The rapid dissemination of the macrolide resistance gene erm(B) will likely compromise the efficacy of macrolides as the treatment of choice for campylobacteriosis. More importantly, erm(B) is always associated with several multidrug resistance genomic islands (MDRGIs), which confer resistance to multiple other antimicrobials.
KEYWORDS: Campylobacter, MDRGIs, erm(B), macrolide resistance
ABSTRACT
The rapid dissemination of the macrolide resistance gene erm(B) will likely compromise the efficacy of macrolides as the treatment of choice for campylobacteriosis. More importantly, erm(B) is always associated with several multidrug resistance genomic islands (MDRGIs), which confer resistance to multiple other antimicrobials. Continuous monitoring of the emergence of erm(B) and analysis of its associated genetic environments are crucial for our understanding of macrolide resistance in Campylobacter. In this study, 290 Campylobacter isolates (216 Campylobacter coli isolates and 74 Campylobacter jejuni isolates) were obtained from 1,039 fecal samples collected in 2016 from pigs and chickens from three regions of China (344 samples from Guangdong, 335 samples from Shanghai, and 360 samples from Shandong). Overall, 74 isolates (72 C. coli isolates and 2 C. jejuni isolates) were PCR positive for erm(B). Combined with data from previous years, we observed a trend of increasing prevalence of erm(B) in C. coli. Pulsed-field gel electrophoresis analyses suggested that both clonal expansion and horizontal transmission were involved in the dissemination of erm(B) in C. coli, and three novel types of erm(B)-associated MDRGIs were identified among the isolates. Furthermore, 2 erm(B)-harboring C. jejuni isolates also contained an aminoglycoside resistance genomic island and a multidrug-resistance-enhancing efflux pump, encoded by RE-cmeABC. Antimicrobial susceptibility testing showed that most of the isolates were resistant to all clinically important antimicrobial agents used for the treatment of campylobacteriosis. These findings suggest that the increasing prevalence of erm(B)-associated MDRGIs might further limit treatment options for campylobacteriosis.
INTRODUCTION
Campylobacter species, especially Campylobacter jejuni and Campylobacter coli, are the leading bacterial foodborne pathogens (1). Therefore, the World Health Organization (WHO) has listed Campylobacter as one of four key causes of diarrheal diseases worldwide (2). In clinical settings, macrolides are the drugs of choice for the treatment of campylobacteriosis (3); however, the widespread use of antimicrobial agents in medical science and veterinary practice has hastened the emergence of macrolide resistance among Campylobacter strains in the past few years (4–6). Tylosin, tilmicosin, tulathromycin, and the new drug tildipirosin are macrolides that have been widely used in the livestock and poultry industries in China, Europe, and the United States (7, 8). Macrolide resistance rates are much higher in China than in most developed countries, in which rates have remained below 10% (6, 9–11). The three major mechanisms of macrolide resistance in Campylobacter include mutations in target genes (23S rRNA and ribosomal proteins L4 and L22), antibiotic efflux pumps (CmeABC and resistance-enhancing CmeABC [RE-CmeABC]), and ribosomal methylation carried out by an erm(B)-encoded ribosomal methylase (12, 13). The erm(B)-based mechanism is of particular concern because the gene is horizontally transferable among Campylobacter strains and confers high-level resistance (14). Additionally, most erm(B) loci in Campylobacter are associated with multidrug resistance genomic islands (MDRGIs), which mediate resistance to multiple classes of antibiotics, including aminoglycosides, tetracyclines, and fosfomycin (15).
Interestingly, worldwide macrolide resistance rates for C. jejuni isolates are much lower than those for C. coli (16). Similarly, while nine classes of erm(B)-harboring MDRGIs have been detected in Campylobacter strains from China, Spain, and the United States, only two of those classes (types VII and IX) were detected in C. jejuni, with the remaining seven classes all being identified in C. coli isolates (15, 17, 18). Of note, novel erm(B) genetic environments are continually emerging in Campylobacter. Therefore, determining the structure of erm(B)-harboring MDRGIs is key to understanding the mechanisms of resistance gene transmission in erm(B)-carrying isolates.
In this study, we analyzed the prevalence of erm(B)-harboring MDRGIs in Campylobacter strains isolated from pigs and chickens in Guangzhou, Shanghai, and Shandong, China, in 2016. We identified three novel erm(B)-harboring MDRGI types in Campylobacter isolates from chickens, with one of the types, designated type X, being identified in 2 C. jejuni isolates. Sequence analysis revealed that those 2 isolates not only contained the erm(B)-harboring MDRGI but also harbored aminoglycoside resistance genomic islands (ARGIs) and the enhanced multidrug efflux pump-encoding locus RE-cmeABC (19). Thus, the isolates exhibited resistance to all antibiotics commonly used for clinical treatment of Campylobacter infections, including macrolides, fluoroquinolones, aminoglycosides, tetracyclines, lincosamides, and amphenicols.
RESULTS AND DISCUSSION
Prevalence of erm(B) in Campylobacter.
A total of 290 Campylobacter isolates (216 C. coli isolates and 74 C. jejuni isolates) were obtained from 1,039 samples collected from the three regions of China (344 samples from Guangdong, 335 samples from Shanghai, and 360 samples from Shandong). Seventy-four erm(B)-positive isolates, including 72 C. coli isolates and 2 C. jejuni isolates, were isolated from chicken samples, while no erm(B)-positive isolates were obtained from swine samples (Table 1). The percentages of erm(B)-positive Campylobacter isolates from the three regions are shown in Table 1. Compared with our previous study examining Campylobacter isolates collected between 2013 and 2016 (20), the prevalence of erm(B) among C. coli isolates from Guangdong Province increased from 4.2% (6/144 isolates) in 2013 to 37.3% (57/153 isolates) in 2016. A chi-square test revealed a significant linear trend in prevalence from 2013 through 2016 (P < 0.001), while the prevalence rate among isolates from Shanghai significantly increased from 5.9% (6/102 isolates) in 2015 to 25.9% (15/58 isolates) in 2016 (chi-square test, P < 0.001). The prevalence of erm(B) among isolates from Shandong Province remained low across the examined time period (chi-square test, P > 0.05). These results strongly support the hypothesis that erm(B)-positive Campylobacter strains are more extensively disseminated in Guangdong Province, particularly as both erm(B)-positive C. jejuni isolates were obtained from samples from Guangdong.
TABLE 1.
Prevalence of erm(B) in Campylobacter isolates from livestock and poultry collected over 4 successive years (2013 to 2016) (20)a
| Location and year | No. of samples | No. of isolates (% [95% CI]) |
|||
|---|---|---|---|---|---|
| C. coli | erm(B) in C. coli | C. jejuni | erm(B) in C. jejuni | ||
| Guangdong | |||||
| 2013 | 524 | 144 (27.5 [23.7–31.5]) | 6 (4.2 [1.5–8.8]) | 16 (3.1 [1.8–4.9]) | 0 |
| 2014 | 176 | 119 (67.6 [60.2–74.5]) | 28 (23.5 [16.2–32.2]) | 16 (9.1 [5.3–14.3]) | 0 |
| 2015 | 250 | 132 (52.8 [46.4–59.1]) | 31 (23.5 [16.5–31.6]) | 4 (1.6 [0.4–4.0]) | 0 |
| 2016 | 344 | 153 (44.5 [39.1–49.9]) | 57 (37.3 [29.6–45.4]) | 17 (4.9 [2.9–7.8]) | 2 (11.8 [1.5–36.4]) |
| Shanghai | |||||
| 2013 | 490 | 105 (21.4 [17.9–25.3]) | 4 (3.8 [1.0–9.5]) | 27 (5.5 [3.7–7.9]) | 0 |
| 2014 | 163 | 104 (63.8 [55.9–71.2]) | 3 (2.9 [0.6–8.2]) | 25 (15.3 [10.2–21.8]) | 0 |
| 2015 | 467 | 102 (21.8 [18.2–25.9]) | 6 (5.9 [2.2–12.4]) | 32 (6.9 [4.7–9.5]) | 0 |
| 2016 | 335 | 58 (17.3 [13.4–21.8]) | 15 (25.9 [15.3–39.0]) | 11 (3.3 [1.7–5.8]) | 0 |
| Shandong | |||||
| 2013 | 460 | 140 (30.4 [26.3–34.9]) | 3 (2.1 [0.4–6.1]) | 142 (30.9 [26.7–35.3]) | 0 |
| 2014 | 431 | 163 (37.8 [33.2 42.6]) | 4 (2.5 [0.7–6.2]) | 1 (0.2 [0–1.3]) | 0 |
| 2015 | 417 | 98 (23.5 [19.5–27.9]) | 0 | 0 | 0 |
| 2016 | 360 | 5 (1.4 [0.5–3.2]) | 0 | 46 (12.8 [9.5–16.7]) | 0 |
| Total | |||||
| 2013 | 1,474 | 389 (26.4 [24.2–28.7]) | 13 (3.3 [1.8–5.6]) | 185 (12.6 [10.9–14.4]) | 0 |
| 2014 | 770 | 386 (50.1 [46.5–53.7]) | 35 (9.1 [6.4–12.4]) | 42 (5.5 [4.0–7.3]) | 0 |
| 2015 | 1,134 | 332 (29.3 [26.6–32.0]) | 37 (11.1 [8.0–15.0]) | 36 (3.2 [2.2–4.4]) | 0 |
| 2016 | 1,039 | 216 (20.8 [18.4–23.4]) | 72 (33.3 [27.1–40.0]) | 74 (7.1 [5.6–8.9]) | 2 (2.7 [0.3–9.4]) |
| All | 4,417 | 1,323 (29.9 [28.6–31.3]) | 157 (11.9 [10.2–13.7]) | 337 (7.6 [6.9–8.5]) | 2 (0.6 [0.07–1.1]) |
Data from both our previous study and the current study are included. Bold type indicates data from the current study.
Genotyping.
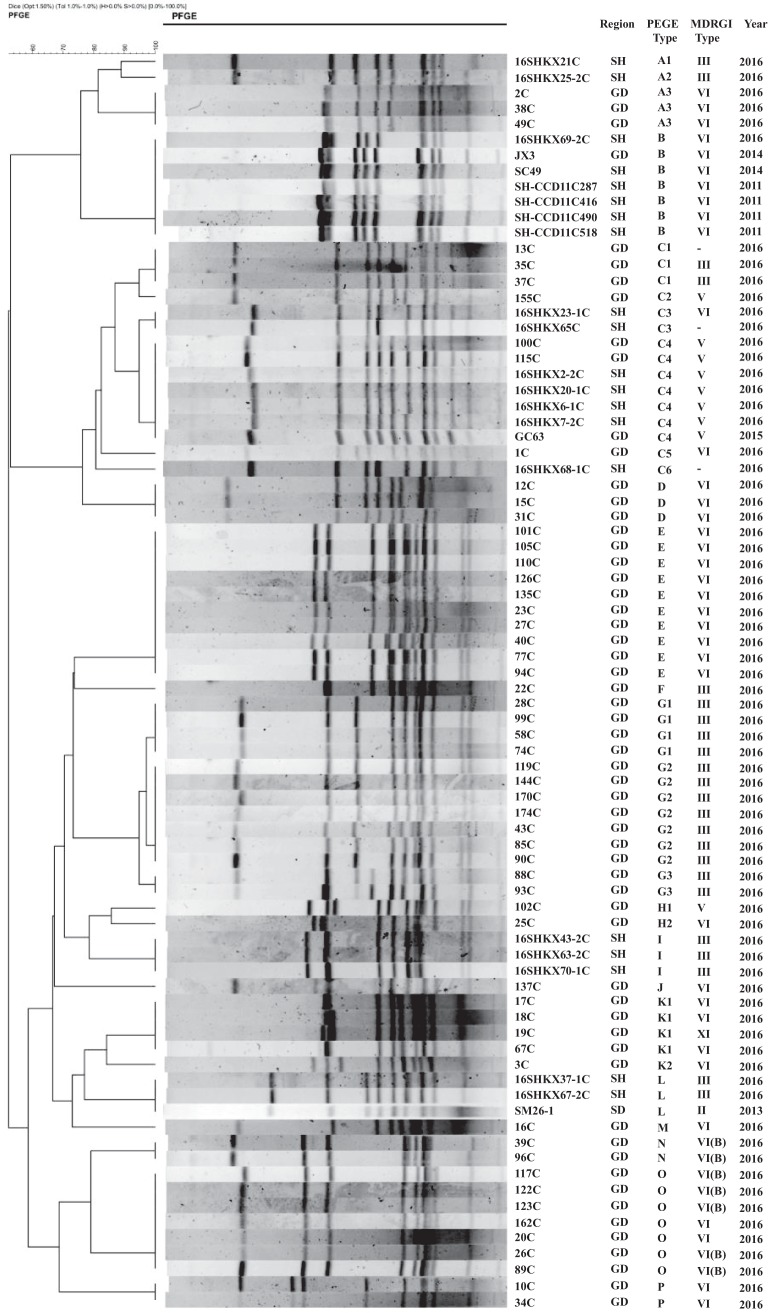
Pulsed-field gel electrophoresis (PFGE) analysis was performed on the 72 erm(B)-positive C. coli isolates and 2 erm(B)-positive C. jejuni isolates collected in 2016. We also analyzed 4 human isolates obtained from clinical gastroenteritis cases in Shanghai in 2011 and 1 reference isolate each from pigs and chickens collected from Shanghai, Guangdong, and Shandong in 2013 and 2014 in our previous study (15, 20). Using a cutoff value of 80% pattern similarity, the erm(B)-positive C. coli isolates were clustered into 16 PFGE patterns, including 3 unique patterns and 13 clusters (Fig. 1). The isolates from Guangdong showed the greatest number of common patterns (14 patterns, including 3 unique patterns), while isolates from Shanghai showed only 5 patterns. Fewer patterns were identified than in our previous study, with 4 predominant clones (C, E, G, and O) accounting for the majority of isolates (62.5% [45/72 isolates]). Interestingly, strain 16SHKX69-2C, isolated from Shanghai, showed PFGE pattern B and had 100% homology to animal and human isolates collected from Shanghai in 2011 and 2014 and to animal isolates collected from Guangdong in 2014. This finding is consistent with our previous study showing that erm(B) was disseminated among Campylobacter isolates from humans and food-producing animals (20). Additionally, the 2 C. jejuni isolates, 8C and 36C, showed 100% homology, indicating that they belonged to the same clonal type (see Fig. S1 in the supplemental material). These results suggest that both regional expansion of a particular clone and horizontal transmission were involved in the dissemination of erm(B) among Campylobacter strains, although clonal expansion appears to have accounted for a larger proportion of the dissemination.
FIG 1.
PFGE typing of erm(B)-positive C. coli isolates. Seventy-two isolates were collected in 2016, while 8 reference isolates were collected between 2011 and 2015. SmaI was used for PFGE analysis. Regions included Shandong (SD), Shanghai (SH), and Guangdong (GD). A minus sign indicates that the MDRGI type could not be assigned.
Identification of erm(B)-harboring MDRGIs.
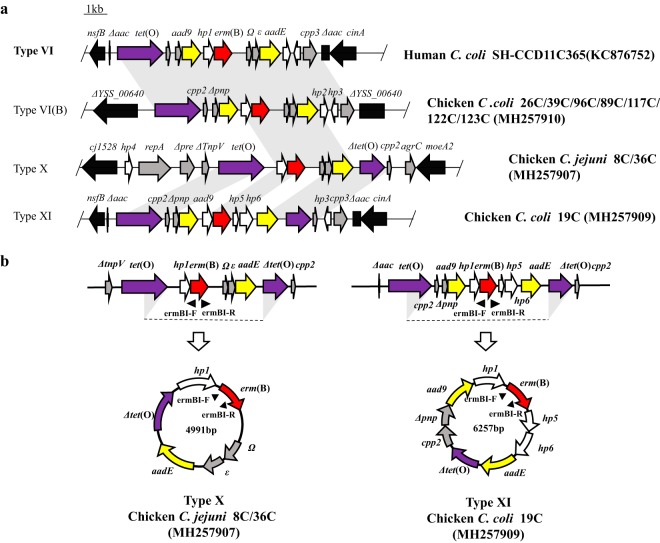
Long-range PCR assays based on integration sites and the genetic environment in 8 different MDRGIs identified in erm(B)-positive Campylobacter isolates were performed as described previously (21). Sequencing of the PCR products revealed that 40.54% of the erm(B)-positive isolates (30/74 isolates) contained regions that resembled a type VI MDRGI (>98% identity), while type III MDRGIs were detected in 23 C. coli isolates (31.08% [23/74 isolates]). Only 8 isolates contained a type V MDRGI, which was the predominant type in our previous study (20). It is noteworthy that types VI and III were identified as the predominant MDRGI types in this study, as this is consistent with the profiles of erm(B)-positive Campylobacter isolates from humans (15, 22). Moreover, PFGE pattern B, which was detected in isolates from animals and humans from different regions and years, was associated with the type VI MDRGI (Fig. 2a). These findings suggest that type VI MDRGIs may be frequently transferred between animal- and human-derived Campylobacter strains.
FIG 2.
(a) Chromosomal organization and comparison of three novel MDRGI types in erm(B)-positive C. coli isolates. erm(B) is in red, aminoglycoside resistance genes are in yellow, tetracycline resistance gene tet(O) is in purple, genes with predicted functions are in gray, and genes coding for hypothetical proteins are in white. Genes flanking the MDRGIs are depicted by black arrows. Gray shading indicates regions with >98% nucleotide sequence identity. Representative strains for each type of MDRGI are indicated at the right. (b) Circular products. Circular products were detected by inverse PCR, while sequence analysis confirmed that the intermediates of both the type X and type XI MDRGIs contained the regions between the two triangular shadows for tet(O).
Overall, 11 C. coli isolates contained MDRGIs that did not belong to any currently recognized type. Therefore, we used Illumina HiSeq sequencing and primer walking to obtain the sequences of the complete MDRGI segments. Sequence alignment revealed that erm(B) was associated with MDRGIs in the 11 C. coli isolates. In 8 of the isolates, we identified the genetic boundaries of the MDRGIs in the chromosome; however, we were unable to determine the complete genetic environment of the MDRGIs in the remaining 3 isolates. Two novel classes of MDRGIs were detected in the C. coli isolates. Interestingly, an 8,870-bp segment showing 100% nucleotide sequence identity to type VI MDRGIs was inserted into filamentous-hemagglutinin-encoding gene YSS_00640 in 7 of the C. coli isolates. Thus, we designated this novel MDRGI type subtype VI(B). In C. coli isolate 19C, the erm(B) gene was located between nfsB and cinA, as is observed in MDRGI types V and VI. Therefore, the entire 10,541-bp segment in this isolate was designated MDRGI type XI. BLAST analysis revealed that the two MDRGIs also shared a common origin of similar nucleotide sequences with Gram-positive bacteria, implying that the two MDRGIs in Campylobacter were derived from Gram-positive bacteria, as described previously (15). Furthermore, the type VI(B) and type XI MDRGIs were flanked by identical conserved genes or had the same gene arrangement as type VI MDRGIs, the predominant type in human- and animal-derived Campylobacter isolates, hinting at the possibility that these MDRGIs are stably transferrable and highly adaptable.
Characterization of erm(B)-carrying C. jejuni isolates.
To determine the genetic environment of erm(B) in C. jejuni isolates 8C and 36C, we sequenced and analyzed the genomes of the isolates using a hybrid PacBio (8C only) and Illumina (8C and 36C) sequencing approach. Sequence analysis indicated that the two isolates belonged to the same epidemic clonal lineage. In both isolates, erm(B) was located within an 11,766-bp MDRGI (type X) on the chromosome, which was inserted into the intergenic region between cj1528 and moeA2 (Fig. 2a). The MDRGIs had a G+C content of 38.9%, similar to those of other MDRGIs but higher than that of the genome of reference C. jejuni strain NTCT 11168 (G+C content of 30.5%). The MDRGI contained 10 complete open reading frames (ORFs) and 3 truncated ORFs, 3 of which corresponded to antibiotic resistance genes, including tet(O), erm(B), and aadE. Intriguingly, repA, which was located upstream of a complete tet(O) locus, shared a high degree of homology (60% coverage and 99% identity) with the corresponding gene in a type VII MDRGI from another C. jejuni isolate (23). It is noteworthy that, since the first report of erm(B) in Campylobacter (14), novel genetic environments have continuously been described. Analysis of all published studies of erm(B) in Campylobacter (14, 15, 17, 18, 22–25) shows that type VI and type III are the predominant MDRGI types in this genus, with both types consistently being represented in isolates from humans and animals. This finding suggests that these two MDRGI types are widely disseminated among Campylobacter isolates (Table 2).
TABLE 2.
Types of erm(B)-harboring MDRGIs in Campylobacter isolates from all reported studies
| MDRGI type | No. of isolates belonging to type [% of erm(B)-positive isolates] |
||||
|---|---|---|---|---|---|
| This study, animal | Previous studiesa
|
Total |
|||
| Animal | Human | Animal | Human | ||
| Totalb | 74 | 153 | 28 | 227 | 28 |
| I | 0 | 2 (1.3) | 0 | 2 (0.9) | 0 |
| II | 0 | 2 (1.3) | 0 | 2 (0.9) | 0 |
| III | 23 (31.1) | 20 (13.1) | 2 (7.1) | 43 (18.9) | 2 (7.1) |
| IV | 0 | 0 | 1 (3.6) | 0 | 1 (3.6) |
| V | 8 (10.8) | 44 (28.8) | 1 (3.6) | 52 (22.9) | 1 (3.6) |
| VI | 30 (40.5) | 30 (19.6) | 9 (32.1) | 60 (26.4) | 9 (32.1) |
| VI(B) | 7 (9.5) | 0 | 0 | 7 (3.1) | 0 |
| VII | 0 | 1 (0.7) | 0 | 1 (0.4) | 0 |
| VIII | 0 | 1 (0.7) | 0 | 1 (0.4) | 0 |
| IX | 0 | 0 | 1 (3.6) | 0 | 1 (3.6) |
| X | 2 (2.7) | 0 | 0 | 2 (0.9) | 0 |
| XI | 1 (1.4) | 0 | 0 | 1 (0.4) | 0 |
| Unknown | 3 (4.1) | 44 (28.8) | 4 (14.3) | 47 (20.7) | 4 (14.3) |
Sequence analysis of C. jejuni isolates 8C and 36C showed that, along with an erm(B)-harboring MDRGI, both isolates contained an ARGI and the enhanced multidrug efflux pump-encoding locus RE-cmeABC. The ARGI showed 99% nucleotide sequence identity to the ARGI in C. coli strain HS11B (which was also isolated from Guangdong Province) and was flanked by cj0073c and cj0072c (GenBank accession number MH257908) in both isolates (Fig. S2) (26). The horizontally transferable enhanced multidrug efflux pump gene RE-cmeABC, which was first identified in 2016, mediates resistance to multiple classes of antibiotics, including fluoroquinolones, amphenicols, tetracyclines, and macrolides (19). Thus, C. jejuni isolates 8C and 36C contained a multitude of antimicrobial resistance genes, including RE-cmeABC, erm(B), tet(O), aadE, cat, aphA3, aad9, aph2, aadA, aac, and aph(2ʺ)-If, and exhibited high-level resistance to all major antimicrobials, including fluoroquinolone, macrolide, tetracycline, and aminoglycoside drugs that are currently being used in the clinical treatment of Campylobacter infections (Table S1) (27). Therefore, these Campylobacter isolates could be considered “superbugs” and represent a significant threat to public health.
Mode of horizontal transfer of erm(B) in Campylobacter.
To assess the transferability of the novel MDRGIs, natural transformation and reverse PCR were performed. Although we were unable to obtain transformants after repeated attempts, two circular intermediates were detected, i.e., a 4,991-bp circular intermediate in C. jejuni isolates 8C and 36C and a 6,257-bp intermediate in C. coli 19C (Fig. 2b). In all cases, the formation of the circular intermediates occurred via the two tet(O) direct repeats (one complete gene and one truncated gene) located at the ends of the MDRGIs. The presence of the circular intermediate in C. jejuni 8C/36C and C. coli 19C indicates that erm(B) could change its location in the chromosome along with other resistance genes, such as aadE, aad9, and Δtet(O). Upon integration of the circular intermediate into a plasmid, erm(B) could be disseminated via the plasmid and could be coselected by streptomycin and tetracycline. Additionally, because the circular intermediate is formed by recombination between the tet(O) genes, it is likely to be able to integrate into other plasmids or chromosomal regions containing homologous sequences. tet(O) is one of the most prevalent resistance genes in Campylobacter; therefore, tet(O) may serve as a key integration site for horizontal transfer of other resistance genes, as well as increasing the risk of erm(B) acquisition (28).
In summary, this study investigated the prevalence of erm(B) in Campylobacter isolates from pigs and chickens in China and then classified the erm(B)-carrying MDRGIs. The results confirmed that erm(B) is widely prevalent among Campylobacter strains in Guangdong Province, with an obvious trend of increasing prevalence over the past few years. PFGE analysis suggested that both clonal expansion and horizontal transmission were involved in the dissemination of erm(B) in Campylobacter, although clonal expansion accounted for a greater proportion of the dissemination. Examination of the genetic environment of the erm(B) genes showed that type VI and type III MDRGIs are the predominant types in isolates from both humans and animals, hinting at the possibility of wide dissemination of these MDRGIs among Campylobacter isolates. We also identified three novel erm(B)-carrying MDRGIs, two of which were found in C. coli isolates, while one was identified in a C. jejuni isolate. Significantly, this appears to be the first report of the coexistence of erm(B)-harboring MDRGIs, ARGIs, and RE-cmeABC in C. jejuni isolated from chickens. These isolates were resistant to all antimicrobial agents frequently used in clinical therapy of campylobacteriosis and thus pose a significant threat to public health.
MATERIALS AND METHODS
Campylobacter isolates and detection of erm(B).
In total, 290 Campylobacter isolates (74 C. jejuni isolates and 216 C. coli isolates) were isolated from 1,039 chicken cecal and swine fecal samples collected from Shanghai and from Guangdong and Shandong provinces in 2016. Detailed information on the isolates is provided in Table S1 in the supplemental material. All Campylobacter isolates were cultured on Mueller-Hinton agar (Oxoid, Hampshire, UK) at 42°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2). All isolates were subsequently screened for erm(B) by PCR, as described previously (15), and the resulting PCR products were sequenced. Data regarding erm(B)-positive isolates recorded in previous studies were also obtained, to allow composite analysis. Collected data included prevalence, PFGE profiling, and MDRGI clustering information (14, 15, 17, 18, 22–25).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing of erm(B)-carrying Campylobacter isolates was carried out using the agar dilution method, as described by the Clinical and Laboratory Standards Institute and the National Antimicrobial Resistance Monitoring System (29, 30). The Campylobacter isolates were tested to determine their resistance to six classes of antimicrobials, including macrolides, lincosamides, aminoglycosides, tetracycline, fluoroquinolone, and amphenicol. C. jejuni ATCC 33560 was used as the quality control strain.
Molecular typing.
All erm(B)-positive Campylobacter isolates were genotyped by PFGE, which was conducted with a CHEF-DR III apparatus (Bio-Rad Laboratories, Hercules, CA, USA) according to the protocol for Campylobacter (31). Genomic DNA extracted from the Campylobacter isolates was digested with SmaI, while XbaI-digested genomic DNA from Salmonella enterica serotype Braenderup strain H9812 was used as the reference marker. Results were analyzed according to the Dice coefficient method, using InfoQuest FP version 4.5 (Bio-Rad Laboratories).
Identification of MDRGIs and whole-genome sequencing.
Except for type IX (17), which may have been located within a plasmid, all classes of erm(B)-carrying MDRGIs in Campylobacter (types I to VIII) have been chromosomally located. PCR primers, the sequences of which are listed in Table S2, were designed to amplify all type I to VIII MDRGIs. Because of difficulties with obtaining complete sequences, erm(B) primers were used in conjunction with primers specific for each MDRGI type, to obtain two half segments. The resulting amplicons were visualized by electrophoresis and used to confirm the MDRGI type. However, this method did not allow precise discrimination of types IV, V, and VI. Therefore, specific reverse primers were designed for these three MDRGI types, which were used with the forward erm(B) primer (Table S2). The genomes of erm(B)-positive type X and type XI isolates that could not be assigned to any class of MDRGIs were then sequenced using the Illumina HiSeq 2500 system (Illumina, San Diego, CA, USA). CLC Genomics Workbench version 9 (CLC bio, Aarhus, Denmark) was used to de novo assemble the generated reads into contigs, and the regions flanking the erm(B)-carrying contigs were identified using a primer walking strategy. To assess the genomic background of erm(B)-positive C. jejuni isolate 8C, a hybrid of both the PacBio and Illumina sequencing methods was used. The assembly statistics for the PacBio genome are shown in Table S3. Whole-fragment analysis was conducted using the RAST annotation server (http://rast.nmpdr.org) and BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Natural transformation was performed to assess the transferability in 10 isolates carrying the 3 novel MDRGIs, as described previously (14). Primer sequences for inverse PCR, which was performed to detect the existence of a circular intermediate, as described previously (32), are listed in Table S2.
Accession number(s).
Whole-genome sequencing data that support the findings of this study have been deposited in the NCBI BioProject database under accession number PRJNA492384. The sequences of novel MDRGIs described in this paper have been deposited in the GenBank database under accession numbers MH257910, MH257907, and MH257909.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Key Research and Development Program of China (grant 2017YFC1601501), the Sanming Project of Medicine in Shenzhen (grant SZSM201611068), and the National Natural Science Foundation of China (grants 31761133004 and 31802247).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00153-19.
REFERENCES
- 1.Ruiz-Palacios GM. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 23 January 2018. Campylobacter. https://www.who.int/en/news-room/fact-sheets/detail/campylobacter.
- 3.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. 2015. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima C, Kishimoto M, Ezaki T. 2015. Distribution of antimicrobial resistance in Campylobacter strains isolated from poultry at a slaughterhouse and supermarkets in Japan. Biocontrol Sci 20:179–184. doi: 10.4265/bio.20.179. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TN, Hotzel H, El-Adawy H, Tran HT, Le MT, Tomaso H, Neubauer H, Hafez HM. 2016. Genotyping and antibiotic resistance of thermophilic Campylobacter isolated from chicken and pig meat in Vietnam. Gut Pathog 8:19. doi: 10.1186/s13099-016-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Dong Y, Deng F, Liu D, Yao H, Zhang Q, Shen J, Liu Z, Gao Y, Wu C, Shen Z. 2016. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J Antimicrob Chemother 71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- 7.Poehlsgaard J, Andersen NM, Warrass R, Douthwaite S. 2012. Visualizing the 16-membered ring macrolides tildipirosin and tilmicosin bound to their ribosomal site. ACS Chem Biol 7:1351–1355. doi: 10.1021/cb300105p. [DOI] [PubMed] [Google Scholar]
- 8.McEwen SA, Collignon PJ. 2018. Antimicrobial resistance: a One Health perspective. Microbiol Spectr 6:ARBA-0009-2017. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PubMed] [Google Scholar]
- 9.Bardon J, Pudova V, Kolackova I, Karpiskova R, Roderova M, Kolar M. 2017. Virulence and antibiotic resistance genes in Campylobacter spp. in the Czech Republic. Epidemiol Mikrobiol Imunol 66:59–66. [PubMed] [Google Scholar]
- 10.Cha W, Mosci R, Wengert SL, Singh P, Newton DW, Salimnia H, Lephart P, Khalife W, Mansfield LS, Rudrik JT, Manning SD. 2016. Antimicrobial susceptibility profiles of human Campylobacter jejuni isolates and association with phylogenetic lineages. Front Microbiol 7:589. doi: 10.3389/fmicb.2016.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B, Ma L, Li Y, Jia H, Wei J, Shao D, Liu K, Shi Y, Qiu Y, Ma Z. 2017. Antimicrobial resistance of Campylobacter species isolated from broilers in live bird markets in Shanghai, China. Foodborne Pathog Dis 14:96–102. doi: 10.1089/fpd.2016.2186. [DOI] [PubMed] [Google Scholar]
- 12.Bolinger H, Kathariou S. 2017. The current state of macrolide resistance in Campylobacter spp.: trends and impacts of resistance mechanisms. Appl Environ Microbiol 83:e00416-17. doi: 10.1128/AEM.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell DB, Wang Y, Lin J. 2008. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob Agents Chemother 52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J, Shen J. 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug-resistant Campylobacter coli. J Antimicrob Chemother 69:964–968. doi: 10.1093/jac/dkt492. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q, Shen J. 2014. Emergence of multidrug-resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrob Agents Chemother 58:5405–5412. doi: 10.1128/AAC.03039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Fang L, Xu C, Zhang Q. 2017. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim Health Res Rev 18:87–98. doi: 10.1017/S1466252317000135. [DOI] [PubMed] [Google Scholar]
- 17.Chen JC, Tagg KA, Joung YJ, Bennett C, Francois Watkins L, Eikmeier D, Folster JP. 2018. Report of erm(B)+ Campylobacter jejuni in the United States. Antimicrob Agents Chemother 62:e02615-17. doi: 10.1128/AAC.02615-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florez-Cuadrado D, Ugarte-Ruiz M, Quesada A, Palomo G, Domínguez L, Porrero MC. 2016. Description of an erm(B)-carrying Campylobacter coli isolate in Europe. J Antimicrob Chemother 71:841–843. doi: 10.1093/jac/dkv383. [DOI] [PubMed] [Google Scholar]
- 19.Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su CC, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J. 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 7:e01543-16. doi: 10.1128/mBio.01543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Deng F, Gao Y, Yao H, Shen Z, Wu C, Wang Y, Shen J. 2017. Dissemination of erm(B) and its associated multidrug-resistance genomic islands in Campylobacter from 2013 to 2015. Vet Microbiol 204:20–24. doi: 10.1016/j.vetmic.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Shen Z, Wang Y, Zhang Q, Shen J. 2018. Antimicrobial resistance in Campylobacter spp. Microbiol Spectr 6:ARBA-0013-2017. doi: 10.1128/microbiolspec.ARBA-0013-2017. [DOI] [PubMed] [Google Scholar]
- 22.Zhang A, Song L, Liang H, Gu Y, Zhang C, Liu X, Zhang J, Zhang M. 2016. Molecular subtyping and erythromycin resistance of Campylobacter in China. J Appl Microbiol 121:287–293. doi: 10.1111/jam.13135. [DOI] [PubMed] [Google Scholar]
- 23.Deng F, Wang Y, Zhang Y, Shen Z. 2015. Characterization of the genetic environment of the ribosomal RNA methylase gene erm(B) in Campylobacter jejuni. J Antimicrob Chemother 70:613–615. doi: 10.1093/jac/dku418. [DOI] [PubMed] [Google Scholar]
- 24.Florez-Cuadrado D, Ugarte-Ruiz M, Meric G, Quesada A, Porrero MC, Pascoe B, Sáez-Llorente JL, Orozco GL, Domínguez L, Sheppard SK. 2017. Genome comparison of erythromycin resistant Campylobacter from turkeys identifies hosts and pathways for horizontal spread of erm(B) genes. Front Microbiol 8:2240. doi: 10.3389/fmicb.2017.02240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Zhang M, Yang W, Fang Y, Wang G, Hou F. 2016. A seventeen-year observation of the antimicrobial susceptibility of clinical Campylobacter jejuni and the molecular mechanisms of erythromycin-resistant isolates in Beijing, China. Int J Infect Dis 42:28–33. doi: 10.1016/j.ijid.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Yao H, Liu D, Wang Y, Zhang Q, Shen Z. 2017. High prevalence and predominance of the aph(2'')-If gene conferring aminoglycoside resistance in Campylobacter. Antimicrob Agents Chemother 61:e00112-17. doi: 10.1128/AAC.00112-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 28.Friis LM, Pin C, Taylor DE, Pearson BM, Wells JM. 2007. A role for the tet(O) plasmid in maintaining Campylobacter plasticity. Plasmid 57:18–28. doi: 10.1016/j.plasmid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated and fastidious bacteria, 3rd ed CLSI publication M45. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 2019. Antibiotics tested by NARMS. https://www.cdc.gov/narms/antibiotics-tested.html.
- 31.Ribot EM, Fitzgerald C, Kubota K, Swaminathan B, Barrett TJ. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J Clin Microbiol 39:1889–1894. doi: 10.1128/JCM.39.5.1889-1894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Wang Y, Wu C, Shen Z, Schwarz S, Du XD, Dai L, Zhang W, Zhang Q, Shen J. 2012. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother 56:1650–1654. doi: 10.1128/AAC.06091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.