We assessed the antimicrobial-inactivation capability of BacT/Alert (FA Plus and FN Plus) or Bactec (Plus Aerobic/F and Plus Anaerobic/F) media for 40 antibiotic-bacterium combinations in simulated adult blood cultures. Aside from high recovery rates (93.2% and 88.4%, respectively), we showed that at the lowest but clinically relevant antibiotic concentrations, both BacT/Alert and Bactec media recovered all the organisms tested with drugs except for Escherichia coli, which was tested in the presence of meropenem.
KEYWORDS: blood culture system, drug inactivation, personalized therapy
ABSTRACT
We assessed the antimicrobial-inactivation capability of BacT/Alert (FA Plus and FN Plus) or Bactec (Plus Aerobic/F and Plus Anaerobic/F) media for 40 antibiotic-bacterium combinations in simulated adult blood cultures. Aside from high recovery rates (93.2% and 88.4%, respectively), we showed that at the lowest but clinically relevant antibiotic concentrations, both BacT/Alert and Bactec media recovered all the organisms tested with drugs except for Escherichia coli, which was tested in the presence of meropenem. Delayed recoveries were mainly associated with vancomycin.
TEXT
Bloodstream infections (BSIs) are associated with high rates of morbidity and mortality (1). Thus, timely and accurate diagnoses of BSIs are crucial to correctly manage infected patients (2), particularly those with infection-complicating sepsis and septic shock (3). Often, the physician in charge empirically administers broad-spectrum antimicrobial therapy before obtaining a microbiological diagnosis of the infection because of the patient’s critical status (4). Blood culture (BC) is still the gold standard for BSI detection (5). Time to detection (TTD) with today’s automated BC systems (BacT/Alert Virtuo [bioMérieux, Marcy l’Étoile, France] and BactecFX [Becton Dickinson, Sparks, MD]) may be significantly delayed or even unmeasurable because of slow or absent microbial growth due to antimicrobial activity in BCs (6).
In the early 1990s, BacT/Alert FAN bottles (Organon Teknika, Durham, NC) containing Fuller’s earth and absorbent charcoal were invented to enhance recovery of microorganisms. The bottles were later improved and launched as charcoal-containing BacT/Alert FA and FN bottles (bioMérieux). Resin-containing media such as Bactec Plus Aerobic/F and Plus Anaerobic/F (Becton Dickinson) and, since 2013, BacT/Alert FA Plus (aerobic) and FN Plus (anaerobic) (bioMérieux) further improved the recovery of microorganisms from patient samples. Numerous studies evaluated a single bottle type or compared the performance of charcoal-containing bottles with that of resin-containing bottles in the presence of antimicrobials (7–10). In particular, a range of antimicrobial agents that were potentially active in BCs of patients treated for BSI was neutralized in vitro (11, 12).
Until now, few BC simulation studies (carried out using spiked BCs with whole blood, bacteria, and antibiotics) evaluated the antimicrobial-neutralizing capability of BacT/Alert FAN Plus media in comparison with Bactec Plus media in both anaerobic and aerobic BC bottles. In two independent studies (13, 14), researchers compared aerobic media performances of BacT/Alert FA Plus bottles with those of Bactec Plus Aerobic/F bottles by testing bacteria and antibiotics (Pseudomonas aeruginosa and antipseudomonal β-lactams, in one case [14]). However, investigating both aerobic and anaerobic BC bottles is important for improving treatment outcomes in septic patients (15). Therefore, provision of more in vitro data on the capability of BC bottles to overcome the inhibitory effect of preadministered antibiotics in contrived clinical scenarios is essential (16). With this in mind, we aimed to assess the effects of antimicrobial inactivation in BacT/Alert FAN Plus or Bactec Plus media by measuring the TTD and organism growth for several antibiotic-bacterium combinations in simulated adult aerobic and anaerobic BCs. Specifically, we mimicked antibiotic blood concentrations of patients under antimicrobial drug treatment.
(Part of this study was presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Madrid, Spain, 21 to 24 April 2018 [17].)
The ethics committee of the Fondazione Policlinico Universitario A. Gemelli IRCCS approved the study (approval number 48781/17). We used resin-containing aerobic and anaerobic bottles in both BacT/Alert Virtuo (BacT/Alert FA Plus and FN Plus) and Bactec FX (Bactec Plus Aerobic/F and Plus Anaerobic/F) systems. We chose the 13 most commonly used antimicrobial drugs to treat specific BSIs and paired them with the most frequently isolated Gram-negative (n = 4) and Gram-positive (n = 4) bacterial species, for which we showed full susceptibility against specific antibiotics tested (Table 1). We obtained antibiotics from their respective manufacturers and the bacterial species from the American Type Culture Collection (ATCC) as reference strains. Each antibiotic was diluted in sterile water from freshly prepared stock solutions, so that 0.5 ml of a given dilution provided enough of the drug to reach the intended drug concentration in a bottle spiked with whole blood (see below). Cmax corresponded to the peak plasma concentrations achievable after standard dosage of the drug in a 70-kg adult with normal renal function (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf), and two other concentrations corresponded to one-half and one-quarter of the Cmax (C/2 and C/4, respectively) (Table 1). The Cmax concentrations were estimated and did not exactly reflect the actual concentrations in vivo (e.g., in patients with renal failure). Before testing, the bacterial strains were subcultured twice overnight on standard solid media at 37°C, and their MICs were determined with the broth microdilution method according to the ISO 20776-1/2006 procedure as recommended by EUCAST (18). In our BC simulation model (19), each bottle was spiked with 8-ml banked whole blood (obtained from the Division of Transfusion Medicine at our institution), 0.5-ml antibiotic solution (obtained as described above), and 0.5-ml bacterial suspension (50 to 100 CFU, achieved after diluting a suspension of ∼5 × 108 CFU/ml in phosphate-buffered saline). Resins are equally useful in the presence of lower and higher microbe quantities, as those present in blood during BSIs (20), but we decided on a more stringent experimental approach and used only one inoculum in the study. We included a positive (i.e., antibiotic-free bottle) and a negative (i.e., only blood-containing bottle) control for each simulated BC. The latter controls served to confirm the sterility of the blood, which we previously checked with a culture. In total, 40 antibiotic-organism combinations were tested in triplicate with each of the three different drug concentrations in both aerobic and anaerobic bottles, except for Bacteroides fragilis and P. aeruginosa, which were only tested under anaerobic or aerobic conditions, respectively. We immediately loaded the spiked bottles into the respective BC instruments and incubated them up to 5 days (120 h) or until they showed positive results. The positive (growth detection) or negative (no growth detection) signal was the endpoint used to calculate the TDD for each bottle (i.e., calculated from when the bottle entered into the BC system). After that, we subcultured the BC broth onto standard solid media to confirm positive or negative detections and/or to exclude contaminations. Statistical analyses were done using the Intercooled Stata program version 11 and GraphPad Prism 7. In each BC simulation, we calculated the number of positive bottles detected with both BC systems by the total number of bottles tested, as well as the mean TDD for the positive bottles. The TTD for bottles that were negative at 5 days was expressed as >120 h. Thus, we compared the rates of detection and the differences in TTDs between BC systems and between aerobic (BacT/Alert FA Plus and Bactec Plus Aerobic/F) and anaerobic (BacT/Alert FN Plus and Bactec Plus Anaerobic/F) bottles with the McNemar’s test or the paired t test, as appropriate. We considered all differences with P values of <0.05 statistically significant.
TABLE 1.
Organism and antimicrobial drug combinations tested by the simulated adult blood culture model
| Species (strain) | Drug | MIC (μg/ml)a | Drug concentration (μg/ml)b
|
||
|---|---|---|---|---|---|
| Cmax | C/2 | C/4 | |||
| Gram-negative organism | |||||
| Bacteroides fragilis (ATCC 25285) | Meropenem | 0.12 | 50 | 25 | 12.5 |
| Metronidazole | 0.5 | 25 | 12.5 | 6.3 | |
| Piperacillin-tazobactam | 0.06 | 368/23 | 184/11.5 | 92/5.8 | |
| Escherichia coli (ATCC 25922) | Ceftaroline | 0.06 | 39 | 19.5 | 9.75 |
| Ciprofloxacin | 0.016 | 3.9 | 1.95 | 0.97 | |
| Gentamicin | 0.5 | 12 | 6 | 3 | |
| Levofloxacin | 0.06 | 9.5 | 4.75 | 2.38 | |
| Meropenem | 0.06 | 50 | 25 | 12.5 | |
| Piperacillin-tazobactam | 2 | 368/23 | 184/11.5 | 92/5.8 | |
| Tigecycline | 0.12 | 1.5 | 0.75 | 0.37 | |
| Klebsiella pneumoniae (ATCC 700603) | Ceftaroline | 0.5 | 39 | 19.5 | 9.75 |
| Ciprofloxacin | 0.25 | 3.9 | 1.95 | 0.97 | |
| Gentamicin | 2 | 12 | 6 | 3 | |
| Levofloxacin | 0.06 | 9.5 | 4.75 | 2.38 | |
| Meropenem | 0.12 | 50 | 25 | 12.5 | |
| Piperacillin-tazobactam | 8 | 368/23 | 184/11.5 | 92/5.8 | |
| Tigecycline | 0.5 | 1.5 | 0.75 | 0.37 | |
| Pseudomonas aeruginosa (ATCC 27853) | Ciprofloxacin | 0.5 | 3.9 | 1.95 | 0.97 |
| Gentamicin | 1 | 12 | 6 | 3 | |
| Meropenem | 0.25 | 50 | 25 | 12.5 | |
| Piperacillin-tazobactam | 4 | 368/23 | 184/11.5 | 92/5.75 | |
| Gram-positive organism | |||||
| Enterococcus faecalis (ATCC 29212) | Ampicillin | 0.5 | 47 | 23.5 | 11.75 |
| Linezolid | 2 | 20 | 10 | 5 | |
| Tigecycline | 0.12 | 1.5 | 0.75 | 0.37 | |
| Vancomycin | 2 | 50 | 25 | 12.5 | |
| Methicillin-susceptible Staphylococcus aureus (ATCC 29213) | Ceftaroline | 0.12 | 39 | 19.5 | 9.75 |
| Daptomycin | 0.25 | 57 | 28.5 | 14.25 | |
| Linezolid | 2 | 20 | 10 | 5 | |
| Tigecycline | 0.06 | 1.5 | 0.75 | 0.37 | |
| Vancomycin | 0.5 | 50 | 25 | 12.5 | |
| Methicillin-resistant Staphylococcus aureus (ATCC 43300) | Ceftaroline | 0.5 | 39 | 19.5 | 9.75 |
| Ciprofloxacin | 0.5 | 3.9 | 1.95 | 0.97 | |
| Gentamicin | 1 | 12 | 6 | 3 | |
| Linezolid | 2 | 20 | 10 | 5 | |
| Tigecycline | 0.25 | 1.5 | 0.75 | 0.37 | |
| Vancomycin | 1 | 50 | 25 | 12.5 | |
| Streptococcus pneumoniae (ATCC 49619) | Ciprofloxacin | 0.12 | 3.9 | 1.95 | 0.97 |
| Linezolid | 0.5 | 20 | 10 | 5 | |
| Penicillin G | 0.25 | 40 | 20 | 10 | |
| Vancomycin | 0.12 | 50 | 25 | 12.5 | |
MICs of the antimicrobial drugs for each organism tested were determined by the EUCAST broth microdilution method according to standard procedures (18).
For each antimicrobial drug tested, concentrations corresponding to the plasma peak (Cmax), half (C/2), and quarter (C/4) levels are indicated. The Cmax values were those reported in online documents by EUCAST (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf).
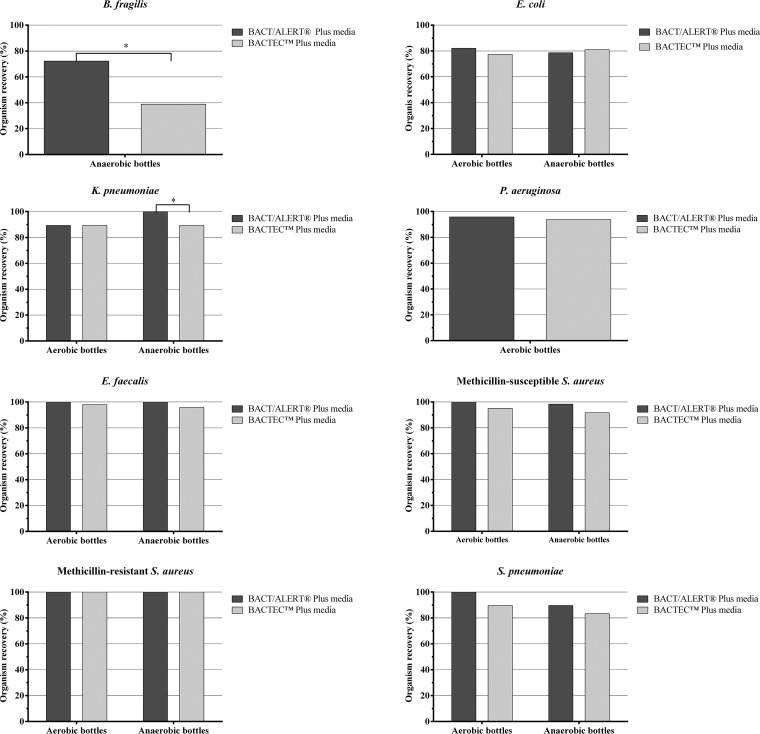
We obtained 1,752 BacT/Alert (FA Plus and FN Plus) or Bactec (Aerobic/F Plus and Anaerobic/F Plus) media-containing BC bottles to study 13 antibiotic and 8 bacterial organism combinations (Table 1). Of the bottles, 888 were aerobic (spiked with all the organisms except for B. fragilis), and 864 were anaerobic (spiked with all the organisms except for P. aeruginosa). The overall percent recovery of organisms with BacT/Alert Plus media was 93.2% (816/876 bottles) and with Bactec Plus media was 88.4% (774/876 bottles). This overall difference was statistically significant (P < 0.001). Statistically significant differences were also evident between BacT/Alert and Bactec media in aerobic bottles (94.2% [418/444] versus 90.9% [404/444]; P < 0.001) and anaerobic bottles (92.1% [398/432] versus 85.6% [370/432]; P < 0.001), respectively. Of BC bottles without antibiotics (drug-free controls), 100% (480/480) yielded all the organisms tested. We further stratified the results by organism type (Fig. 1). Differences were statistically significant in favor of BacT/Alert Plus media, most notably for B. fragilis and Klebsiella pneumoniae in anaerobic BC bottles (P < 0.05). We did not observe statistically significant differences for Escherichia coli, P. aeruginosa, Enterococcus faecalis, methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), or Streptococcus pneumoniae organisms.
FIG 1.
Percent recovery in aerobic (BacT/Alert FA Plus and Bactec Aerobic/F Plus) or anaerobic (BacT/Alert FN Plus and Bactec Anaerobic/F Plus) bottles containing antibiotics by organism.
Tables 2 and 3 summarize the TTDs for the aforementioned Gram-negative and Gram-positive bacterial species, respectively, in both BacT/Alert and Bactec aerobic and anaerobic Plus media with antibiotics compared with the same media without antibiotics. The highest ΔTTDs (≥3 h) were noticed for meropenem-P. aeruginosa (aerobic), meropenem-K. pneumoniae (anaerobic), and piperacillin-tazobactam-B. fragilis (anaerobic) in BacT/Alert FAN Plus media and for meropenem-P. aeruginosa (aerobic) and ceftaroline-E. coli (anaerobic) in Bactec Plus media for Gram-negative organisms recovered at the antibiotic Cmax (Table 2). The highest ΔTTDs (≥3 h) in BacT/Alert or Bactec media, respectively, were noted for ampicillin-E. faecalis (aerobic/anaerobic), vancomycin-MSSA (aerobic/anaerobic), ceftaroline-MRSA (anaerobic or aerobic/anaerobic), tigecycline-MRSA (anaerobic or aerobic), and vancomycin-MRSA (aerobic/anaerobic) for Gram-positive organisms recovered at the antibiotic Cmax (Table 3). Furthermore, the highest ΔTTDs were evident for linezolid-S. pneumoniae (anaerobic), gentamicin-MRSA (anaerobic), and ciprofloxacin-MRSA (anaerobic) in only BacT/Alert FAN Plus media and for linezolid-MRSA (aerobic/anaerobic) in only Bactec Plus media. Interestingly, at the antibiotic C/4 (1/4 Cmax), ΔTTDs for the aforementioned antibiotic-Gram-negative organism combinations were nearly zero (Table 2), whereas ΔTTDs for the aforementioned antibiotic-Gram-positive organism combinations had values much higher than zero (Table 3). The only exceptions were ampicillin-E. faecalis and linezolid-S. pneumoniae combinations. More interestingly, only ΔTTDs for meropenem tested with E. coli and (only in aerobic media) K. pneumoniae at the three drug concentrations remained unmeasured, because no growth was detected in both BacT/Alert and Bactec media (Table 2). Finally, overall TTDs (aerobic/anaerobic) in BacT/Alert FAN Plus media were significantly shorter than in Bactec Plus media for both Gram-negative (11.08/11.85 h versus 13.28/14.77 h; P < 0.0001, for all comparisons) and Gram-positive (11.86/12.06 h versus 14.30/15.36 h; P < 0.0001, for all comparisons) bacterial species.
TABLE 2.
Times to detection of Gram-negative organisms recovered from BacT/Alert or Bactec aerobic (AE) and anaerobic (ANA) Plus media at each antimicrobial drug concentration tested in blood culture triplicates
| Organism and drug | Concentrationa | BacT/Alert Plus media |
Bactec Plus media |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (no. of replicates/total) |
Mean TTDb (h) |
ΔTTDc (h) |
Recovery |
Mean TTDb (h) |
ΔTTDc (h) |
||||||||
| AE | ANA | AE | ANA | AE | ANA | AE | ANA | AE | ANA | AE | ANA | ||
| B. fragilis | |||||||||||||
| Meropenem | Cmax | –d | 3/3 | – | 30.87 | – | 2.35 | – | 0/3 | – | >120 | – | NAe |
| C/2 | – | 3/3 | – | 29.32 | – | 0.80 | – | 0/3 | – | >120 | – | NA | |
| C/4 | – | 3/3 | – | 27.49 | – | −1.03 | – | 1/3 | – | 33.26 | – | 1.03 | |
| No drug | – | 3/3 | – | 28.52 | – | – | 3/3 | – | 32.23 | ||||
| Metronidazole | Cmax | – | 0/3 | – | >120 | – | NA | – | 0/3 | – | >120 | – | NA |
| C/2 | – | 0/3 | – | >120 | – | NA | – | 0/3 | – | >120 | – | NA | |
| C/4 | – | 1/3 | – | 36.08 | – | 5.62 | – | 0/3 | – | >120 | – | NA | |
| No drug | – | 3/3 | – | 30.46 | – | – | 3/3 | – | 33.11 | – | |||
| Piperacillin-tazobactam | Cmax | – | 1/3 | – | 32.59 | – | 3.57 | – | 0/3 | – | >120 | – | NA |
| C/2 | – | 3/3 | – | 29.54 | – | 0.52 | – | 1/3 | – | 48.00 | – | 14.36 | |
| C/4 | – | 3/3 | – | 28.52 | – | −0.5 | – | 3/3 | – | 46.30 | – | 12.66 | |
| No drug | – | 3/3 | – | 29.02 | – | – | 3/3 | – | 33.64 | ||||
| E. coli | |||||||||||||
| Ceftaroline | Cmax | 3/3 | 1/3 | 11.11 | 11.02 | 1.75 | 2.07 | 0/3 | 1/3 | >120 | 13.56 | NA | 3.24 |
| C/2 | 3/3 | 3/3 | 9.49 | 10.04 | 0.13 | 1.09 | 1/3 | 3/3 | 13.46 | 12.28 | 3.00 | 1.96 | |
| C/4 | 3/3 | 3/3 | 9.28 | 9.37 | −0.08 | 0.42 | 3/3 | 3/3 | 11.95 | 10.91 | 1.49 | 0.59 | |
| No drug | 3/3 | 3/3 | 9.36 | 8.95 | 3/3 | 3/3 | 10.46 | 10.32 | |||||
| Gentamicin | Cmax | 3/3 | 2/3 | 9.27 | 9.20 | 0.07 | −0.08 | 3/3 | 3/3 | 11.15 | 12.96 | 0.46 | 1.71 |
| C/2 | 3/3 | 3/3 | 9.59 | 8.75 | 0.39 | −0.53 | 3/3 | 3/3 | 11.12 | 11.43 | 0.43 | 0.18 | |
| C/4 | 3/3 | 3/3 | 9.28 | 8.38 | 0.08 | −0.9 | 3/3 | 3/3 | 10.72 | 11.18 | 0.03 | −0.07 | |
| No drug | 3/3 | 3/3 | 9.20 | 9.28 | 3/3 | 3/3 | 10.69 | 11.25 | |||||
| Meropenem | Cmax | 0/3 | 0/3 | >120 | >120 | NA | NA | 0/3 | 0/3 | >120 | >120 | NA | NA |
| C/2 | 0/3 | 0/3 | >120 | >120 | NA | NA | 0/3 | 0/3 | >120 | >120 | NA | NA | |
| C/4 | 0/3 | 0/3 | >120 | >120 | NA | NA | 0/3 | 0/3 | >120 | >120 | NA | NA | |
| No drug | 3/3 | 3/3 | 9.31 | 8.89 | 3/3 | 3/3 | 11.19 | 11.93 | |||||
| Piperacillin-tazobactam | Cmax | 3/3 | 3/3 | 10.35 | 9.95 | 0.64 | 0.80 | 3/3 | 2/3 | 13.69 | 14.50 | 2.40 | 2.14 |
| C/2 | 3/3 | 3/3 | 10.22 | 9.42 | 0.51 | 0.27 | 3/3 | 3/3 | 13.07 | 13.43 | 1.78 | 1.07 | |
| C/4 | 3/3 | 3/3 | 10.30 | 9.31 | 0.59 | 0.16 | 3/3 | 3/3 | 12.37 | 12.43 | 1.08 | 0.07 | |
| No drug | 3/3 | 3/3 | 9.71 | 9.15 | 3/3 | 3/3 | 11.29 | 12.36 | |||||
| Tigecycline | Cmax | 0/3 | 0/3 | >120 | >120 | NA | NA | 0/3 | 0/3 | >120 | >120 | NA | NA |
| C/2 | 1/3 | 1/3 | 10.14 | 10.30 | 0.66 | 1.31 | 2/3 | 2/3 | 12.34 | 12.92 | 0.69 | 0.75 | |
| C/4 | 2/3 | 2/3 | 9.50 | 10.15 | 0.02 | 1.16 | 2/3 | 3/3 | 11.95 | 12.48 | 0.30 | 0.31 | |
| No drug | 3/3 | 3/3 | 9.48 | 8.99 | 3/3 | 3/3 | 11.65 | 12.17 | |||||
| Ciprofloxacin | Cmax | 3/3 | 3/3 | 10.08 | 10.14 | −0.01 | 1.52 | 3/3 | 3/3 | 11.40 | 11.32 | 0.19 | 0.58 |
| C/2 | 3/3 | 3/3 | 9.53 | 9.40 | −0.56 | 0.78 | 3/3 | 3/3 | 11.18 | 11.26 | −0.03 | 0.52 | |
| C/4 | 3/3 | 3/3 | 10.09 | 8.91 | 0.00 | 0.29 | 3/3 | 3/3 | 11.18 | 11.19 | −0.03 | 0.45 | |
| No drug | 3/3 | 3/3 | 10.09 | 8.62 | 3/3 | 3/3 | 11.21 | 10.74 | |||||
| Levofloxacin | Cmax | 3/3 | 3/3 | 10.21 | 9.34 | 0.37 | 0.28 | 3/3 | 3/3 | 12.15 | 12.10 | 0.74 | 0.73 |
| C/2 | 3/3 | 3/3 | 10.15 | 9.35 | 0.31 | 0.29 | 3/3 | 3/3 | 11.45 | 11.45 | 0.04 | 0.08 | |
| C/4 | 3/3 | 3/3 | 9.46 | 8.69 | −0.38 | −0.37 | 3/3 | 3/3 | 11.48 | 11.32 | 0.07 | −0.05 | |
| No drug | 3/3 | 3/3 | 9.84 | 9.06 | 3/3 | 3/3 | 11.41 | 11.37 | |||||
| K. pneumoniae | |||||||||||||
| Ceftaroline | Cmax | 3/3 | 3/3 | 11.16 | 11.20 | 0.12 | 0.47 | 3/3 | 3/3 | 13.15 | 13.08 | 0.98 | 0.23 |
| C/2 | 3/3 | 3/3 | 11.22 | 10.81 | 0.18 | 0.08 | 3/3 | 3/3 | 13.26 | 13.20 | 1.09 | 0.35 | |
| C/4 | 3/3 | 3/3 | 11.07 | 10.57 | 0.03 | −0.16 | 3/3 | 3/3 | 12.52 | 12.48 | 0.35 | −0.37 | |
| No drug | 3/3 | 3/3 | 11.04 | 10.73 | 3/3 | 3/3 | 12.17 | 12.85 | |||||
| Ciprofloxacin | Cmax | 3/3 | 3/3 | 10.32 | 10.45 | 1.17 | 0.99 | 3/3 | 3/3 | 11.46 | 11.26 | 0.07 | −0.11 |
| C/2 | 3/3 | 3/3 | 10.12 | 10.19 | 0.97 | 0.73 | 3/3 | 3/3 | 11.29 | 11.30 | −0.10 | −0.07 | |
| C/4 | 3/3 | 3/3 | 9.57 | 9.90 | 0.42 | 0.44 | 3/3 | 3/3 | 11.38 | 11.28 | −0.01 | −0.09 | |
| No drug | 3/3 | 3/3 | 9.15 | 9.46 | 3/3 | 3/3 | 11.39 | 11.37 | |||||
| Gentamicin | Cmax | 3/3 | 3/3 | 10.10 | 9.85 | 0.25 | 0.53 | 3/3 | 3/3 | 11.43 | 11.39 | 0.24 | 0.11 |
| C/2 | 3/3 | 3/3 | 10.08 | 9.31 | 0.23 | −0.01 | 3/3 | 3/3 | 11.49 | 11.38 | 0.30 | 0.10 | |
| C/4 | 3/3 | 3/3 | 9.42 | 9.42 | −0.43 | 0.10 | 3/3 | 3/3 | 11.21 | 11.19 | 0.02 | −0.09 | |
| No drug | 3/3 | 3/3 | 9.85 | 9.32 | 3/3 | 3/3 | 11.19 | 11.28 | |||||
| Levofloxacin | Cmax | 3/3 | 3/3 | 10.61 | 10.30 | 0.33 | −0.10 | 3/3 | 3/3 | 12.40 | 12.88 | −0.01 | 0.53 |
| C/2 | 3/3 | 3/3 | 10.31 | 10.30 | 0.03 | −0.10 | 3/3 | 3/3 | 12.37 | 12.43 | −0.04 | 0.08 | |
| C/4 | 3/3 | 3/3 | 10.16 | 10.39 | −0.12 | −0.01 | 3/3 | 3/3 | 12.19 | 12.35 | −0.22 | 0.00 | |
| No drug | 3/3 | 3/3 | 10.28 | 10.40 | 3/3 | 3/3 | 12.41 | 12.35 | |||||
| Meropenem | Cmax | 0/3 | 3/3 | >120 | 14.92 | NA | 4.67 | 0/3 | 0/3 | >120 | >120 | NA | NA |
| C/2 | 0/3 | 3/3 | >120 | 12.75 | NA | 2.50 | 0/3 | 0/3 | >120 | >120 | NA | NA | |
| C/4 | 0/3 | 3/3 | >120 | 11.60 | NA | 1.35 | 0/3 | 0/3 | >120 | >120 | NA | NA | |
| No drug | 3/3 | 3/3 | 10.14 | 10.25 | 3/3 | 3/3 | 12.28 | 12.55 | |||||
| Piperacillin-tazobactam | Cmax | 3/3 | 3/3 | 9.46 | 9.43 | 0.07 | 0.11 | 3/3 | 3/3 | 11.37 | 10.87 | 1.08 | −0.08 |
| C/2 | 3/3 | 3/3 | 9.92 | 9.44 | 0.53 | 0.12 | 3/3 | 3/3 | 10.84 | 10.87 | 0.55 | −0.08 | |
| C/4 | 3/3 | 3/3 | 9.27 | 9.13 | −0.12 | −0.19 | 3/3 | 3/3 | 10.42 | 10.75 | 0.13 | −0.20 | |
| No drug | 3/3 | 3/3 | 9.39 | 9.32 | 3/3 | 3/3 | 10.29 | 10.95 | |||||
| Tigecycline | Cmax | 3/3 | 3/3 | 11.16 | 11.20 | 0.28 | 0.36 | 3/3 | 3/3 | 13.15 | 13.08 | 0.87 | 0.81 |
| C/2 | 3/3 | 3/3 | 11.22 | 10.81 | 0.34 | −0.03 | 3/3 | 3/3 | 13.26 | 13.20 | 0.98 | 0.93 | |
| C/4 | 3/3 | 3/3 | 11.07 | 10.57 | 0.19 | −0.27 | 3/3 | 3/3 | 12.52 | 12.48 | 0.24 | 0.21 | |
| No drug | 3/3 | 3/3 | 10.88 | 10.84 | 3/3 | 3/3 | 12.28 | 12.27 | |||||
| P. aeruginosa | |||||||||||||
| Ciprofloxacin | Cmax | 3/3 | – | 15.33 | – | 1.89 | – | 1/3 | – | 17.15 | – | 1.23 | – |
| C/2 | 3/3 | – | 14.86 | – | 1.42 | – | 3/3 | – | 16.40 | – | 0.48 | – | |
| C/4 | 3/3 | – | 14.75 | – | 1.31 | – | 3/3 | – | 16.07 | – | 0.15 | – | |
| No drug | 3/3 | – | 13.44 | – | 3/3 | – | 15.92 | – | |||||
| Gentamicin | Cmax | 1/3 | – | 14.36 | – | 1.22 | – | 2/3 | – | 16.20 | – | 0.54 | – |
| C/2 | 3/3 | – | 13.54 | – | 0.40 | – | 3/3 | – | 15.33 | – | −0.33 | – | |
| C/4 | 3/3 | – | 13.19 | – | 0.05 | – | 3/3 | – | 15.07 | – | −0.59 | – | |
| No drug | 3/3 | – | 13.14 | – | 3/3 | – | 15.66 | – | |||||
| Meropenem | Cmax | 3/3 | – | 19.48 | – | 5.91 | – | 3/3 | – | 37.01 | – | 19.77 | – |
| C/2 | 3/3 | – | 14.32 | – | 0.75 | – | 3/3 | – | 20.14 | – | 2.90 | – | |
| C/4 | 3/3 | – | 13.59 | – | 0.02 | – | 3/3 | – | 17.27 | – | 0.03 | – | |
| No drug | 3/3 | – | 13.57 | – | 3/3 | – | 17.24 | – | |||||
| Piperacillin-tazobactam | Cmax | 3/3 | – | 14.20 | – | 1.03 | – | 3/3 | – | 16.80 | – | 1.55 | – |
| C/2 | 3/3 | – | 13.96 | – | 0.79 | – | 3/3 | – | 16.26 | – | 1.01 | – | |
| C/4 | 3/3 | – | 13.34 | – | 0.17 | – | 3/3 | – | 16.14 | – | 0.89 | – | |
| No drug | 3/3 | – | 13.17 | – | 3/3 | – | 15.25 | – | |||||
For each antibiotic tested, Cmax, C/2, and C/4 drug concentrations are those specified in Table 1.
For each organism-antibiotic combination tested, the mean time to detection (TTD) was calculated by summing the TTDs of single blood culture replicates. TTDs values are rounded to the nearest decimal.
For each organism-antibiotic combination tested, ΔTTD is the difference of TTD of the antibiotic-containing blood culture by that of the antibiotic-free blood culture (no-drug control). Boldface values are the clinically significant ΔTTD values (≥3 h) obtained for some organism-antibiotic combinations at the Cmax, C/2, and/or C/4 tested.
–, Lack of data because the organism was cultured only in anaerobic (ANA) (B. fragilis) or aerobic (AE) (P. aeruginosa) bottles.
NA, not applicable (no organism growth in any bottle).
TABLE 3.
Times to detection of Gram-positive organisms recovered from BacT/Alert or Bactec aerobic (AE) and anaerobic (ANA) Plus media at each antimicrobial drug concentration tested in blood culture triplicates
| Organism and drug | Concentrationa | BacT/Alert Plus media |
Bactec Plus media |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (no. of replicates/total) |
Mean TTDb
|
ΔTTDc
|
Recovery |
Mean TTDb
|
ΔTTDc
|
||||||||
| AE | ANA | AE | ANA | AE | ANA | AE | ANA | AE | ANA | AE | ANA | ||
| E. faecalis | |||||||||||||
| Ampicillin | Cmax | 3/3 | 3/3 | 18.75 | 17.79 | 9.08 | 8.06 | 2/3 | 1/3 | 15.28 | 16.50 | 3.17 | 4.23 |
| C/2 | 3/3 | 3/3 | 11.28 | 11.30 | 1.61 | 1.57 | 3/3 | 3/3 | 13.97 | 14.35 | 1.86 | 2.08 | |
| C/4 | 3/3 | 3/3 | 10.95 | 10.32 | 1.28 | 0.59 | 3/3 | 3/3 | 12.28 | 13.29 | 0.17 | 1.02 | |
| No drug | 3/3 | 3/3 | 9.67 | 9.73 | 3/3 | 3/3 | 12.11 | 12.27 | |||||
| Linezolid | Cmax | 3/3 | 3/3 | 9.66 | 9.81 | 0.20 | −0.11 | 3/3 | 3/3 | 11.30 | 11.52 | −0.01 | 0.14 |
| C/2 | 3/3 | 3/3 | 9.28 | 9.27 | −0.18 | −0.65 | 3/3 | 3/3 | 11.25 | 11.54 | −0.06 | 0.16 | |
| C/4 | 3/3 | 3/3 | 9.15 | 9.24 | −0.31 | −0.68 | 3/3 | 3/3 | 10.50 | 11.38 | −0.81 | 0.00 | |
| No drug | 3/3 | 3/3 | 9.46 | 9.92 | 3/3 | 3/3 | 11.31 | 11.38 | |||||
| Tigecycline | Cmax | 3/3 | 3/3 | 10.53 | 11.38 | 1.04 | 2.16 | 3/3 | 3/3 | 13.39 | 13.45 | 2.03 | 2.26 |
| C/2 | 3/3 | 3/3 | 9.32 | 9.84 | −0.17 | 0.62 | 3/3 | 3/3 | 11.93 | 12.29 | 0.57 | 1.10 | |
| C/4 | 3/3 | 3/3 | 9.21 | 9.27 | −0.28 | 0.05 | 3/3 | 3/3 | 11.09 | 11.44 | −0.27 | 0.25 | |
| No drug | 3/3 | 3/3 | 9.49 | 9.22 | 3/3 | 3/3 | 11.36 | 11.19 | |||||
| Vancomycin | Cmax | 3/3 | 3/3 | 10.99 | 10.70 | 1.31 | 0.72 | 3/3 | 3/3 | 12.85 | 13.71 | 0.75 | 1.62 |
| C/2 | 3/3 | 3/3 | 10.86 | 10.42 | 1.18 | 0.44 | 3/3 | 3/3 | 12.39 | 13.40 | 0.29 | 1.31 | |
| C/4 | 3/3 | 3/3 | 10.90 | 10.66 | 1.22 | 0.68 | 3/3 | 3/3 | 12.28 | 13.20 | 0.18 | 1.11 | |
| No drug | 3/3 | 3/3 | 9.68 | 9.98 | 3/3 | 3/3 | 12.10 | 12.09 | |||||
| Methicillin-susceptible S. aureus | |||||||||||||
| Ceftaroline | Cmax | 3/3 | 3/3 | 11.82 | 12.20 | 0.89 | 2.73 | 0/3 | 0/3 | >120 | >120 | NAd | NA |
| C/2 | 3/3 | 3/3 | 10.94 | 10.45 | 0.01 | 0.98 | 3/3 | 3/3 | 16.93 | 16.77 | 5.45 | 4.32 | |
| C/4 | 3/3 | 3/3 | 10.36 | 9.71 | −0.57 | 0.24 | 3/3 | 3/3 | 14.34 | 13.78 | 2.86 | 1.33 | |
| No drug | 3/3 | 3/3 | 10.93 | 9.47 | 3/3 | 3/3 | 11.48 | 12.45 | |||||
| Daptomycin | Cmax | 3/3 | 3/3 | 10.20 | 9.54 | −0.02 | 0.48 | 3/3 | 3/3 | 12.76 | 12.62 | 1.33 | 0.29 |
| C/2 | 3/3 | 3/3 | 10.30 | 9.25 | 0.08 | 0.19 | 3/3 | 3/3 | 11.77 | 12.33 | 0.34 | 0.00 | |
| C/4 | 3/3 | 3/3 | 10.40 | 9.16 | 0.18 | 0.10 | 3/3 | 3/3 | 11.43 | 12.40 | 0.00 | 0.07 | |
| No drug | 3/3 | 3/3 | 10.22 | 9.06 | 3/3 | 3/3 | 11.43 | 12.33 | |||||
| Linezolid | Cmax | 3/3 | 3/3 | 11.70 | 10.49 | 1.52 | 1.24 | 3/3 | 3/3 | 11.97 | 13.08 | 0.61 | 1.08 |
| C/2 | 3/3 | 3/3 | 11.90 | 10.17 | 1.72 | 0.92 | 3/3 | 3/3 | 12.11 | 13.21 | 0.75 | 1.21 | |
| C/4 | 3/3 | 3/3 | 10.46 | 9.83 | 0.28 | 0.58 | 3/3 | 3/3 | 11.46 | 12.38 | 0.10 | 0.38 | |
| No drug | 3/3 | 3/3 | 10.18 | 9.25 | 3/3 | 3/3 | 11.36 | 12.00 | |||||
| Tigecycline | Cmax | 3/3 | 3/3 | 10.94 | 10.90 | −0.18 | 0.88 | 3/3 | 2/3 | 12.28 | 12.85 | 0.66 | 0.29 |
| C/2 | 3/3 | 3/3 | 10.89 | 9.90 | −0.23 | −0.12 | 3/3 | 3/3 | 11.49 | 13.30 | −0.13 | 0.74 | |
| C/4 | 3/3 | 3/3 | 11.07 | 9.89 | −0.05 | −0.13 | 3/3 | 3/3 | 11.34 | 12.95 | −0.28 | 0.39 | |
| No drug | 3/3 | 3/3 | 11.12 | 10.02 | 3/3 | 3/3 | 11.62 | 12.56 | |||||
| Vancomycin | Cmax | 3/3 | 2/3 | 15.52 | 13.41 | 3.51 | 3.51 | 3/3 | 2/3 | 25.49 | 36.32 | 12.53 | 23.71 |
| C/2 | 3/3 | 3/3 | 14.57 | 13.24 | 3.34 | 3.34 | 3/3 | 3/3 | 21.45 | 23.94 | 8.49 | 11.33 | |
| C/4 | 3/3 | 3/3 | 13.92 | 12.05 | 2.15 | 2.15 | 3/3 | 3/3 | 18.23 | 18.82 | 5.27 | 6.21 | |
| No drug | 3/3 | 3/3 | 11.19 | 9.90 | 3/3 | 3/3 | 12.96 | 12.61 | |||||
| Methicillin-resistant S. aureus | |||||||||||||
| Ceftaroline | Cmax | 3/3 | 3/3 | 13.88 | 19.84 | 1.32 | 9.21 | 3/3 | 3/3 | 17.64 | 21.78 | 4.34 | 7.27 |
| C/2 | 3/3 | 3/3 | 13.84 | 17.60 | 1.28 | 6.97 | 3/3 | 3/3 | 17.35 | 18.28 | 4.05 | 3.77 | |
| C/4 | 3/3 | 3/3 | 13.23 | 16.14 | 0.67 | 5.51 | 3/3 | 3/3 | 15.96 | 16.80 | 2.66 | 2.29 | |
| No drug | 3/3 | 3/3 | 12.56 | 10.63 | 3/3 | 3/3 | 13.30 | 14.51 | |||||
| Ciprofloxacin | Cmax | 3/3 | 3/3 | 13.62 | 16.32 | 1.93 | 6.00 | 3/3 | 3/3 | 13.63 | 14.84 | 0.08 | −0.06 |
| C/2 | 3/3 | 3/3 | 12.51 | 15.08 | 0.82 | 4.76 | 3/3 | 3/3 | 13.37 | 14.77 | −0.18 | −0.13 | |
| C/4 | 3/3 | 3/3 | 12.55 | 14.20 | 0.86 | 3.88 | 3/3 | 3/3 | 13.32 | 14.32 | −0.23 | −0.58 | |
| No drug | 3/3 | 3/3 | 11.69 | 10.32 | 3/3 | 3/3 | 13.55 | 14.90 | |||||
| Gentamicin | Cmax | 3/3 | 3/3 | 13.85 | 16.66 | 2.10 | 6.29 | 3/3 | 3/3 | 13.17 | 14.29 | −0.37 | 0.10 |
| C/2 | 3/3 | 3/3 | 12.68 | 16.40 | 0.93 | 6.03 | 3/3 | 3/3 | 13.29 | 14.07 | −0.25 | −0.12 | |
| C/4 | 3/3 | 3/3 | 12.55 | 15.40 | 0.80 | 5.03 | 3/3 | 3/3 | 13.20 | 14.08 | −0.34 | −0.11 | |
| No drug | 3/3 | 3/3 | 11.75 | 10.37 | 3/3 | 3/3 | 13.54 | 14.19 | |||||
| Linezolid | Cmax | 3/3 | 3/3 | 12.80 | 12.02 | 1.37 | 1.37 | 3/3 | 3/3 | 18.57 | 19.52 | 5.27 | 4.44 |
| C/2 | 3/3 | 3/3 | 12.90 | 11.00 | 0.35 | 0.35 | 3/3 | 3/3 | 17.65 | 18.54 | 4.35 | 3.46 | |
| C/4 | 3/3 | 3/3 | 11.46 | 11.09 | 0.44 | 0.44 | 3/3 | 3/3 | 16.20 | 18.47 | 2.90 | 3.39 | |
| No drug | 3/3 | 3/3 | 11.98 | 10.65 | 3/3 | 3/3 | 13.30 | 15.08 | |||||
| Tigecycline | Cmax | 3/3 | 3/3 | 15.25 | 14.55 | 2.33 | 4.15 | 3/3 | 3/3 | 17.94 | 18.01 | 4.27 | 2.90 |
| C/2 | 3/3 | 3/3 | 14.46 | 13.88 | 1.54 | 3.48 | 3/3 | 3/3 | 18.15 | 17.34 | 4.48 | 2.23 | |
| C/4 | 3/3 | 3/3 | 13.92 | 13.22 | 1.00 | 2.82 | 3/3 | 3/3 | 17.12 | 15.42 | 3.45 | 0.31 | |
| No drug | 3/3 | 3/3 | 12.92 | 10.40 | 3/3 | 3/3 | 13.67 | 15.11 | |||||
| Vancomycin | Cmax | 3/3 | 3/3 | 16.19 | 20.27 | 4.15 | 9.69 | 3/3 | 3/3 | 25.26 | 38.05 | 11.63 | 22.48 |
| C/2 | 3/3 | 3/3 | 15.46 | 14.80 | 3.42 | 4.22 | 3/3 | 3/3 | 22.44 | 26.61 | 8.81 | 11.04 | |
| C/4 | 3/3 | 3/3 | 14.00 | 13.89 | 1.96 | 3.31 | 3/3 | 3/3 | 20.33 | 19.22 | 6.70 | 3.65 | |
| No drug | 3/3 | 3/3 | 12.04 | 10.58 | 3/3 | 3/3 | 13.63 | 15.57 | |||||
| S. pneumoniae | |||||||||||||
| Ciprofloxacin | Cmax | 3/3 | 3/3 | 12.42 | 12.24 | 1.19 | 0.99 | 3/3 | 3/3 | 13.55 | 13.51 | 0.48 | 0.13 |
| C/2 | 3/3 | 3/3 | 12.20 | 11.26 | 0.97 | 0.01 | 3/3 | 3/3 | 13.17 | 13.47 | 0.10 | 0.09 | |
| C/4 | 3/3 | 3/3 | 11.88 | 11.24 | 0.65 | −0.01 | 3/3 | 3/3 | 13.05 | 13.36 | −0.02 | −0.02 | |
| No drug | 3/3 | 3/3 | 11.23 | 11.25 | 3/3 | 3/3 | 13.07 | 13.38 | |||||
| Linezolid | Cmax | 3/3 | 3/3 | 11.26 | 19.51 | 0.19 | 7.13 | 3/3 | 3/3 | 13.66 | 16.40 | 0.53 | 2.42 |
| C/2 | 3/3 | 3/3 | 11.89 | 13.83 | 0.82 | 1.45 | 3/3 | 3/3 | 13.29 | 14.81 | 0.16 | 0.83 | |
| C/4 | 3/3 | 3/3 | 11.74 | 12.41 | 0.67 | 0.03 | 3/3 | 3/3 | 13.19 | 15.12 | 0.06 | 1.14 | |
| No drug | 3/3 | 3/3 | 11.07 | 12.38 | 3/3 | 3/3 | 13.13 | 13.98 | |||||
| Penicillin G | Cmax | 3/3 | 3/3 | 12.50 | 14.95 | 1.63 | 2.52 | 3/3 | 2/3 | 16.71 | 17.40 | 2.60 | 2.95 |
| C/2 | 3/3 | 3/3 | 11.32 | 12.52 | 0.45 | 0.09 | 3/3 | 3/3 | 15.13 | 15.88 | 1.02 | 1.43 | |
| C/4 | 3/3 | 3/3 | 11.63 | 12.26 | 0.76 | −0.17 | 3/3 | 3/3 | 15.03 | 15.30 | 0.92 | 0.85 | |
| No drug | 3/3 | 3/3 | 10.87 | 12.43 | 3/3 | 3/3 | 14.11 | 14.45 | |||||
| Vancomycin | Cmax | 3/3 | 0/3 | 11.32 | >120 | 0.14 | NA | 0/3 | 0/3 | >120 | >120 | NA | NA |
| C/2 | 3/3 | 2/3 | 11.22 | 12.25 | 0.04 | 0.22 | 1/3 | 0/3 | 19.50 | >120 | 7.06 | NA | |
| C/4 | 3/3 | 2/3 | 11.28 | 12.19 | 0.10 | 0.16 | 3/3 | 2/3 | 16.43 | 17.35 | 3.99 | 4.25 | |
| No drug | 3/3 | 3/3 | 11.18 | 12.03 | 3/3 | 3/3 | 12.44 | 13.10 | |||||
For each antibiotic tested, Cmax, C/2, and C/4 are those specified in Table 1.
For each organism-antibiotic combination tested, the mean time to detection (TTD) was calculated by summing the TTDs of single blood culture replicates. TTDs are rounded to the nearest decimal.
For each organism-antibiotic combination tested, ΔTTD is the difference of TTD of the antibiotic-containing blood culture by that of the antibiotic-free blood culture (no-drug control). Boldface values are the clinically significant ΔTTD values (≥3 h) obtained for some organism-antibiotic combinations at the Cmax, C/2, and/or C/4 tested.
NA, not applicable (no organism growth in any bottles).
Our findings agree with the recent findings of other researchers that the present ex vivo (using human blood and simulated antibiotic concentrations)/in vitro study provides further support for a worthwhile use of BacT/Alert (FA Plus and FN Plus) or Bactec (Plus Aerobic/F and Plus Anaerobic/F) antimicrobial-inactivating BC media (13, 14). We tested a range of Gram-negative and Gram-positive bacterial species against clinically effective concentrations of three to seven antibiotics per species. Apart from the peak (P) concentration (i.e., Cmax), we selected for each antibiotic two descending concentrations (i.e., C/2 and C/4) that were higher than the midpoint (M) and/or through (T) concentrations tested elsewhere (8, 9, 14). Therefore, consistent with the study by Mitteregger et al. (10), we ensured that the antibiotic levels of C/2- or C/4-spiked BCs were still higher than the expected antibiotic-inactivating capability for aerobic/anaerobic BC media so that organism recoveries could occur in BC bottles containing still-active (above the MIC) antibiotic concentrations. Importantly, aside from the overall high recovery rates by both systems (93.2% and 88.4%, respectively), we showed that either BacT/Alert (FA Plus and FN Plus) or Bactec (Plus Aerobic/F and Plus Anaerobic/F) media allowed for the recovery of all organisms tested at the antibiotic C/4 in ≥1 replicate of 40 antibiotic-organism combinations (Tables 2 and 3). The only exceptions were that E. coli in the presence of C/4 meropenem failed to grow in any aerobic or anaerobic bottle, and K. pneumoniae in the presence of C/4 meropenem and B. fragilis in the presence of C/4 metronidazole grew only in BacT/Alert FN Plus. In fact, BacT/Alert FN Plus was able to recover K. pneumoniae isolates at all levels of meropenem, not just at C/4. In the study by Grupper et al. (14), P. aeruginosa ATCC 27853, the sole bacterial species tested and able to be compared, grew in <26 h in all BacT/Alert FA Plus and Bactec Plus Aerobic/F bottles in the presence of meropenem T concentrations. In our study, the same species grew in ∼13 and ∼17 h in all BacT/Alert FA Plus and Bactec Plus Aerobic/F bottles, respectively in the presence of meropenem C/4 concentrations. Despite differences in the bacterial inoculum between that study and ours (7 to 30 and 50 to 100 CFU/bottle, respectively), we could equitably compare our results with those of Grupper et al. (14). Conversely, Mitteregger et al. (10) showed the recovery of meropenem-susceptible P. aeruginosa isolates (MIC, 0.094 μg/ml) in BacT/Alert FA Plus bottles containing meropenem P concentrations. If a larger inoculum (up to 100 CFU/ml) in the study by Mitteregger et al. contributed to these conflicting findings, it remains unknown. In one clinical study, Zadroga et al. (11) assessed the recovery of bacteria in patient BCs in relation to prior antimicrobial administration. Surprisingly, the researchers reported residual piperacillin concentrations above the MIC in BacT/Alert FAN bottles, which contain activated charcoal instead of the polymeric beads found in the FA Plus and FN Plus bottles, sampled near the P or M and not in those sampled near the T concentration. Notably, Zadroga et al. (11) observed the recovery of bacteria in Bactec Plus BC bottles from the same patients who were administered piperacillin-tazobactam before collection. Taken together, these data suggest that the variability in BC performances across the studies may not only depend on antimicrobial-binding media but also on combinations of bacteria and antibiotics in BCs (16).
In terms of TTD, the other parameter evaluated here, at the antibiotic C/4, delays ≥3 h with respect to detection in control (antibiotic-free) bottles were noticed in 12 bacterium-antibiotic pairs across all Gram-positive bacteria except for E. faecalis. Among Gram-negative bacteria, B. fragilis with metronidazole (BacT/Alert FN Plus) and piperacillin-tazobactam (Bactec Plus Anaerobic/F) also showed delays of ≥3 h. Eight (66.6%) of the 12 Gram-positive cases included MRSA tested with ceftaroline, ciprofloxacin, gentamicin, or vancomycin in BacT/Alert FN Plus; linezolid or vancomycin in Bactec Plus Anaerobic/F; tigecycline in Bactec Plus Aerobic/F; and vancomycin in both Bactec Plus Aerobic/F and Plus Anaerobic/F. Two (16.7%) cases involved MSSA, and 2 (16.7%) involved S. pneumoniae, all tested with vancomycin in both Bactec Plus Aerobic/F and Plus Anaerobic/F. Looking at these cases, differences in TTD did not favor the Bactec Plus Aerobic/F and/or Plus Anaerobic/F media for 8 drug-organism combinations and BacT/Alert FN Plus media for 4 combinations. Notably, 7 (58.3%) of 12 cases involved vancomycin, i.e., 2 with MSSA, 3 with MRSA, and 2 with S. pneumoniae. Thus, it is plausible that the type/species of bacterium and/or its interaction with a specific antibiotic in a complex milieu such as that of the BC bottle can affect the outcome of antibiotic inactivation by the resin within the BC bottle media.
In conclusion, within the limitations of a simulated study (16), we showed a relative rather than an absolute advantage for BacT/Alert (FA Plus and FN Plus) media compared with Bactec (Plus Aerobic/F and Plus Anaerobic/F) media. At concentrations almost equivalent to the lowest possible levels of antibiotics in plasma, both BacT/Alert and Bactec Plus media were efficient against 13 antibiotics, with the exception of E. coli with meropenem in all bottles and K. pneumoniae with meropenem in BacT/Alert FA Plus and Bactec Plus Aerobic/F and Plus Anaerobic/F bottles. Clinically relevant delays in TTD (≥3 h) were noticed with vancomycin, particularly when tested in Bactec Plus media. Ultimately, optimizing the time of draw for and the collection of BCs (i.e., just before the next antibiotic dose and with complete aerobic and anaerobic sets) may help maximize the efficiency of BacT/Alert or Bactec Plus media for the recovery of bloodstream bacterial pathogens.
ACKNOWLEDGMENTS
We thank Federica Ventriglia for technical assistance and Franziska Lohmeyer for English review of this study.
In addition to providing reagents and funding for this study, bioMérieux participated in the study design and reviewed and commented on the manuscript before submission.
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Pien BC, Sundaram P, Raoof N, Costa SF, Mirrett S, Woods CW, Reller LB, Weinstein MP. 2010. The clinical and prognostic importance of positive blood cultures in adults. Am J Med 123:819–828. doi: 10.1016/j.amjmed.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 4.Reddy P. 2016. Empiric antibiotic therapy of nosocomial bacterial infections. Am J Ther 23:e982–e994. doi: 10.1097/MJT.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 5.Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. 2016. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol 7:697. doi: 10.3389/fmicb.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamy B, Ferroni A, Henning C, Cattoen C, Laudat P. 2018. How to: accreditation of blood cultures' proceedings. A clinical microbiology approach for adding value to patient care. Clin Microbiol Infect 24:933–934. doi: 10.1016/j.cmi.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Spaargaren J, van Boven CP, Voorn GP. 1998. Effectiveness of resins in neutralizing antibiotic activities in Bactec Plus Aerobic/F culture medium. J Clin Microbiol 36:3731–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. 2007. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol 45:816–821. doi: 10.1128/JCM.02064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller NS, Rogan D, Orr BL, Whitney D. 2011. Comparison of BD Bactec Plus blood culture media to VersaTREK Redox blood culture media for detection of bacterial pathogens in simulated adult blood cultures containing therapeutic concentrations of antibiotics. J Clin Microbiol 49:1624–1627. doi: 10.1128/JCM.01958-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitteregger D, Barousch W, Nehr M, Kundi M, Zeitlinger M, Makristathis A, Hirschl AM. 2013. Neutralization of antimicrobial substances in new BacT/Alert FA and FN Plus blood culture bottles. J Clin Microbiol 51:1534–1540. doi: 10.1128/JCM.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zadroga R, Williams DN, Gottschall R, Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP, Sutherland C, Hansen GT. 2013. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis 56:790–797. doi: 10.1093/cid/cis1021. [DOI] [PubMed] [Google Scholar]
- 12.Kirn TJ, Mirrett S, Reller LB, Weinstein MP. 2014. Controlled clinical comparison of BacT/Alert FA Plus and FN Plus blood culture media with BacT/Alert FA and FN blood culture media. J Clin Microbiol 52:839–843. doi: 10.1128/JCM.03063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovern D, Katzin B, Johnson K, Broadwell D, Miller E, Gates A, Deol P, Doing K, van Belkum A, Marshall C, Mathias E, Dunne WM Jr. 2016. Antimicrobial binding and growth kinetics in BacT/ALERT FA Plus and BACTEC Aerobic/F Plus blood culture media. Eur J Clin Microbiol Infect Dis 35:2033–2036. doi: 10.1007/s10096-016-2759-9. [DOI] [PubMed] [Google Scholar]
- 14.Grupper M, Nicolau DP, Aslanzadeh J, Tanner LK, Kuti JL. 2017. Effects of clinically meaningful concentrations of antipseudomonal β-lactams on time to detection and organism growth in blood culture bottles. J Clin Microbiol 55:3502–3512. doi: 10.1128/JCM.01241-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 16.Grupper M, Kuti JL, Nicolau DP. 2017. In vitro blood culture bottle inoculation of whole blood with clinically relevant antibiotic concentrations: a word of caution. Eur J Clin Microbiol Infect Dis 36:917–919. doi: 10.1007/s10096-016-2874-7. [DOI] [PubMed] [Google Scholar]
- 17.Menchinelli G, Liotti FM, Giordano L, D'Inzeo T, Fiori B, Sanguinetti M, Spanu T. 2018. Abstr 28th Eur Congr Clin Microbiol Infect Dis [ECCMID], Madrid, Spain, 21 to 24 April 2018, poster P1950. [Google Scholar]
- 18.International Organization for Standardization (ISO). 2006. Clinical laboratory testing and in vitro diagnostic test systems—susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices—Part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. ISO 20776-1 ISO, Geneva, Switzerland: http://www.eucast.org/ast_of_bacteria/mic_determination/. [Google Scholar]
- 19.Menchinelli G, Liotti FM, Fiori B, De Angelis G, D'Inzeo T, Giordano L, Posteraro B, Sabatucci M, Sanguinetti M, Spanu T. 2019. In vitro evaluation of BacT/Alert VIRTUO, BacT/Alert 3D, and Bactec FX automated blood culture systems for detection of microbial pathogens using simulated human blood samples. Front Microbiol 10. doi: 10.3389/fmicb.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opota O, Croxatto A, Prod'hom G, Greub G. 2015. Blood culture-based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect 21:313–322. doi: 10.1016/j.cmi.2015.01.003. [DOI] [PubMed] [Google Scholar]