We describe the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) exposure against multidrug-resistant Pseudomonas aeruginosa (meropenem MICs 8 to >256 μg/ml) in a neutropenic murine thigh infection model. WCK 5222 MICs ranged from 4 to 32 μg/ml.
KEYWORDS: human simulated, pharmacodynamics, pharmacokinetics
ABSTRACT
We describe the in vivo efficacy of human-simulated WCK 5222 (cefepime-zidebactam) exposure against multidrug-resistant Pseudomonas aeruginosa (meropenem MICs 8 to >256 μg/ml) in a neutropenic murine thigh infection model. WCK 5222 MICs ranged from 4 to 32 μg/ml. Substantial in vivo WCK 5222 activity was observed against all isolates, further enhancing the efficacy of zidebactam alone in 11/16 isolates (WCK 5222 mean reduction, –1.62 ± 0.58 log10 CFU/thigh), and a lack of activity was observed with cefepime monotherapy.
TEXT
Pseudomonas aeruginosa is a leading cause of health care-associated infections (HAIs), ranking in the top five causative organisms for ventilator-associated pneumonia (VAP) (no. 2), catheter-associated urinary tract infections (no. 3), and surgical-site infections (no. 5) between the years 2011 and 2014 (1). Among VAP P. aeruginosa isolates, approximately 28% were carbapenem resistant, and 20% were deemed multidrug resistant (MDR) (1). Multidrug resistance among P. aeruginosa is often multifactorial due to the organism’s ability to acquire multiple resistance mechanisms, including β-lactamase production, overexpression of efflux pumps, downregulation of porin production, and target site modifications (2). These properties render P. aeruginosa infections a serious threat, as clinicians are often compelled to adopt more aggressive treatment strategies, such as the use polymyxins, despite their toxicity.
Zidebactam is a non-β-lactam, bicyclo-acyl hydrazide antibiotic derived from diazabicyclooctane scaffold, with dual mechanisms of action, demonstrating high affinity for penicillin-binding protein 2 (PBP2) binding in Gram-negative bacteria, including P. aeruginosa isolates, and exhibiting β-lactamase inhibition against classes A and C enzymes (3). When paired with an appropriate β-lactam (i.e., cefepime), zidebactam has been shown to function as a β-lactam enhancer (4). In vivo, a human-simulated regimen (HSR) of the combination of cefepime and zidebactam (WCK 5222) caused extensive (i.e., >1 to 2 log) eradication of carbapenem-resistant Acinetobacter spp. from neutropenic mouse lungs and thighs (5, 6). In vitro, WCK 5222 inhibited 99.5% of P. aeruginosa isolates at concentrations of ≤8 μg/ml (1:1 ratio), including carbapenem-resistant P. aeruginosa isolates (WCK 5222 MIC90s against P. aeruginosa of 4 μg/ml and against AmpC-overproducing and metallo-β-lactamase-producing P. aeruginosa of 8 μg/ml); however, in vivo data against P. aeruginosa are currently lacking (7, 8).
We sought here to evaluate the in vivo efficacy of the WCK 5222 HSR compared to cefepime HSR or zidebactam HSR alone against MDR P. aeruginosa isolates in the neutropenic murine thigh infection model. In order to demonstrate the robustness of the WCK 5222 combination against the most challenging P. aeruginosa strains, we were specifically interested in examining the efficacy against isolates with a WCK 5222 MIC of ≥MIC90 against P. aeruginosa (4 μg/ml). To guide the isolate selection, a large collection of clinical P. aeruginosa isolates (n = 70) were initially screened for the WCK 5222 MICs (cefepime-zidebactam at a 1:1 ratio), and 16 isolates were then selected for the in vivo efficacy assessment. All 16 isolates were MDR (defined as nonsusceptibility to at least one agent in three or more antimicrobial classes) and meropenem resistant (meropenem MICs of 8 to >256 μg/ml). Several isolates produced β-lactamases, including Pseudomonas-derived cephalosporinase (PDC), metallo-β-lactamases (NDM and VIM), and extended-spectrum β-lactamases (VEB and CTX-M).
The MICs of cefepime, zidebactam WCK 5222, meropenem, piperacillin-tazobactam, ceftolozane-tazobactam, aztreonam, amikacin, and ciprofloxacin were determined in triplicate using the broth microdilution as outlined by the Clinical and Laboratory Standards Institute (9).
Female ICR mice weighing 20 to 22 g (Charles River Laboratories, Inc., Wilmington, MA) were utilized in the study, and the protocol was approved by the Hartford Hospital Institutional Animal Care and Use Committee. The neutropenic murine thigh model was used as previously published (5). Treatment and control groups were composed of three mice each. Cefepime (Qilu Antibiotics, Jinan, China), zidebactam (Wockhardt Bio AG, Switzerland), and WCK 5222 treatment groups received previously established HSRs administered by subcutaneous injections equivalent to clinical doses of 2 g every 8 h (q8h; 1-h infusion), 1 g q8h (1-h infusion), or cefepime HSR coadministered with zidebactam HSR, respectively (6, 10). A confirmatory pharmacokinetic study was completed to validate human WCK 5222 exposures according to a published regimen (6). Control mice were vehicle dosed. The initial CFU burden was assessed prior to dose administration (0 h) for each isolate as mice were euthanized and their thighs harvested, as previously described (11). Efficacy was quantified by the change in bacterial density (Δlog10 CFU/thigh) obtained in the treatment mice after 24 h relative to the 0-h untreated controls. The values corresponding to the log10 change in CFU at 24 h for zidebactam and WCK 5222 were compared using a Student t test (equal variances were assumed) using SigmaPlot 14.0 (Systat Software, Inc., San Jose, CA). A P value of ≤0.05 was considered significant.
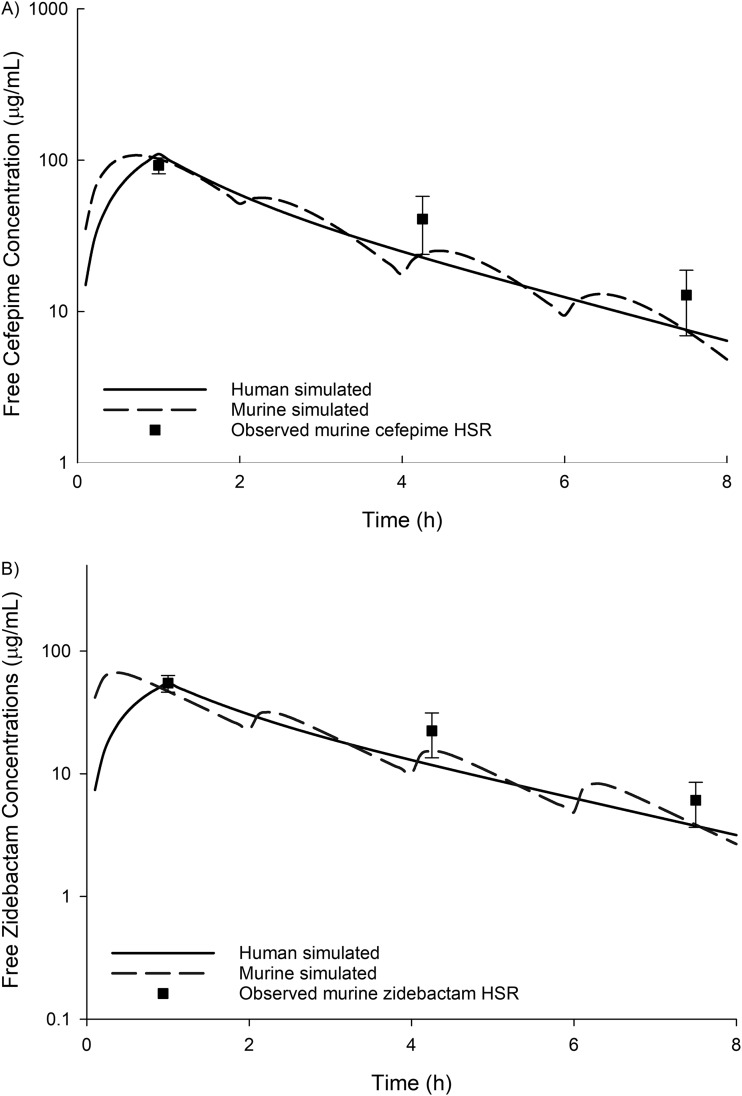
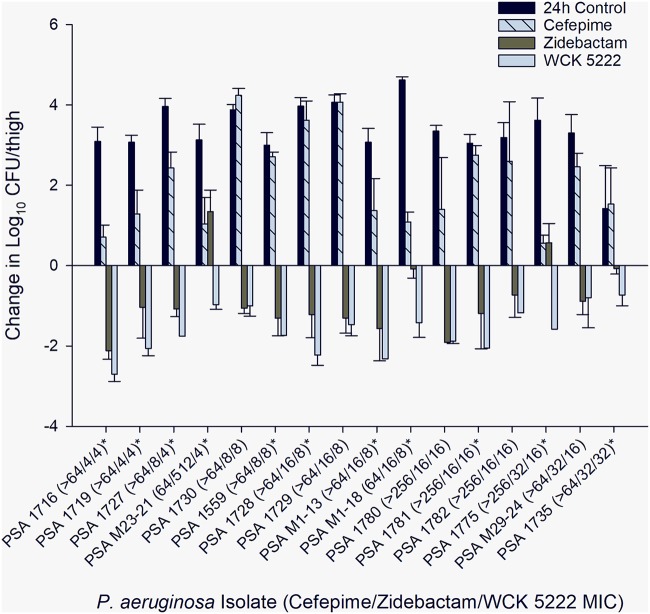
For the 16 MDR-P aeruginosa studied, the cefepime, zidebactam, and WCK 5222 MICs were ≥64, 4 to 512, and 4 to 32 μg/ml, respectively (Table 1). For one isolate (M23-21), WCK 5222 showed considerably improved in vitro potency compared to the use of zidebactam alone, with zidebactam and WCK 5222 MICs of 512 and 4 μg/ml, respectively. The results from the confirmatory pharmacokinetic study are presented in Fig. 1. The mean log10 CFU/thigh at 0 h was 4.75±0.43. Compared to the 0-h control, the changes in mean bacterial density at 24 h in untreated control mice and in mice treated with cefepime, zidebactam, and WCK 5222 arms were +3.36±0.71, +2.12±1.17, –0.85±0.90, and –1.62±0.58 log10 CFU/thigh, respectively (Fig. 2). A reduction in bacterial burden was observed with the WCK 5222 HSR for all isolates. Compared to treatment with zidebactam alone, WCK 5222 demonstrated enhanced antibacterial activity in 11/16 isolates (P < 0.05), most notably for M23-21 (+1.34±0.54 log10 CFU bacterial growth with zidebactam HSR, –0.97±0.12 log10 CFU bacterial kill with WCK 5222 HSR) and 1775 (+0.57±0.48 log10 CFU bacterial growth with zidebactam HSR, –1.58±0.00 log10 CFU bacterial kill with WCK 5222 HSR). For isolates with a WCK 5222 MIC of <32 μg/ml, WCK 5222 achieved >1 log10 CFU kill against 13/15 isolates inclusive of 4/5 with MICs of 16 μg/ml.
TABLE 1.
Phenotypic profiles and resistance mechanisms of the isolates selected for the in vivo efficacy studies
| Organism | MIC (μg/ml) |
β-Lactamase(s) encoded | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cefepime | Zidebactam | WCK 5222 | Meropenem | Piperacillin-tazobactam | Ceftolozane-tazobactam | Aztreonam | Amikacin | Ciprofloxacin | ||
| PSA 1716 | >64 | 4 | 4 | ≥64 | ≥128 | ≥64 | 32 | >32 | ≥8 | VIM-28 |
| PSA 1719 | >64 | 4 | 4 | ≥8 | ≥128 | ≥64 | ≥16 | >32 | ≥8 | VIM-1 |
| PSA 1727 | >64 | 8 | 4 | ≥8 | ≥64 | ≥64 | 8 | >32 | ≥8 | PDC-3, VIM-2 |
| PSA M23-21 | 64 | 512 | 4 | 8 | 128 | 8 | 128 | 256 | 1 | NAa |
| PSA 1730 | >64 | 8 | 8 | ≥64 | ≥128 | ≥64 | ≥16 | >32 | ≥8 | VEB-14, VIM-49 |
| PSA 1559 | >64 | 8 | 8 | 16 | >32 | 64 | >128 | 32 | >16 | NA |
| PSA 1728 | >64 | 16 | 8 | ≥64 | ≥128 | ≥64 | 4 | >32 | ≥8 | VIM-2 |
| PSA 1729 | >64 | 16 | 8 | ≥64 | ≥64 | ≥64 | ≥16 | >32 | ≥8 | PDC-11, VEB-14, VIM-49 |
| PSA M1-13 | >64 | 16 | 8 | 32 | 128 | 128 | 128 | 128 | 32 | NA |
| PSA M1-18 | 64 | 16 | 8 | 16 | 512 | 8 | 128 | 32 | 0.5 | NA |
| PSA 1780 | >256 | 16 | 16 | >256 | 256 | >256 | >64 | 128 | >16 | NDM, VEB, CTX-M |
| PSA 1781 | >256 | 16 | 16 | >256 | >256 | >256 | >64 | >256 | >16 | VIM, OXA-4, CTX-M |
| PSA 1782 | >256 | 16 | 16 | >256 | >256 | >256 | >64 | >256 | >16 | NDM, CTX-M |
| PSA 1775 | >256 | 32 | 16 | >256 | >256 | >256 | 32 | >256 | >16 | NDM, CTX-M |
| PSA M29-24 | >64 | 32 | 16 | 32 | 256 | 2 | 32 | 256 | 4 | NA |
| PSA 1735 | >64 | 32 | 32 | ≥64 | ≥64 | 64 | ≥16 | >32 | 4 | PDC-3 |
NA, not available.
FIG 1.
Observed cefepime (A) and zidebactam (B) free plasma concentrations in the neutropenic thigh infection model following human-simulated regimens.
FIG 2.
Mean bacterial changes in log10 CFU/thigh ± the standard deviations at 24 h relative to the starting inoculum in a neutropenic murine thigh infection model. MICs are displayed in μg/ml. *, statistically significant difference (P < 0.05) between zidebactam and WCK 5222.
Effective antimicrobials against MDR P. aeruginosa remain scarce, highlighting the need for agents with novel mechanisms of action. In our study, HSR of zidebactam alone demonstrated in vivo activity against MDR P. aeruginosa, inclusive of metallo-β-lactamase-producing high-risk clones, which reflected the compound’s PBP binding capability in P. aeruginosa. WCK 5222 demonstrated enhanced efficacy over zidebactam alone in the majority of isolates up to an MIC of 32 μg/ml, a finding attributed to the β-lactam enhancing effect of zidebactam, driven by the complementary PBP binding of cefepime (PBP1 and PBP3) and zidebactam (PBP2) (3). Our findings support the clinical translatability of previous in vitro data demonstrating zidebactam's enhancement of the activity of β-lactams against P. aeruginosa exhibiting various resistance mechanisms, including isolates resistant to ceftolozane-tazobactam, an agent often reserved for infections caused by MDR P. aeruginosa (3). Furthermore, our data demonstrated potent WCK 5222 in vivo activity against P. aeruginosa isolates at the top 10% of the WCK 5222 MIC distribution (7, 8). In summary, data derived in the present study display the in vitro and in vivo activities of WCK 5222 against MDR and metallo-β-lactamase-producing P. aeruginosa, as well as assist with the delineation of the MIC susceptibility breakpoints. These results support the clinical evaluation of WCK 5222 for the management of infections due to MDR P. aeruginosa.
ACKNOWLEDGMENTS
This study was funded by Wockhardt Bio AG, Switzerland.
We acknowledge Sara Giovagnoli, Janice Cunningham, Elizabeth Cyr, Kim Greenwood, Michelle Insignares, James Kidd, Lauren McLellan, Alissa Padgett, Debora Santini, Christina Sutherland, Courtney Bouchard, Nicole DeRosa, Elias Mullane, Tomefa Asempa, Lindsay Avery, and Iris Chen from the Center for Anti-Infective Research and Development, Hartford, CT, for their dedication to exceptional technical quality and their assistance with this study.
REFERENCES
- 1.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol l37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch EB, Tam VH. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res 10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Ravikumar T, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, van den Akker F, Bonomo RA. 2018. Strategic approaches to overcome resistance against Gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61:4067–4086. doi: 10.1021/acs.jmedchem.8b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abuhussain SSA, Avery LM, Abdelraouf K, Nicolau DP. 2018. In vivo efficacy of humanized WCK 5222 (cefepime-zidebactam) exposures against carbapenem-resistant Acinetobacter baumannii in the neutropenic thigh model. Antimicrob Agents Chemother 63:e01931-18. doi: 10.1128/AAC.01931-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avery LM, Abdelraouf K, Nicolau DP. 2018. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother 62:e00948-18. doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, M100, 28th ed. CLSI, Wayne, PA. [Google Scholar]
- 10.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Patel A, Chavan R, Yeole R, Friedland HD, Bhatia A. 2018. Plasma and intrapulmonary concentrations of cefepime and zidebactam following intravenous administration of WCK 5222 to healthy adult subjects. Antimicrob Agents Chemother 62:e00682-18. doi: 10.1128/AAC.00682-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]