Despite appropriate antibiotic therapy, pneumococcal meningitis (PM) is associated with a case fatality rate of up to 30% in high-income countries. Survivors often suffer from severe lifelong disabilities.
KEYWORDS: Streptococcus pneumoniae, brain infection, hearing loss, meningitis, neuroinflammation
ABSTRACT
Despite appropriate antibiotic therapy, pneumococcal meningitis (PM) is associated with a case fatality rate of up to 30% in high-income countries. Survivors often suffer from severe lifelong disabilities. An excessive inflammatory reaction drives the pathophysiology, leading to brain damage and neurologic sequelae. We aimed to improve the outcome of experimental PM by simultaneously targeting different pathophysiological mechanisms with combined adjunctive therapies previously shown to be neuroprotective. In vitro, the anti-inflammatory effects of doxycycline and daptomycin were evaluated on primary rat astroglial cells stimulated with Streptococcus pneumoniae. Eleven-day-old infant Wistar rats were infected intracisternally with S. pneumoniae and randomized for treatment with ceftriaxone or combination adjuvant therapy consisting of ceftriaxone, daptomycin, and doxycycline. During acute PM, combined-adjuvant therapy with ceftriaxone, daptomycin, and doxycycline increased the survival rate from 64.1% to 85.8% (P < 0.01) and alleviated weight loss compared to ceftriaxone monotherapy (P < 0.01). Levels of inflammatory cytokines were significantly reduced by combined-adjuvant therapy in vitro (P < 0.0001) and in cerebrospinal fluid in vivo (P < 0.05). In infected animals treated with combined adjunctive therapy, cortical damage was significantly reduced (P < 0.05), and animals showed a trend toward better hearing capacity 3 weeks after the infection (P = 0.089), an effect which was significant in mildly infected animals (48 decibels [dB] versus 67.22 dB; P < 0.05). These mildly infected animals showed significantly reduced cochlear fibrous occlusion (P < 0.01). By combining nonbacteriolytic daptomycin and anti-inflammatory doxycycline with ceftriaxone, the previously reported beneficial effects of the drugs were cumulated and identified the triple-antibiotic therapy as a promising therapeutic option for pediatric PM.
INTRODUCTION
Acute bacterial meningitis is a severe illness with high mortality and morbidity, especially when acquired during infancy or childhood, and causes long-lasting neurofunctional deficits (e.g., hearing loss, epilepsy, cerebral palsy, and cognitive deficits), which tremendously influence quality of life in affected children (1–6). Currently, Neisseria meningitidis and Streptococcus pneumoniae are the most prevalent etiological agents for childhood meningitis beyond the neonatal age, as Haemophilus influenzae type b has been nearly eradicated since vaccine introduction (7). In high-income countries, meningitis caused by S. pneumoniae and N. meningitidis presents case fatality rates of 30% and 7%, respectively (6, 8). Fatality rates are reported to be as high as 50% in resource-poor settings (9). The risk for neurologic sequelae is especially high after pneumococcal meningitis (PM) (3), which causes a massive infection of the central nervous system (CNS), with associated cortical necrosis and apoptosis of dentate gyrus granular cell progenitors in the hippocampus, as found in human patients (10) and animal models (11–14). Neural cell death is caused by multiple factors, including bacterial toxins and an excessive inflammatory reaction from the host (15–17). Together with the recruited neutrophils, activated brain-resident microglia are able to produce large quantities of inflammatory cytokines and reactive oxygen and nitrogen species (ROS and RNS), helping to eradicate the pathogen but also contributing to the development of neuronal damage (15, 18, 19). Pathological cell death in the hippocampus during acute PM correlates with learning and memory deficits (20–23). Furthermore, PM induces damage in the peripheral nervous system (PNS), characterized by sensorineural hearing loss caused by damage to hair cells and spiral ganglion neurons (SGN) in the inner ear (24–26), provoking hearing impairments in up to 30% of survivors (3, 4, 6).
Clinical guidelines recommend the use of adjunctive dexamethasone, an anti-inflammatory corticosteroid, for adult PM in high-income countries (27, 28). However, adjuvant dexamethasone failed to provide a beneficial effect on PM-induced mortality and hearing loss in children (27, 29) and even aggravated mortality, acute brain injury, and long-term learning deficits in different experimental models of bacterial meningitis (23, 30, 31). Over the last few decades, alternative adjuvant therapies, including antioxidants, complement inhibitors, nonbacteriolytic antibiotics, or matrix metalloproteinase (MMP) inhibitors, which target different pathophysiological mechanisms during acute PM, were tested and have shown promising results in PM animal models (32–39). These therapies were mostly evaluated as single-adjuvant therapies or in combination with dexamethasone (40, 41), which is less relevant in pediatric meningitis. We recently postulated combining successful single adjuvants to more effectively reduce CNS and PNS damage, thereby improving long-term outcomes by reducing neurologic sequelae after pediatric PM. This strategy was successfully tested in the same experimental model as described in this present study by combining daptomycin (DAP) and the matrix metalloproteinase inhibitor cipemastat (Trocade) (42). An independent experimental study also reported beneficial effects of combining adjuvant therapies to improve acute and neurofunctional outcomes after murine PM (43).
DAP has previously been shown to clear pneumococci from cerebrospinal fluid (CSF) more rapidly than ceftriaxone (CRO) without inducing bacterial lysis, thereby lowering the overall inflammatory burden in animal models (44, 45). DAP penetrates into the CSF, especially after neurological infection (46, 47), and reaches 5 to 11.5% concentration in serum, mediating bactericidal effects (46–48). In infant rat PM, adjunctive daptomycin reduced neuroinflammatory cytokines in the CSF and decreased brain injury and hearing loss (32, 42). Apart from its antimicrobial activity, doxycycline (DOX), which is known to penetrate well into the brain and CSF (49), also has multiple anti-inflammatory effects by reducing cytokine release and inhibiting MMP activity (50–56). Adjuvant DOX reduced mortality and injury to the brain and cochlea in experimental infant rat PM (56), similarly to other MMP inhibitors that were shown to reduce blood-brain barrier (BBB) permeability, inflammatory cytokines in CSF, brain injury, and mortality during acute bacterial meningitis (34, 42, 57, 58).
By combining adjunctive DAP and DOX therapies, we intend to target multiple pathophysiological mechanisms responsible for brain injury during acute bacterial meningitis with the aim to integrate the beneficial effects of both substances and improve the neurofunctional outcome after pediatric PM.
RESULTS
Antibiotic susceptibility of S. pneumoniae serotype 3.
The MIC of ceftriaxone for the S. pneumoniae serotype 3 used was determined to be 0.003 mg/liter. MICs of daptomycin and doxycycline were both at 0.064 mg/liter. Determined MICs revealed susceptibility of our S. pneumoniae serotype 3 strain to assessed antibiotics according to published data (59, 60).
Reduced release of inflammatory cytokines and nitric oxide in vitro.
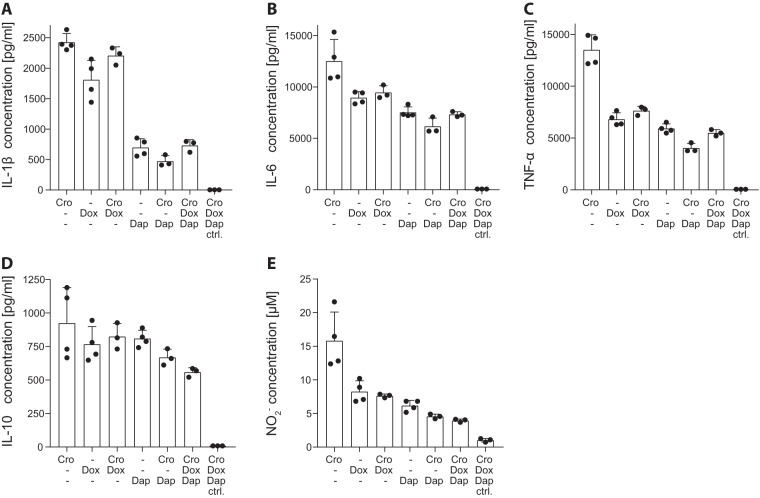
Inflammatory cytokines and nitric oxide (NO) were measured in astroglial cell culture supernatant 24 h after concomitant application of living S. pneumoniae bacteria and antibiotics. Antibacterial but bacteriolytic therapy with CRO induced a strong release of inflammatory cytokines (interleukin-1β [IL-1β], IL-6, IL-10, and tumor necrosis factor alpha [TNF-α]) (Fig. 1A to D) and NO (Fig. 1E). After adjustment of data for multiple testing, DOX monotherapy significantly reduced the release of IL-1β (P < 0.002), IL-6 (P < 0.002), TNF-α (P < 0.0001), and NO (P < 0.001) compared to levels with CRO monotherapy. Accordingly, CRO combined with DOX (CRO+DOX) significantly decreased IL-6 (P < 0.02), TNF-α (P < 0.0001), and NO (P < 0.001) levels. DAP monotherapy and combination therapy with CRO+DAP reduced the inflammatory reaction to a greater extent, with clearly lowered inflammatory cytokines, except for IL-10 (all, P < 0.0001 for CRO versus DAP and CRO versus CRO+DAP), and NO release (P < 0.0001 for all combinations containing DAP). Notably, combined triple therapy with CRO+DAP+DOX significantly decreased the release of IL-1β, IL-6, TNF-α, and NO (all, P < 0.0001 for CRO+DAP+DOX versus CRO) and was the only therapy to significantly reduce IL-10 concentrations compared to levels with CRO monotherapy (P < 0.03). S. pneumoniae exposure did not induce a visible cytopathic effect on astroglial cells in any of the treatment groups, as determined by the observation of intact monolayers (data not shown). Therefore, the reported reduction of NO and cytokine production upon DOX and/or DAP therapy cannot be associated with increased cell death within these groups.
FIG 1.
Inflammatory cytokines and nitric oxide (NO) production upon in vitro stimulation of astroglial cells with living S. pneumoniae bacteria. Cytokines and NO were measured in cell culture supernatant 24 h after concomitant application of living S. pneumoniae bacteria and antibiotics. Infection and bacteriolysis induced by ceftriaxone (CRO) resulted in high levels of IL-1β (A), IL-6 (B), TNF-α (D), and NO (E) release. Compared to CRO monotherapy, DOX significantly reduced levels of IL-1β (P < 0.002), IL-6 (P < 0.002), TNF-α (P < 0.0001), and NO (P < 0.001); CRO+DOX significantly decreased levels of IL-6 (P < 0.02), TNF-α (P < 0.0001), and NO (P < 0.001); DAP, CRO+DAP, and CRO+DAP+DOX reduced levels of IL-1β (P < 0.0001), IL-6 (P < 0.0001), TNF-α (P < 0.0001), and NO (P < 0.0001). CRO+DAP+DOX was the only regimen to significantly reduce IL-10 levels (P < 0.03). Significance levels are not indicated in the graphs for clearer representation. Statistical differences were assessed by one-way ANOVA using Tukey’s multiple comparison to adjust for multiple testing.
Improved survival and increased weight gain in infant rats with PM.
A total of 156 infant Wistar rats were enrolled for this study. One animal was excluded after unsuccessful infection with no detectable bacterial CSF titer at 18 h postinfection (hpi). All other infected animals were included after having fulfilled at least one criterion for successful infection (positive bacterial CSF titer of >106 CFU/ml, reduced clinical score, weight loss, or changes in posture).
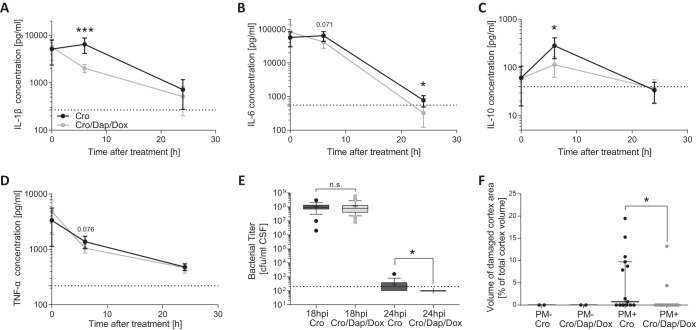
Infected rats showed reduced survival compared to survival of uninfected controls (Fig. 2A). Animals receiving the combination adjuvant therapy demonstrated a significantly better survival rate than infected rats receiving CRO monotherapy (85.8% versus 64.1%; log rank P = 0.0052). Compared to uninfected controls, which constantly gained weight within 42 h after mock infection, animals with PM showed only a slight weight increase within the first 18 h (Fig. 2B). Thereafter, infected animals treated with CRO monotherapy lost weight (2.81% weight loss at 42 hpi compared to weight at time of infection). This weight loss was reduced with combined-adjuvant therapy (0.8% weight gain at 42 hpi compared to weight at time of infection), resulting in a significantly increased weight compared to infected animals with CRO monotherapy (P = 0.0022). At all measured time points, uninfected animals gained significantly more weight than their infected counterparts, independent of treatment modality (P < 0.0001). Upon infection, clinical scores were reduced and reached a minimum at 24 hpi, whereas uninfected animals did not show any changes in clinical scores at any time point (Fig. 2C). Clinical scores of infected animals treated with combination adjuvant therapy showed a trend to be higher than those of infected animals receiving CRO monotherapy at 24 hpi (P = 0.0884). From 24 hpi on, infected rats started to recover and showed an improvement in clinical scores at 42 hpi, which were still significantly lower than those of uninfected control animals (P = 0.0015 for CRO and P = 0.0053 for CRO+DAP+DOX).
FIG 2.
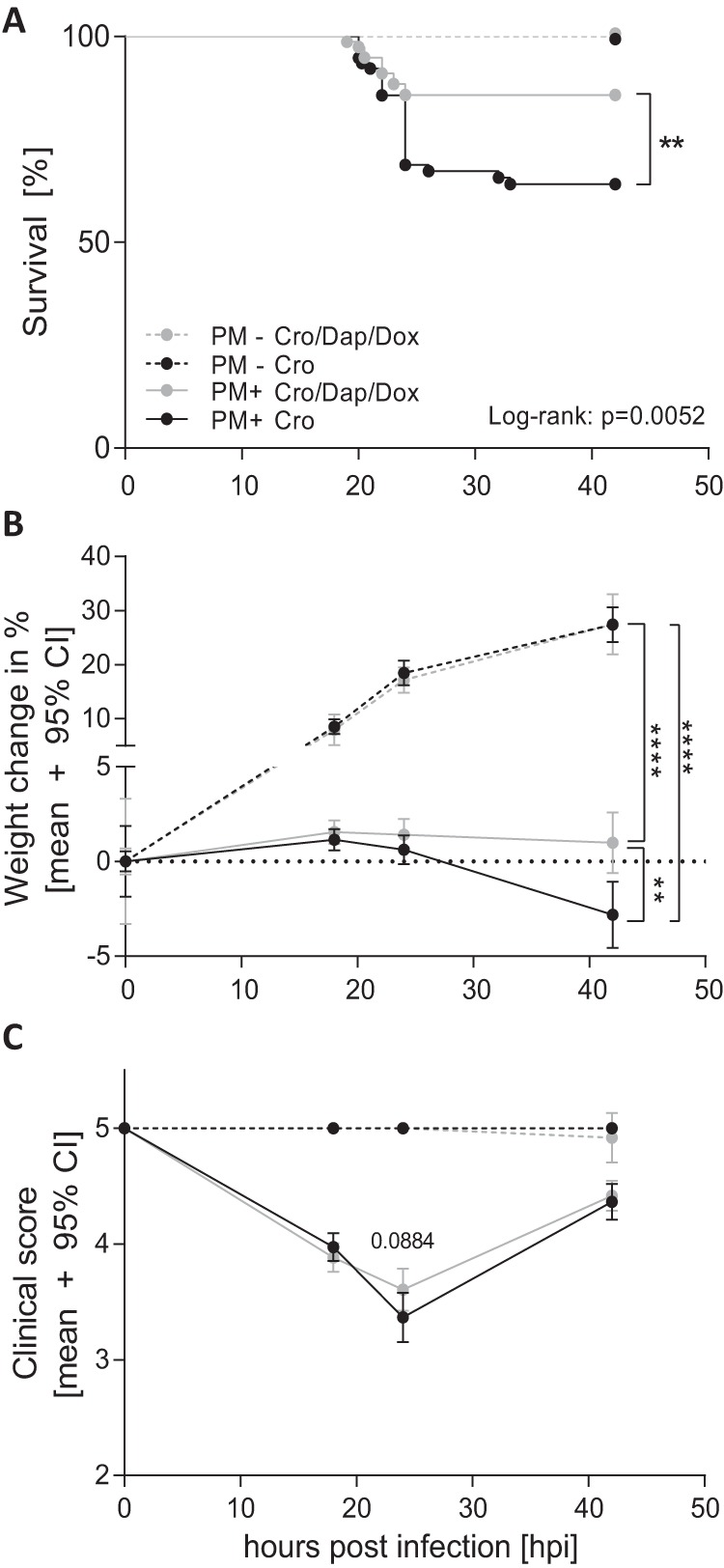
Clinical data of infant rats during acute pneumococcal meningitis. Combined antibiotic therapy (n = 72) with daptomycin (DAP), doxycycline (DOX), and ceftriaxone (CRO) significantly improved survival compared to that with CRO monotherapy (n = 71) during acute PM (A). Relative weight change during acute PM indicates reduced weight gain in infected (PM+) compared to that of mock-infected (PM−) animals (n = 6 per treatment group). Infected animals with combined-adjuvant therapy presented significantly increased weight at 42 hpi compared to that of animals receiving CRO monotherapy (B). Clinical scoring revealed reduced clinical scores in all infected animals compared to those of mock-infected animals (C). Differences were assessed by an unpaired t test in for data in panels B and C and with a log rank test to compare survival rates in panel A. **, P < 0.01; ****, P < 0.0001.
Reduction of inflammatory parameters in CSF in vivo.
Inflammatory cytokines were measured in the CSF of infected animals before treatment and at 6 and 24 h after treatment initiation (representing 18, 24, and 42 hpi). Before treatment, cytokine levels were comparable between the two infected groups. Compared to levels in animals treated with CRO monotherapy, IL-1β and IL-10 CSF levels were significantly reduced in rats receiving the combination adjuvant therapy 6 h after treatment initiation (P = 0.0004 and P = 0.0128) (Fig. 3A and C). IL-6 and TNF-α revealed a trend toward reduced levels in CSF (P = 0.071 and P = 0.076) (Fig. 3B and D). Twenty-four hours after therapy start, IL-6 was significantly lower in animals receiving combined-adjuvant interventions (P = 0.0139) (Fig. 3B). Gamma interferon (IFN-γ) levels were similar between the two groups at all time points (data not shown). Before therapy initiation, bacterial CSF titers were not different, with 1.09 × 108 ± 0.71 × 108 CFU/ml in the CRO group and 1.15 × 108 ± 1.26 × 108 CFU/ml in the CRO+DAP+DOX group (P = 0.772). Six hours after therapy began, all animals treated with the combined-adjuvant intervention had undetectable bacterial titers (<102 ± 0 CFU/ml), whereas half of the animals receiving CRO monotherapy still showed detectable but very low bacterial titers in CSF (3.06 × 102 ± 0.89 × 102 CFU/ml), representing a significantly faster bacterial clearance in CSF with combined-adjuvant therapy (P = 0.0236) (Fig. 3E).
FIG 3.
Inflammatory CSF cytokine levels, cortical necrosis, and bacterial clearance from CSF during acute PM. Cytokine levels are represented by means ±95% confidence intervals starting before treatment initiation and at 6 h and 24 h after treatment start (representing 18, 24, and 42 hpi) for IL-1β (A), IL-6 (B), IL-10 (C), and TNF-α (D). Combination adjuvant therapy (n = 11) significantly reduced IL-1β, IL-6, and IL-10 CSF levels compared to those with CRO monotherapy (n = 11) while showing a trend toward a reduced TNF-α CSF level 6 h after treatment start. Bacterial titers in the CSF were similar in the two treatment groups before the start of therapy (at 18 hpi; n = 53 for CRO+DAP+DOX; n = 49 for CRO), with a faster bacterial clearance in animals receiving the combined-adjuvant therapy (n = 17) than that with CRO monotherapy (n = 18) (E). Cortical necrosis was found only in animals with PM and was significantly reduced by combined-adjuvant therapy (n = 15) compared to the level with CRO monotherapy (n = 15) (F). Statistical differences were assessed using an unpaired t test for cytokines and bacterial titer at 18 hpi. For necrotic cortex volume and bacterial titer at 24 hpi, a Mann-Whitney test was used as data were not normally distributed. *, P < 0.05; ***, P < 0.001; n.s., not significant.
Reduced cerebral complication in vivo.
PM-induced cerebral damage was assessed in all animals sacrificed at 42 hpi. Cortical necrosis was found only in animals with PM. Its extent was significantly higher in the group treated with CRO monotherapy than in animals receiving combined-adjuvant therapy (0.73% [interquartile range (IQR), 0 to 9.7%; n = 15] versus 0% [IQR, 0 to 0%; n = 15]; P = 0.0302) (Fig. 3F). Elevated levels of hippocampal apoptosis were found only in infected animals (data not shown). Here, however, no differences between the two treatment groups were found (0.89 ± 0.91 apoptotic cells per visual field in CRO monotherapy [n = 13] vs. 0.92 ± 1.26 in CRO+DAP+DOX therapy [n = 15], P = 0.943).
Improvement of hearing capacity 3 weeks after infection.
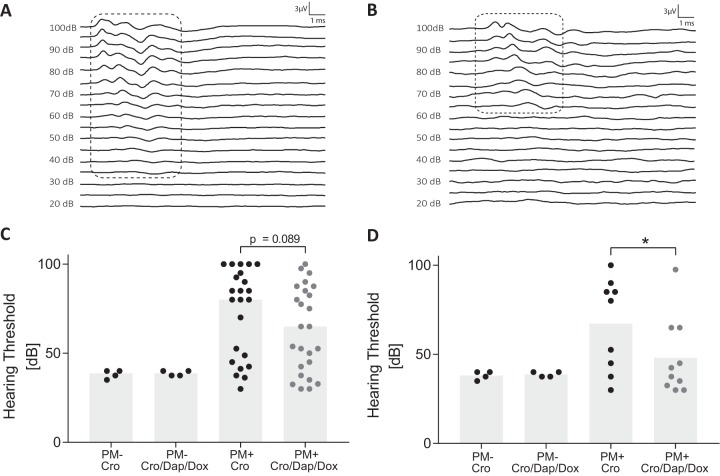
To investigate long-term neurologic sequelae induced by PM, hearing capacity was assessed by auditory-evoked brainstem response (ABR) 3 weeks after infection. Representative ABR recordings from a control animal and an infected rat are shown in Fig. 4A and B, respectively. Control animals showed detectable responses down to 35 decibels (dB), whereas the infected animal did not show any measurable signal below 65 dB (Fig. 4A and B). The average hearing threshold was 38.13 ± 2.39 dB or 38.75 ± 1.44 dB in uninfected animals that received CRO monotherapy or combined-adjuvant therapy, respectively (Fig. 4C). Infected animals showed significantly higher average hearing thresholds than uninfected rats, irrespective of therapy regimen. Treatment with combined-adjuvant therapy showed a trend for improved hearing capacity compared to that with CRO monotherapy (63.26 ± 23.9 dB versus 72.88 ± 24.8 dB; P = 0.0891) (Fig. 4C) in infected rats. In experiments with severely infected animals (defined as an in-litter mortality of >15%), severe hearing loss (average hearing threshold of ≥80 dB) was observed, with no difference between therapies. When only experiments with an in-litter mortality of less than 15% (mild infection experiments) were considered, combination adjuvant therapy with DAP and DOX, compared to CRO monotherapy, significantly reduced PM-induced hearing loss (48.00 ± 21.7 dB [n = 10] versus 67.22 ± 25.9 dB [n = 9]; P = 0.0482).
FIG 4.
Hearing capacity assessed 3 weeks after acute pneumococcal meningitis. Representative ABR recordings from a mock-infected rat (A) with a hearing threshold of 35 dB compared to that of an infected rat (B) with an elevated hearing threshold of 65 dB. Perceived sound intensities are indicated with dashed lines. Infected animals receiving combined-adjuvant therapy (n = 25) showed a trend toward improved hearing thresholds compared to those of animals receiving CRO monotherapy (n = 23) (C). Combined-adjuvant therapy significantly reduced hearing loss in mildly infected animals (n = 10) compared to results with CRO monotherapy (n = 9) (D). *, P < 0.05.
Univariate linear regression (Table 1) analysis revealed a significantly increased hearing threshold with increasing bacterial inoculum (70.5 dB per additional log of infection inoculum; P < 0.001). Low in-litter mortality and a higher clinical score at 24 hpi were both found to predict attenuation of PM-induced hearing impairments (−18.1 dB in the case of mildly infected animals, P = 0.011; −26.7 dB per additional point of activity score at 24 hpi, P < 0.001). Multivariate linear regression modeling (Table 1), including infection inoculum, litter mortality, and treatment regimen, displayed comparable findings. After data were adjusted for in-litter mortality and bacterial inoculum, combination adjuvant therapy was found to significantly reduce PM-induced hearing loss by 10.3 dB (P = 0.037).
TABLE 1.
Univariate and multivariate linear regression for click hearing thresholds 3 weeks after survival of an episode of acute pneumococcal meningitis
| Variable | Univariate regression model (n = 48) |
Multivariate regression model (n = 48) |
||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Bacterial inoculuma | 70.5 | 45.6 to 95.5 | <0.001 | 69.9 | 48.2 to 91.6 | <0.001 |
| Combined adjuvant therapy | −9.7 | −23.8 to 4.4 | 0.172 | −10.3 | −19.9 to −0.6 | 0.037 |
| Low in-litter mortality | −18.1 | −31.8 to −4.3 | 0.011 | −17.1 | −26.9 to −7.3 | 0.001 |
| Activity at 24 hpib | −26.7 | −39.5 to −14.02 | <0.001 | |||
Per additional log of infection inoculum.
Per additional point of activity score.
Improved hearing is associated with reduced fibrous occlusion of the perilymphatic space.
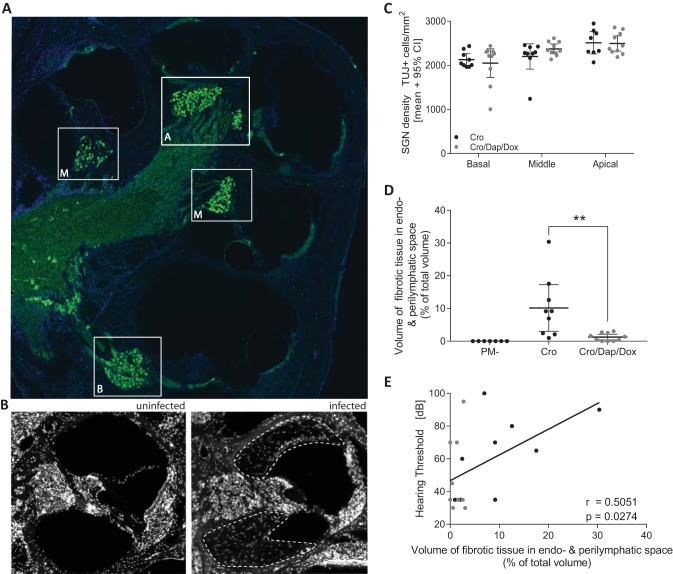
To investigate the reason for improved hearing capacity in mildly infected rats treated with combination adjuvant therapy, histomorphological damage in inner ear was assessed by immunofluorescence. Previous work showed that PM-induced hearing loss was accompanied by reduced spiral ganglion neuron density in the cochlea, which is a critical predictor for the efficacy of a cochlear implant (25). Spiral ganglion neurons were quantified in the basal, middle, and apical turns of mildly infected animals, as indicated in Fig. 5A. No statistical differences were found between the two treatment groups at any of the cochlear turns (Fig. 5C). In the present study, only a few animals showed a reduction in SGN density (<2,000 β-III tubulin-positive [TUJ+] cells/mm2) (e.g., left middle turn in Fig. 5A), compared to SGN density previously reported in mock-infected animals (25). Histomophological assessment of the cochlear turns revealed extensive fibrous occlusion of the perilymphatic space in infected animals (Fig. 5B). Fibrous occlusion was significantly reduced in infected animals treated with the combined-adjuvant intervention compared to that in animals receiving CRO monotherapy (Fig. 5D). In mildly infected animals, a significant positive correlation was found for fibrous occlusion of the perilymphatic space and hearing threshold (r = 0.5051, P = 0.0274) (Fig. 5E).
FIG 5.
Immunohistological analysis of spiral ganglion neuron density and cochlear occlusion in mildly infected animals. (A) Midmodiolar section immunostained for neurons (β-III tubulin; green) and cell nuclei (DAPI; blue) showing the basal (B), middle (M), and apical (A) turns of an infected rat 3 weeks after PM. (B) The absence and presence of fibrous occlusion in the perilymphatic space in a mock-infected control animal and in an infected animal receiving standard CRO monotherapy are represented in immunofluorescence pictures. The area with fibrous occlusion in the perilymphatic space is indicated by white dashed lines. (C) Spiral ganglion neuron (SGN) density levels in any of the cochlear turns in infected animals were not significantly different between the two treatment groups. (D) Combined-adjuvant therapy (n = 10) significantly reduced the amount of fibrous tissue in the perilymphatic space compared to that with CRO monotherapy (n = 9). (E) A significant positive correlation for fibrous occlusion of the perilymphatic space and hearing threshold was found. **, P < 0.01.
DISCUSSION
Observational clinical studies repetitively found neurologic sequelae in patients surviving bacterial meningitis, with especially high risk after PM (3). A profound inflammatory reaction in the CNS and PNS is associated with cortical necrosis, hippocampal apoptosis, and cochlear damage (11–13, 24–26). In addition to bacterial toxins, the host’s excessive inflammatory reaction contributes to the observed neural cell death (15–17). Bacterial autolysis and also bactericidal antibiotics with antibiotic-dependent lysis of bacteria provoke a release of highly immunogenic products, promoting inflammatory processes and disease severity (15, 61–64). During PM, secreted MMPs from activated immune cells are crucially upregulated and associated with brain injury (15, 34, 65), with higher MMP-9 levels being associated with the development of hearing impairment or secondary epilepsy in infected children (65). By degrading basal lamina components and tight junction proteins, MMP-9 weakens the blood-brain barrier (BBB) (58, 66–68) and facilitates leukocyte extravasation and BBB leakage (33, 69). Additionally, MMPs contribute to the inflammatory reaction and brain injury via their sheddase and convertase activities and are thus able to cleave and activate inflammatory cytokines and chemokines (33, 70–72).
DOX has multiple anti-inflammatory effects by reducing cytokine release and inhibiting MMP activity (50–56). Pharmacologic inhibition of MMPs during acute bacterial meningitis resulted in the reduction of blood-brain barrier permeability, inflammatory cytokine levels in CSF, brain injury, and mortality (34, 42, 57, 58). Selective MMP inhibitors (e.g., BB-94, BB-1101, GM-6001, or TNF-484) have not yet been successfully tested in clinical studies. DOX, on the other hand, has a well-characterized clinical safety profile (56). The dosage of DOX used here was previously shown by other investigators to suppress cerebral MMP-9 activity and reduce ischemic brain damage in rodents (73, 74). In experimental infant rat PM, adjuvant DOX reduced mortality and injury to the brain and cochlea (56).
DAP, a nonbacteriolytic but bactericidal antibiotic, integrates itself into the bacterial cell membrane and induces depolarization and biosynthesis inhibition (75–77). In previous work, we demonstrated a faster bacterial clearance from CSF with DAP, leading to reduced inflammatory parameters and decreased cortical complication compared to results with standard CRO therapy in infant rat PM (45, 78). In the same infant rat PM model, CRO with adjunctive DAP reduced PM-associated cerebral damage and inflammatory cytokine levels in the CSF and improved hearing capacity (32, 42). In adult rats with PM, DAP treatment attenuated cognitive impairment in surviving rats compared to that with CRO (79).
Our previous work on the experimental model reported here, as well as other independent experimental work, confirmed the potential of combined-adjuvant interventions targeting multiple pathophysiological mechanisms during PM, thereby improving acute and neurofunctional outcomes (42, 43). With this study, we were able to show that combined-adjuvant therapies in experimental pediatric PM cumulate previously reported beneficial effects of single adjuvants and significantly improve acute neuropathology and neuroinflammation, thereby improving neurofunctional outcomes compared to those with standard CRO monotherapy. Combined-adjuvant therapy with DAP and DOX was able to improve survival and weight gain in infant rats infected with S. pneumoniae (Fig. 2A and B). Adjuvant DOX, but not DAP, previously demonstrated its capacity to significantly improve survival among infant rats with PM (32, 56). Tetracyclines also demonstrated mortality reduction in experimental sepsis models (80). On the other hand, adjuvant DAP was previously shown to improve weight change after treatment initiation (32). Here, adjuvant combination therapy with DOX and DAP significantly reduced inflammatory cytokines in CSF in vitro and in vivo (Fig. 1 and 3). Despite previous findings, which showed reduced CSF levels of TNF-α upon DOX-induced TNF-α-converting enzyme (TACE) inhibition (56) and reduced CSF IL-6 levels with adjuvant DAP (32), we found statistical trends toward lower CSF levels of these cytokines only 6 h after treatment initiation (Fig. 3B and D). Of note, animals treated with combined-adjuvant therapy showed slightly higher initial TNF-α and IL-6 levels (18 hpi) with faster subsequent reduction after therapy initiation. In other experimental inflammatory diseases, DOX was shown to inhibit interleukin-converting enzyme, thereby lowering the bioavailability of IL-1β (56, 81). In accordance with these data, we found significantly reduced IL-1β levels 6 h after initiating therapy in vivo with combined-adjuvant intervention compared to levels with CRO monotherapy. Therapy initiation with CRO monotherapy caused increased CSF levels of IL-1β and IL-6, indicating that bacterial burst by lytic antibiotic further aggravates cerebral inflammation (44, 45, 63), which we were able to inhibit with adjuvant nonbacteriolytic DAP in vitro and in vivo. Our in vitro model of neuroinfection and neuroinflammation with primary rat astroglial cells supported the concept of induced neuroinflammation upon therapy initiation with a bacteriolytic antibiotic, with clearly reduced inflammatory cytokines and NO release by treatment with nonbacteriolytic antibiotics (Fig. 1). The reduced induction of neuroinflammation in vitro by simultaneous application of CRO+DAP (and CRO+DAP+DOX) underlines previous findings showing fast antibacterial action of DAP (45) and thereby supports the concept of DAP’s anti-inflammatory actions even when DAP is applied concomitantly with CRO.
Animals receiving combined CRO+DAP+DOX showed significantly reduced cortical damage at 42 hpi (Fig. 3F), without showing a better outcome in terms of hippocampal apoptosis (data not shown). The reduced cortical complication might be attributed to the overall reduction of CSF inflammation, as previously found by single-adjuvant DAP and DOX therapy (32, 56).
Combined-adjuvant therapy with CRO+DAP+DOX improved hearing thresholds in mildly infected infant rats (Fig. 4D), whereas severely infected animals showed profound hearing loss (average hearing threshold of ≥80 dB), which might be beyond potential protection. These data were confirmed by a multivariate linear regression showing that, after data were adjusted for infection inoculum and high in-litter mortality, rats with combined adjuvant therapy revealed improved hearing capacity by 10.3 dB (Table 1). Histologic analysis revealed that spiral ganglion neuron density was not significantly affected in mildly infected rats (Fig. 5C) compared to previously published data of mock-infected infant rats (25). Improved hearing capacity in animals with combined adjuvant therapy was correlated to a significant reduction of fibrous occlusion of the perilymphatic space (Fig. 5D and E). Fibrous obliteration of the perilymphatic space after experimental PM has been described previously in a mouse model of PM and positively correlated with increased hearing loss (37). During acute infection, leukocytes enter the perilymphatic space, contributing to the inflammatory processes (26). Resolution of the granulocytic inflammation is expected to cause occlusion of the perilymphatic space with connective tissue, potentially leading to cochlear ossification (26). As cochlear ossification can limit the access for cochlear implantations (82), a reduction in fibrous obliteration with associated cochlear ossification has important clinical consequences. Of note, cochlear hair cells and presynaptic ribbons of surviving inner hair cells were not analyzed during this study. The detected improvement in hearing capacity with combined-adjuvant DAP and DOX might also be attributed to a protection of cochlear hair cells or their connectivity to the SGN. In previous work, we demonstrated that connectivity of inner hair cells to SGN was already reduced after mild infection, whereas the inner hair cells themselves remained unaffected (25). In mildly infected animals with low-level fibrotic occlusion, loss of inner hair cell connectivity might explain elevated hearing thresholds (Fig. 5E).
In a comprehensive meta-analysis, adjunctive anti-inflammatory corticosteroids were shown to improve the outcome of adults with PM in high-income countries and of children with meningitis caused by Haemophilus influenzae type b, without showing a clear benefit of corticosteroids in children with PM (27). Multiple experimental models of bacterial meningitis with adjunctive dexamethasone demonstrated aggravated mortality and acute hippocampal injury with subsequent learning and memory deficits, especially in infant rodents (23, 30, 31, 83, 84). As there is no substantial evidence supporting the use of dexamethasone in children with PM, we did not include it in our study focusing on pediatric PM. Further limitations of this study include the lack of direct comparison to single-adjuvant therapies. As the respective single-adjuvant therapies were already tested in our laboratory on the same model of PM, we decided not to include these therapy groups but to compare results to previously reported data (32, 56). Additionally, we note the limitation of direct intracisternal inoculation of pneumococci to induce PM, which does not represent the pathophysiologic route of infection via the bloodstream (15). Yet development of meningitis after intranasal inoculation with bloodstream spread and hematogenous CNS infection is obtained only in a small proportion of the infected animals, even after experimentally induced extracellular matrix degradation (85), thus limiting the use of this inoculation method for specific research on meningitis. Furthermore, animals in our experiments were treated for 5 days and not 10 to 14 days, as recommended for humans (28). Previous experiments showed, however, that this treatment duration resulted in complete bacterial clearance with associated recovery of clinical scores and weight loss (25, 32, 42). Last, resulting CSF daptomycin levels of 0.5 mg/liter in patients with neurological infections (47, 86) are just slightly above the MIC for S. pneumoniae (60), and this may affect the beneficial effects seen in our animal model.
Conclusion.
Combination adjuvant therapy with nonbacteriolytic DAP and DOX, with its MMP-inhibitory and anti-inflammatory properties, caused faster bacterial clearance and reduced inflammatory CSF cytokine levels, known to be mediators of brain damage during acute PM. Previously reported beneficial effects of these single adjuvants were merged by combined intervention and improved survival, weight loss, cerebral complication, and neurologic sequelae such as hearing loss. Therefore, we conclude that combining adjuvant DAP and DOX with CRO is a promising therapeutic option to improve the outcome of PM.
MATERIALS AND METHODS
Infecting organism.
A strain of Streptococcus pneumoniae (serotype 3) that has previously been used in experimental PM (12-14, 21, 23-25, 31-35, 42, 45, 56, 58, 78, 83) was cultured overnight in brain heart infusion (BHI) medium, diluted 10-fold in fresh, prewarmed BHI medium, and grown for 5 h to reach the logarithmic phase, as reported earlier (32, 58). The bacteria were centrifuged for 10 min at 3,100 × g, washed twice, and resuspended in sterile, pyrogen-free saline (0.85% NaCl). Bacteria were further diluted to the desired density by measuring the optical density at 570 nm (OD570). Inoculum accuracy was determined by serial dilutions and plating on Columbia sheep blood agar (CSBA) plates.
Antibiotic susceptibility testing.
For MIC determination, blood agar plates were inoculated with S. pneumoniae serotype 3, the strain used in this study. MICs of ceftriaxone (0.002 to 32 mg/liter) (Liofilchem srl, Italy), doxycycline (0.016 to 256 mg/liter) (bioMérieux, USA), and daptomycin (0.016 to 256 mg/liter) (bioMérieux, USA) were determined by using antibiotic gradient strips, according to the manufacturer’s protocol and as described before (45).
In vitro model of neuroinfection and neuroinflammation.
Primary rat astroglial cells, isolated from the cortex and hippocampus of infant rats on postnatal day 3 (P3), were kept in culture medium, consisting of Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, Merck Switzerland) with 5% fetal calf serum (FCS; Biochrom, Germany), GlutaMAX (ThermoFisher, Switzerland), and antibiotic-antimycotic (ThermoFisher, Switzerland), for 11 days at 37°C with 5% CO2, as reported elsewhere (18, 87). For stimulation assays, cells were seeded in poly-l-ornithine-coated 24-well plates and kept for an additional 3 days. Subsequently, cell culture medium was replaced with phenol red-free and pyruvate-free DMEM (Gibco/ThermoFisher, Switzerland) with 5% FCS and GlutaMAX. Cells were stimulated with 3 × 107 CFU/ml S. pneumoniae in logarithmic growth phase. Directly after cells were stimulated with living bacteria, antibiotics were added to the cell culture medium (10 μg/ml CRO [Rocephine; Roche)], 1 μg/ml DAP [Cubicin; Cubist Pharmaceuticals], 3 μg/ml DOX [doxycycline hyclate; Sigma]), at levels reflecting CSF concentrations found in patients or experimental studies focusing on neuroinfections (46–49, 86, 88, 89). Nitric oxide production was measured using Griess reagent (Sigma-Aldrich/Merck, Switzerland). Briefly, 100 μl of Griess reagent was mixed with 100 μl of cell culture medium in 96-well plates. Absorbance was measured at 550 nm with a microplate reader (THERMOmax; Molecular Devices, USA). Nitrite concentrations were calculated from a NaNO2 standard curve.
Infant rat model of pneumococcal meningitis.
All animal studies were approved by the Animal Care and Experimentation Committee of the Canton of Bern, Switzerland (license BE 129/14) and followed the Swiss national guidelines for the performance of animal experiments. A well-established infant rat model of PM was used for the experiments as previously described (32, 58). Eleven-day-old Wistar rats together with their dams were obtained from Charles River Laboratories (Sulzfeld, Germany). The dams were provided with tap water and pellet diet ad libitum. Litters were kept in rooms at a controlled temperature of 22 ± 2°C. During the acute phase of the disease, animals were housed in conventional cages in a room with natural light. For long-term experiments after bacterial curing, animals were transferred to individually ventilated cages (IVC) in a room with controlled 12-h light/dark cycles. Intracisternal infections were performed by injection of 10 μl of saline containing 7.33 × 105 ± 3.4 × 105 CFU/ml living S. pneumoniae bacteria. Mock-infected control animals received an equivalent volume of saline. Pneumococcal meningitis was confirmed 18 h postinfection (hpi) by quantitative analysis of bacterial titers in CSF samples, when the animals developed symptomatic disease. For this, 5 μl of CSF was collected by puncture of the cisterna magna, followed by serial dilution and cultivation on Columbia sheep blood agar (CSBA) plates. All infected animals were treated with CRO (100 mg/kg [Rocephine; Roche] twice daily [b.i.d.]) and randomized for (i) combination adjuvant therapy (n = 72) consisting of DAP (10 mg·kg−1 day−1, subcutaneously [s.c.] in saline in a single dose [Cubicin; Cubist Pharmaceuticals]) plus DOX (30 mg/kg, intraperitoneally [i.p.] once daily [Sigma]) or (ii) saline in the control group (n = 72). Antibiotic therapy with CRO was started at 18 hpi in all animals. Therapies involving DAP were started by the application of DAP followed by a 15-min-delayed application (i.e., at 18:15 hpi) of other therapies. For long-term experiments assessing neurofunctional outcomes, CRO and DOX therapies were continued until day 5 after infection. Mock-infected animals either received the combination adjuvant therapy (n = 6) or the CRO monotherapy with vehicles (n = 6). All animals received the same amount of fluids during the experiments.
The rats were weighed and examined clinically at 0, 18, and 24 hpi and before sacrifice at 42 hpi, as previously described (58). Activity scores represent the following: 1, coma; 2, animal does not turn upright; 3, animal turns upright within 30 s; 4, animal has minimal ambulatory activity, turns upright in <5 s; 5, normal. Weight was assessed relative to weight at time of infection (percent increase). Investigators were blinded for treatment modalities. Spontaneous mortality was documented. Punctures of the cisterna magna were performed using a 30-gauge needle to obtain CSF samples at 18, 24, and 42 hpi. CSF samples not used for bacterial titer determination were centrifuged (16,000 × g at 4°C for 10 min), and the supernatants were frozen at −80°C until further use. Animals were sacrificed with an overdose of pentobarbital (150 mg/kg of body weight, i.p.) (Esconarkon; Streuli Pharma AG, Uznach, Switzerland) at 42 hpi and perfused with ice-cold 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) before their brains were removed and fixed in 4% PFA for histological analysis.
Histomorphometric assessment of cortical damage and hippocampal apoptosis.
Damage to cerebral structures was quantified as previously described in all animals sacrificed at 42 hpi (33, 90, 91). Briefly, brains were fixed in 4% PFA and cryopreserved in 18% sucrose in PBS at 4°C overnight. Coronal brain cryosections (45 μm thick) obtained by systematic uniform sampling were stained for Nissl substance with cresyl violet. Cortical damage was defined as areas of decreased neuronal density. Histological features of apoptosis were quantified in 48 systematic visual fields spanning the hippocampus of both hemispheres. Histologic assessments were performed and evaluated by a person blinded to treatment modalities.
Quantitative analysis of cytokine levels in the CSF.
A panel of cytokines previously found to be upregulated in PM (15, 92), i.e., IL-1β, IL-6, TNF-α, IL-10, and IFN-γ, was assessed using a magnetic multiplex assay (Rat Magnetic Luminex Assay; R&D Systems/Bio-Techne) and a Bio-Plex 200 station (Bio-Rad Laboratories) as previously described (25, 33). Undiluted cell culture supernatant or 5 μl of CSF harvested and centrifuged at 18, 24, and 42 hpi was diluted to a final volume of 50 μl using the provided assay buffer. At least 50 beads were measured for each analyte. Calibration curves from recombinant standards were calculated with Bio-Plex Manager software (version 4.1.1) using five-parameter logistic curve fitting. For samples below the detection limit, the value of the detection limit provided by the manufacturer (TNF-α, 22.1 pg/ml; IL-6, 56.0 pg/ml; IL-1β, 26.7 pg/ml; IL-10, 18.6 pg/ml; IFN-γ, 70.5 pg/ml) was multiplied by the dilution factor.
Click- and pure-tone-evoked auditory brainstem response.
Auditory brainstem responses (ABRs) were recorded in response to click stimulations and pure tones on both ears using a SmartEP system (Intelligent Hearing Systems, Miami, FL, USA), as previously described (25). Animals were anesthetized with isoflurane (5% for induction and 2% for maintenance) using a Combi-Vet Vaporizer System equipped with a digital flowmeter (Rothacher Medical, Switzerland). Click stimuli at 100 μs and 5-ms pure-tone pips (Blackman envelope; polarity alternating) were presented at a rate of 21.1 s−1, ranging from 100 to 20 dB sound pressure level (SPL) in 10-dB decrements (5-dB decrements close to threshold). A total of 1,024 responses were averaged at each sound level and filtered between 100 and 1,500 Hz. The hearing threshold was defined as the lowest intensity that induced the appearance of a visually detectable first peak. ABRs were recorded between P30 and P34. Hearing thresholds were independently analyzed and discussed by multiple blinded investigators.
Immunohistological analysis of spiral ganglion neuron density and cochlear occlusion.
Immunohistological analysis of the cochlea was performed as reported earlier (25). In summary, 3 weeks after infection, the animals were sacrificed and perfused with 4% paraformaldehyde (PFA). Cochleas were dissected and isolated, followed by overnight fixation in 4% PFA at 4°C. Samples were decalcified with Osteosoft (Merck) for 10 days before dehydration and cryopreservation in 30% sucrose, followed by cryosectioning. Fourteen-micrometer midmodiolar sections were cut and mounted on Superfrost Plus microscopy slides (ThermoFisher Scientific). The immunofluorescence procedure was performed using a Shandon Sequenza staining rack (ThermoFisher Scientific). Sections were permeabilized for 5 min with 0.1% Triton X-100 and then blocked with a blocking solution (2% bovine serum albumin [BSA] plus 0.01% Triton X-100 in PBS) for 1 h at room temperature. The neuron-specific primary antibody against β-III tubulin ([TUJ] monoclonal mouse anti β-III tubulin; R&D Systems) was diluted 1:500 in blocking solution and incubated overnight at 4°C. The slides were rinsed three times with PBS and incubated with the secondary antibody (Alexa Fluor 488 conjugated; Invitrogen) diluted 1:500 in blocking solution for 2 h at room temperature. After being washed three times with PBS, the samples were mounted with a coverslip using Fluoroshield containing 4′,6′-diamidino-2-phenylindole (DAPI; Sigma). Images of the spiral ganglion were acquired with a Zeiss Axio Imager M1 with Zeiss EC Plan‐Neofluar objectives using AxioVision software (AxioVs40, version 4.8.2.0; Carl Zeiss MicroImagin GmbH).
Statistical analysis.
Statistical analyses were performed with GraphPad Prism software (Prism 7 for Windows; GraphPad Software, Inc., San Diego, CA). If not stated otherwise, results are presented as mean values ± standard deviations. To compare data between two groups, an unpaired Student's t test was used for parametric data. For nonparametric data, a Mann-Whitney test was used. For in vitro cytokines and NO release comparing multiple different groups, Tukey’s multiple-comparison test was applied to adjust for multiple testing. Mortality rates were calculated using a log rank (Mantel-Cox) test for significance based on all infected animals and numbers of animals sacrificed for ethical reasons (clinical score of 2) or dying spontaneously. In box plots, the horizontal line within the box represents the median, and the top and bottom of the box mark the 75th and the 25th percentiles, respectively. The upper and lower bounds of the whiskers represent the range of the data. A Pearson correlation was performed to correlate cochlear occlusion with hearing capacity. A two-tailed P value of <0.05 was considered statistically significant.
For exploratory data analysis, a multivariate linear regression model was used to estimate predictors and determinants for bacterial meningitis-induced hearing loss. The linear coefficients and a 95% confidence interval (CI) were calculated for each variable. Statistical analyses were performed using STATA, version 12 (STATA Corp., College Station, TX).
ACKNOWLEDGMENTS
We thank Franziska Simon, Michelle Buri, Sabrina Hupp, and Robert Lukesch for excellent technical support. Productive discussions and input from the ESCMID Study Group for Infectious Diseases of the Brain (ESGIB) are greatly appreciated.
This work was supported by a grant from the Swiss National Science Foundation (grant 310030-162583).
We declare that we have no conflicts of interest regarding the publication of this paper.
REFERENCES
- 1.Koedel U, Scheld WM, Pfister H-W. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2:721–736. doi: 10.1016/S1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer MC, Heckenberg SGB, de Gans J, Spanjaard L, Reitsma JB, van de Beek D. 2010. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology 75:1533–1539. doi: 10.1212/WNL.0b013e3181f96297. [DOI] [PubMed] [Google Scholar]
- 3.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. 2010. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 10:317–328. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- 4.Chandran A, Herbert H, Misurski D, Santosham M. 2011. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J 30:3–6. doi: 10.1097/INF.0b013e3181ef25f7. [DOI] [PubMed] [Google Scholar]
- 5.Baraff LJ, Lee SI, Schriger DL. 1993. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J 12:389–394. doi: 10.1097/00006454-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 6.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. 2004. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre PB, O’Brien KL, Greenwood B, van de Beek D, Booy R, Heath P. 2012. Effect of vaccines on bacterial meningitis worldwide. Lancet 380:1703–1711. doi: 10.1016/S0140-6736(12)61187-8. [DOI] [PubMed] [Google Scholar]
- 8.van de Beek D. 2012. Progress and challenges in bacterial meningitis. Lancet 380:1623–1624. doi: 10.1016/S0140-6736(12)61808-X. [DOI] [PubMed] [Google Scholar]
- 9.van de Beek D, Farrar JJ, de Gans J, Mai NTH, Molyneux EM, Peltola H, Peto TE, Roine I, Scarborough M, Schultsz C, Thwaites GE, Tuan PQ, Zwinderman A. 2010. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 9:254–263. doi: 10.1016/S1474-4422(10)70023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nau R, Soto A, Bruck W. 1999. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol 58:265–274. doi: 10.1097/00005072-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A, Brück W, Nau R. 2001. A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol 101:499–508. [DOI] [PubMed] [Google Scholar]
- 12.Grandgirard D, Steiner O, Täuber MG, Leib SL. 2007. An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol 114:609–617. doi: 10.1007/s00401-007-0304-8. [DOI] [PubMed] [Google Scholar]
- 13.Bifrare Y-D, Gianinazzi C, Imboden H, Leib SL, Täuber MG. 2003. Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 13:481–488. doi: 10.1002/hipo.10142. [DOI] [PubMed] [Google Scholar]
- 14.Grandgirard D, Bifrare Y-D, Pleasure SJ, Kummer J, Leib SL, Täuber MG. 2007. Pneumococcal meningitis induces apoptosis in recently postmitotic immature neurons in the dentate gyrus of neonatal rats. Dev Neurosci 29:134–142. doi: 10.1159/000096218. [DOI] [PubMed] [Google Scholar]
- 15.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. 2011. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 24:557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell L, Smith SH, Braun JS, Herzog K, Weber JR, Tuomanen EI. 2004. dual phases of apoptosis in pneumococcal meningitis. J Infect Dis 190:2039–2046. doi: 10.1086/425520. [DOI] [PubMed] [Google Scholar]
- 17.Agyeman P, Grandgirard D, Leib SL. 2014. Pathogenesis and pathophysiology of bacterial infections, p 341–364. In Scheld MW, Marra CM, Whitley RJ (ed), Infections of the central nervous system. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 18.Iliev AI, Stringaris AK, Nau R, Neumann H. 2004. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9). FASEB J 18:412–414. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- 19.Marques CP, Cheeran M-J, Palmquist JM, Hu S, Lokensgard JR. 2008. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J Neurovirol 14:229–238. doi: 10.1080/13550280802093927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellmer A, Noeske C, Gerber J, Munzel U, Nau R. 2000. Spatial memory and learning deficits after experimental pneumococcal meningitis in mice. Neurosci Lett 296:137–140. doi: 10.1016/S0304-3940(00)01645-1. [DOI] [PubMed] [Google Scholar]
- 21.Loeffler JM, Ringer R, Hablützel M, Täuber MG, Leib SL. 2001. The free radical scavenger α-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J Infect Dis 183:247–252. doi: 10.1086/317921. [DOI] [PubMed] [Google Scholar]
- 22.Nau R, Brück W. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci 25:38–45. doi: 10.1016/S0166-2236(00)02024-5. [DOI] [PubMed] [Google Scholar]
- 23.Leib SL, Heimgartner C, Bifrare Y-D, Loeffler JM, Täuber MG. 2003. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr Res 54:353–357. doi: 10.1203/01.PDR.0000079185.67878.72. [DOI] [PubMed] [Google Scholar]
- 24.Perny M, Solyga M, Grandgirard D, Roccio M, Leib SL, Senn P. 2017. Streptococcus pneumoniae-induced ototoxicity in organ of Corti explant cultures. Hear Res 350:100–109. doi: 10.1016/j.heares.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Perny M, Roccio M, Grandgirard D, Solyga M, Senn P, Leib SL. 2016. The severity of infection determines the localization of damage and extent of sensorineural hearing loss in experimental pneumococcal meningitis. J Neurosci 36:7740–7749. doi: 10.1523/JNEUROSCI.0554-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein M, Koedel U, Pfister H-W, Kastenbauer S. 2003. Morphological correlates of acute and permanent hearing loss during experimental pneumococcal meningitis. Brain Pathol 13:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer MC, McIntyre P, Prasad K, van de Beek D. 2015. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 9:CD004405. doi: 10.1002/14651858.CD004405.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard C, Pagliano P, Pfister HW, Read RC, Sipahi OR, Brouwer MC, ESCMID Study Group for Infections of the Brain (ESGIB). 2016. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22:S37–S62. doi: 10.1016/j.cmi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Peltola H, Roine I, Fernandez J, Gonzalez Mata A, Zavala I, Gonzalez Ayala S, Arbo A, Bologna R, Goyo J, Lopez E, Mino G, Dourado de Andrade S, Sarna S, Jauhiainen T. 2010. Hearing impairment in childhood bacterial meningitis is little relieved by dexamethasone or glycerol. Pediatrics 125:e1–e8. doi: 10.1542/peds.2009-0395. [DOI] [PubMed] [Google Scholar]
- 30.Spreer A, Gerber J, Hanssen M, Schindler S, Hermann C, Lange P, Eiffert H, Nau R. 2006. Dexamethasone increases hippocampal neuronal apoptosis in a rabbit model of Escherichia coli meningitis. Pediatr Res 60:210–215. doi: 10.1203/01.pdr.0000227553.47378.9f. [DOI] [PubMed] [Google Scholar]
- 31.Coimbra RS, Loquet G, Leib SL. 2007. Limited efficacy of adjuvant therapy with dexamethasone in preventing hearing loss due to experimental pneumococcal meningitis in the infant rat. Pediatr Res 62:291–294. doi: 10.1203/PDR.0b013e318123fb7c. [DOI] [PubMed] [Google Scholar]
- 32.Grandgirard D, Burri M, Agyeman P, Leib SL. 2012. Adjunctive daptomycin attenuates brain damage and hearing loss more efficiently than rifampin in infant rat pneumococcal meningitis. Antimicrob Agents Chemother 56:4289–4295. doi: 10.1128/AAC.00674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liechti FD, Grandgirard D, Leppert D, Leib SL. 2014. Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infect Immun 82:1710–1718. doi: 10.1128/IAI.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leib SL, Clements JM, Lindberg RLP, Heimgartner C, Loeffler JM, Pfister L-A, Täuber MG, Leppert D. 2001. Inhibition of matrix metalloproteinases and tumour necrosis factor α converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734–1742. doi: 10.1093/brain/124.9.1734. [DOI] [PubMed] [Google Scholar]
- 35.Auer M, Pfister LA, Leppert D, Täuber MG, Leib SL. 2000. Effects of clinically used antioxidants in experimental pneumococcal meningitis. J Infect Dis 182:347–350. doi: 10.1086/315658. [DOI] [PubMed] [Google Scholar]
- 36.Högen T, Demel C, Giese A, Angele B, Pfister H-W, Koedel U, Klein M. 2013. Adjunctive N-acetyl-l-cysteine in treatment of murine pneumococcal meningitis. Antimicrob Agents Chemother 57:4825–4830. doi: 10.1128/AAC.00148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein M, Koedel U, Pfister H-W, Kastenbauer S. 2003. Meningitis-associated hearing loss: Protection by adjunctive antioxidant therapy. Ann Neurol 54:451–458. doi: 10.1002/ana.10684. [DOI] [PubMed] [Google Scholar]
- 38.Masouris I, Klein M, Dyckhoff S, Angele B, Pfister HW, Koedel U. 2017. Inhibition of DAMP signaling as an effective adjunctive treatment strategy in pneumococcal meningitis. J Neuroinflammation 14:214. doi: 10.1186/s12974-017-0989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woehrl B, Brouwer MC, Murr C, Heckenberg SGB, Baas F, Pfister HW, Zwinderman AH, Morgan BP, Barnum SR, van der Ende A, Koedel U, van de Beek D. 2011. Complement component 5 contributes to poor disease outcome in humans and mice with pneumococcal meningitis. J Clin Invest 121:3943–3953. doi: 10.1172/JCI57522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mook-Kanamori BB, Rouse MS, Kang C-I, van de Beek D, Steckelberg JM, Patel R. 2009. Daptomycin in experimental murine pneumococcal meningitis. BMC Infect Dis 9:50. doi: 10.1186/1471-2334-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasanmoentalib ES, Valls Seron M, Morgan BP, Brouwer MC, van de Beek D. 2015. Adjuvant treatment with dexamethasone plus anti-C5 antibodies improves outcome of experimental pneumococcal meningitis: a randomized controlled trial. J Neuroinflammation 12:149. doi: 10.1186/s12974-015-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muri L, Grandgirard D, Buri M, Perny M, Leib SL. 2018. Combined effect of non-bacteriolytic antibiotic and inhibition of matrix metalloproteinases prevents brain injury and preserves learning, memory and hearing function in experimental paediatric pneumococcal meningitis. J Neuroinflammation 15:233. doi: 10.1186/s12974-018-1272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein M, Höhne C, Angele B, Högen T, Pfister HW, Tüfekci H, Koedel U. 2018. Adjuvant non-bacteriolytic and anti-inflammatory combination therapy in pneumococcal meningitis: an investigation in a mouse model. Clin Microbiol Infect 25:108.e9–108.e15. doi: 10.1016/j.cmi.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 44.Stucki A, Cottagnoud M, Winkelmann V, Schaffner T, Cottagnoud P. 2007. Daptomycin produces an enhanced bactericidal activity compared to ceftriaxone, measured by [3H]choline release in the cerebrospinal fluid, in experimental meningitis due to a penicillin-resistant pneumococcal strain without lysing its cell wall. Antimicrob Agents Chemother 51:2249–2252. doi: 10.1128/AAC.01000-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grandgirard D, Schürch C, Cottagnoud P, Leib SL. 2007. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob Agents Chemother 51:2173–2178. doi: 10.1128/AAC.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber P, Stucki A, Acosta F, Cottagnoud M, Cottagnoud P. 2006. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J Antimicrob Chemother 57:720–723. doi: 10.1093/jac/dkl007. [DOI] [PubMed] [Google Scholar]
- 47.Kullar R, Chin JN, Edwards DJ, Parker D, Coplin WM, Rybak MJ. 2011. Pharmacokinetics of single-dose daptomycin in patients with suspected or confirmed neurological infections. Antimicrob Agents Chemother 55:3505–3509. doi: 10.1128/AAC.01741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cottagnoud P, Pfister M, Acosta F, Cottagnoud M, Flatz L, Kuhn F, Muller H-P, Stucki A. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob Agents Chemother 48:3928–3933. doi: 10.1128/AAC.48.10.3928-3933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yim CW, Flynn NM, Fitzgerald FT. 1985. Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis. Antimicrob Agents Chemother 28:347–348. doi: 10.1128/AAC.28.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee H-M, Golub LM. 2004. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol 24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 51.Roach D, Fitridge R, Laws P, Millard S, Varelias A, Cowled P. 2002. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur J Vasc Endovasc Surg 23:260–269. doi: 10.1053/ejvs.2002.1598. [DOI] [PubMed] [Google Scholar]
- 52.Golub LM, Lee H-M, Ryan ME, Giannobile WV, Payne J, Sorsa T. 1998. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 53.Pasquale TR, Tan JS. 2005. Nonantimicrobial effects of antibacterial agents. Clin Infect Dis 40:127–135. doi: 10.1086/426545. [DOI] [PubMed] [Google Scholar]
- 54.Gabler WL, Creamer HR. 1991. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res 26:52–58. doi: 10.1111/j.1600-0765.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 55.Suomalainen K, Sorsa T, Golub LM, Ramamurthy N, Lee HM, Uitto VJ, Saari H, Konttinen YT. 1992. Specificity of the anticollagenase action of tetracyclines: relevance to their anti-inflammatory potential. Antimicrob Agents Chemother 36:227–229. doi: 10.1128/AAC.36.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meli DN, Coimbra RS, Erhart DG, Loquet G, Bellac CL, Täuber MG, Neumann U, Leib SL. 2006. Doxycycline reduces mortality and injury to the brain and cochlea in experimental pneumococcal meningitis. Infect Immun 74:3890–3896. doi: 10.1128/IAI.01949-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paul R, Lorenzl S, Koedel U, Sporer B, Vogel U, Frosch M, Pfister H-W. 1998. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann Neurol 44:592–600. doi: 10.1002/ana.410440404. [DOI] [PubMed] [Google Scholar]
- 58.Leib SL, Leppert D, Clements J, Täuber MG. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 68:615–620. doi: 10.1128/IAI.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org/clinical_breakpoints/.
- 60.Pankuch GA, Jacobs MR, Appelbaum PC. 2003. Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials. J Antimicrob Chemother 51:443–446. doi: 10.1093/jac/dkg091. [DOI] [PubMed] [Google Scholar]
- 61.Tomasz A, Moreillon P, Pozzi G. 1988. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J Bacteriol 170:5931–5934. doi: 10.1128/jb.170.12.5931-5934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis 151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 63.Nau R, Eiffert H. 2005. Minimizing the release of proinflammatory and toxic bacterial products within the host: A promising approach to improve outcome in life-threatening infections. FEMS Immunol Med Microbiol 44:1–16. doi: 10.1016/j.femsim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Nau R, Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. 2000. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis 31:80–84. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- 66.Rosenberg GA. 2002. Matrix metalloproteinases in neuroinflammation. Glia 39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. 2007. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 68.McColl BW, Rothwell NJ, Allan SM. 2008. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci 28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellner J, Leib SL. 2006. In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol Dis 21:647–656. doi: 10.1016/j.nbd.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Leppert D, Lindberg RLP, Kappos L, Leib SL. 2001. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Rev 36:249–257. doi: 10.1016/S0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 71.Khokha R, Murthy A, Weiss A. 2013. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 72.Song J, Wu C, Zhang X, Sorokin LM. 2013. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1 -induced peritonitis. J Immunol 190:401–410. doi: 10.4049/jimmunol.1202286. [DOI] [PubMed] [Google Scholar]
- 73.Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang G-Y, Young WL. 2004. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- 74.Clark WM, Lessov N, Lauten JD, Hazel K. 1997. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J Mol Neurosci 9:103–108. doi: 10.1007/BF02736854. [DOI] [PubMed] [Google Scholar]
- 75.Steenbergen JN, Alder J, Thorne GM, Tally FP. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother 55:283–288. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 76.Sauermann R, Rothenburger M, Graninger W, Joukhadar C. 2008. Daptomycin: a review 4 years after first approval. Pharmacology 81:79–91. doi: 10.1159/000109868. [DOI] [PubMed] [Google Scholar]
- 77.Baltz RH. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr Opin Chem Biol 13:144–151. doi: 10.1016/j.cbpa.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 78.Grandgirard D, Oberson K, Bühlmann A, Gäumann R, Leib SL. 2010. Attenuation of cerebrospinal fluid inflammation by the nonbacteriolytic antibiotic daptomycin versus that by ceftriaxone in experimental pneumococcal meningitis. Antimicrob Agents Chemother 54:1323–1326. doi: 10.1128/AAC.00812-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barichello T, Gonçalves JCN, Generoso JS, Milioli GL, Silvestre C, Costa CS, da Rosa Coelho J, Comim CM, Quevedo J. 2013. Attenuation of cognitive impairment by the nonbacteriolytic antibiotic daptomycin in Wistar rats submitted to pneumococcal meningitis. BMC Neurosci 14:42. doi: 10.1186/1471-2202-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maitra SR, Bhaduri S, Valane PD, Tervahartiala T, Sorsa T, Ramamurthy N. 2003. Inhibition of matrix metalloproteinases by chemically modified tetracyclines in sepsis. Shock 20:280–285. doi: 10.1097/00024382-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Solomon A, Rosenblatt M, Li DQ, Liu Z, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. 2000. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci 41:2544–2557. [PubMed] [Google Scholar]
- 82.Senn P, Rostetter C, Arnold A, Kompis M, Vischer M, Häusler R, Ozdoba C, Mantokoudis G, Caversaccio M. 2012. Retrograde cochlear implantation in postmeningitic basal turn ossification. Laryngoscope 122:2043–2050. doi: 10.1002/lary.23397. [DOI] [PubMed] [Google Scholar]
- 83.Bally L, Grandgirard D, Leib SL. 2016. Inhibition of hippocampal regeneration by adjuvant dexamethasone in experimental infant rat pneumococcal meningitis. Antimicrob Agents Chemother 60:1841–1846. doi: 10.1128/AAC.02429-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zysk G, Brück W, Gerber J, Brück Y, Prange HW, Nau R. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol 55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Zwijnenburg PJG, van der Poll T, Florquin S, van Deventer SJH, Roord JJ, van Furth AM. 2001. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J Infect Dis 183:1143–1146. doi: 10.1086/319271. [DOI] [PubMed] [Google Scholar]
- 86.Piva S, Di Paolo A, Galeotti L, Ceccherini F, Cordoni F, Signorini L, Togni T, De Nicolò A, Rasulo FA, Fagoni N, Latronico N, D’Avolio A. 3 January 2019. Daptomycin plasma and CSF levels in patients with healthcare-associated meningitis. Neurocrit Care. doi: 10.1007/s12028-018-0657-y. [DOI] [PubMed] [Google Scholar]
- 87.Hupp S, Heimeroth V, Wippel C, Förtsch C, Ma J, Mitchell TJ, Iliev AI. 2012. Astrocytic tissue remodeling by the meningitis neurotoxin pneumolysin facilitates pathogen tissue penetration and produces interstitial brain edema. Glia 60:137–146. doi: 10.1002/glia.21256. [DOI] [PubMed] [Google Scholar]
- 88.Klugman KP, Friedland IR, Bradley JS. 1995. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 39:1988–1992. doi: 10.1128/AAC.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karlsson M, Hammers S, Nilsson-Ehle I, Malmborg AS, Wretlind B. 1996. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob Agents Chemother 40:1104–1107. doi: 10.1128/AAC.40.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gianinazzi C, Grandgirard D, Imboden H, Egger L, Meli DN, Bifrare Y-D, Joss PC, Täuber MG, Borner C, Leib SL. 2003. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol 105:499–507. doi: 10.1007/s00401-003-0672-7. [DOI] [PubMed] [Google Scholar]
- 91.Gehre F, Leib SL, Grandgirard D, Kummer J, Bhlmann A, Simon F, Gumann R, Kharat AS, Tuber MG, Tomasz A. 2008. Essential role of choline for pneumococcal virulence in an experimental model of meningitis. J Intern Med 264:143–154. doi: 10.1111/j.1365-2796.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- 92.van Furth AM, Roord JJ, van Furth R. 1996. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun 64:4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]