Pseudomonas aeruginosa is a Gram-negative opportunistic bacterial pathogen that can cause chronic lung infections in patients with cystic fibrosis (CF). The current preferred treatment for CF lung infections includes inhaled tobramycin (TOB); however, studies suggest TOB cannot effectively inhibit biofilm formation.
KEYWORDS: Pseudomonas aeruginosa, alginate, antibiofilms, antimicrobial activity, mouse pneumonia, multidrug resistance, nebulization, rifaximin, tobramycin
ABSTRACT
Pseudomonas aeruginosa is a Gram-negative opportunistic bacterial pathogen that can cause chronic lung infections in patients with cystic fibrosis (CF). The current preferred treatment for CF lung infections includes inhaled tobramycin (TOB); however, studies suggest TOB cannot effectively inhibit biofilm formation. Using an NIH small compounds drug library approved for safe use in humans, we identified rifaximin (RFX), a semisynthetic, rifamycin family, nonsystemic antibiotic that inhibits alginate production and growth in P. aeruginosa. Inhibition of alginate production was further analyzed using the uronic acid carbazole assay and a promoter reporter assay that measures the transcription of the alginate biosynthetic operon. Compared to TOB, RFX significantly reduced alginate production in laboratory and CF sputum isolates of P. aeruginosa. In addition, RFX showed a narrow range of MICs when measured with multidrug-resistant bacterial species of clinical relevance, synergistic activities with TOB or amikacin against clinical isolates, as well as reduction toward in vitro preformed biofilms. In C57BL/6 mice, penetration of nebulized TOB into the lungs was shown at a higher level than that of RFX. Further, in vivo assessment using a DBA/2 mouse lung infection model found increased survival rates with a single-dose treatment of nebulized RFX and decreased P. aeruginosa PAO1 bioburden with a multiple-dose treatment of RFX plus TOB. In addition, mice treated with a single exposure to dimethyl sulfoxide (DMSO), a solvent that dissolves RFX, showed no apparent toxicity. In summary, RFX may be used to supplement TOB inhalation therapy to increase efficacy against P. aeruginosa biofilm infections.
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic bacterial pathogen responsible for hospital-acquired infections and ventilator-associated pneumonia (1). The primary cause of mortality in patients with cystic fibrosis (CF) is sequelae from chronic respiratory infections with P. aeruginosa (2). Factors such as biofilm formation and multidrug resistance make P. aeruginosa particularly difficult to treat (3).
Biofilms are formed when colonies aggregate and produce an extracellular matrix that provides protection from antibiotics and immune responses (4). P. aeruginosa produces a matrix that contains a polysaccharide called alginate, which consists of a linear copolymer of guluronic and mannuronic acid (4). The formation of P. aeruginosa biofilms leads to a poor prognosis in patients by decreasing lung function and increasing morbidity and mortality (5). The phenotype of alginate overproduction by P. aeruginosa is known as mucoidy and is a prevalent phenotype in CF sputum isolates (6).
The current treatment strategy for CF patients with chronic P. aeruginosa infections is daily administration of inhaled tobramycin (TOB) for a period of 4 weeks. Tobramycin has been formulated for inhalational therapy (Tobi). TOB is an aminoglycoside antibiotic with a broad spectrum against Gram-negative bacteria, including P. aeruginosa (7). However, studies have suggested that TOB alone may not be an optimal treatment for CF chronic lung infections with P. aeruginosa, especially biofilm-producing strains. In vitro studies have shown that diffusion of TOB is inhibited by the binding of TOB to alginate (8). Furthermore, the penetration of TOB through alginate biofilms and CF sputa is decreased as biofilms develop, especially at lower concentrations (9). Additionally, the use of multiple doses of TOB therapy has resulted in a decrease in the susceptibility of P. aeruginosa strains to aminoglycosides due to the increase in drug impermeability (9, 10). Therefore, an alteration in the treatment strategy is needed to aid in targeting biofilm formation.
Rifaximin (RFX; Xifaxan) is a broad-spectrum RNA polymerase inhibitor commonly used to treat traveler’s diarrhea and hepatic encephalopathy (11–13). RFX is not water soluble and can be delivered in the gastrointestinal tract in high dosages with little risk of uptake and absorption into the body. Furthermore, RFX shows activity against P. aeruginosa and blocks alginate production (14, 15).
In this study, we screened a NIH drug library (16, 17) and identified RFX as a possible antibiotic adjuvant to treat P. aeruginosa lung infections. We also determined that with TOB, RFX was more efficacious against multidrug resistant (MDR) P. aeruginosa isolates from CF patients. We further showed that RFX was significantly better at inhibiting alginate production in P. aeruginosa than TOB and that the addition of RFX to TOB helped decrease preformed biofilms. Moreover, we showed that we could deliver RFX to the lungs via nebulization and efficiently treat respiratory infections in a mouse pneumonia model. These results suggest that RFX may be a suitable supplement to TOB in treating P. aeruginosa biofilm respiratory infections.
RESULTS
Screen of NIH drug library for inhibition of alginate production by P. aeruginosa identified RFX.
Treatment with TOB cannot completely eradicate P. aeruginosa lung infections in CF, as shown by various recent studies (5, 7, 9, 10, 18, 19). To identify an alternative treatment strategy, we screened a NIH drug library (16, 17), consisting of the 1,171 pharmacologically active compounds currently used in pharmaceuticals and/or clinical trials for inhibition of alginate production, as well as growth inhibition activity against P. aeruginosa. We tested three concentrations of drugs (50, 10, and 2 μM). A reporter plasmid containing promoters for the alginate biosynthetic operon (PalgD) fused to the lacZ operon was introduced into a stable mucoid laboratory strain of P. aeruginosa, PAO581 (20). Using this construct, we screened for growth and mucoidy inhibition, as well as effects on alginate biosynthetic promoter activity. Fifteen of the drugs were identified as inhibitors of mucoidy of P. aeruginosa (see Table S1 and Fig. S1 in the supplemental material). We further tested these drugs using a clinical mucoid isolate from a CF patient and determined that RFX was the most effective drug to inhibit growth and mucoidy (data not shown).
RFX is effective against clinical isolates of Gram-negative and Gram-positive bacteria and has synergistic activity with TOB or amikacin.
Because RFX is not a traditional treatment for P. aeruginosa infections, we first wanted to test whether it was effective in vitro against P. aeruginosa and other bacterial pathogens seen in CF. Since RFX is not used for treatment of ventilator-associated pneumonia (VAP), we thought the isolates of tracheal aspirates from VAP patients in the intensive care unit (ICU) may be sensitive to RFX. To determine the susceptibility to RFX, we chose the resistant isolates from Cabell Huntington Hospital (Huntington, WV) to measure the MICs (21) for RFX (see Table S2 in the supplemental material). The MIC50 and MIC90 for MDR Staphylococcus aureus including methicillin-resistant S. aureus (MRSA) were determined to be 2 and 16 μg/ml, respectively (Table 1). The MIC50 and MIC90 for MDR P. aeruginosa were determined to be 8 and 16 μg/ml, respectively (Table 1). The MICs of RFX were also determined for other drug-resistant pathogens that were associated with CF and VAP. We found that RFX effectively inhibited the growth of clinical isolates of Burkholderia cepacia, Acinetobacter baumannii, and Stenotrophomonas maltophilia (Table 1).
TABLE 1.
Determination of RFX MICs against clinical isolates of MDR bacterial speciesa
| Bacterial species | No. of strains tested | MIC50 (mg/liter) | MIC90 (mg/liter) |
|---|---|---|---|
| Pseudomonas aeruginosa | 26 | 8 | 16 |
| Staphylococcus aureus | 22 | 2 | 16 |
| Acinetobacter baumannii | 9 | <0.5 | <0.5 |
| Burkholderia cepacia | 4 | 16 | 32 |
| Stenotrophomonas maltophilia | 1 | 16 | 16 |
Sources of clinical isolates are listed in Table S2 in the supplemental material. MIC50 and MIC90 are the concentrations of RFX required for the growth inhibition of 50% and 90% of strains, respectively.
RFX is a member of the rifamycins family of antibiotics. Its function is to inhibit transcription by bacterial RNA polymerase; however, mutations in the gene encoding the bacterial RNA polymerase frequently emerge. To increase the efficacy of this group of antibiotics, we considered whether RFX could be used in combination with antipseudomonal antibiotics to reduce the frequency of resistant mutations. Checkerboard (isobologram) analysis with RFX and TOB combinations showed synergistic (summation of the fractional inhibition concentration [ΣFIC] ≤ 0.5) or additive (ΣFIC 0.5 < to ≤ 1) effects (22) in all of the strains tested (Table 2). In the final analysis, RFX was found to be a good candidate for further study as an antibiotic adjuvant for the treatment of respiratory infections due to MDR pathogens.
TABLE 2.
MICs and synergy activity of RFX and TOB against P. aeruginosa strains
| Strain | Typea | MIC RFX | MIC RFX in combination | FIC RFX | MIC TOB | MIC TOB in combination | FIC TOB | ΣFIC | Activityb |
|---|---|---|---|---|---|---|---|---|---|
| PAO1 | NM | 16 | 4 | 0.25 | 2 | 0.25 | 0.125 | 0.375 | ++ |
| PAO581 | M | 16 | 4 | 0.25 | 2 | 0.5 | 0.25 | 0.5 | + |
| PAO581 | NM revertant | 16 | 4 | 0.25 | 2 | 0.5 | 0.25 | 0.5 | + |
| CF001 | M | 16 | 4 | 0.25 | 2 | 0.5 | 0.25 | 0.5 | + |
| CF010 | M | 8 | 2 | 0.25 | 2 | 0.5 | 0.25 | 0.5 | + |
| CF003 | NM | 4 | 1 | 0.25 | 2 | 0.25 | 0.125 | 0.375 | + |
NM, nonmucoid; M, mucoid.
Lowest ΣFIC measure: synergy (++) ≤ 0.5; additive (+) 0.5 < to ≤ 1; indifferent (±) 1< to ≤ 4; antagonism (−) > 4 (22).
RFX is more effective than TOB in inhibiting the alginate production in P. aeruginosa.
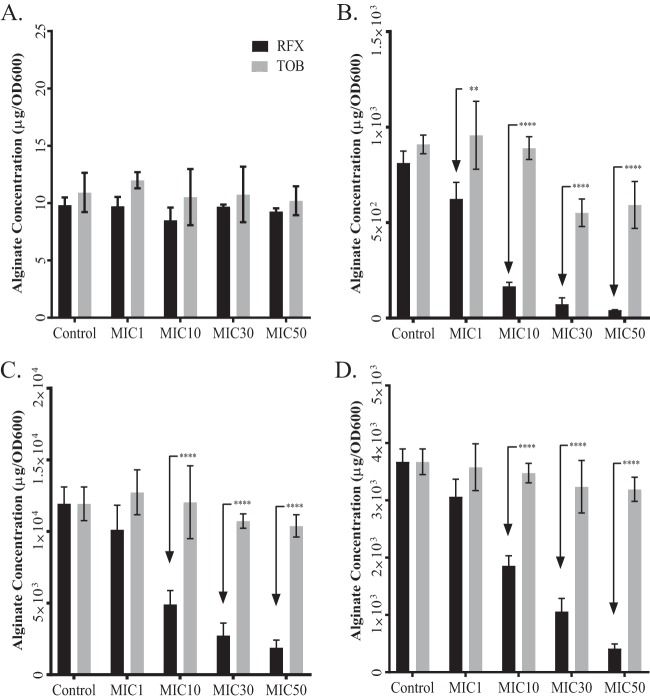
The results of the preliminary drug screening indicated that RFX may inhibit alginate biosynthesis. To investigate the extent of this inhibition, we grew PAO1, PAO581, and two mucoid P. aeruginosa CF isolates (CF001 and CF010) (23) on Pseudomonas isolation agar (PIA) plates containing sub-MICs of either TOB or RFX and measured alginate production with the carbazole assay. We chose these isolates because they had a similar MIC range for RFX and TOB and were still sensitive to TOB. We used MIC normalization to compare the effect of the two drugs on inhibition of alginate production, as this allows the measurement of alginate made by the same number of bacterial cells. Using this method, there was no significant difference in alginate production by the nonmucoid strain PAO1 when treated with TOB or RFX at any concentration (Fig. 1A). Conversely, RFX significantly decreased alginate production in the mucoid PAO581 strain at all concentrations tested compared to that with TOB (Fig. 1B). We also observed a significant decrease in alginate production in the mucoid CF isolates CF001 and CF010 with RFX compared to that with TOB (Fig. 1C and D). These results indicate that RFX is more effective than TOB at inhibiting the alginate production in mucoid strains of P. aeruginosa.
FIG 1.
RFX is more potent than TOB for inhibition of alginate production of laboratory and CF sputum isolates of P. aeruginosa. Alginate production for nonmucoid reference strain PAO1 (A), PAO581 (PAO1mucA25) (B), and stable mucoid clinical isolates CF001 (C) and CF010 (D) when grown on PIA at 37°C for 24 h with increasing concentrations of RFX or TOB according to the predetermined MIC. Data shown represent means of 5 replicates per group ± standard error (SE). Two-way ANOVA analysis was used with statistical significance set as a P value of <0.01 (****, P < 0.0001; **, P < 0.001; *, P < 0.01).
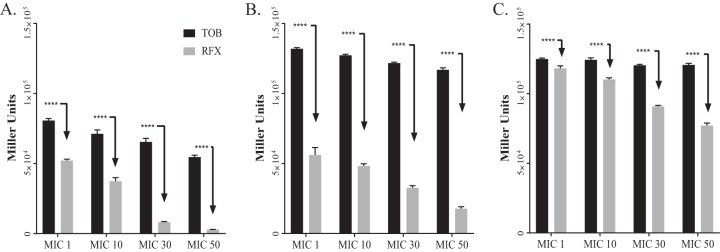
While RFX inhibits alginate production, we wanted to determine whether the inhibition was specific to alginate or whether it was a global suppression of transcription. To test this, we used the promoter reporter construct PalgD_lacZ in pLP170 (20) to quantify promoter activity of the alginate biosynthetic operon PalgD. A promoterless empty vector pLP170-lacZ was used as a negative control. Using the β-galactosidase reporter activity, we quantified the activity of PalgD in PAO581 (Fig. 2A) and two mucoid isolates (CF001 and CF010) from CF patients. When grown in PIA containing various concentrations of RFX or TOB (Fig. 2B and C), RFX had a more pronounced effect on promoter activity than TOB throughout the concentration range in all three tested strains. Based on these data, it was concluded that RFX inhibits alginate biosynthesis by antagonizing transcription.
FIG 2.
RFX versus TOB mediated inhibition of the promoter PalgD activity of the alginate biosynthetic operon in laboratory and clinical isolates of P. aeruginosa. The β-galactosidase activity of PalgD was measured using the pLP170-PalgD-lacZ reporter construct in PAO581 (A), CF001 (B), and CF010 (C) grown on PIA plates with increasing concentrations of RFX or TOB (MIC1, MIC10, MIC30, and MIC50 as being 1%, 10%, 30%, and 50% MIC, respectively) at 37°C for 24 h. Relative expression mean values are shown ± SE of triplicate determinations. Two-way ANOVA was used to determine statistical significance set at a P value of <0.01 (****, P < 0.0001).
TOB with the addition of RFX reduces the biofilm aggregates and viable cell counts.
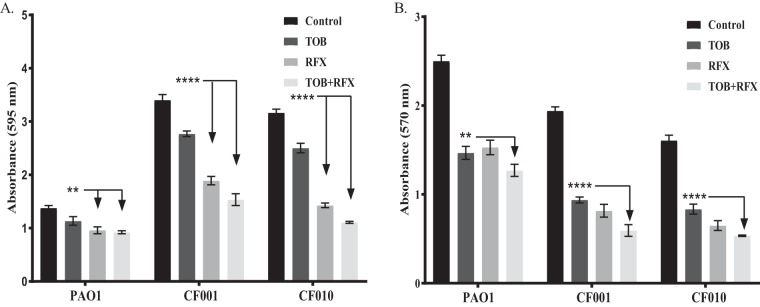
After determining that RFX inhibited alginate production, we then tested the ability of RFX to reduce biofilm aggregates, defined as a decrease in preformed biofilm, as well as the biofilm viable cell counts. We performed an established biofilm reduction assay (24) and viable cell counts using PAO1 and mucoid clinical isolates CF001 and CF010. TOB significantly reduced biofilm compared to that of the untreated control (Fig. 3A). However, TOB with the addition of RFX demonstrated increased ability to reduce the biofilm aggregates compared to those of TOB alone. Furthermore, TOB significantly reduced the number of viable cells in a preformed biofilm compared to that of the untreated control. The addition of RFX with TOB significantly reduced this number further (Fig. 3B), suggesting that the addition of RFX helps TOB penetrate biofilms and decrease biofilm viable cell counts.
FIG 3.
Biofilm and cell viability reduction from treatment with TOB and/or RFX. (A) Biofilms were grown for 24 h in a 96-well plate and then treated with TOB, RFX, or TOB and RFX at a concentration of 3× MIC. (B) Biofilms were grown for 24 h in a 96-well plate and then treated with TOB, RFX, or TOB and RFX at concentration of 3× MIC. Two-way ANOVA analysis was used with statistical significance set as a P value of <0.01 (****, P < 0.0001; **, P < 0.001; *, P < 0.01).
RFX can be delivered to the lungs via nebulization.
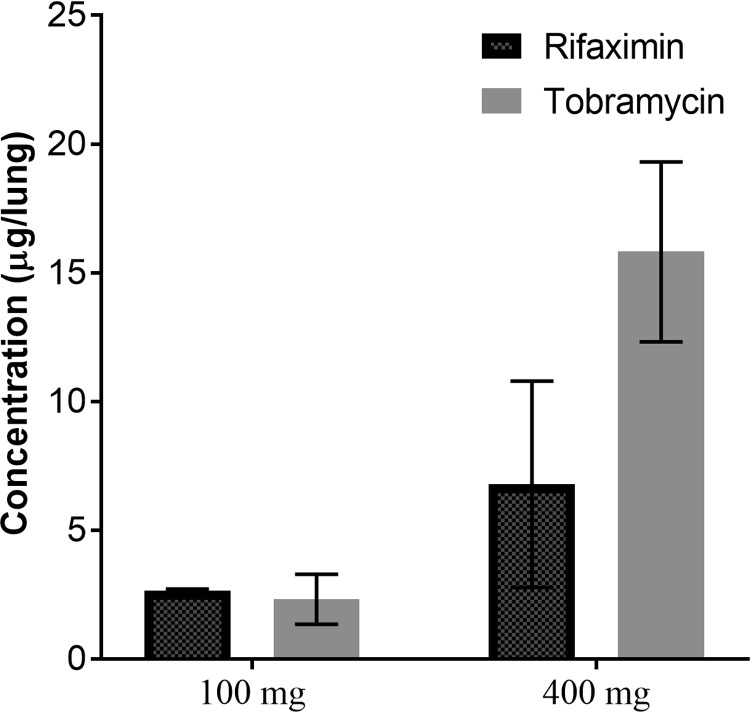
As treatment for traveler’s diarrhea, RFX is a drug optimized for oral use. For RFX to be useful as a treatment for CF patients, we wanted to test whether it could be delivered to the lungs through nebulization. The physiochemical properties make RFX only sparingly soluble in water; therefore, dimethyl sulfoxide (DMSO) was evaluated as a nebulization vehicle. The potential adverse effects were first assessed by nebulizing DMSO alone to a group of C57BL/6 mice (n = 8). We used this strain of mice due to their natural resistance against infection based on our previous studies (25). Exposure to a single dose of 5 ml of 100% DMSO caused no adverse effects. One month after exposure, all exposed mice were alive with body weights comparable to those of the phosphate-buffered saline (PBS)-treated control mice (data not shown). Next, we optimized the delivery of both RFX and TOB via nebulization. RFX or TOB was delivered to the lungs of C57BL/6 mice (n = 16) using a Schuco nebulizer and whole-body exposure chamber with targeted delivery concentrations of 100 mg or 400 mg per mouse (estimated to be 200 mg per mouse lung). These two concentrations were chosen, as the current approved clinical treatment for nebulization therapy in human CF is 300 mg TOB nebulized in 5 ml 0.225% saline as the drug delivery chamber is roughly the volume of an average human lung. Thus, both RFX and TOB were naturally respired by the mice. To determine the dosage of each drug received by the mice, the lungs were homogenized and drug concentrations of TOB and RFX were determined via enzyme-linked immunosorbent assay (ELISA) and high-pressure liquid chromatography (HPLC), respectively. RFX and TOB were present in the lungs at similar concentrations after the 100-mg nebulization (Fig. 4); however, at a 400-mg nebulization, TOB exhibited a non-statistically significant increase in lung concentration over RFX. These results indicate that nebulization can deliver both drugs to the mouse lung at a low dose (100 mg), but TOB may penetrate the lung tissues more efficiently than RFX at a high dose (400 mg). To determine the solvent effect on the particle size of nebulized drugs, the droplet size for the two formulations were measured with the median droplet size for TOB and RFX being 5.07 μm and 5.02 μm, respectively (see Fig. S2 in the supplemental material).
FIG 4.
Comparison of the levels of RFX and TOB delivered via nebulization in mouse lungs. The concentration of RFX or TOB present in lungs of C57BL/6 mice immediately after treatment with 100 or 400 mg of the respective antibiotics. Data shown represent means of 4 lung samples with replicates totaling 8 samples per group ± SE. One-way ANOVA was used to analyze the data with statistical significance set at a P value of <0.01. No significant differences were found between the RFX versus TOB concentrations present in the mouse lungs.
RFX effectively treats P. aeruginosa infection in mice.
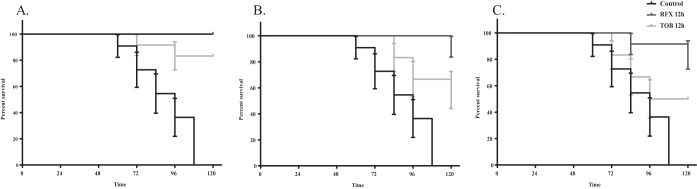
After establishing the dosing of TOB or RFX to efficiently reach the lungs, we compared the efficacy of both drugs against P. aeruginosa lung infections using an established mouse model (25). DBA/2 (n = 84) male mice were first infected with aerosolized P. aeruginosa PAO1 in a whole-body exposure chamber as previously described (26). The mice were then separated into four groups. One group was left untreated to determine the rate of mortality (control). The remaining three groups were treated with RFX or TOB using a Schuco nebulizer and whole-body exposure chamber at a concentration of 300 mg at 0 h, 6 h, or 12 h postinfection. All control mice died within 5 days (Fig. 5), consistent with previous reports (25). Treatment with RFX or TOB at 0 h after infection resulted in 100% and 92% survival, respectively (Fig. 5A). At 6 h postinfection, treatment of RFX resulted in 92% survival while 58% of mice treated with TOB survived (Fig. 5B). In the 12 h treatment group, 83% of mice treated with RFX survived while only 50% of mice treated with TOB survived (Fig. 5C). Thus, RFX treatment after P. aeruginosa lung infection resulted in a higher survival and recovery rate than TOB treatment in a mouse lung infection model.
FIG 5.
RFX is more effective than TOB in the treatment of P. aeruginosa infection in mice. Kaplan-Meier curves showing percent survival of DBA/2 mice with P. aeruginosa PAO1 infection after treatment with RFX or TOB at 0 h postinfection (A), 6 h postinfection (B), and 12 h postinfection (C). Data shown represent means of 12 mice per group. The Mantel-Cox test was used with significance set at a P value of <0.01 and showing standard error (SE) bars.
The addition of RFX to multiple treatments of TOB reduces P. aeruginosa growth in lungs of mice.
Based on the efficacy of RFX observed with the single-dose treatment, we used a multiple-dose study to replicate clinical treatment of CF infections. The efficacy of TOB in multiple doses with or without RFX was determined using the established lung infection model. DBA/2 (n = 24) female mice were exposed to aerosolized P. aeruginosa PAO1 with a chromosomal bioluminescent operon (luxCDABE) as described above (22). A bioluminescent PAO1 was used to track the respiratory bacterial load, as well as to determine the presence of the lung normal flora. The mice were then separated into three groups of 8 as follows: one group was untreated, one group received three TOB treatments, and one group received an initial RFX treatment followed by two TOB treatments. The group without treatment exhibited the highest amount of bacterial load with growth increasing to a maximum of 109 CFU at 24 h postinfection (Fig. 6). Multiple treatments of TOB reduced the amount of bacterial growth in the lungs, with growth leveling out around 108 CFU. With the addition of RFX to the doses of TOB, growth was further reduced and kept consistent at 106 CFU. All colonies isolated from the lung homogenates of the infected mice were bioluminescent (see Fig. S3 in the supplemental material), suggesting that the total bacterial load resulted from the initial infection.
FIG 6.
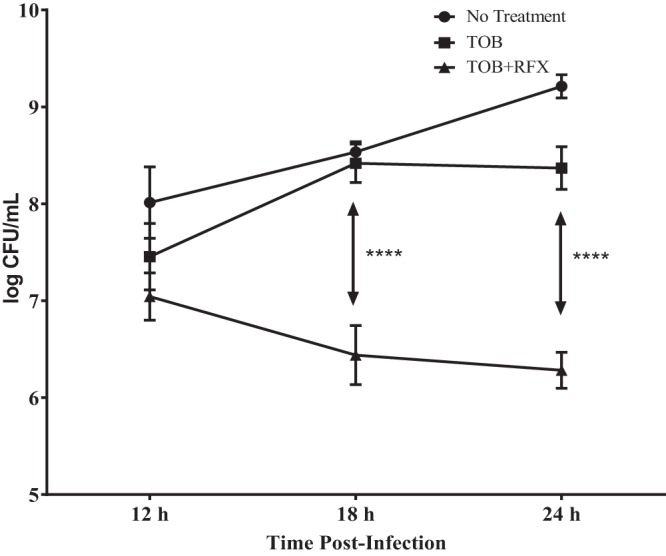
Addition of RFX to multiple treatments of TOB results in a decrease of CFU per milliliter of P. aeruginosa in mouse lungs. Plot showing the CFU per milliliter counts of mice after being infected with P. aeruginosa and left untreated, treated with multiple doses of TOB, or treated with multiple doses of TOB with addition of RFX. Data shown represent average of 2 mice per time point per group. Two-way ANOVA was used to test for statistical significance with a P value of <0.01 (****, P < 0.0001).
DISCUSSION
Biofilm formation by P. aeruginosa is a major risk factor for people suffering CF (27). Current treatment includes the repeated use of inhaled TOB. This antibiotic may not continue to be an effective treatment strategy due to emerging aminoglycoside resistance in P. aeruginosa from the biofilm formation that results in decreased permeability of TOB. In this study, we found that RFX, which has been approved for use in the treatment of traveler's diarrhea, irritable bowel syndrome, and hepatic encephalopathy, has antibacterial activity against P. aeruginosa and other MDR bacterial pathogens and can inhibit alginate production. Furthermore, RFX was effective against other bacterial pathogens associated with respiratory infections in CF and patients with VAP. These data suggest that RFX may be an effective antibiotic adjuvant for respiratory infections in CF patients.
Previous research has shown that the efficacy of TOB is hindered by its inability to penetrate and inhibit biofilm production (19). Although a biofilm is a general description of the matrices produced by P. aeruginosa and has many components (27), we used alginate production as a representation of biofilm production in this study. Carbazole assays were performed using media containing either RFX or TOB to measure the inhibitory effect on alginate production. In all mucoid strains tested, RFX significantly decreased alginate production compared to that with TOB. This decrease in alginate production suggests that RFX may inhibit alginate biofilm production and enhance the bacterial uptake of TOB by P. aeruginosa in CF patients.
After determining that TOB and/or RFX inhibited alginate and decreased biofilm production, we next investigated whether these treatments could reduce preformed biofilms and viable cell counts with P. aeruginosa strains PAO1, CF001, and CF010. TOB significantly reduced biofilm aggregates and viable cell counts compared to untreated samples. However, TOB was more effective in combination with RFX, as treatment with both drugs reduced biofilm aggregates and viable cell counts compared to those with TOB alone. This suggests that the addition of RFX may be a suitable supplement to treating biofilm producing strains of P. aeruginosa by enhancing TOB biofilm penetration. Thus, patients with complications from biofilm-producing species may benefit from the addition of RFX to current treatment plans.
The unique physiochemical and pharmacokinetic (PK) properties of RFX compared to those of other rifamycins led us to focus on RFX for in vivo studies. Oral RFX has low solubility in water and achieves high intestinal concentrations due to poor absorption. We found that RFX is readily soluble in DMSO, which is a solvent that is used at low concentrations in chronic obstructive pulmonary disease (COPD) patients to alleviate tar build up due to smoking (28). However, recent studies suggest DMSO to be toxic and produce cellular apoptosis at low concentrations (29). To examine the possible toxicity associated with DMSO inhalation, mice were exposed to 100% DMSO prior to testing the drugs, and no physical adverse effects (irritation, activity level, body weight, or death) were observed. Delivery of nebulized RFX in DMSO and TOB in saline was then compared in mice via inhalation in a whole-body exposure chamber. The nebulization exhibited low efficiency in that the concentrations of bacteria and drugs nebulized were significantly higher than the amount delivered into the lungs, but this was expected as the system relies on delivery of drugs via natural respiration. This method was preferred over intratracheal delivery due to the possibility of injury with the insertion of tracheal tubes that could be misinterpreted as adverse effects of RFX as well as the change in respiratory patterns while anesthetized. Importantly, we optimized the inhalational delivery of drugs by measuring the tissue level of drugs via HPLC and ELISA. At lower dosages, the drugs were delivered into the lung at a similar concentration; however, as the amount of the drug nebulized increased, TOB was found to be more concentrated than RFX in mouse lungs. This may be due to the low solubility and absorption of RFX in the lung, but the exact cause is not known at this time. Droplet size analysis revealed that DMSO has a minimal effect on the particle size of nebulized RFX because the TOB-saline formulation produced almost identical particle size. Furthermore, the two drug formulations were able to deposit through the airway and lung with the nebulizer used in this study to have a therapeutic effect. Moreover, no adverse effects were observed in the RFX-treated mice. The mice behaved normally and survived for several months after the nebulization until euthanasia. Our data therefore indicated that RFX can be delivered to the lungs via nebulization at measurable levels. Future studies should focus on optimizing the drug delivery method and determining the cause of the difference in concentration between the drugs at higher dosages.
After determining that RFX was deliverable via inhalation, we tested for therapeutic efficacy using a P. aeruginosa pneumonia model (25). The ideal test would use an animal model with chronic lung infection caused by mucoid strains of P. aeruginosa to correlate the clinical condition of a CF patient; however, a model has not been developed to date. As an alternative, we used an acute lung infection mouse model that was caused through natural respiration of P. aeruginosa aerosol in mice. We previously found that DBA/2 mice are highly susceptible to lung colonization by P. aeruginosa compared to other inbred mouse strains. Lung infections in DBA/2 mice with nonmucoid strains of P. aeruginosa PAO1 resulted in excessive infiltration of neutrophils to the mouse lungs followed by massive formation of edema (25). The mice typically die within 120 h after lung infection. In this study, this model was used to test the drug efficacy for treating P. aeruginosa respiratory infections in mouse. Overall, more mice survived after treatment with RFX than in all other experimental groups. While the survival rate following the 6-h administration of TOB was less than that following the 12-h administration, this difference is attributable to only one less mouse surviving between test groups. Although multiple treatments are used in clinical settings, a single-dose treatment was used in this study to test the preliminary efficacy of TOB versus RFX. Future studies will focus on determining if alginate production is inhibited in a chronic lung infection mouse model.
Once a single-dose study determined the efficacy of TOB versus RFX, a multiple-dose study was used to determine the effect of adding RFX to multiple treatments of TOB, which would more closely represent current clinical treatment methods. The animal model for the multiple-dose experiment was similar to that of the single-dose experiment, with the exception that the P. aeruginosa strain PAO1 used had a stably expressed bioluminescent marker to track the bacteria and to determine presence in a native microbiome. In this study, we observed that the addition of RFX reduced bacterial growth compared to that with TOB alone. Bioluminescence was also conserved and observed in all colonies grown from lung homogenates, suggesting that no native bacteria were culturable. These results strengthen the idea that the addition of RFX could aid TOB in penetration of P. aeruginosa biofilm infections.
In conclusion, we have shown that RFX is a potential candidate for use against known lung pathogens as a supplementary additive to the currently established TOB treatment. RFX was shown to efficiently reach the lungs and increase survival rates in P. aeruginosa lung infections in mice. Since RFX is being repurposed for use in the lungs, further testing is required to better understand the pharmacokinetics and toxicity of RFX when delivered in the lungs. In addition, further testing will be conducted to replace DMSO with a water-based solvent for RFX to avoid possible adverse effects of DMSO.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains used in this study are listed in Table 3. P. aeruginosa strains were all grown on Pseudomonas isolation agar (PIA) plates (Difco, Detroit, MI) or in Pseudomonas isolation broth (PIB) (Difco, Detroit, MI) at 37°C, prepared by adding 20 ml of glycerol/liter of medium as recommended by the manufacturer. PIA was supplemented with 300 μg/ml of carbenicillin when necessary.
TABLE 3.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype, phenotype, or descriptiond | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Prototroph, NM | P. Phibbsa |
| PAO581 | PAO1 mucA25, M | J. Govanb |
| CF001 | CF Isolate, M | M. Ansteadc |
| CF003 | CF Isolate, NM | M. Ansteadc |
| CF010 | CF Isolate, M | 4 |
| Plasmids | ||
| pRK2013 | Kmr, Tra Mob ColE1 | 33 |
| pLP170 | 8.3-kb, promoterless-lacZ, Apr, multiple cloning site | 34 |
| pLP170-PalgD | Complete PalgD promoter (989 bp upstream of ATG) fused with lacZ in pLP170 BamHI/HindII | 20 |
| pUC18-mini-Tn7T-lux | Pseudomonas suicide vector that carries luxCDABE operon for insertion into attTn7 sites | 35 |
P. Phibbs, East Carolina University, Greenville, NC.
J. Govan, University of Edinburgh, Scotland, UK.
M. Anstead, University of Kentucky Department of Pediatrics, Lexington, KY.
NM, nonmucoid; M, mucoid.
Screen for drug candidates that inhibit growth and alginate production in P. aeruginosa.
The drug collection library was assembled by the National Institutes of Health (NIH) through the Molecular Libraries Roadmap Initiative. It is called NIH clinical collections 1 and 2 (NCC plates 105 and 106) and contains 446 and 725 compounds, respectively. The library was arrayed in 96-well plates as an approximately 10 mM solution in 100% DMSO. These low-molecular-weight compounds all have a history of safe use in human clinical trials and were curated and supplied by Evotec. Three concentrations of drugs (50, 10, and 2 μM) were used to screen for the inhibition of growth and alginate production in P. aeruginosa. A mucoid strain of P. aeruginosa PAO581 (PAO1mucA25) was introduced with plasmid pLP170 carrying the alginate biosynthetic promoter PalgD fused with a promoterless lacZ operon. This strain was grown with about the same number of cells as adjusted by optical density for the screen on Pseudomonas isolation agar (PIA) supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) to a final concentration of 20 μg/ml.
MIC testing.
MIC testing was performed as previously described (21). Briefly, an overnight culture of bacteria was diluted in 1:100 of a 0.5 McFarland standard as inoculum. The same number of cells was grown in LB broth containing serial, 2-fold dilutions of RFX or TOB. Growth was measured via optical density (OD) at 600 nm after 24 h of incubation at 37°C. Concentrations of antibiotic required for inhibition of 50% and 90% of strains were determined.
Synergy testing.
Synergistic potential of RFX and TOB was performed by isobologram (checkerboard) analysis in a 96-well plate format (21). Briefly, PIB containing serial dilutions of each drug was plated in 64 wells of a 96-well plate with one drug vertically plated and the other horizontally plated. Bacterial cells of P. aeruginosa were grown for 4 to 6 h to an early log phase and then added to the wells of the plate. Growth in the wells was measured via OD at 600 nm after 24 h of incubation at 37°C. The MIC and fractional inhibition concentration (FIC) were calculated to determine synergism, antagonism, or additivity between the following tested strains of P. aeruginosa: PAO1, PAO581, PAO581 isogenic mutant, CF001, and CF010. The summative ΣFIC was calculated from the FICs and interpreted according to the standard index values as follows: synergy, ≤0.5; indifferent, 0.5 < to ≤4; antagonism, >4 (22).
Uronic acid carbazole assay.
Alginate production was measured via carbazole assay (30). Laboratory and clinical isolate strains were grown in PIB from a single colony isolation. The strains were then plated on 150-mm agar plates containing no antibiotic; RFX at concentrations of 10 μM (MIC50), 6 μM (MIC30), 2 μM (MIC10), and 0.2 μM (MIC1); or TOB at concentrations of 2 μM (MIC50), 1.2 μM (MIC30), 0.4 μM (MIC10), and 0.04 μM (MIC1) and incubated at 37°C for 48 h. Sterile PBS (30 ml) was added to the plates, and the cells plus their mucoid film were collected into 50-ml conical tubes. A 1-ml sample was taken to measure growth via OD at 600 nm. A 700-μl sample was then added to tubes containing 6 ml of a sulfuric acid-borate solution. A 200-μl aliquot of 0.1% carbazole was then added to the tubes and incubated for 30 m at 55°C. The alginate was then measured via OD at 530 nm and plotted against a standard curve made with d-mannuronic acid (Sigma-Aldrich). The reported values represent an average from three independent alginate collections with standard deviation.
Biofilm reduction assay.
A biofilm reduction assay developed by Sheppard et al. (24) was used to determine the reduction of biofilms in P. aeruginosa strains by TOB and/or RFX. Briefly, biofilms were grown in a flat bottom 96-well microplate (Corning) using 200 μl LB of 1.0 × 105 to 1.5 × 105 inoculum of lab and clinical strains of nonmucoid and mucoid P. aeruginosa. After 24 to 30 h of incubation at 37°C, the medium was removed, and plates were washed with PBS. New medium containing 1× to 5× the MIC90 of TOB and/or RFX was added to the plate in a checkerboard pattern to determine synergy of biofilm reduction. After 24 h of incubation at 37°C, the medium was removed and 200 μl of 0.1% aqueous crystal violet was added and left at room temperature 0.5 h. The solution was then removed, plates were washed with PBS, and 200 μl of 30% acetic acid was added. The plates were then read via OD at 595 nm using a SpectraMax i3x plate reader to compare biofilm dispersion between TOB and/or RFX.
Viable cell assay.
Viable cell counts were done to determine the reduction of viable cells in preformed biofilms treated with TOB and/or RFX. Briefly, following biofilm reduction protocol, biofilms were grown in a flat bottom 96-well microplate (Corning) using 200 μl LB of 1.0 × 105 to 1.5 × 105 inoculum of lab and clinical strains of nonmucoid and mucoid P. aeruginosa for 24 h at 37°C. After incubation, the medium was removed, and the plate was washed with PBS twice. New LB medium containing 1× to 5× the MIC90 of TOB and/or RFX was added to the plate in a checkerboard pattern. After 24 h of growth at 37°C, the medium was removed and replaced with methylthiazol tetrazolium (MTT; Sigma). After 1 h of incubation, DMSO was added and plates were read via OD at 570 nm using a SpectraMax i3x plate reader to determine viable cell differences between TOB and/or RFX.
Mice.
Protocols for murine lung infections and in vivo drug efficacy testing were approved by Marshall University Institutional Animal Care and Use Committee (IACUC). Animals used in this study were obtained from Charles River Laboratories (Wilmington, MA). C57BL/6 mice were used for the drug nebulization studies, and DBA/2 mice were used for the murine P. aeruginosa pneumonia model (25). Mice were housed in the Marshall University Animal Facility under veterinary supervision. The ages of mice tested were between 8 and 12 weeks. The weight of each mouse was taken prior to testing.
Drug nebulization.
C57BL/6 mice (n = 8) were first exposed to 5 ml DMSO for 10 min to determine any adverse effects that may occur from using DMSO. Mice (n = 16) were then exposed to TOB in 0.225% saline or RFX in DMSO via nebulization until the 5-ml drug mixture was depleted—typically 10 to 15 min. Nebulization was conducted using the Schuco nebulizer unit with small volume median diameter of droplet particle size and whole-body exposure chamber purchased from Kent Scientific (Torrington, CT). TOB was nebulized at concentrations of 100 mg (n = 4) or 400 mg (n = 4) in 5 ml of 0.225% saline. RFX was nebulized at concentrations of 100 mg (n = 4) or 400 mg (n = 4) in 5 ml of 100% dimethyl sulfoxide. Mice were sacrificed immediately after nebulization via CO2 chamber. Lungs were then collected and homogenized. TOB concentrations were measured using ELISA. RFX concentrations were measured via HPLC.
ELISA analysis of TOB tissue concentration.
TOB concentration in the lung tissue was measured via ELISA following the protocol established by Sachetelli et al. (31). Briefly, a 96-well flat bottom microtiter plate was coated with 0.1% alcian blue in 3% acetic acid and incubated for 30 min at 37°C. The plates were then washed twice with PBS and dried at room temperature (RT). After drying, the plates were coated with TOB in carbonate buffer for generating the standard curve or with lung homogenate samples overnight at RT. The plates were then washed twice with PBS containing 0.05% Tween 20 (PBS-T). Skim milk in PBS-T was then added to the wells and incubated overnight at 37°C. The plates were washed with PBS-T, and sheep IgG anti-TOB polyclonal antibody (catalog number PA1-75463; Thermo Scientific) was added and incubated 1 h at 37°C. The plates were then washed three times with PBS-T, and horseradish peroxidase (HRP)-conjugated rabbit anti-sheep IgG polyclonal antibody (Thermo Scientific) was added and incubated 1 h at 37°C. The plates were then washed three times with 0.05% PBS Tween 20, and TMB (3,3',5,5'-tetramethylbenzidine) solution was added and incubated for 30 min at RT. Acid stop solution was added and absorbance was read on a SpectraMax spectrophotometer at 450 nm and 540 nm. Concentrations of TOB in lung samples were determined by reference to a standard curve generated with the known amount of HPLC grade TOB.
HPLC analysis of RFX tissue concentration.
HPLC analysis of RFX in the lung homogenates was performed to determine the amount of RFX retained in the mouse lungs using an adaptation of a previously published method (32). Briefly, we used a Beckman HPLC system gold or a Waters Alliance HPLC system. The samples were centrifuged at 1,000 × g for 10 min and the supernatant collected. An aliquot of the supernatant was injected on a 5-μm Waters XTerra R18 column (250 mm × 4.6 mm). The mobile phase was a 45:55 mix of 0.1% acetic acid/acetonitrile with a flow rate of 1 ml/min. The detector was a Beckman photodiode array at 239 nm. The amount of RFX in lung samples was determined by reference to RFX standards analyzed by the same HPLC method.
Particle size analysis of nebulized drugs.
Droplet size analysis of drugs used for inhalational therapy was conducted by Spray Analytics (Lincolnshire, IL). Samples of TOB in 0.225% saline and RFX in 100% DMSO at the treatment dose of 60 mg/ml for both were prepared and submitted along with a Schuco nebulizer unit used for drug nebulization. A Malvern Spraytec was used for analysis with the tested product sprayed horizontally at less than 5 cm from the detector beam. The samples were measured in triplicates for 20 to 30 s of stable operation of the nebulizer. Refractive index and absorption values used were those of DMSO for RFX and water for TOB.
Murine pneumonia model treatment.
TOB or RFX was used to treat P. aeruginosa respiratory infection in a DBA/2 mouse model as established by Wilson et al. (25). Lung infection was induced by a single exposure to a total of 84 male DBA/2 mice that were 8 weeks old (Charles River Laboratories) to P. aeruginosa PAO1 aerosols, which were prepared as previously reported. Immediately after infection, the mice were separated into seven groups (n = 12 per group) as follows: (i) control with no treatment, (ii) treatment with 300 mg TOB immediately after infection, (iii) treatment with 300 mg RFX immediately after infection, (iv) treatment with 300 mg TOB 6 h after infection, (v) treatment with 300 mg RFX 6 h after infection, (vi) treatment with 300 mg TOB 12 h after infection, or (vii) treatment with 300 mg RFX 12 h after infection. Each experimental group was treated with TOB or RFX with a control group treated with PBS following the nebulization protocol as described above. The mice were monitored for survival at 12-h intervals for 72 h and then at 24-h intervals for 6 weeks.
Multiple doses of TOB with or without RFX were used to treat a P. aeruginosa respiratory infection in a DBA/2 mouse model established by Wilson et al. (25); however, the PAO1 strain used had a bioluminescent operon (luxCDABE). Female DBA/2 mice were infected at 0 h and split into 3 experimental groups of 8 as follows: no treatment; treatment with 300 mg TOB at 6, 8, and 10 h postinfection; or treatment with 300 mg RFX at 6 h and 300 mg TOB at 8 and 10 h postinfection. Two mice from each group were sacrificed at 12, 18, and 24 h postinfection. Lungs from sacrificed mice were homogenized in 1% peptone phosphate buffer saline (PPBS) and tested for bacterial growth to determine CFU load per mouse. Bacteria grown were also imaged for evidence of bioluminescence. Two mice from each group were left to observe for survival.
Statistical analysis.
The one-way and two-way analysis of variance (ANOVA), Kaplan-Meier plots, and Mantel-Cox test were used to analyze the data, with statistical significance set at a P value of <0.01. All statistical analysis was performed using GraphPad Prism version 7.02 for windows (GraphPad Software, La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants R44GM113545 and P20GM103434.
H.D.Y. is also the cofounder and chief science officer (CSO) of Progenesis Technologies, LLC.
We thank Kyle Lehosit, Alexandria Carter, and Emily Fedukovich, summer undergraduate interns sponsored by the WV-INBRE program, for the initial drug library screens, and Richard M. Niles from Progenesis Technologies for critical review of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02341-18.
REFERENCES
- 1.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. 2006. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher JC, Yu H, Mudd MH, Deretic V. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun 65:3838–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasirmoghadas P, Yadegari S, Moghim S, Esfahani BN, Fazeli H, Poursina F, Hosseininassab SA, Safaei HG. 2018. Evaluation of biofilm formation and frequency of multidrug-resistant and extended drug-resistant strain in Pseudomonas aeruginosa isolated from burn patients in Isfahan. Adv Biomed Res 7:61. doi: 10.4103/abr.abr_37_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head NE, Yu H. 2004. Cross-sectional analysis of clinical and environmental isolates of Pseudomonas aeruginosa: biofilm formation, virulence, and genome diversity. Infect Immun 72:133–144. doi: 10.1128/IAI.72.1.133-144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciofu O, Tolker-Nielsen T, Jensen PØ, Wang H, Høiby N. 2015. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev 85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Hanes M, Chrisp CE, Boucher JC, Deretic V. 1998. Microbial pathogenesis in cystic fibrosis: pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect Immun 66:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GG, Moreau-Marquis S, Stanton BA, O'Toole GA. 2008. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols WW, Dorrington SM, Slack MP, Walmsley HL. 1988. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother 32:518–523. doi: 10.1128/AAC.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod DL, Nelson LE, Shawar RM, Lin BB, Lockwood LG, Dirk JE, Miller GH, Burns JL, Garber RL. 2000. Aminoglycoside-resistance mechanisms for cystic fibrosis Pseudomonas aeruginosa isolates are unchanged by long-term, intermittent, inhaled tobramycin treatment. J Infect Dis 181:1180–1184. doi: 10.1086/315312. [DOI] [PubMed] [Google Scholar]
- 11.Iorio N, Malik Z, Schey R. 2015. Profile of rifaximin and its potential in the treatment of irritable bowel syndrome. Clin Exp Gastroenterol 8:159–167. doi: 10.2147/CEG.S67231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullen K, Prakash R. 2010. Rifaximin for the treatment of hepatic encephalopathy. Expert Rev Gastroenterol Hepatol 4:665–677. doi: 10.1586/egh.10.78. [DOI] [PubMed] [Google Scholar]
- 13.Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. 2014. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 12:1390–1397. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Brown EL, Xue Q, Jiang ZD, Xu Y, Dupont HL. 2010. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother 54:388–396. doi: 10.1128/AAC.00691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci A, Coppo E, Barbieri R, Debbia EA, Marchese A. 2017. The effect of sub-inhibitory concentrations of rifaximin on urease production and on other virulence factors expressed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus. J Chemother 29:67–73. doi: 10.1080/1120009X.2016.1195069. [DOI] [PubMed] [Google Scholar]
- 16.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. 2005. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 17.Stavrovskaya IG, Narayanan MV, Zhang W, Krasnikov BF, Heemskerk J, Young SS, Blass JP, Brown AM, Beal MF, Friedlander RM, Kristal BS. 2004. Clinically approved heterocyclics act on a mitochondrial target and reduce stroke-induced pathology. J Exp Med 200:211–222. doi: 10.1084/jem.20032053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao B, Christophersen L, Kolpen M, Jensen PO, Sneppen K, Hoiby N, Moser C, Sams T. 2016. Diffusion retardation by binding of tobramycin in an alginate biofilm model. PLoS One 11:e0153616. doi: 10.1371/journal.pone.0153616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long TE, Keding LC, Lewis DD, Anstead MI, Withers TR, Yu HD. 2016. Anionic fluoroquinolones as antibacterials against biofilm-producing Pseudomonas aeruginosa. Bioorg Med Chem Lett 26:1305–1309. doi: 10.1016/j.bmcl.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan Withers T, Heath Damron F, Yin Y, Yu HD. 2013. Truncation of type IV pilin induces mucoidy in Pseudomonas aeruginosa strain PAO579. Microbiologyopen 2:459–470. doi: 10.1002/mbo3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isenberg HD, American Society for Microbiology. 2004. Clinical microbiology procedures handbook, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 22.Moody J. 1992. Synergy testing: broth microdilution checkerboard and broth macrodilution methods, p 5.18.1–5.18.23. In Eisenberg H. (ed), Clinical microbiology procedures handbook. ASM Press, Washington, DC. [Google Scholar]
- 23.Al Ahmar R, Kirby BD, Yu HD. 2018. Pyrimidine biosynthesis regulates small-colony variant and mucoidy in Pseudomonas aeruginosa through sigma factor competition. J Bacteriol 201:e00575-18. doi: 10.1128/JB.00575-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard JG, McAleer JP, Saralkar P, Geldenhuys WJ, Long TE. 2018. Allicin-inspired pyridyl disulfides as antimicrobial agents for multidrug-resistant Staphylococcus aureus. Eur J Med Chem 143:1185–1195. doi: 10.1016/j.ejmech.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson KR, Napper JM, Denvir J, Sollars VE, Yu HD. 2007. Defect in early lung defence against Pseudomonas aeruginosa in DBA/2 mice is associated with acute inflammatory lung injury and reduced bactericidal activity in naive macrophages. Microbiology 153:968–979. doi: 10.1099/mic.0.2006/002261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Head NE. 2002. Persistent infections and immunity in cystic fibrosis. Front Biosci 7:d442–d457. doi: 10.2741/A787. [DOI] [PubMed] [Google Scholar]
- 27.Hoiby N, Ciofu O, Bjarnsholt T. 2010. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 28.Kimura R, Traber LD, Herndon DN, Neuhaus GD, Traber DL. 1988. Treatment of smoke-induced pulmonary injury with nebulized dimethylsulfoxide. Circ Shock 25:333–341. [PubMed] [Google Scholar]
- 29.Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. 2014. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 30.Knutson CA, Jeanes A. 1968. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem 24:470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- 31.Sachetelli S, Beaulac C, Lagace J. 1998. Aminoglycoside detection using a universal ELISA binding procedure onto polystyrene microtiter plates in comparison with HPLC analysis and microbiological agar-diffusion assay. Biochim Biophys Acta 1379:35–41. doi: 10.1016/S0304-4165(97)00079-2. [DOI] [PubMed] [Google Scholar]
- 32.Rao RN, Vali RM, Rao AV. 2012. Determination of rifaximin in rat serum by ionic liquid based dispersive liquid-liquid microextraction combined with RP-HPLC. J Sep Sci 35:1945–1952. doi: 10.1002/jssc.201200202. [DOI] [PubMed] [Google Scholar]
- 33.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, Goldberg JB, Pier GB. 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun 65:3086–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi KH, Schweizer HP. 2006. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.