As increasing numbers of colistin-resistant bacteria emerge, new therapies are urgently needed to treat infections caused by these pathogens. The discovery of new combination therapies is one important way to solve such problems.
KEYWORDS: PFK-158, colistin resistance, combination, mcr-1
ABSTRACT
As increasing numbers of colistin-resistant bacteria emerge, new therapies are urgently needed to treat infections caused by these pathogens. The discovery of new combination therapies is one important way to solve such problems. Here, we report that the antitumor drug PFK-158 and its analogs PFK-015 and 3PO can exert synergistic effects with colistin against colistin-resistant Enterobacteriaceae, including mcr-1-positive or high-level-colistin-resistant (HLCR) isolates, as shown by a checkerboard assay. The results of a time-kill assay revealed that colistin combined with PFK-158 continuously eliminated colistin-resistant Escherichia coli 13-43, Klebsiella pneumoniae H04, and Enterobacter cloacae D01 in 24 h. Images from scanning electron microscopy (SEM) at 5 h postinoculation confirmed the killing effect of the combination. Finally, in vivo treatment showed that PFK-158 had a better synergistic effect than its analogs. Compared to the corresponding rates after colistin monotherapy, the survival rates of systemically infected mice were significantly increased 30% or 60% when the mice received an intravenous injection of colistin in combination with 15 mg/kg of body weight PFK-158. These results have important implications for repurposing PFK-158 to combat colistin resistance.
INTRODUCTION
Polymyxins, including polymyxin B and colistin, have been considered the last resort for carbapenem-resistant Gram-negative pathogens. In past decades, mutations in the pmrAB, phoPQ, and mgrB genes located in the chromosome of bacteria were considered the main causes of high-level resistance to polymyxins (1). However, in 2015, Jianzhong Shen in China discovered that plasmids carrying the mcr-1 gene could easily be transferred among bacteria and result in polymyxin resistance (2). Subsequently, increasing numbers of polymyxin-resistant bacteria carrying mcr-1 were reported in different countries (3).
Due to the narrow therapeutic window of polymyxins, combination therapy is a useful strategy to lower the effective concentration and nephrotoxicity of polymyxins. To cope with polymyxin-resistant bacterial infections, physicians have generally carried out polymyxin-based combination therapy with other existing antibiotics (4–9). However, as polymyxin-resistant bacteria always exhibit multidrug resistance, it is not always effective to combine polymyxins with other antibiotics, and new combinations are needed. Because of the lengthy process and a low success rate of drug discovery, one effective way is repurposing drugs that have been launched or are in clinical trials for antibacterial use (10, 11). Therefore, we screened a clinical compound library and discovered that PFK-158 potentiated polymyxin efficacy. PFK-158 and its analogs PFK-015 and 3PO were originally applied to treat cancer by inhibiting 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) activity (12–14). A phase I clinical trial of PFK-158 was successfully completed in July 2016 (Advanced Cancer Therapeutics), while 3PO and PFK-015 were still in the preclinical phase. In this study, we evaluated colistin-based combinations with PFK-158, PFK-015, or 3PO both in vitro and in vivo. This work may shed light on a new therapy to combat colistin-resistant pathogens.
RESULTS
In vitro evaluation of synergy.
The MICs of different isolates are shown in Table 1. Due to the various clinical treatments through which they had been processed, all mcr-1-positive and high-level-colistin-resistant (HLCR) Enterobacteriaceae showed multidrug-resistant phenotypes, and aztreonam (ATM), levofloxacin (LVX), nitrofurantoin (NIT), colistin (CST), and polymyxin B (PMB) resistance simultaneously existed in most of the isolates.
TABLE 1.
Representative MIC values against colistin-resistant clinical isolatesa
| Strain | MIC (μg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CST | PMB | TGC | CAZ | FEP | ATM | MEM | AMK | LVX | NIT | |
| E. coli 08-85 | 8 | 8 | 0.25 | 0.5 | 32 | 64 | ≤0.03 | 4 | 32 | 64 |
| E. coli 13-43 | 8 | 8 | 1 | 2 | 8 | 64 | ≤0.03 | 4 | 64 | 128 |
| E. coli 13-66 | 8 | 8 | 1 | 2 | 4 | 16 | ≤0.03 | 2 | 64 | 128 |
| E. coli 13-68 | 8 | 8 | 0.5 | 1 | 8 | 4 | ≤0.03 | 32 | 8 | 128 |
| E. coli H67 | 4 | 4 | 0.25 | 64 | 128 | 64 | 4 | 2 | 16 | 256 |
| K. pneumoniae 09-20 | 16 | 16 | 2 | 1 | 0.5 | 0.5 | ≤0.03 | >1,024 | 32 | 256 |
| K. pneumoniae H04 | 32 | 32 | 1 | >256 | 8 | 32 | 2 | 256 | 32 | ≥512 |
| E. cloacae D01 | 256 | 256 | 2 | 256 | 2 | 64 | 1 | 2 | 0.125 | 64 |
| E. cloacae L09 | 32 | 32 | 1 | >256 | 32 | 64 | 4 | 16 | 32 | 256 |
| E. cloacae R31 | 64 | 128 | 0.5 | 1 | 0.064 | 64 | 1 | 2 | 1 | 64 |
CST, colistin; PMB, polymyxin B; TGC, tigecycline; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; MEM, meropenem; AMK, amikacin; LVX, levofloxacin; NIT, nitrofurantoin. According to CLSI supplement M100 (28th edition), breakpoints for resistance are listed as follows: CST/PMB/MEM, 4 μg/ml; LVX, 8 μg/ml; CAZ/FEP/ATM, 16 μg/ml; AMK, 64 μg/ml; NIT, 128 μg/ml (27).
The results of the checkerboard assay showed that PFK-158 had a significant synergistic effect with polymyxins (PMB or CST). To explore the best potentiator of polymyxins, the PFK-158 analogs 3PO and PFK-015 were also evaluated in this study (Fig. 1). As shown in Table 2, the fractional inhibitory concentration indices (FICIs) of the polymyxin-based combination were all below 0.5 in colistin-resistant Enterobacteriaceae, indicating synergistic effects of PFK-158 or its analogs with polymyxins. The MICs of all colistin-resistant bacteria, regardless of whether they were mcr-1 positive or HLCR, could be decreased to 2 μg/ml or lower. For the HLCR isolate Enterobacter cloacae D01, these compounds could decrease their MIC values by at least 128-fold to a susceptible level. Compared with 3PO, PFK-158 and PFK-015 showed lower FICIs in all colistin-resistant isolates. However, FICIs of the three combinations were >0.5 in all tested colistin-susceptible strains, indicating the indifferent effect of the combinations.
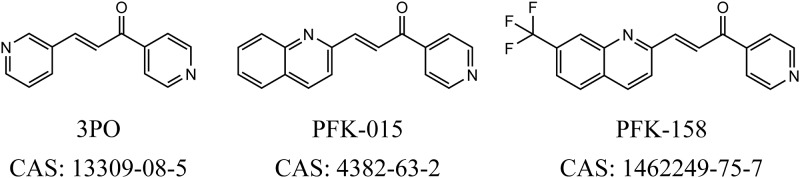
FIG 1.
Chemical structures and Chemical Abstracts Service (CAS) registry numbers of 3PO, PFK-015, and PFK-158.
TABLE 2.
Checkerboard assay results of the polymyxin-based combination with 3PO, PFK-015, or PFK-158a
| Strain | Source | mcr-1 status | 3PO combination |
PFK-015 combination |
PFK-158 combination |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | MIC (μg/ml) (fold change) |
FICI | Drug | MIC (μg/ml) |
FICI | Drug | MIC (μg/ml) |
FICI | ||||||
| Alone | Combined | Alone | Combined | Alone | Combined | |||||||||
| E. coli 08-85 | Human blood | + | CST | 8 | 2 (4) | 0.313 | CST | 8 | 2 (4) | 0.258 | CST | 8 | 2 (4) | 0.266 |
| 3PO | 512 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 16 | ||||||
| PMB | 8 | 2 (4) | 0.313 | PMB | 8 | 2 (4) | 0.258 | PMB | 8 | 2 (4) | 0.258 | |||
| 3PO | 512 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 8 | ||||||
| E. coli 13-43 | Human urine | + | CST | 8 | 2 (4) | 0.313 | CST | 8 | 2 (4) | 0.254 | CST | 8 | 2 (4) | 0.266 |
| 3PO | 512 | 32 | PFK-015 | 1,024 | 4 | PFK-158 | 1,024 | 16 | ||||||
| PMB | 8 | 2 (4) | 0.313 | PMB | 8 | 2 (4) | 0.258 | PMB | 8 | 2 (4) | 0.252 | |||
| 3PO | 512 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 2 | ||||||
| E. coli 13-66 | Human | + | CST | 8 | 2 (4) | 0.313 | CST | 8 | 2 (4) | 0.258 | CST | 8 | 2 (4) | 0.254 |
| 3PO | 512 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 4 | ||||||
| PMB | 8 | 2 (4) | 0.313 | PMB | 8 | 2 (4) | 0.258 | PMB | 8 | 2 (4) | 0.252 | |||
| 3PO | 512 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 2 | ||||||
| E. coli 13-68 | Human | + | CST | 8 | 1 (8) | 0.375 | CST | 8 | 2 (4) | 0.258 | CST | 8 | 2 (4) | 0.266 |
| 3PO | 256 | 64 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 16 | ||||||
| PMB | 8 | 1 (8) | 0.375 | PMB | 8 | 2 (4) | 0.258 | PMB | 8 | 2 (4) | 0.252 | |||
| 3PO | 256 | 64 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 2 | ||||||
| E. coli H67 | Human liver | + | CST | 4 | 1(4) | 0.375 | CST | 4 | 1 (4) | 0.258 | CST | 4 | 1 (4) | 0.281 |
| 3PO | 256 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 32 | ||||||
| PMB | 4 | 1 (4) | 0.375 | PMB | 4 | 1 (4) | 0.258 | PMB | 4 | 1 (4) | 0.254 | |||
| 3PO | 256 | 32 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 4 | ||||||
| E. coli ATCC 25922 | ATCC | − | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.25 (2) | 0.5 |
| 3PO | 256 | 0.125 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
| PMB | 0.5 | 0.25 (2) | 0.5 | PMB | 0.5 | 0.25 (2) | 0.5 | PMB | 0.5 | 0.25 (2) | 0.5 | |||
| 3PO | 256 | 0.125 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
| K. pneumoniae 09-20 | Human blood | + | CST | 16 | 1 (16) | 0.188 | CST | 16 | 2 (8) | 0.141 | CST | 16 | 2 (8) | 0.141 |
| 3PO | 512 | 64 | PFK-015 | 1,024 | 16 | PFK-158 | 1,024 | 16 | ||||||
| PMB | 16 | 1 (16) | 0.188 | PMB | 16 | 2 (8) | 0.156 | PMB | 16 | 2 (8) | 0.133 | |||
| 3PO | 512 | 64 | PFK-015 | 1,024 | 32 | PFK-158 | 1,024 | 8 | ||||||
| K. pneumoniae H04 | Human liver | − | CST | 32 | 1 (32) | 0.156 | CST | 32 | 2 (16) | 0.07 | CST | 32 | 2 (16) | 0.07 |
| 3PO | 512 | 64 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 8 | ||||||
| PMB | 32 | 1 (32) | 0.156 | PMB | 32 | 2 (16) | 0.066 | PMB | 32 | 2 (16) | 0.064 | |||
| 3PO | 512 | 64 | PFK-015 | 1,024 | 4 | PFK-158 | 1,024 | 2 | ||||||
| K. pneumoniae ATCC 700603 | ATCC | − | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.5 (1) | 1 |
| 3PO | 1,024 | 0.125 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
| PMB | 0.5 | 0.25 (2) | 0.5 | PMB | 0.5 | 0.25 (2) | 0.5 | PMB | 0.5 | 0.25 (2) | 0.5 | |||
| 3PO | 1,024 | 0.125 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
| E. cloacae D01 | Human blood | − | CST | 256 | 2 (128) | 0.039 | CST | 256 | 1 (256) | 0.02 | CST | 256 | 2 (128) | 0.012 |
| 3PO | 512 | 16 | PFK-015 | 1,024 | 16 | PFK-158 | 1,024 | 4 | ||||||
| PMB | 256 | 2 (128) | 0.039 | PMB | 256 | 2 (128) | 0.01 | PMB | 256 | 2 (128) | 0.008 | |||
| 3PO | 512 | 16 | PFK-015 | 1,024 | 2 | PFK-158 | 1,024 | 0.5 | ||||||
| E. cloacae L09 | Human sputum | − | CST | 32 | 1 (32) | 0.047 | CST | 32 | 1 (32) | 0.033 | CST | 32 | 2 (16) | 0.078 |
| 3PO | 512 | 8 | PFK-015 | 1,024 | 2 | PFK-158 | 1,024 | 16 | ||||||
| PMB | 32 | 2 (16) | 0.078 | PMB | 32 | 2 (16) | 0.064 | PMB | 32 | 2 (16) | 0.064 | |||
| 3PO | 512 | 8 | PFK-015 | 1,024 | 2 | PFK-158 | 1,024 | 2 | ||||||
| E. cloacae R31 | Human urine | − | CST | 64 | 1 (64) | 0.032 | CST | 64 | 1 (64) | 0.023 | CST | 64 | 2 (32) | 0.031 |
| 3PO | 512 | 8 | PFK-015 | 1,024 | 8 | PFK-158 | 1,024 | 0.125 | ||||||
| PMB | 128 | 2 (64) | 0.047 | PMB | 128 | 1 (128) | 0.023 | PMB | 128 | 2 (64) | 0.016 | |||
| 3PO | 512 | 16 | PFK-015 | 1,024 | 16 | PFK-158 | 1,024 | 0.25 | ||||||
| E. cloacae ATCC 49141 | ATCC | − | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.25 (2) | 0.5 | CST | 0.5 | 0.25 (2) | 0.5 |
| 3PO | 512 | 0.125 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
| PMB | 0.5 | 0.25 (2) | 0.51 | PMB | 0.5 | 0.25 (2) | 0.5 | PMB | 0.5 | 0.25 (2) | 0.5 | |||
| 3PO | 512 | 4 | PFK-015 | 1,024 | 0.125 | PFK-158 | 1,024 | 0.125 | ||||||
Fold changes are shown in parentheses. +, mcr-1-positive isolate; −, mcr-1-negative isolate; CST, colistin; PMB, polymyxin B; FICI, fractional inhibitory concentration index.
Time-kill study of the combinations.
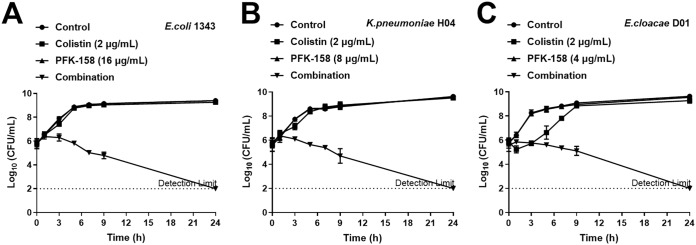
Time-kill assays were performed using mcr-1-positive Escherichia coli 13-43, HLCR Klebsiella pneumoniae H04, and HLCR Enterobacter cloacae D01. As shown in Fig. 2, colistin combined with PFK-158 showed constant bactericidal effects for 24 h with a minimum of a ∼4-log10 CFU/ml reduction in all three isolates, and the bacterial counts for all isolates were reduced to below the limit of detection within 24 h. For E. coli 13-43 and K. pneumoniae H04, no bactericidal effect was observed with colistin or PFK-158 monotherapy (Fig. 2A and B). Although the bacterial count for E. cloacae D01 was reduced in the first hour after colistin (2 μg/ml) monotherapy, it could regrow in 9 h to the control group level (Fig. 2C).
FIG 2.
Time-kill curves of colistin and PFK-158 alone or in combination against E. coli 13-43 (A), K. pneumoniae H04 (B), or E. cloacae D01 (C).
Impact of the combination therapy on cellular morphology.
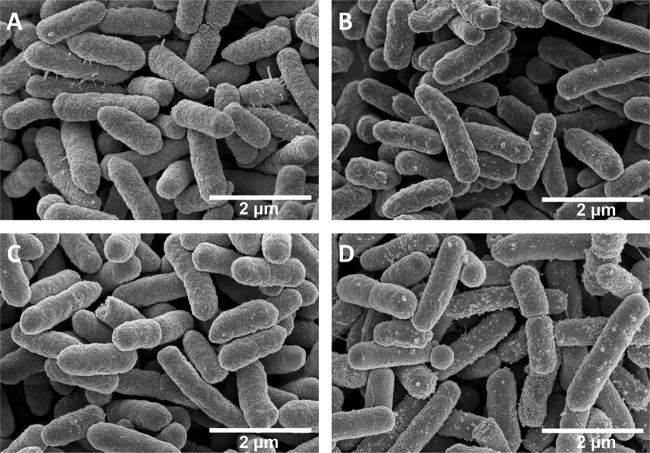
To investigate whether PFK-158 or combination therapy could change the cellular surface morphology of bacteria to exert its synergistic effect, a scanning electron microscope was used. The results showed that treatment with PFK-158 alone could not affect the integrity of the cellular surface (Fig. 3C) compared with that of the control group (Fig. 3A), and colistin monotherapy caused a few micelles to form (Fig. 3B). The combination treatment, however, caused more micelle formation (Fig. 3D) and subsequently led to bacterial cell lysis. As demonstrated in Fig. 3, colistin was the main reason for the generation of micelles, and although PFK-158 alone could not change the morphology of the bacteria, it could potentialize the killing effect of colistin and cause more micelle formation. Transmission electron microscopy (TEM) was also used to investigate the cellular morphology influenced by the combination, and the results confirmed what we observed in scanning electron microscopy (SEM) experiments (see Fig. S1 in the supplemental material).
FIG 3.
Images from SEM for high-level-colistin-resistant E. cloacae D01 after different treatments for 5 h. (A) Control; (B) 2 μg/ml colistin alone; (C) 4 μg/ml PFK-158 alone; (D) 2 μg/ml colistin and 4 μg/ml PFK-158 in combination.
In vivo evaluation of synergy.
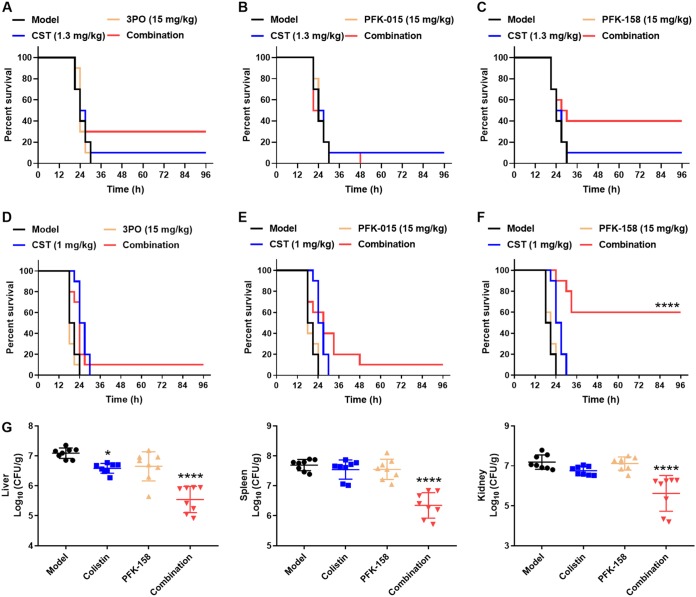
First, we investigated the maximum tolerated doses (MTDs) of PFK-158 and its analogs or combination therapies in mice (Table S1). The results showed that the MTDs of 3PO, PFK-015, and PFK-158 were greater than or equal to 75 mg/kg, 30 mg/kg, and 60 mg/kg, respectively, when ICR mice received an intravenous (i.v.) injection. Additionally, the body weight gain of mice was not influenced by the compounds under MTDs or combination therapies (Fig. S2). Thus, a dose of 15 mg/kg (i.v.) was chosen as a safe dose for in vivo study. Mouse survival rates were monitored until day 3 postinfection. Compared with its analogs, PFK-158 showed a significant synergistic effect on colistin in systemically infected mice (Fig. 4C and F). In the K. pneumoniae H04 infection mouse model, colistin combined with PFK-158 treatment improved the survival rate from 10% (for 15 mg/kg PFK-158 or 1.3 mg/kg colistin monotherapy) to 40% (for the combination of 15 mg/kg PFK-158 and 1.3 mg/kg). The survival rate could also be improved from 10% to 30% when treatment with colistin combined with 3PO was administered (Fig. 4A). However, colistin combined with PFK-015 showed no influence on the survival rate in this model (Fig. 4B). In the HLCR E. cloacae D01 infection mouse model, the survival rate was significantly increased from 0% to 60% when colistin was combined with 15 mg/kg PFK-158 treatment. Colistin combined with 3PO or PFK-015 slightly improved the animal survival rates to 10% in the E. cloacae D01 infection model (Fig. 4C and D).
FIG 4.
Synergistic effect of colistin (CST) combined with 3PO, PFK-015, or PFK-158 in vivo. (A to F) Mice were intraperitoneally infected with HLCR clinical isolate E. cloacae D01 (A to C) or K. pneumoniae H04 (D to F). A total of 15 mg/kg 3PO (A and D), PFK-015 (B and E), or PFK-158 (C and F), alone or combined with colistin, was intravenously injected at 1 h postinfection (n = 10 per group). A solvent was injected into the model group. (G) Bacterial loads in organs (liver, spleen, and kidney) influenced by colistin, PFK-158, or combination therapy in the E. cloacae D01 infection model were determined at 16 h postinfection (n = 8 per group). *, P ≤ 0.05; ****, P ≤ 0.0001 (compared with the model group).
As PFK-158 showed a firmly synergistic effect both in vitro and in vivo, we decided to further explore whether the increase in the survival rate was caused by the antibacterial effect of the combination therapy. The bacterial loads in the liver, spleen, and kidney of mice were determined by colony counting. As shown in Fig. 4G, colistin combined with PFK-158 significantly decreased bacterial loads in the organs of mice (P ≤ 0.0001). On average, CFU counts were reduced by more than 1 order of magnitude in the combination therapy groups; whereas colistin at 1 mg/kg showed no effect on bacterial growth in the spleen and kidney, it slightly reduced bacterial loads in the liver.
DISCUSSION
The rapid emergence of multidrug-resistant Gram-negative bacteria over the last 2 decades has led to the reuse of polymyxins, which were nearly abandoned in the clinic because of their various side effects (15). However, the discovery of the mcr-1-carrying plasmid in 2015 broke the last barrier between superbugs and humans, and new therapies or drugs are urgently needed. Although several nonantibiotics that are synergistic with polymyxins were reported recently, most of them were evaluated in vitro (10, 16–18), and only a few compounds have been evaluated in animal models (11, 19–21). Hence, we screened a clinical compound library to search for new compounds that can potentiate colistin efficacy and discovered that one class of chemicals, PFK-158 and its analogs, could fight against polymyxin-resistant pathogens when used together with colistin. In this study, we evaluated the synergistic antibacterial effect of these compounds (especially PFK-158) with colistin both in vitro and in vivo and explored the possible reasons behind these effects.
First, we compared the synergistic effects of PFK-158, PFK-015, and 3PO in combination with polymyxins in vitro. To confirm the applicability of the combination in the clinic, three kinds of polymyxin-resistant Enterobacteriaceae (E. coli, K. pneumoniae, and E. cloacae) with different resistant mechanisms (mcr-1 positive or HLCR) were selected for the initial antimicrobial activity evaluation. PFK-158, PFK-015, or 3PO exhibited significant synergy (FICI of ≤0.5) with polymyxins. Compared with PFK-015 or PFK-158, 3PO showed weak synergy, while most FICIs were high in its combination group. According to the differences among the structures of all three compounds (Fig. 1), quinolyl might be the key group for improving the synergistic effect. As PFK-015 and PFK-158 showed no bactericidal effect when used singly, the survival pressure of bacteria would be greatly relieved. Therefore, it might take a long time for resistance to the combination therapies to emerge.
In vivo studies showed that PFK-158 in combination with colistin yielded significant improvements in mouse survival rates and reductions in the bacterial burdens in the livers, spleens, and kidneys of mice. An increasing dose of colistin to combat against colistin-resistant bacteria could be avoided due to combination therapy. The reduction of bacterial burdens in the kidney and other organs might also alleviate the organ injury caused by bacterial infection and nephrotoxicity of colistin. Taking all in vitro results, in vivo results, and previously reported data into consideration, we further studied PFK-158 as a promising potentiator of colistin. Unlike the other potentiators reported previously (7, 11, 16, 17, 21), colistin in combination with PFK-158 showed a constant killing effect, which eliminated the bacteria below the detection limit in time-kill studies. This finding reminded us that PFK-158 might have a unique mechanism of action.
According to SEM and TEM imaging results, PFK-158 had no influence on cellular morphology when used singly. However, bacteria in the combination group generated more micelles than bacteria in the colistin monotherapy group. These results suggested that PFK-158 might directly enhance the bacterium-killing effect of colistin to exert its synergy and might influence the permeability of the membrane. The micelle generation that we observed was in accordance with the carpet model hypothesis, which is often used to explain the mechanism of antimicrobial peptides (AMPs) (22). This hypothesis suggested that AMPs might be associated with the acidic lipid-rich regions of the membrane and “carpeting” the surface, creating transient holes in the membrane and finally leading to the formation of micelles and cell lysis. This means that micelles are precursors of cell death. More micelles were generated, and more cell deaths occurred.
PFK-158 and its analogs PFK-015 and 3PO were antitumor drugs targeting PFKFB3. Although complete results of the phase I clinical trial of PFK-158 remain unreleased, the literature showed that compared with PFK-015 or 3PO, PFK-158 exhibited superior pharmacokinetic properties and safety in preclinical research (14, 23, 24). The MTDs in the patents were similar to what we obtained in our safety study. According to the patents, when BALB/c mice were intravenously administered 5 mg/kg of each compound, the t1/2 (half-life) and Cmax (maximum concentration) of PFK-158 were 10.5 h and 4,176 ng/ml, respectively, while the parameters for PFK-015 (t1/2 of 5.0 h and Cmax of 3,053 ng/ml) were both significantly lower (15). Therefore, the variability of the correlation between in vitro and in vivo efficacies of 3PO and PFK-015 may be associated with their pharmacokinetic properties. For instance, the synergy of PFK-015 was equivalent to that of PFK-158 against K. pneumoniae H04 in vitro. But PFK-015 might not reach an adequate plasma concentration or might be eliminated quickly in vivo. As PFK-158 and its analogs belong to the same class of chemicals, the parental structure could be further modified to generate more diverse potentiators of polymyxins for improving their pharmacodynamic or pharmacokinetic properties.
In conclusion, one new class of potentiators of colistin was evaluated both in vitro and in vivo. PFK-158 not only decreased the MICs of colistin to eradicate both colistin-susceptible and colistin-resistant (mcr-1 or HLCR) Enterobacteriaceae in vitro but also enhanced the efficacy of colistin in vivo. This finding might shed light on the discovery of combination therapies for infections caused by colistin-resistant pathogens.
MATERIALS AND METHODS
Bacterial isolates.
All colistin-resistant bacterial strains were obtained from the CAMS Collection Center of Pathogen Microorganisms (CAMS-CCPM-A) in China. E. coli 08-85, E. coli 13-43, E. coli 13-66, E. coli 13-68, E. coli H67, and K. pneumoniae 09-20 were mcr-1-positive isolates. K. pneumoniae H04, E. cloacae D01, E. cloacae L09, and E. cloacae R31 were high-level-colistin-resistant isolates. The colistin-susceptible isolates E. coli ATCC 25922, K. pneumoniae ATCC 700603, and E. cloacae ATCC 49141 were obtained from the American Type Culture Collection (ATCC). All isolates were frozen at −80°C until they were used. PCR amplification was conducted to detect the existence of the mcr-1 gene as previously reported (2). The sequences of PCR products were determined by Sangon Biotech Company (Shanghai, China).
Antimicrobial agents and susceptibility test.
All antibiotics, including colistin, polymyxin B, tigecycline, ceftazidime, cefepime, aztreonam, meropenem, amikacin, levofloxacin, and nitrofurantoin, were purchased from the National Institutes for Food and Drug Control (Beijing, China). Solvents and diluents for the preparation of antibiotics complied with Clinical and Laboratory Standards Institute (CLSI) guidelines (25). 3PO, PFK-015, and PFK-158 were purchased from Target Molecule Company (Boston, MA, USA). The MICs of representative antibiotics were determined by the broth microdilution method in accordance with CLSI guidelines. The final inoculum in each well was approximately 5 × 105 CFU/ml. The microtiter plates were incubated at 37°C for 18 h, and the results were observed by the naked eye. The experiments were performed in triplicate on different days.
Checkerboard analysis of combination effects.
A clinical compound library (n = 688) was screened to identify the potentiators of colistin by a broth microdilution checkerboard assay. We examined the antibacterial activities of these combination therapies against both mcr-1-positive and mcr-1-negative colistin-resistant Enterobacteriaceae. Polymyxin-based combinations were prepared using 96-well round-bottom microtiter plates with drug concentrations that were 2-fold serially diluted (26). The two drugs were mixed in a 96-well plate, followed by the addition of a standard bacterial suspension at a final concentration of 5 × 105 CFU/ml in cation-adjusted Mueller-Hinton (CAMH) broth. After incubation for 18 h at 37°C, the results were also observed by the naked eye. The combination effects of polymyxins (PMB or CST) with PFK-158 or its analogs were determined by calculating the fractional inhibitory concentration index (FICI) using the concentration combinations with the highest combination effects: FICI = (MIC of drug A in the combination/MIC of drug A alone) + (MIC of drug B in the combination/MIC of drug B alone). The antimicrobial combination was defined as synergistic when the FICI was ≤0.5; indifferent when 0.5 < FICI < 4, and antagonistic when the FICI was ≥4. The experiments were performed in triplicate on different days.
Time-kill assays.
Time-kill curve assays were performed with E. coli 13-43, K. pneumoniae H04, and E. cloacae D01, according to a method described previously by Lu et al. (27), with minor modifications. Briefly, a culture of each isolate grown overnight was diluted with 3 ml CAMH broth to a final concentration of ∼106 CFU/ml. Next, colistin (at 2 μg/ml) and PFK-158 (at the lowest concentrations that can show synergistic effects when combined with colistin), singly or in combinations, were added. Viable cell counts were determined 0, 1, 3, 5, 7, 9, and 24 h after incubation at 37°C by plating 10-μl serially diluted samples onto LB agar plates in triplicate. The results were recorded as log10 CFU per milliliter. The combination of colistin and PFK-158 was considered synergistic if bacterial killing was 2 log10 units higher than the most active monotherapy.
Scanning electron microscopy and transmission electron microscopy.
SEM and TEM were employed to examine the effect of the colistin–PFK-158 combination on the cellular morphology of the HLCR isolate E. cloacae D01. A log-phase culture was treated with 2 μg/ml colistin, 4 μg/ml PFK-158, or both for 4 h in CAMH broth as described above for the time-kill studies. For the SEM and TEM studies, samples were transferred to 15-ml polypropylene tubes (Corning, USA) and centrifuged at 6,000 × g for 3 min. Supernatants were discarded, and bacterial pellets were resuspended and washed in 1 ml 2.5% glutaraldehyde in phosphate-buffered saline (PBS). The tubes were fixed overnight at 4°C. Once fixed, the tubes were centrifuged again at 6,000 × g for 3 min, the fixatives were removed, and bacterial pellets were finally resuspended in 1 ml PBS. SEM was conducted by using a scanning electron microscope (Hitachi SU8020), and TEM was conducted by using a transmission electron microscope (JEOL JEM-1200EX).
In vivo treatment evaluation.
All compounds were prepared in 5% ethanol, 5% Cremophor, and 90% D5W (5% dextrose in water). All mice were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. The safety study was conducted with female ICR mice. Mice were intravenously injected with a solvent, 75 mg/kg 3PO, 30 mg/kg PFK-015, or 60 mg/kg PFK-158 (n = 8 per group). The body weight changes of the mice were monitored for a week postinjection. Female ICR mice (body weights of 18 to 20 g) were also used for the mouse systemic infection model. Mice were infected intraperitoneally with a 0.5-ml K. pneumoniae H04 or E. cloacae D01 bacterial suspension (100% minimum lethal dose) in 5% mucin. After 1 h of infection, colistin (1 mg/kg for K. pneumoniae H04 infection and 1.3 mg/kg for E. cloacae D01 infection), 3PO, PFK-015, or PFK-158 (15 mg/kg), singly or in combination, was injected intravenously into the mice (n = 10 per group). A group of mice was treated with the same solvent as a control group. The experiments were performed in triplicate on different days. A log rank test was applied to compare the survival distributions of different samples.
When colony counting of different organs was conducted, 8 mice in each group were sacrificed 16 h after infection (n = 8 per group). The livers, spleens, and kidneys were aseptically excised, homogenized, serially diluted, and plated on LB agar plates for CFU counts. Bacterial colony counts were expressed as the mean log10 CFU per milliliter (± standard error of the mean [SEM]). Animal husbandry and all animal experiments were performed according to national standards for laboratory animals in China (GB/T 35892-2018) (28), with approval from the Laboratory Animal Welfare and Ethics Committee of the Institute of Medicinal Biotechnology, Peking Union Medical College. Statistical significance for the bacterial killing of different treatment groups was calculated with one-way analysis of variance (ANOVA) and Bonferroni’s multiple comparisons.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the CAMS Initiative for Innovative Medicine (grant number 2016-I2M-3-014), the National Mega-project for Innovative Drugs (grant number 2018ZX09721001), and the National Natural Science Foundation of China (grant numbers 81621064, 81361138020, and 81573475).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00271-19.
REFERENCES
- 1.Olaitan AO, Morand S, Rolain J-M. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 3.Giamarellou H. 2016. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents 48:614–621. doi: 10.1016/j.ijantimicag.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Lin X, Yao X, Huang Y, Liu W, Ma T, Fang B. 2018. Synergistic antimicrobial activity of colistin in combination with rifampin and azithromycin against Escherichia coli producing MCR-1. Antimicrob Agents Chemother 62:e01631-18. doi: 10.1128/AAC.01631-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacNair CR, Stokes JM, Carfrae LA, Fiebig-Comyn AA, Coombes BK, Mulvey MR, Brown ED. 2018. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun 9:458. doi: 10.1038/s41467-018-02875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Patel S, Abboud C, Diep J, Ly NS, Pogue JM, Kaye KS, Li J, Rao GG. 2017. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: pharmacodynamics and morphological changes. Int J Antimicrob Agents 49:224–232. doi: 10.1016/j.ijantimicag.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran TB, Cheah S-E, Yu HH, Bergen PJ, Nation RL, Creek DJ, Purcell A, Forrest A, Doi Y, Song J, Velkov T, Li J. 2016. Anthelmintic closantel enhances bacterial killing of polymyxin B against multidrug-resistant Acinetobacter baumannii. J Antibiot (Tokyo) 69:415–421. doi: 10.1038/ja.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidaillac C, Benichou L, Duval RE. 2012. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 56:4856–4861. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Walsh TR, Yang RS, Zheng M, Wei MC, Tyrrell JM, Wang Y, Liao XP, Sun J, Liu YH. 2019. Novel partners with colistin to increase its in vivo therapeutic effectiveness and prevent the occurrence of colistin resistance in NDM- and MCR-co-producing Escherichia coli in a murine infection model. J Antimicrob Chemother 74:87–95. doi: 10.1093/jac/dky413. [DOI] [PubMed] [Google Scholar]
- 10.Torres NS, Montelongo-Jauregui D, Abercrombie JJ, Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK, Leung KP. 2018. Antimicrobial and antibiofilm activity of synergistic combinations of a commercially available small compound library with colistin against Pseudomonas aeruginosa. Front Microbiol 9:2541. doi: 10.3389/fmicb.2018.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TB, Wang J, Doi Y, Velkov T, Bergen PJ, Li J. 2018. Novel polymyxin combination with antineoplastic mitotane improved the bacterial killing against polymyxin-resistant multidrug-resistant Gram-negative pathogens. Front Microbiol 9:721. doi: 10.3389/fmicb.2018.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J. 2008. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 13.Seo M, Kim J-D, Neau D, Sehgal I, Lee Y-H. 2011. Structure-based development of small molecule PFKFB3 inhibitors: a framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS One 6:e24179. doi: 10.1371/journal.pone.0024179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapolsky GH, Chand P. May 2017. PFKFB3 inhibit and methods of use as an anti-cancer therapeutic. US patent 10,010,542B2.
- 15.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 16.Baron SA, Rolain JM. 2018. Efflux pump inhibitor CCCP to rescue colistin susceptibility in mcr-1 plasmid-mediated colistin-resistant strains and Gram-negative bacteria. J Antimicrob Chemother 73:1862–1871. doi: 10.1093/jac/dky134. [DOI] [PubMed] [Google Scholar]
- 17.Cannatelli A, Principato S, Colavecchio OL, Pallecchi L, Rossolini GM. 2018. Synergistic activity of colistin in combination with resveratrol against colistin-resistant Gram-negative pathogens. Front Microbiol 9:1808. doi: 10.3389/fmicb.2018.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, Tyers M, Brown ED, Wright GD. 2011. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat Chem Biol 7:348–350. doi: 10.1038/nchembio.559. [DOI] [PubMed] [Google Scholar]
- 19.Daly SM, Sturge CR, Felder-Scott CF, Geller BL, Greenberg DE. 2017. MCR-1 inhibition with peptide-conjugated phosphorodiamidate morpholino oligomers restores sensitivity to polymyxin in Escherichia coli. mBio 8:e01315-17. doi: 10.1128/mBio.01315-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Liu S, Wang T, Li H, Tang S, Wang J, Wang Y, Deng X. 2018. Pterostilbene, a potential MCR-1 inhibitor that enhances the efficacy of polymyxin B. Antimicrob Agents Chemother 62:e02146-17. doi: 10.1128/AAC.02146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Wang T, Guo Y, Liu S, Wang J, Shen Y, Tang S, Wang Y, Deng X. 2018. In vitro/vivo activity of potential MCR-1 inhibitor in combination with colistin against mcr-1-positive Klebsiella pneumoniae. Front Microbiol 9:1615. doi: 10.3389/fmicb.2018.01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciumac D, Gong H, Hu X, Lu JR. 2019. Membrane targeting cationic antimicrobial peptides. J Colloid Interface Sci 537:163–185. doi: 10.1016/j.jcis.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 23.Chesney J, John OT, Telang S, Clem B, Meier J. March 2009. Family of pfkfb3 inhibitors with anti-neoplastic activities. US patent 20,090,074,884A1.
- 24.Shi L, Pan H, Liu Z, Xie J, Han W. 2017. Roles of PFKFB3 in cancer. Signal Transduct Target Ther 2:17044. doi: 10.1038/sigtrans.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Stokes JM, MacNair CR, Ilyas B, French S, Cote JP, Bouwman C, Farha MA, Sieron AO, Whitfield C, Coombes BK, Brown ED. 2017. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol 2:17028. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X, Yang X, Li X, Lu Y, Ren Z, Zhao L, Hu X, Jiang J, You X. 2013. In vitro activity of sodium new houttuyfonate alone and in combination with oxacillin or netilmicin against methicillin-resistant Staphylococcus aureus. PLoS One 8:e68053. doi: 10.1371/journal.pone.0068053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standardization Administration of the People’s Republic of China. 2018. Laboratory animal—guideline for ethical review of animal welfare. GB/T 35892-2018 Standardization Administration of the People’s Republic of China, Beijing, China. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.