Urinary biomarkers are superior to serum creatinine for defining onset and extent of kidney injury. This study classifies the temporal predictive ability of biomarkers for vancomycin-induced kidney injury (VIKI) as defined by histopathologic damage.
KEYWORDS: KIM-1, PK/PD, PK/TD, biomarker, histopathology, injury, kidney, toxicity, urinary, vancomycin
ABSTRACT
Urinary biomarkers are superior to serum creatinine for defining onset and extent of kidney injury. This study classifies the temporal predictive ability of biomarkers for vancomycin-induced kidney injury (VIKI) as defined by histopathologic damage. Male Sprague-Dawley rats (n = 125) were randomized to receive 150 to 400 mg/kg of body weight/day vancomycin via once or twice daily intraperitoneal injection over 1, 3, or 6 days. Urine was collected once during the 24 h prior to euthanasia or twice for rats treated for 6 days. Receiver operating characteristic (ROC) curves were employed to assess the urinary biomarker performances of kidney injury molecule 1 (KIM-1), clusterin, osteopontin (OPN), cystatin C, and neutrophil gelatinase-associated lipocalin (NGAL) to predict histopathologically defined VIKI (using a national standard pathological assessment scheme from hematoxylin and eosin stained kidneys). Urinary KIM-1, clusterin, and OPN outperformed cystatin C and NGAL with regard to sensitivity and specificity. For the earliest injury, urinary KIM-1 (area under the receiver operating characteristic curve [AUC], 0.662; P < 0.001) and clusterin (AUC, 0.706; P < 0.001) were the most sensitive for predicting even low-level histopathologic damage at 24 h compared to NGAL. KIM-1 and clusterin are the earliest and most sensitive predictors of VIKI. As injury progresses, KIM-1, clusterin, and OPN best define the extent of damage.
TEXT
Vancomycin is among the most frequently prescribed antibiotics in U.S. hospitals (1–4) and the gold standard for treating methicillin-resistant Staphylococcus aureus (MRSA) infections (5). Despite its common use, vancomycin-induced kidney injury (VIKI) is a serious yet potentially avoidable adverse drug event. Rates of VIKI have been estimated to be between 5 and 43% (6–10). VIKI is clinically defined as a minimum of two elevations in serum creatinine concentration (i.e., rise of 0.5 mg/dl or a ≥50% increase from baseline, whichever is greater) after several days of vancomycin therapy (11). A single randomized controlled trial between vancomycin and linezolid demonstrated an attributable rate of acute kidney injury of ∼10% (12). With over 3 million patients (3, 4, 13) estimated to receive vancomycin annually, the attributable harm of VIKI annually is approximately 300,000 in the United States alone.
We have previously demonstrated that urinary biomarkers, such as kidney injury molecule-1 (KIM-1) and osteopontin (OPN), are sensitive predictors for histopathologic damage in VIKI (14, 15), specifically proximal tubule injury (14). Traditional markers, such as elevations in serum creatinine (SCr) and development of oliguria, are delayed events and are downstream effects from a reduction in glomerular filtration rather than early indicators of the tubular injury itself (16). Since diagnosis of acute kidney injury using traditional markers is delayed in onset until injury has progressed, dosing of vancomycin can continue well beyond the time of the initial insult, further compounding injury. Thus, it is desirable to understand both the onset and magnitude of injury so that therapy can be stopped or modified or an alternative therapy can be initiated. This study builds on previous work (14, 15) and classifies the temporal predictive ability of various urinary biomarkers for VIKI as defined by histopathologic damage using a translational rat model of kidney disease.
RESULTS
Characteristics of animal cohort.
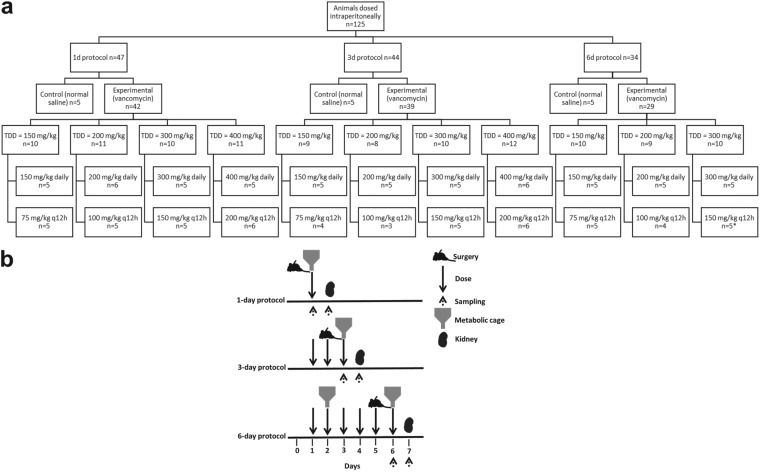
The distribution of animals according to treatment group, fractionation scheme, and duration of exposure is outlined in Fig. 1a. Of the 22 unique treatment groups, animal numbers were consistent with a median of 5 animals per group and 21/22 groups contributing >4 animals with complete urinary biomarker data. Protocols were followed as described in the Materials and Methods with an exception that all five animals receiving vancomycin 150 mg/kg of body weight every 12 h (q12h) in the 6-day protocol missed the second dose on day 6 but contributed urinary biomarker and histopathological data. Differences in animal weight loss postcatheterization according to vancomycin treatment or control status, protocol duration, and daily dose were measured. Mean weight loss was not significantly different between vancomycin-treated and control animals overall (21.6 g versus 15.3 g; P = 0.10). Likewise, animal weight loss did not differ between vancomycin-treated and control animals according to protocol duration for the 1-day (P = 0.95) and 3-day (P = 0.14) protocol groups. However, a significant difference in weight loss was observed between vancomycin-treated and control animals in the 6-day protocol group (23.6 ± 5.1 g versus −0.6 ± 1.4 g; P < 0.0001). This weight loss did not increase with an increase in total daily dose (P = 0.99).
FIG 1.
(a) Flow chart for animal dosing. TDD, total daily dose; q12h, every 12 h; *, missed second dose on day 6. (b) Timeline of the experiments.
Urinary output, histopathology, immunohistochemistry, and urinary biomarker concentrations after exposure to vancomycin or normal saline.
Urinary output measurements were stratified according to vancomycin treatment or control status, protocol duration, and daily dose. The overall mean ± standard deviation (SD) urine output over a 24-h collection period was 12.6 ± 5.4 ml (n = 125). Mean total urine output did not differ between vancomycin-treated and control animals overall (12.5 versus 14.0 ml; P = 0.19) or in the subgroups stratified by days of study (data not shown). Similarly, changes in urine output were not associated with differences in total daily dose within each protocol group (P = 0.90).
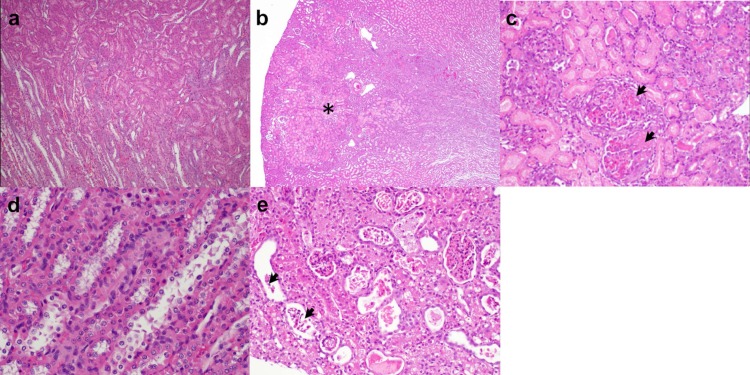
The median worst ordinal histopathological scores were significantly increased in the vancomycin-treated animals compared to those of the controls overall (1 versus 0; P = 0.04) and in subsets of the 3-day (1 versus 0; P = 0.03) and 6-day (1 versus 0; P = 0.03) protocol groups. Intraperitoneal administration of vancomycin was associated with dose-responsive nephrotoxicity with intratubular casts and sloughed cells in the cortical tubules and tubular cell degeneration/necrosis/apoptosis in the outer medulla that became pronounced after 72 h of treatment (Fig. 2b, c, and e) and showed additional progressive changes in the outer cortex through day 7. No KIM-1 labeling was evident in sections of renal cortex from control animals (see Fig. S1a in the supplemental material). By contrast, samples from vancomycin-treated animals (see Fig. S1b) showed high levels of KIM-1 labeling (brown color) in the cytoplasm and on the apical surface of the epithelial cells in most of the proximal tubules (arrows).
FIG 2.
Representative photomicrographs of hematoxylin and eosin (H&E) stained rat kidney sections. (a and d) Normal histology of kidney tissue in control rats administered normal saline intraperitoneally once daily for 24 h (×40) and 72 h (×200), respectively. (b) Classic wedge-shaped region of infarct (asterisk, ×20) with (c) necrosis of tubular epithelium and portions of glomeruli (arrowheads, ×100) in cortex of rats treated with vancomycin 200 mg/kg once daily for 24 h. (e) Multiple tubules containing sloughed cells (arrowheads) in cortex of rats treated with vancomycin 400 mg/kg once daily for 72 h (×100).
Urinary KIM-1 was significantly higher in the vancomycin-treated animals than in the controls (P < 0.03) and increased with protocol duration from 1.3 to 1.5 to 8.5 to 9.3 ng/ml after 1, 2, 3, and 6 days of vancomycin treatment, respectively (data not shown). We ran a forward (data not shown) and backward, stepwise, multivariate logistic regression model with similar results. In the multivariate model across all study days, only KIM-1 (P < 0.001) and daily dose of vancomycin (P = 0.0135) were retained in the model to predict histopathologic damage (see Table S1 in the supplemental material). Every 1 ng/ml increase of KIM-1 resulted in a 1.3-fold increased likelihood of having a histopathologic score of ≥2 (see Fig. S2 in the supplemental material).
ROC analyses.
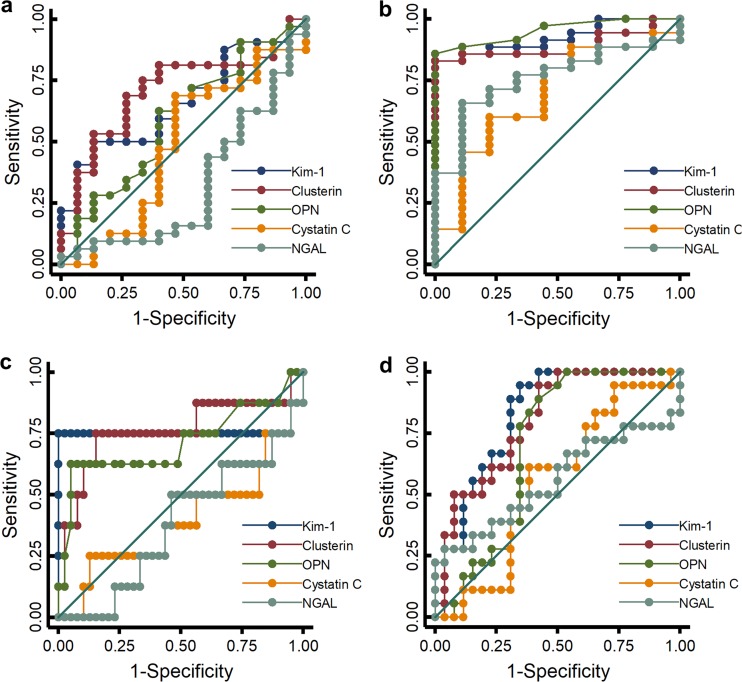
A visual display of the predictive performance of biomarkers stratified by histopathologic score and protocol length is shown in Fig. 3. Urinary KIM-1, clusterin, and OPN generally outperformed cystatin C and neutrophil gelatinase-associated lipocalin (NGAL) with regard to sensitivity and specificity. For the earliest injury, urinary KIM-1 (area under the receiver operating characteristic curve [AUC], 0.662; P = 0.003) (Table 1; see also Fig. S3 in the supplemental material) and clusterin (AUC, 0.706; P < 0.001) were most sensitive for predicting even low-level histopathologic damage (≥1) at 24 h when compared to NGAL. In the same time frame, OPN was the next most predictive of histopathology (≥1; AUC, 0.59; P = 0.051). Results were similar for more significant histopathologic damage at day 1 (i.e., score of ≥2) but did not reach statistical significance. After 3 days of vancomycin treatment, histopathology of ≥2 was best predicted by KIM-1 (AUC, 0.822; P = 0.037 versus NGAL) though clusterin approached significance (AUC, 0.801; P = 0.06 versus NGAL). KIM-1 concentrations of 0.62 ng/ml, 6.11 ng/ml, and 19.08 ng/ml were the cut points for classifying histopathologic damage of ≥1, ≥2, and ≥3, respectively (see Table S2 in the supplemental material). Thus, KIM-1 and clusterin were the best predictors of early damage and performed well from minor (histopathology score of ≥1) to more severe (histopathology score of ≥3) damage. Cystatin C and NGAL showed poor correlation with histopathologic damage (see Fig. S3). Results were similar when the total amount of urinary KIM-1, clusterin, OPN, cystatin C, or NGAL excreted per 24 h was used as an endpoint (instead of urinary concentrations). In the 1-day protocol, we were missing 5 urine final volumes for the controls, so our data set contains n = 42 for the total amount of biomarkers excreted in 24 h compared to n = 47 for the biomarker concentrations. Receiver operating characteristic (ROC) curves were repeated with total biomarker amounts (see Fig. S4 and Table S3 in the supplemental material). KIM-1 and clusterin still remained the best predictors of early damage.
FIG 3.
ROC analysis for vancomycin studies. (a to d) ROC curves demonstrating sensitivity and specificity of urinary KIM-1, clusterin, OPN, cystatin C, and NGAL with respect to a histopathology grade of ≥1 on day 1 (a) and on day 3 (b) and a histopathology grade of ≥2 on day 1 (c) and on day 3 (d). Animal numbers, n; histopath = 0, n = 32; histopath ≥ 1, n = 93; histopath ≥ 2, n = 37.
TABLE 1.
Comparative analysis of diagnostic performance characteristics of urinary biomarkers with different severity grades of histopathology using area under the receiver operating characteristic curvesa
| Subgroup | Biomarker (ng/ml) | Day 1 biomarkers predicting 24 h histopath (AUC [95% CI]) | Day 3 biomarkers predicting 72 h histopath (AUC [95% CI]) | Day 2 biomarkers predicting 144 h histopath (AUC [95% CI]) | Day 6 biomarkers predicting 144 h histopath (AUC [95% CI]) |
|---|---|---|---|---|---|
| Histopath of ≥1 | NGAL | 0.320 (0.14–0.50) | 0.758 (0.61–0.91) | 0.649 (0.39–0.91) | 0.760 (0.56–0.96) |
| KIM-1 | 0.661 (0.50–0.82) | 0.923 (0.85–1.00) | 0.752 (0.59–0.91) | 0.918 (0.82–1.00) | |
| Clusterin | 0.706 (0.55–0.86) | 0.892 (0.80–0.99) | 0.754 (0.59–0.92) | 0.889 (0.78–1.00) | |
| OPN | 0.590 (0.41–0.77) | 0.952 (0.90–1.00) | 0.615 (0.41–0.82) | 0.757 (0.58–0.94) | |
| Cystatin C | 0.483 (0.29–0.68) | 0.688 (0.49–0.89) | 0.692 (0.47–0.91) | 0.538 (0.29–0.78) | |
| Histopath of ≥2 | NGAL | 0.381 (0.16–0.60) | 0.555 (0.36–0.75) | 0.668 (0.44–0.90) | 0.71 (0.53–0.89) |
| KIM-1 | 0.762 (0.46–1.00) | 0.822 (0.70–0.95) | 0.913 (0.79–1.00) | 0.90 (0.80–1.00) | |
| Clusterin | 0.762 (0.52–1.00) | 0.801 (0.67–0.93) | 0.861 (0.74–1.00) | 0.82 (0.68–0.96) | |
| OPN | 0.714 (0.46–0.97) | 0.695 (0.53–0.86) | 0.828 (0.68–0.98) | 0.85 (0.72–0.99) | |
| Cystatin C | 0.394 (0.14–0.65) | 0.549 (0.37–0.72) | 0.695 (0.49–0.90) | 0.55 (0.34–0.76) | |
| Histopath of ≥3 | NGAL | Outcome does not vary | 0.571 (0.37–0.77) | 0.931 (0.84–1.00) | 0.58 (0.40–0.76) |
| KIM-1 | Outcome does not vary | 0.476 (0.21–0.74) | 0.986 (0.95–1.00) | 0.83 (0.68–0.99) | |
| Clusterin | Outcome does not vary | 0.381 (0.23–0.53) | 0.793 (0.63–0.96) | 0.72 (0.53–0.92) | |
| OPN | Outcome does not vary | 0.511 (0.18–0.84) | 0.755 (0.60–0.91) | 0.69 (0.52–0.86) | |
| Cystatin C | Outcome does not vary | 0.488 (0.00–1.00) | 0.813 (0.48–1.00) | 0.37 (0.16–0.57) |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; OPN, osteopontin.
DISCUSSION
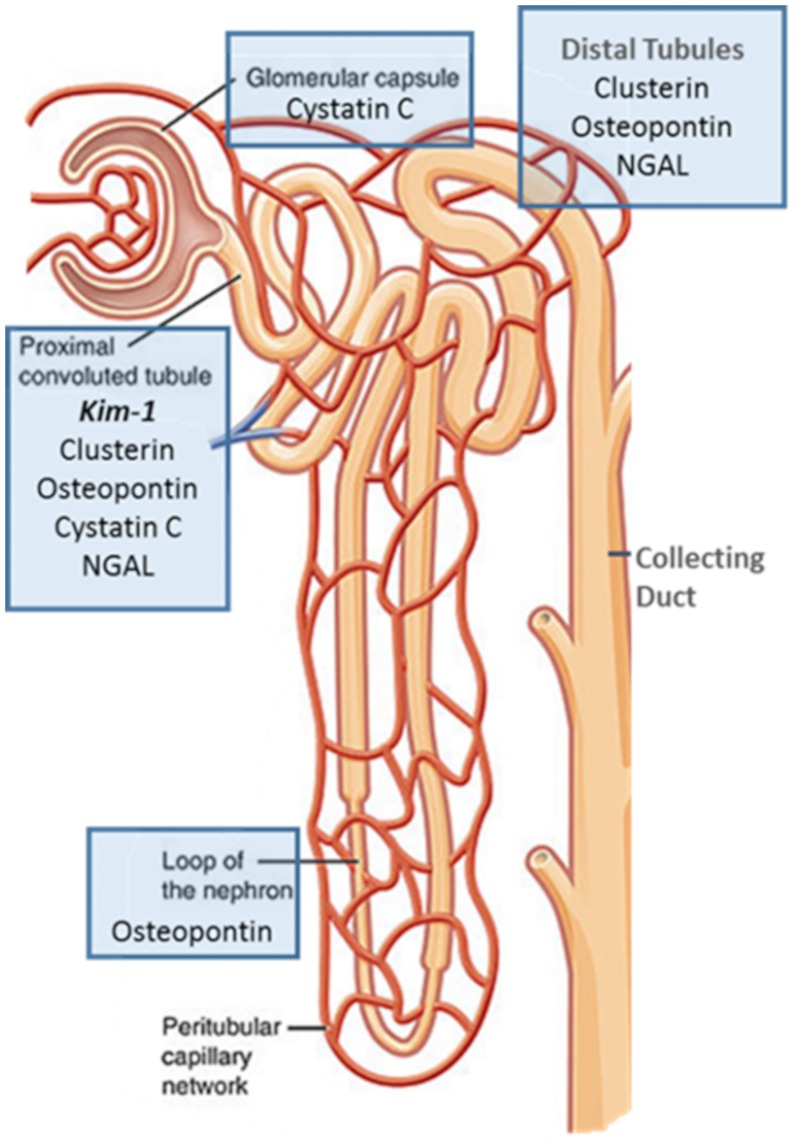
We identified KIM-1 and clusterin as the most sensitive biomarkers for early and minor histopathologic damage for kidney injury caused by vancomycin. Notably, several biomarkers have been issued letters of support for detection of xenobiotic-induced kidney injury (17) by the European Medicines Agency and U.S. Food and Drug Administration (18) for preclinical studies. Specifically, change from baseline is supported for the following biomarkers: KIM-1, clusterin, cystatin C, NGAL, and OPN. Additionally, alpha-glutathione S-transferase, albumin, and total protein are supported, but they were not studied here. Histopathologic and urinary biomarker studies by our group (14, 15, 19) and others (20–23) have confirmed locale and quantified damage to the nephron in laboratory-based and clinical studies. Since biomarkers are reasonably specific for different anatomic portions of the nephron (Fig. 4), understanding the best biomarker for each xenobiotic, such as vancomycin, is important. For vancomycin, our previous studies demonstrated the low utility of changes in SCr and blood urea nitrogen (BUN) for identifying VIKI; hence, we focused on newer biomarkers of injury in this analysis (14, 15).
FIG 4.
Schematic of the nephron (51) and urinary biomarker colocalization with injury (21). Bolded biomarker will aid in discretizing injury location.
Mechanistically, vancomycin causes oxidative stress on proximal tubule cells (24) as a function of free radical generation that leads to nephron death (25, 26). Identification of renal injury before it becomes profound is highly important clinically; thus, we sought to clarify the best biomarkers for identifying early VIKI. The traditional biomarker used to clinically identify acute kidney injury (AKI) is SCr (27, 28); however, using SCr as a biomarker requires 30 to 50% direct parenchymal damage before measurable changes indicate that a renal insult has occurred (29). In certain disease states (e.g., cirrhosis, muscle wasting, etc.) where muscle mass is low, SCr is not useful for detecting acute kidney injury with traditional thresholds (30). Notably, Vaidya et al. (39) found KIM-1 to be a more sensitive marker of kidney injury than SCr across a range of nephrotoxicants; however, KIM-1 was not specifically analyzed for vancomycin alone. In our current study, KIM-1 and clusterin measured at day 1 significantly outperformed other biomarkers of AKI in predicting histopathological damage at day 2 (Fig. 3). At days 3 and 6, clusterin and KIM-1 were the best biomarkers for detecting any histopathologic change (i.e., any score of ≥1) and more substantial injury (scores of ≥2), whereas NGAL was the poorest marker of these early injuries. OPN, which is a more general marker of damage throughout the nephron (20, 21), performed best when damage was severe (i.e., any score of ≥3) or the injury was sustained over a longer time period.
The early rise of KIM-1 and clusterin, markers of proximal tubule injury, support that VIKI initiates in the proximal tubule (14, 21, 28, 31, 32). Taken together, these results may indicate that the early damage begins in the proximal tubule but progresses to impact the entire nephron. KIM-1 is highly conserved between rats and humans; thus, the predictive capacity of rat KIM-1 for VIKI is highly compelling as a preclinical model. A human homolog, KIM-1b, is structurally similar except for the cytoplasmic domain (33).
In the multivariate model, KIM-1 and daily dose of vancomycin were most predictive of histopathologic damage (see Table S1 in the supplemental material). Each rise of 1 ng/ml of KIM-1 was associated with a 1.3-fold increase of likelihood of a histopathologic score of ≥2 (Fig. S2). Additional work will be needed to understand the full allometric links between the rat and human as well as to define thresholds for KIM-1 that are associated with meaningful changes in humans. In the rat model, histopathology is a valid and attainable endpoint, whereas this is much more difficult in studies of patients where biopsies are rare. Clinically, changes will ultimately need to be linked with SCr rise (e.g., threshold of biomarkers in early days of treatment that predict the SCr-based endpoints). In most clinical studies, VIKI has generally manifested 6 to 7 days into therapy, but these studies have relied on traditional and insensitive markers of AKI such as SCr and BUN (7, 8, 12). A recent report (34) assessed urinary KIM-1 and NGAL for clinical VIKI and validates that injury may occur and be detectable very early. These authors demonstrated that differences in creatinine by day 2 predicted VIKI (defined in their study as an increase in SCr of ≥0.3 mg/dl [26.5 μmol/liter] within 48 h or an SCr level of ≥1.5 times the baseline level within 7 days). However, urinary KIM-1 and NGAL predicted VIKI as early as day 0 and persisted through day 3 of vancomycin treatment. These results suggest that subclinical differences in kidney function may predispose to VIKI and that VIKI is predictable very early in therapy with biomarkers. Notably, this study only assessed KIM-1 and NGAL. High urinary NGAL levels are reported in infection (35–37) and were less predictive in our VIKI models. Thus, the role of NGAL is uncertain.
We acknowledge limitations to this study. Notably, a rat model was used; however, the urinary biomarkers studied are conserved in humans. Clinical studies will ultimately be needed to define the link between urinary biomarkers and clinical markers of injury (i.e., RIFLE [risk, injury, failure, loss of kidney function, and end-stage kidney disease] and AKIN [Acute Kidney Injury Network] criteria [27, 28]) for VIKI. For this study, we used allometrically scaled doses but did not analyze vancomycin exposures associated with the biomarker response. The longer duration of this study (i.e., 6 days) meant that many animals were already in renal failure at the time that vancomycin concentration monitoring was performed. Defining antecedent exposures, as we have done in previous studies (14, 15), is needed to link exposure metrics to toxicity, and those results have already been reported. The goal of this study was to identify the relationship between biomarkers and histopathologic change (i.e., the gold standard methodology for kidney damage in animal studies). Thus, exposure/response would not have been more informative than dose/response.
In conclusion, KIM-1 and clusterin are the earliest and most sensitive predictors of VIKI. As injury progresses, KIM-1 and clusterin remain the most predictive biomarkers, but OPN also predicts histopathologic damage. As these biomarkers are shared with humans, these findings will be useful in translational study. Urinary biomarkers may facilitate monitoring plans that detect subclinical kidney injury and prevent VIKI.
MATERIALS AND METHODS
Ethics.
This toxicology study was conducted at Midwestern University in Downers Grove, IL. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC; protocol number 2295) and conducted in compliance with the National Research Council’s publication, Guide for the Care and Use of Laboratory Animals, 8th edition (38).
Materials.
Treatments were clinical grade vancomycin (lot numbers 343748E03, 447358E02, 52130DD; Hospira, Lake Forrest, IL) or normal saline (veterinary 0.9% sodium chloride injection, USP; Abbott Laboratories, North Chicago, IL). Other materials were similar to our previous reports (14, 15).
Experimental design and animals.
Male Sprague-Dawley rats (n = 125) were randomized into a treatment group of vancomycin or a control group of normal saline. Animals were categorized according to duration of treatment (1-day, 3-day, or 6-day protocol). This analysis included some animals from previous reports (i.e., control animals, 1-day treated animals, and those receiving vancomycin at low doses for 3 days) (14, 15). The Rhodes study was carried out between November 2014 and February 2015, and the O’Donnell study was carried out between June 2015 and August 2015. Experimental design was similar to those reports with the following notable exception: 56 rats were additionally studied to expand the duration of study and dose range. Specifically, the added animals were treated for 3 days (n = 22) with high dose vancomycin (300 to 400 mg/kg) and for 6 days with 150, 200, or 300 mg/kg vancomycin (n = 29) or normal saline (n = 5, control group) (Fig. 1a). These cohorts were completed between June and August 2015.
In brief, all treatments were administered by intraperitoneal injection. Vancomycin-treated rats received total daily doses of 150, 200, 300, or 400 mg/kg either as a single or as a twice daily divided dose (e.g., 150 mg/kg was given as a single injection or as 75 mg/kg twice daily). Twice daily doses were given approximately 12 h apart. Thus, the study utilized a range of dose magnitudes for each treatment length in order to understand how biomarker concentrations would correspond to histopathologic damage while ensuring that a range of low to high biomarker concentrations (vis-à-vis VIKI continuum) would be observed for each treatment length. Due to the high rate of nephrotoxicity observed with the 400-mg/kg dose in the 3-day protocol, animals in the 6-day protocol were not administered the 400-mg/kg dose. The dosing range was chosen based on previous studies (14, 20, 39) and to span the human range after allometric scaling. The clinical kidney injury threshold for vancomycin in adult patients is ≥4 g/day (40). Assuming a 70-kg patient (i.e., 57 mg/kg), the rat equivalent is 345 mg/kg in a 0.3-kg rat based on the following equation: human equivalent dose = animal dose in mg/kg × (animal weight in kg/human weight in kg)0.33 (40, 41).
Rats were housed in a light- and temperature-controlled room for the duration of the study and allowed free access to water and food except during a period of time in which they resided in metabolic cages (catalog number 650-0350; Nalgene, Rochester, NY). Food was restricted during the metabolic cage period. The metabolic cage period spanned the 24 h prior to euthanasia, with the exception that animals treated for 6 days were placed in the metabolic cage on day 2 and day 6 (for 24 h per each occurrence). For example, animals that received 3 days of therapy had urine collected between hours 72 and 96, and histopathologic endpoints were at 96 h (i.e., the start of day 4) (Fig. 1b). Data were analyzed for all animals that had urinary biomarkers available for assay.
Blood and urine collection.
Blood samples were drawn from a single right-sided internal jugular vein catheter in a sedation-free manner, when possible, for the pharmacokinetic experiments as described elsewhere (14, 15). Blood samples were collected 24 h prior to the protocol end with a maximum of 8 samples per animal for all vancomycin- and control-treated animals (Fig. 1b). However, this study does not report on exposures, as they are not representative of the exposures that led to renal failure. Urine collection was completed while the animals resided in the metabolic cages (i.e., 24-h residence). Urine volume was totaled over this interval. Urine was centrifuged at 400 × g for 5 min, and supernatant was stored at −80°C until batch analysis.
Determination of urinary biomarkers of AKI.
Urine samples were analyzed in batch to determine concentrations of KIM-1, clusterin, OPN, cystatin C, and neutrophil gelatinase-associated lipocalin (NGAL). Microsphere-based Luminex xMAP technology was used for the determination of all biomarker concentrations as previously described (42, 43). Urine samples were aliquoted into 96-well plates supplied with Milliplex MAP rat kidney toxicity magnetic bead panels 1 and 2 (EMD Millipore Corporation, Charles, MO) and analyzed according to the manufacturer’s protocol. A separate standard curve was run with every assay plate per the manufacturer’s instructions. Raw data from our previous studies (14, 15) were fit using standard curves in Microsoft Excel (15) and GraphPad (14). To standardize for this combined work, we utilized a unified mathematical approach within GraphPad (i.e., the log-transformed mean fluorescence intensity versus log biomarker concentration in ng/ml fit as a 4-parameter logistic sigmoidal curve) and created individual standard curves for each plate in GraphPad.
Histopathological evaluation.
Animals were euthanized via exsanguination through the right atrium while under anesthesia with ketamine/xylazine (100/10 mg/kg, by intraperitoneal injection). Kidneys were then harvested, washed in cold isotonic saline, and preserved in 10% formalin solution. Histopathological analysis was conducted by Charles River Pathology Associates on paraffin-embedded hematoxylin and eosin-stained kidney sections as previously described (15). In brief, a longitudinal transverse section was obtained from the midportion of the left kidney, and a coronal section was obtained from the midportion of the right kidney. Categorical scoring was done according to Predictive Safety Testing Consortium guidelines and utilized grades from 0 to 5, where the grades for pathological lesions were 0 for no observable pathology, 1 for minimal pathology, 2 for mild pathology, 3 for moderate pathology, 4 for marked pathology, and 5 for severe pathology (39, 44–48). The composite score for an individual animal was calculated as the highest ordinal score from any kidney site (48).
Immunohistochemical visualization of KIM-1.
Formalin-fixed, paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene, rehydrated with a series of alcohol washes, and then incubated for 20 min at 95°C in a citrate buffer solution (number 00-5000; Zymed Laboratories, South San Francisco, CA, USA). The samples were then processed for visualization of KIM-1 using a monoclonal anti-rat KIM-1 antibody and the same labeling protocol for paraffin-embedded tissue samples described previously (49).
Receiver operating characteristic curves.
Receiver operating characteristic (ROC) comparative analyses were performed using the “roccomp” and “rocgold” commands in Stata/IC 13.1 (StataCorp, College Station, TX). Predictors were the value of each urinary biomarker (as urinary concentration or total amount excreted per 24 h) for the ultimate histopathologic classification. Histopathologic classification was treated as binary with outcome groupings of ≥1, ≥2, and ≥3. The initial ROC outcome was any histopathologic damage dichotomized as grade 0 versus ≥1. To evaluate the severity of histopathologic damage, we performed additional ROC analyses dichotomizing histopathologic scores as follows: grade 0 to 1 versus ≥2 and grades 0 to 2 versus ≥3. Thus, all positive grades of histopathology (grades 1 to 5) were treated with equal weight for the ROC analysis. Only samples that had nonmissing values were used for the analyses. To define the temporal predictive capacity of biomarkers, areas under the receiver operating characteristic curves (AUCs) were calculated for each biomarker and histopathologic score, stratified by duration of study protocol. Comparisons between AUCs were facilitated by comparing each score to a referent group (i.e., the urinary biomarker with the smallest area from 1-day protocols). Significance levels were adjusted using Sidak’s test for multiple comparisons. Our cut point for biomarkers was based on the highest possible correct classification (i.e., true positive and true negative). In the case of ties for cut points having identical correct classification values, the highest sensitivity was utilized to determine the cut point (50).
Statistical analysis.
Statistical analyses were performed using Stata/IC 13.1. Body weight loss, median worst ordinal histopathological score, urine output, and urinary biomarker concentrations were compared across vancomycin total daily dose and dosing frequency groups for each dosing protocol. Differences were evaluated either using Student’s t test, one-way analysis of variance (ANOVA), Mann-Whitney U test, or the Wilcoxon rank sum test as appropriate. As an exploratory analysis, forward and backwards, stepwise, multivariate logistic regressions (variable removal P > 0.2 and retention P < 0.05 by likelihood ratio test) were performed to assess the impact of urinary biomarkers, divided daily dosing scheme, study duration, and milligrams per kilograms per day on histopathologic damage. A histopathology score of 2 or greater defined VIKI in this analysis. Log and non-log transformed biomarker predictors were explored. Since two urinary results were obtained for animals in the 6-day protocol, both the 2-day and the 6-day biomarkers were explored to predict the 6-day histopathology. All tests were two-tailed, with an a priori level of statistical significance set at an alpha of 0.05.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases under award number R15-AI105742.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Marc Scheetz reports a research grant with Nevakar. No other relevant conflicts of interest exist for any author.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00079-19.
REFERENCES
- 1.Pakyz AL, MacDougall C, Oinonen M, Polk RE. 2008. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 168:2254–2260. doi: 10.1001/archinte.168.20.2254. [DOI] [PubMed] [Google Scholar]
- 2.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. 2011. Benchmarking risk–adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 53:1100–1110. doi: 10.1093/cid/cir672. [DOI] [PubMed] [Google Scholar]
- 3.Kelesidis T, Braykov N, Uslan DZ, Morgan DJ, Gandra S, Johannsson B, Schweizer ML, Weisenberg SA, Young H, Cantey J, Perencevich E, Septimus E, Srinivasan A, Laxminarayan R. 2016. Indications and types of antibiotic agents used in 6 acute care hospitals, 2009–2010: a pragmatic retrospective observational study. Infect Control Hosp Epidemiol 37:70–79. doi: 10.1017/ice.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. 2016. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 176:1639–1648. doi: 10.1001/jamainternmed.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodvold KA, McConeghy KW. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58:S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 6.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 7.Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. 2011. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 55:3278–3283. doi: 10.1128/AAC.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 9.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 10.Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, Kett DH, Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia Study Group. 2012. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther 34:149–157. doi: 10.1016/j.clinthera.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 12.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 13.Weiss AJ, Elixhauser A. 2014. HCUP Statistical Brief #180. Overview of hospital stays in the Unites States, 2012. Agency for Healthcare Reserach and Quality, Rockville, MD: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. [PubMed] [Google Scholar]
- 14.O'Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, Venkatesan N, Pais G, Cluff C, Lamar PC, Briyal S, Day JZ, Gulati A, Scheetz MH. 2017. 24-Hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e00416-17. doi: 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Askenazi DJ, Ambalavanan N, Goldstein SL. 2009. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol 24:265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Critical Path Institute Predictive Safety Testing Consortium. 2018. Biomarker qualification determination letter. Critical Path Institute, London, UK: https://c-path.org/programs/pstc/. [Google Scholar]
- 18.U.S. Food and Drug Administration. 2016. Letter of support for drug-induced vascular injury (DIVI) biomarker(s). U.S. Food and Drug Administration, Washington, DC: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM530365.pdf. [Google Scholar]
- 19.O'Donnell JN, Rhodes NJ, Miglis CM, Catovic L, Liu J, Cluff C, Pais G, Avedissian S, Joshi MD, Griffin B, Prozialeck W, Gulati A, Lodise TP, Scheetz MH. 2018. Dose, duration, and animal sex predict vancomycin-associated acute kidney injury in preclinical studies. Int J Antimicrob Agents 51:239–243. doi: 10.1016/j.ijantimicag.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. 2012. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 21.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. 2010. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naghibi B, Ghafghazi T, Hajhashemi V, Talebi A. 2007. Vancomycin-induced nephrotoxicity in rats: is enzyme elevation a consistent finding in tubular injury? J Nephrol 20:482–488. [PubMed] [Google Scholar]
- 23.Dieterich C, Puey A, Lin S, Lyn S, Swezey R, Furimsky A, Fairchild D, Mirsalis JC, Ng HH. 2009. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 107:258–269. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamgbola O. 2016. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 7:136–147. doi: 10.1177/2042018816638223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M, Okada S, Kinoshita H. 2003. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 37:373–379. doi: 10.1080/1071576031000061002. [DOI] [PubMed] [Google Scholar]
- 26.Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. [Google Scholar]
- 28.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. 2004. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte CG, Preuss HG. 1993. Assessment of renal function–glomerular and tubular. Clin Lab Med 13:33–52. doi: 10.1016/S0272-2712(18)30459-1. [DOI] [PubMed] [Google Scholar]
- 30.Waikar SS, Bonventre JV. 2009. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King DW, Smith MA. 2004. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro 18:797–803. doi: 10.1016/j.tiv.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. 2002. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 33.Moresco RN, Bochi GV, Stein CS, De Carvalho JAM, Cembranel BM, Bollick YS. 2018. Urinary kidney injury molecule-1 in renal disease. Clin Chim Acta 487:15–21. doi: 10.1016/j.cca.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Pang HM, Qin XL, Liu TT, Wei WX, Cheng DH, Lu H, Guo Q, Jing L. 2017. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: a prospective study. Eur Rev Med Pharmacol Sci 21:4203–4213. [PubMed] [Google Scholar]
- 35.Hjortrup PB, Haase N, Wetterslev M, Perner A. 2013. Clinical review: predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care 17:211. doi: 10.1186/cc11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fjaertoft G, Foucard T, Xu S, Venge P. 2005. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr 94:661–666. doi: 10.1080/08035250510031610. [DOI] [PubMed] [Google Scholar]
- 37.Chakraborty S, Kaur S, Muddana V, Sharma N, Wittel UA, Papachristou GI, Whitcomb D, Brand RE, Batra SK. 2010. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol 105:2050–2059. doi: 10.1038/ajg.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 39.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2005. Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER), Washington, DC. [Google Scholar]
- 42.Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. 2009. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. 2009. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattes WB, Walker EG. 2009. Translational toxicology and the work of the predictive safety testing consortium. Clin Pharmacol Ther 85:327–330. doi: 10.1038/clpt.2008.270. [DOI] [PubMed] [Google Scholar]
- 45.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G. 2010. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 46.Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL. 2010. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL. 2010. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol 28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 48.Sistare FD, Dieterle F, Troth S, Holder DJ, Gerhold D, Andrews-Cleavenger D, Baer W, Betton G, Bounous D, Carl K, Collins N, Goering P, Goodsaid F, Gu YZ, Guilpin V, Harpur E, Hassan A, Jacobson-Kram D, Kasper P, Laurie D, Lima BS, Maciulaitis R, Mattes W, Maurer G, Obert LA, Ozer J, Papaluca-Amati M, Phillips JA, Pinches M, Schipper MJ, Thompson KL, Vamvakas S, Vidal JM, Vonderscher J, Walker E, Webb C, Yu Y. 2010. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat Biotechnol 28:446–454. doi: 10.1038/nbt.1634. [DOI] [PubMed] [Google Scholar]
- 49.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. 2007. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhodes NJ, O'Donnell JN, Lizza BD, McLaughlin MM, Esterly JS, Scheetz MH. 2016. Tree-based models for predicting mortality in gram-negative bacteremia: avoid putting the CART before the horse. Antimicrob Agents Chemother 60:838–844. doi: 10.1128/AAC.01564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.OpenStax College. 19 June 2013. Blood flow in the nephron. Illustration from anatomy & physiology, Connexions Web site. https://commons.wikimedia.org/wiki/File:2611_Blood_Flow_in_the_Nephron.jpg. Accessed 20 February 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.