | 12 | 113 | 10.6 | 28 | 170 | 16.5 | 283 | ||
| Systemic | Allergy | 7 | 271 | 2.6 | 5 | 437 | 1.1 | 708 | |
| Fever | 7 | 247 | 2.8 | 3 | 248 | 1.2 | 495 | ||
| Cutaneous | Rash | 65 | 907 | 7.2 | 66 | 970 | 6.8 | 1,877 | |
| Gastrointestinal | Diarrhea | 22 | 320 | 6.9 | 20 | 335 | 6.0 | 655 | |
| Nausea | 8 | 166 | 4.8 | 10 | 166 | 6.0 | 332 | ||
| Hepatic | Liver dysfunction | 11 | 124 | 8.9 | 13 | 182 | 7.1 | 306 | |
| Liver lab value abnormal | 14 | 111 | 12.6 | 12 | 112 | 10.7 | 223 | ||
| Coagulation | Bleeding | 19 | 162 | 11.7 | 12 | 226 | 5.3 | 388 | 0.0238 |
| PT prolongation | 31 | 166 | 18.7 | 30 | 166 | 18.1 | 332 | ||
| Coagulation abnormality | 79 | 273 | 28.9 | 38 | 244 | 15.6 | 545 | 0.0003 | |
| All coagulation effects | 133 | 629 | 21.1 | 80 | 636 | 12.6 | 1,365 | 0.0001 | |
| Electrolyte | Hypokalemia | 240 | 735 | 32.7 | 224 | 880 | 25.5 | 1,615 | 0.0016 |
| Local | Phlebitis | 14 | 51 | 27.5 | 25 | 103 | 24.3 | 154 | |
The incidence of each adverse drug event was calculated from the number of pooled events divided by the total population evaluated for adverse drug events.
Fisher’s exact test (two-tailed); only P values below 0.05 were reported.
Relatively high incidences of coagulation, hypokalemia, and phlebitis were observed for both DBL and BLAG therapy. Coagulopathy was mostly observed in trials with moxalactam (moxalactam versus no moxalactam, 25% [188 of 752 cases] versus 4.9% [25 of 513 cases]; P < 0.0001; Table S4) (26–28). Hypokalemia was more common for DBL (32.7%) compared to BLAG (25.5%, P = 0.0016). Phlebitis might have been associated with the less advanced quality of early injection formulations. Incidences of superinfection, colonization, and allergy were not significantly higher in the DBL group (Table 3). Other adverse events were relatively minor and comparable between both groups.
DISCUSSION
This meta-analysis showed that empirical, nonoptimized DBL treatments achieved similar clinical and microbiological responses and significantly better safety compared with BLAG therapy (Tables 2 and 3). This is based on thirteen randomized clinical trials reported between 1972 and 1993, before broad-spectrum antibiotics were widely introduced to the clinic. This conclusion was robust in all patients and in patients with febrile neutropenia, and the same trend was observed in two trials studying patients with severe infections (Fig. S3 and S4) (22, 31).
The employed DBL covered a broad range of Gram-negative and Gram-positive species and included at least one β-lactam with antipseudomonal activity; this is still the recommendation in current guidelines for empirical therapy of febrile neutropenia (34). The DBL were chosen empirically. Thus, neither the dose and dosage regimen, nor the PBP receptor binding pattern and resistance mechanisms of the two β-lactams combined were optimized. This highlights the future potential for rationally optimized DBL therapy.
Overall, approximately half of the febrile neutropenia cases had a microbiologically confirmed bacterial infection. Favorable clinical responses may have been partially attributed to less toxicity due to the lack of an aminoglycoside. Only two studies discussed mortality, but neither reported mortality by treatment group (24, 25). The meta-analysis results were robust, considering the large number of cases (Table 2), low heterogeneity, and lack of publication bias. Sensitivity analyses of microbiological responses by pathogen were encouraging despite their relatively small sample size. Typically, antipseudomonal activity was provided by only one of the two β-lactams in DBL therapy but was (at least partially) covered by both the β-lactam and aminoglycoside in BLAG therapy. This may suggest some synergy between the β-lactams used in DBL against P. aeruginosa. Mutations in the active site of PBPs are rare (35, 36). Therefore, we expect synergy due to inactivation of optimal sets of PBPs to remain relevant, as long as the same sets of PBPs are inactivated by DBL based on contemporary β-lactams.
Nowadays, extensive molecular insights and translational pharmacokinetic/pharmacodynamic approaches are available to design and rationally optimize DBL therapy. Binding of PBP4 in P. aeruginosa has been shown to extensively and rapidly upregulate the AmpC β-lactamase (37). The PBP4 is the highest-affinity target of carbapenems, cephaloridine, and cefoxitin (14–16), as well as a high-affinity target of cephalothin and moxalactam (also called latamoxef) (17, 38) in P. aeruginosa. In eight of 13 clinical trials (Table 1), the latter two β-lactams were used in nonoptimal DBL with carbenicillin, ticarcillin, or piperacillin (i.e., β-lactams that are subject to inactivation by the AmpC β-lactamase). To minimize the impact of resistance due to the AmpC β-lactamase, it is likely important to design DBL that include at least one β-lactam that is not inactivated by AmpC. While the chromosomal AmpC β-lactamase, as well as efflux pumps in P. aeruginosa, for example, will remain relevant, many new β-lactamases have emerged and spread over the last decades. These and other new resistance mechanisms will need to be considered when designing and rationally optimizing current DBL regimens.
For microbiological response in Gram-negative species, four of five clinical trials that used ticarcillin in DBL therapy favored BLAG (as shown by risk ratios below 1.0; Fig. 2C). Since 1986, ticarcillin (and carbenicillin) were replaced by piperacillin as the antipseudomonal β-lactam (Table 1). The studies that used piperacillin in DBL but not in BLAG favored DBL therapy, with a pooled risk ratio of 1.17 both for microbiological response in all bacteria and that in Gram-negatives (Fig. S6).
The DBL achieved significantly better microbiological responses in all bacteria and in Gram-negative species compared to those for BLAG with tobramycin (Fig. S5). In these six studies, tobramycin was dosed intermittently at 5 mg/kg/day (or less) or was given as a continuous infusion at 8.9 mg/kg/day (with a small loading dose; Table 1). These tobramycin regimens were expected to yield average peak concentrations in plasma of 8.1 mg/liter or less (Table S3). In contrast, amikacin, netilmicin, and kanamycin were given at higher doses than tobramycin and thus achieved higher peaks in plasma (amikacin, 19 or 36 mg/liter; netilmicin, 18 mg/liter; and kanamycin, 14 mg/liter). However, these peak concentrations cannot be used to directly compare the effects of different aminoglycosides on bacterial killing, resistance prevention, and synergy due to enhancing the target site concentration of β-lactams or due to inhibition of protein synthesis (39–43). Future systematic studies are warranted to dissect and elucidate these mechanisms.
Our conclusions (Table 2) were in good agreement with those of two prior reviews (7, 8); however, the latter reviews did not employ a meta-analysis and assessed a smaller collection of clinical trials. Interestingly, our results also agreed with a recent clinical trial on DBL (ampicillin plus ceftriaxone) compared with ampicillin plus gentamicin against an important Gram-positive pathogen, Enterococcus faecalis (44). Encouragingly, DBL therapy has been recommended by the latest clinical guideline for E. faecalis bloodstream infections and infective endocarditis (45).
The observed safety profile for DBL therapy was generally favorable; the tested combinations had significantly lower renal and ototoxicity compared with BLAG therapy (Table 3). This is attractive for patients with impaired renal function or a risk of toxicity (e.g., due to concomitant use of nephrotoxic agents). Ototoxicity was only reported for a small number of antibiotics (Table 3). Cephalothin is an early β-lactam that is known to cause some nephrotoxicity, especially when combined with aminoglycosides (46, 47). Thus, both cephalothin and the aminoglycoside likely contributed to the overall observed nephrotoxicity. In contrast, double anionic β-lactams, such as ticarcillin and carbenicillin, are dosed as disodium salts. This entails a relatively large sodium concentration that has been shown to provide some protection against aminoglycoside-related nephrotoxicity (48). In agreement with this mechanism, BLAG combinations with ticarcillin or carbenicillin showed a significantly lower risk of nephrotoxicity compared to BLAG with other β-lactams (Table S3). Overall, these nephrotoxicity results need to be interpreted with caution, since the definitions of nephrotoxicity differed between studies.
Most of the reviewed studies from the 1970s to early 1990s (Table 1) used multiple-daily dosing of aminoglycosides, as opposed to contemporary once-daily dosing. The latter is expected to be safer (49–51). Furthermore, therapeutic drug management was likely not widely applied in the reviewed studies and might have improved safety (52–54). Thus, contemporary combination regimens with once-daily aminoglycoside dosing (with or without therapeutic drug management) may show better safety compared to that in the studies reviewed here (Table 3). Moreover, the most popular chemotherapeutic agent at that time (cisplatin) is known to cause renal toxicity and ototoxicity (55, 56). Febrile neutropenia usually occurred 7 to 10 days after anticancer chemotherapy; thus, the toxicity profile of the chemotherapeutic(s) may have overlapped with that of antibiotic therapy.
The relatively high incidence of coagulation abnormalities observed in both groups was associated with moxalactam (Table S4), and possibly also with the hematological toxicity from anticancer chemotherapeutic agents in febrile neutropenic patients. Moxalactam is no longer used clinically. Moxalactam exhibited a significantly higher likelihood of bleeding (odds ratio, 9.9) than other agents in a study with 1,493 patients who received one antibiotic for at least 3 days (57). The underlying mechanisms included inhibition of ADP-induced platelet aggregation and an interference with vitamin K-dependent hepatic metabolism of clotting factors (58, 59).
Hypokalemia might be associated with the high dose of sodium from the β-lactams and may be related to renal potassium loss following the use of β-lactam antibiotics (60). These adverse events are less common for more contemporary β-lactams. Resistance emergence is important both for DBL and BLAG therapy (61); however, only a limited amount of resistance data was published in these early clinical trials. This was in part caused by limited knowledge of the molecular resistance mechanisms for β-lactams at this time. Superinfection and bacterial colonization were reported, and no significant differences were found (Table 3).
Over the last 4 decades, both the clinically prevalent bacterial isolates and the available antibiotics have changed extensively. Thus, caution is required when translating the results from this meta-analysis to the isolates with current resistance mechanisms. While the available aminoglycosides remained unchanged since the 1980s (with exception of the recently approved plazomicin), several newer β-lactams (such as carbapenems and cephalosporins) and newer β-lactamase inhibitors have become available (20). Potential limitations of this meta-analysis further include the time frame of these early, open-label, randomized clinical trials and that some of the studied β-lactams are no longer (widely) clinically used. The quality of reporting of some of these early clinical trials resulted in an unclear risk of bias when evaluated according to contemporary standards. Furthermore, the diversity of these empirical and nonoptimized DBL and BLAG combinations did not allow us to identify optimal combinations. Future translational research should rationally optimize these combinations, leveraging latest pharmacokinetic/pharmacodynamic principles and molecular insights. However, this meta-analysis clearly showed promising safety profiles of DBL therapy in patients that support an in general favorable safety profile of rationally optimized DBL for contemporary β-lactams.
We employed several approaches to enhance the robustness of this meta-analysis. First, we only used randomized clinical trials with a BLAG control group, since BLAG therapy has a long-established status in treating patients with severe infections by Gram-negative pathogens. Second, we put great care into distinguishing the terms “episode,” “patient-trial,” and “subject.” Third, only the evaluable population was considered when calculating clinical response, whereas the overall population who received the dose was included in the safety analysis. Fourth, multiple approaches were used to evaluate microbiological responses (i.e., overall, in all Gram-negative species, and in specific Gram-negative species). Fifth, an exploratory partial least-squares analysis identified potentially influential drugs in DBL and BLAG regimens which led to sensitivity analyses that were underpinned by pharmacokinetic predictions of the aminoglycoside drug exposures.
In summary, this meta-analysis showed comparable clinical and microbiological efficacy and significantly better safety between empirically designed, nonoptimized DBL and BLAG. These conclusions are based on data from thirteen randomized controlled clinical trials. As expected, DBL showed significantly lower renal and ototoxicity compared with BLAG therapy. While empirical, nonoptimized DBL provided promising safety and efficacy, future research will need to design and rationally optimize DBL using newer β-lactams (including carbapenems) and β-lactamase inhibitors. This translational research should leverage mechanistic insights to combat contemporary MDRGN isolates. The latest findings on PBP receptor binding patterns, molecular insights on resistance mechanisms, and translational approaches now enable the rational optimization of innovative DBL dosing strategies. These optimized DBL hold excellent promise to substantially contribute to combating infections by MDRGN and warrant further systematic nonclinical and clinical evaluations.
MATERIALS AND METHODS
Search strategy. (i) Data sources.
An exhaustive literature search was performed in PubMed, Embase, ISI Web of Knowledge, and the Cochrane Central Register of records through 31 July 31 2018, with the search terms “double beta lactam,” “two beta lactam,” and “dual beta lactam.” References cited by these publications identified from this search strategy, relevant reviews, and forward citations were further evaluated.
(ii) Inclusion and exclusion criteria.
We searched for published randomized clinical trials that compared DBL with BLAG therapy. No restrictions were imposed on the patient population, clinical diagnosis, infection type, length of follow-up, or the specific β-lactams and aminoglycosides combined. All studies had to clearly define the clinical diagnosis standards and the evaluation criteria for clinical and microbiological responses in both treatment groups. Mortality data were inspected, but a lack of mortality data did not result in exclusion of the respective study. The diagnosis and treatment response criteria are summarized in the supplemental material (Tables S1 and S2) for quality assessment. Nonclinical studies that did not report clinical data were excluded. Furthermore, studies without sufficient assessment of clinical and microbiological efficacy and DBL studies which lacked a comparator group were excluded. Only studies written in English were included. Two reviewers (Y.J. and M.-J.C.) searched for and examined the identified studies independently.
Data extraction.
Three spreadsheets were developed to extract data from each study for efficacy, safety, and study characteristics. The study characteristics spreadsheet included the last name of the first author, year of publication, diagnosis for antimicrobial treatment in the population, treatment and control regimens, treatment duration, follow-up time, age, and sex. The efficacy spreadsheet included numbers of subjects with positive response and overall numbers of subject for evaluated outcomes. The safety spreadsheet included the last name of the first author, year of publication, numbers of subjects with adverse events, and overall subject number evaluated for the corresponding adverse events. Two reviewers (Y.J. and M.-J.C.) independently extracted the data from the included studies. Disagreements in interpretation were discussed by the two authors, who consulted with the other coauthors (including J.B.B. and G.L.D.) until a consensus was reached.
Outcome.
The primary outcome was clinical response in the clinically assessable population. Clinical response was determined as the improvement of clinical symptoms. Secondary outcomes were microbiological response in all bacteria and in all Gram-negative pathogens. Microbiological response was defined as eradication of pathogens present at baseline in the microbiologically assessable population. The risk ratio was chosen as statistical measure and was calculated as the percentage of clinical or microbiological response for the DBL group divided by the percentage for the BLAG group.
Overall numbers of cases (i.e., episodes, or patient-trials) and overall numbers of patients receiving treatment were provided where available (Table 1). Nevertheless, only evaluable cases were used to calculate the risk ratio for clinical and microbiological responses unless specified otherwise. Definitive nonbacterial pathogen infections (e.g., infections by fungi or virus) were excluded from the clinical assessable cases; however, cases with no evidence of bacterial infection were included (i.e., cases with clinically diagnosed bacterial infection without microbiological evidence).
To assess safety, the overall number of cases (patients) receiving the investigated regimens were taken for evaluation unless specified otherwise. All adverse events reported in the studies were recorded and classified, including superinfection and bacterial colonization. Fisher’s exact test was used to compare the incidence between two groups for each adverse event.
Analysis.
Meta-analyses were performed in the RevMan software (version 5.1) using a random-effects model. Pooled risk ratios and 95% confidence intervals (CIs) were calculated for all primary and secondary outcomes. The DerSimonian-Laird method was used to calculate the between-study variance estimator, τ2 (62). Sensitivity analyses for specific Gram-negative species (i.e., P. aeruginosa, Klebsiella spp., and E. coli) were performed.
For statistical heterogeneity, the between-study variance (τ2) was statistically tested using the Q test, and a P value below 0.10 was considered significant (63). The degree of heterogeneity was assessed by the I2 metric, which denotes the proportion of total variability in the point estimate that could be attributed to statistical heterogeneity; we classified a heterogeneity of 25% to 49% as low, 50% to 74% as moderate, and ≥75% as high. Forest plots and the heterogeneity results were presented.
Risk of bias was assessed using the RevMan software. This included selection bias, performance bias, detection bias, attrition bias, and reporting bias. Publication bias was further explored graphically by funnel plots. The arcsine-Thompson test in the R package meta (version 4.9-2) was used (64) because of its improved power in detecting publication bias for dichotomous data compared to that of other tests in studies with small sample sizes.
An exploratory analysis was performed to identify β-lactams and aminoglycosides that affected the microbiological response in all bacteria and that in Gram-negative species. This analysis was performed for β-lactams and aminoglycosides which were part of the DBL or BLAG regimens in at least three trials. The presence or absence of each drug in DBL or BLAG regimens was used as the independent variables in a partial least-squares analysis using the XLSTAT software (version 19.02; Addinsoft, Long Island City, NY); this analysis weighted the studies according to sample size. The risk ratios for microbiological response in all bacteria and microbiological response in Gram-negative species served as dependent variables. Subsequently, sensitivity analyses were performed for trials with antibiotics that were identified as influential by partial least-squares analysis.
To further inform this analysis, we calculated the average drug exposures (i.e., area under the plasma concentration-time curve from 0 to 24 h) and peak concentrations expected for the studied aminoglycoside dosage regimens based on published PK data (65–68).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by awards R01AI136803 (to J.B.B., A.L., B.M., G.L.D., and R.A.B.) and R01AI130185 (to J.B.B., A.L., G.L.D., J.D.B., and R.A.B.), as well as awards R01AI100560, R01AI063517, R21AI114508, and R01AI072219 (to R.A.B.) from the National Institute of Allergy and Infectious Diseases.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
We have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00425-19.
REFERENCES
- 1.Giske CG, Monnet DL, Cars O, Carmeli Y. 2008. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Freire-Moran L, Aronsson B, Manz C, Gyssens IC, So AD, Monnet DL, Cars O, Group E-E. 2011. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—time to react is now. Drug Resist Updat 14:118–124. doi: 10.1016/j.drup.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzouvelekis L, Markogiannakis A, Psichogiou M, Tassios P, Daikos G. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochado AR, Telzerow A, Bobonis J, Banzhaf M, Mateus A, Selkrig J, Huth E, Bassler S, Zamarreño Beas J, Zietek M, Ng N, Foerster S, Ezraty B, Py B, Barras F, Savitski MM, Bork P, Göttig S, Typas A. 2018. Species-specific activity of antibacterial drug combinations. Nature 559:259–263. doi: 10.1038/s41586-018-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahme C, Butterfield JM, Nicasio AM, Lodise TP. 2014. Dual beta-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagn Microbiol Infect Dis 80:239–259. doi: 10.1016/j.diagmicrobio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Dejace P, Klastersky J. 1986. Comparative review of combination therapy: two beta-lactams versus beta-lactam plus aminoglycoside. Am J Med 80:29–38. doi: 10.1016/0002-9343(86)90476-6. [DOI] [PubMed] [Google Scholar]
- 9.Neu HC. 1985. Amdinocillin: a novel penicillin; antibacterial activity, pharmacology and clinical use. Pharmacotherapy 5:1–10. doi: 10.1002/j.1875-9114.1985.tb04448.x. [DOI] [PubMed] [Google Scholar]
- 10.Denome SA, Elf PK, Henderson TA, Nelson DE, Young KD. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol 181:3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutaria DS, Moya B, Green KB, Kim TH, Tao X, Jiao Y, Louie A, Drusano GL, Bulitta JB. 2018. First penicillin-binding protein occupancy patterns of β-lactams and β-lactamase inhibitors in Klebsiella pneumoniae. Antimicrob Agents Chemother 62:e00282-18. doi: 10.1128/AAC.00282-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutaria DM, Nierychlewski K, Shan J, Boyce JD, Jiao Y, Tao X, Bonomo RA, Velkov T, Louie A, Drusano G, Bulitta JB. 2018. Comprehensive penicillin-binding protein (PBP) occupancy patterns of 18 drugs in Acinetobacter baumannii. ASM/ESCMID, Lisbon, Portugal. [Google Scholar]
- 13.Moya B, Dötsch A, Juan C, Blázquez J, Zamorano L, Haussler S, Oliver A. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog 5:e1000353. doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies TA, Shang W, Bush K, Flamm RK. 2008. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:1510–1512. doi: 10.1128/AAC.01529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Bhachech N, Bush K. 1995. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by β-lactamases. J Antimicrob Chemother 35:75–84. doi: 10.1093/jac/35.1.75. [DOI] [PubMed] [Google Scholar]
- 16.Liao X, Hancock R. 1997. Susceptibility to beta-lactam antibiotics of Pseudomonas aeruginosa overproducing penicillin-binding protein 3. Antimicrob Agents Chemother 41:1158–1161. doi: 10.1128/AAC.41.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez‐Tebár A, Rojo F, Montilla JC, Vázquez D. 1982. Interaction of β-lactam antibiotics with penicillin-binding proteins from Pseudomonas aeruginosa. FEMS Microbiology Lett 14:295–298. doi: 10.1111/j.1574-6968.1982.tb00016.x. [DOI] [Google Scholar]
- 18.Iyobe S, Inoue M, Watanabe M, Mitsuhashi S. 1999. Estimation of outer membrane permeability of carbapenem antibiotics to Pseudomonas aeruginosa. J Infect Chemother 5:168–170. doi: 10.1007/s101569900018. [DOI] [PubMed] [Google Scholar]
- 19.Tao X, Kim TH, Qian Y, Moya B, Zhang L, Jiao Y, Sutaria DS, Bonomo RA, Barth A, Zavascki AP, Drusano GL, Louie A, Bulitta JB. 2018. Novel cassette assay to simultaneously characterize the outer membrane permeability of five beta-lactams in Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Abstr 8462 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Madrid, Spain. [Google Scholar]
- 20.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 21.Middleman E, Watanabe A, Kaizer H, Bodey G. 1972. Antibiotic combinations for infections in neutropenic patients. Evaluation of carbenicillin plus either cephalothin or kanamycin. Cancer 30:573–579. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Klastersky J, Hensgens C, Debusscher L. 1975. Empiric therapy for cancer patients: comparative study of ticarcillin-tobramycin, ticarcillin-cephalothin, and cephalothin-tobramycin. Antimicrob Agents Chemother 7:640–645. doi: 10.1128/AAC.7.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schimpff SC, Landesman S, Hahn DM, Standiford HC, Fortner CL, Young VM, Wiernik PH. 1976. Ticarcillin in combination with cephalothin or gentamicin as empiric antibiotic therapy in granulocytopenic cancer patients. Antimicrob Agents Chemother 10:837–844. doi: 10.1128/AAC.10.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimpff SC, Gaya H, Klastersky J, Tattersall MH, Zinner SH. 1978. Three antibiotic regimens in the treatment of infection in febrile granulocytopenic patients with cancer. J Infect Dis 137:14–29. [PubMed] [Google Scholar]
- 25.Bodey GP, Ketchel SJ, Rodriguez V. 1979. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med 67:608–616. doi: 10.1016/0002-9343(79)90242-0. [DOI] [PubMed] [Google Scholar]
- 26.Winston DJ, Barnes RC, Ho WG, Young LS, Champlin RE, Gale RP. 1984. Moxalactam plus piperacillin versus moxalactam plus amikacin in febrile granulocytopenic patients. Am J Med 77:442–450. doi: 10.1016/0002-9343(84)90100-1. [DOI] [PubMed] [Google Scholar]
- 27.Fainstein V, Bodey GP, Bolivar R, Elting L, McCredie KB, Keating MJ. 1984. Moxalactam plus ticarcillin or tobramycin for treatment of febrile episodes in neutropenic cancer patients. Arch Intern Med 144:1766–1770. doi: 10.1001/archinte.1984.00350210078014. [DOI] [PubMed] [Google Scholar]
- 28.Feld R, Louie TJ, Mandell L, Bow EJ, Robson HG, Chow A, Belch A, Miedzinski L, Rachlis A, Pater J. 1985. A multicenter comparative trial of tobramycin and ticarcillin vs moxalactam and ticarcillin in febrile neutropenic patients. Arch Intern Med 145:1083–1088. doi: 10.1001/archinte.1985.00360060149023. [DOI] [PubMed] [Google Scholar]
- 29.De Jongh C, Joshi J, Thompson B, Newman K, Finley R, Moody M, Salvatore P, Tenney J, Drusano G, Schimpff S. 1986. A double beta-lactam combination versus an aminoglycoside-containing regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Am J Med 80:101–111. [PubMed] [Google Scholar]
- 30.Rotstein C, Cimino M, Winkey K, Cesari C, Fenner J. 1988. Cefoperazone plus piperacillin versus mezlocillin plus tobramycin as empiric therapy for febrile episodes in neutropenic patients. Am J Med 85:36–43. doi: 10.1016/0002-9343(88)90173-8. [DOI] [PubMed] [Google Scholar]
- 31.Torres A, de Celts R, Rabinad E, Marco F, Almela M, Deulofeu R, Rodriguez-Roisin R, Vidal A. 1989. Therapeutic efficacy of the combination of aztreonam with cefotaxime in the treatment of severe nosocomial pneumonia. Chemotherapy 35:15–24. doi: 10.1159/000238716. [DOI] [PubMed] [Google Scholar]
- 32.Kibbler CC, Prentice HG, Sage RJ, Hoffbrand AV, Brenner MK, Mannan P, Warner P, Bhamra A, Noone P. 1989. A comparison of double β-lactam combinations with netilmicin/ureidopenicillin regimens in the empirical therapy of febrile neutropenic patients. J Antimicrob Chemother 23:759–771. doi: 10.1093/jac/23.5.759. [DOI] [PubMed] [Google Scholar]
- 33.Joshi JH, Newman KA, Brown BW, Finley RS, Ruxer RL, Moody MA, Schimpff SC. 1993. Double β-lactam regimen compared to an aminoglycoside/β-lactam regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Support Care Cancer 1:186–194. doi: 10.1007/BF00366445. [DOI] [PubMed] [Google Scholar]
- 34.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-A, Wingard JR. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 35.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 36.Cayô R, Rodríguez M-C, Espinal P, Fernández-Cuenca F, Ocampo-Sosa AA, Pascual A, Ayala JA, Vila J, Martínez-Martínez L. 2011. Analysis of genes encoding penicillin-binding proteins in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 55:5907–5913. doi: 10.1128/AAC.00459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders CC. 1983. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis 147:585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- 38.Livermore D. 1984. Penicillin-binding proteins, porins and outer-membrane permeability of carbenicillin-resistant and-susceptible strains of Pseudomonas aeruginosa. J Med Microbiol 18:261–270. doi: 10.1099/00222615-18-2-261. [DOI] [PubMed] [Google Scholar]
- 39.Drusano GL, Bonomo RA, Bahniuk N, Bulitta JB, Vanscoy B, Defiglio H, Fikes S, Brown D, Drawz SM, Kulawy R, Louie A. 2012. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:231–242. doi: 10.1128/AAC.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav R, Landersdorfer CB, Nation RL, Boyce JD, Bulitta JB. 2015. Novel approach to optimize synergistic carbapenem-aminoglycoside combinations against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 59:2286–2298. doi: 10.1128/AAC.04379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav R, Bulitta JB, Schneider EK, Shin BS, Velkov T, Nation RL, Landersdorfer CB. 2017. Aminoglycoside concentrations required for synergy with carbapenems against Pseudomonas aeruginosa determined via mechanistic studies and modeling. Antimicrob Agents Chemother 61:e00722-17. doi: 10.1128/AAC.00722-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zavascki AP, Klee BO, Bulitta JB. 2017. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15:519–526. doi: 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- 43.Bulitta JB, Ly NS, Landersdorfer CB, Wanigaratne NA, Velkov T, Yadav R, Oliver A, Martin L, Shin BS, Forrest A, Tsuji BT. 2015. Two mechanisms of killing of Pseudomonas aeruginosa by tobramycin assessed at multiple inocula via mechanism-based modeling. Antimicrob Agents Chemother 59:2315–2327. doi: 10.1128/AAC.04099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Hidalgo N, Almirante B, Gavaldà J, Gurgui M, Peña C, de Alarcón A, Ruiz J, Vilacosta I, Montejo M, Vallejo N, López-Medrano F, Plata A, López J, Hidalgo-Tenorio C, Gálvez J, Sáez C, Lomas JM, Falcone M, de la Torre J, Martínez-Lacasa X, Pahissa A. 2013. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 56:1261–1268. doi: 10.1093/cid/cit052. [DOI] [PubMed] [Google Scholar]
- 45.Beganovic M, Luther MK, Rice LB, Arias CA, Rybak MJ, LaPlante KL. 2018. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 67:303–309. doi: 10.1093/cid/ciy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabanillas F, Burgos RC, Rodriguez RC, Baldizón C. 1975. Nephrotoxicity of combined cephalothin-gentamicin regimen. Arch Intern Med 135:850–852. doi: 10.1001/archinte.1975.00330060094013. [DOI] [PubMed] [Google Scholar]
- 47.Wade J, Petty B, Conrad G, Smith C, Lipsky J, Ellner J, Lietman P. 1978. Cephalothin plus an aminoglycoside is more nephrotoxic than methicillin plus an aminoglycoside. Lancet 2:604–606. [DOI] [PubMed] [Google Scholar]
- 48.Sabra R, Branch RA. 1990. Role of sodium in protection by extended-spectrum penicillins against tobramycin-induced nephrotoxicity. Antimicrob Agents Chemother 34:1020–1025. doi: 10.1128/AAC.34.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barclay ML, Kirkpatrick CM, Begg EJ. 1999. Once daily aminoglycoside therapy. Clin Pharmacokinet 36:89–98. doi: 10.2165/00003088-199936020-00001. [DOI] [PubMed] [Google Scholar]
- 50.Freeman CD, Nicolau D, Belliveau P, Nightingale C. 1997. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J Antimicrob Chemother 39:677–686. doi: 10.1093/jac/39.6.677. [DOI] [PubMed] [Google Scholar]
- 51.Begg EJ, Barclay ML, Duffull SB. 1995. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol 39:605–609. doi: 10.1111/j.1365-2125.1995.tb05719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Begg EJ, Barclay ML, Kirkpatrick CJ. 1999. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 47:23–30. doi: 10.1046/j.1365-2125.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkins A, Thomson AH, Brown NM, Semple Y, Sluman C, MacGowan A, Lovering AM, Wiffen PJ. 2016. Amikacin use and therapeutic drug monitoring in adults: do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J Antimicrob Chemother 71:2754–2759. doi: 10.1093/jac/dkw250. [DOI] [PubMed] [Google Scholar]
- 54.Turnidge J. 2003. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 17:503–528. doi: 10.1016/S0891-5520(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 55.Yao X, Panichpisal K, Kurtzman N, Nugent K. 2007. Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 56.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. 2007. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res 226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Brown RB, Klar J, Lemeshow S, Teres D, Pastides H, Sands M. 1986. Enhanced bleeding with cefoxitin or moxalactam: statistical analysis within a defined population of 1493 patients. Arch Intern Med 146:2159–2164. doi: 10.1001/archinte.1986.00360230079013. [DOI] [PubMed] [Google Scholar]
- 58.Bang NU, Tessler SS, Heidenreich RO, Marks CA, Mattler LE. 1982. Effects of moxalactam on blood coagulation and platelet function. Clin Infect Dis 4:S546–S554. doi: 10.1093/clinids/4.Supplement_3.S546. [DOI] [PubMed] [Google Scholar]
- 59.Graninger W, Kurz R, Havel M, Horcher E, Müller M. 1987. Effect of cefotaxime, ceftriaxone and latamoxef on blood coagulation in patients on parenteral nutrition. Chemotherapy 33:452–458. doi: 10.1159/000238535. [DOI] [PubMed] [Google Scholar]
- 60.Veltri KT, Mason C. 2015. Medication-induced hypokalemia. P T 40:185. [PMC free article] [PubMed] [Google Scholar]
- 61.Milatovic D, Braveny I. 1987. Development of resistance during antibiotic therapy. Eur J Clin Microbiol 6:234–244. doi: 10.1007/BF02017607. [DOI] [PubMed] [Google Scholar]
- 62.DerSimonian R, Kacker R. 2007. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarzer G. 2007. meta: an R package for meta-analysis. R News 7:40–45. [Google Scholar]
- 65.Cabana BE, Taggart JG. 1973. Comparative pharmacokinetics of BB-K8 and kanamycin in dogs and humans. Antimicrob Agents Chemother 3:478–483. doi: 10.1128/AAC.3.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Der Auwera PV. 1991. Pharmacokinetic evaluation of single daily dose amikacin. J Antimicrobial Chemotherapy 27:63–71. doi: 10.1093/jac/27.suppl_C.63. [DOI] [PubMed] [Google Scholar]
- 67.Humbert G, Leroy A, Fillastre J, Oksenhendler G. 1978. Pharmacokinetics of netilmicin in the presence of normal or impaired renal function. Antimicrob Agents Chemother 14:40–44. doi: 10.1128/AAC.14.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lode H, Kemmerich B, Koeppe P. 1975. Comparative clinical pharmacology of gentamicin, sisomicin, and tobramycin. Antimicrob Agents Chemother 8:396–401. doi: 10.1128/AAC.8.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.