In this study, a CRISPR/Cas9-mediated genome editing method was used to study the functions of the mgrB, tetA, and ramR genes in mediating colistin and tigecycline resistance in carbapenem-resistant Klebsiella pneumoniae (CRKP). Inactivation of the tetA or ramR gene or the mgrB gene by CRISPR/Cas9 affected bacterial susceptibility to tigecycline or colistin, respectively.
KEYWORDS: CRISPR/Cas9, Klebsiella pneumoniae, drug resistance mechanisms, genome editing, pandrug resistance
ABSTRACT
In this study, a CRISPR/Cas9-mediated genome editing method was used to study the functions of the mgrB, tetA, and ramR genes in mediating colistin and tigecycline resistance in carbapenem-resistant Klebsiella pneumoniae (CRKP). Inactivation of the tetA or ramR gene or the mgrB gene by CRISPR/Cas9 affected bacterial susceptibility to tigecycline or colistin, respectively. This study proved that the CRISPR/Cas9-based genome editing method could be effectively applied to K. pneumoniae and should be further utilized for genetic characterization.
TEXT
Klebsiella pneumoniae is one of the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens which could acquire multiple antimicrobial resistance through either horizontal gene transfer or mutations (1). Tigecycline and polymyxins are last line antibiotics used in clinical settings to treat carbapenem-resistant pathogens (2). Unfortunately, resistance to either one or both tigecycline and colistin has been reported in K. pneumoniae (3, 4). Resistance to these two drugs is commonly mediated by chromosomal gene mutations. Mutations in the ramA, ramR, acrR, and rpsJ genes that lead to increased expression of the AcrAB-TolC, RND (resistance-nodulation-cell division), or OqxAB efflux pump are known to confer tigecycline resistance (5–8), and resistance to polymyxins was shown to be mediated by the mutations in genes encoding the two-component regulatory systems PmrAB, PhoPQ, and CrrAB, leading to further upregulation of the pmrHFIJKLM operon (9–11). Inactivation or downregulation of the mgrB gene via IS insertion, nonsense mutations, or mutations is a common mechanism that confers polymyxin resistance (1, 12, 13). A precise and efficient CRISPR/Cas9-mediated K. pneumoniae genome editing method was recently developed and could be applied to studying pathogens of clinical importance (14). In this work, we studied and confirmed the functions of genes mediating tigecycline and colistin resistance through genome editing in carbapenem-resistant K. pneumoniae (CRKP) strains isolated from a leukemia patient in a previous study (15).
K. pneumoniae strain Y4, which harbored an intact mgrB gene and was susceptible to colistin (MIC = 0.25 mg/liter), was used to study the mechanism of colistin resistance triggered by mgrB mutation. The K. pneumoniae Y17 strain, which was resistant to tigecycline (MIC = 8 mg/liter) and harbored a mutated tetA gene (S251A) and the wild-type ramR gene, was used to study the mechanism of tigecycline resistance.
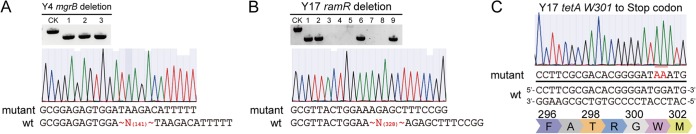
The mgrB gene deletion mutant in the K. pneumoniae Y4 strain and the ramR gene deletion mutant in the K. pneumoniae Y17 strain were successfully obtained using the pCasKP-pSGKP genome editing system (Fig. 1A and B) according to methods described previously (14). Briefly, the pCasKP-apr plasmid was first transformed into the wild-type K. pneumoniae Y4 strain using electroporation (2,500 V, 200 Ω, 25 μF, 2-mm cuvette). After induction of l-arabinose, cells harboring the pCasKP-apr plasmid were then prepared as the competent cells. The annealed mgrB spacer oligonucleotides were inserted into the BsaI sites of the pSGKP-spe plasmid by Golden Gate assembly. The mgrB spacer-introduced pSGKP-spe plasmid and the corresponding 90-nucleotide (nt) single-strand DNA (ssDNA) repair template were then coelectroporated into the aforementioned pCasKP-apr-harboring competent cells. The colonies were selected on an LB agar plate containing 50 mg/liter apramycin and 100 mg/liter spectinomycin at 30°C. The successful mgrB deletion strain (K. pneumoniae Y4-ΔmgrB) was confirmed by PCR and sequencing. After obtaining the mgrB deletion mutant, both the pCasKP-apr and the mgrB spacer-introduced pSGKP-spe plasmids were cured by culturing the cells at 37°C with 5% (wt/vol) sucrose. The K. pneumoniae Y17-ΔramR strain was a ramR gene deletion mutant of the K. pneumoniae Y17 strain created using the same method.
FIG 1.
Genetic manipulation in K. pneumoniae using the pCasKP-pSGKP genome editing system and the pBECKP base editing system. (A and B) Deletion of the mgrB gene in the K. pneumoniae Y4 strain (A) and of the ramR gene in the K. pneumoniae Y17 strain (B) using the pCasKP-pSGKP system. Lane CK is the PCR band from the wild-type (wt) strain as a control. (C) W301 of the tetA gene in the K. pneumoniae Y17 strain was successfully mutated to a stop codon using the pBECKP base editing system.
We used the base editor pBECKP-spe to inactivate the plasmid-borne tetA gene. The pBECKP-spe system executed editing by introduction of a single-stranded DNA break instead of a double-stranded DNA break, which would not result in plasmid removal during editing (14). Briefly, the annealed tetA spacer oligonucleotides were inserted into the BsaI sites of the pBECKP-spe plasmid using Golden Gate assembly. Then, the tetA spacer-introduced pBECKP-spe plasmid was transformed into the wild-type K. pneumoniae Y17 competent cells using electroporation (2,500 V, 200 Ω, 25 μF, 2-mm cuvette). The colonies were selected on an LB agar plate containing 100 mg/liter spectinomycin at 37°C. The successful tetA edited colonies (K. pneumoniae Y17-ΔtetA) were confirmed by PCR and sequencing. The tetA spacer-introduced pBECKP-spe plasmid was cured by culturing the cells in the presence of 5% (wt/vol) sucrose. As shown in Fig. 1C, the tetA gene was successfully mutated by the introduction of a premature stop codon (TAA). Whole-genome sequencing was further utilized to rule out the off-target effects in all three mutants generated by the CRISPR/Cas9 method. The sequencing data accession numbers of the K. pneumoniae Y17-ΔtetA, Y17-ΔramR, and Y4-ΔmgrB strains are SAMN11053200, SAMN11053202, and SAMN11053203 in BioSample (NCBI), respectively.
We examined the tigecycline and colistin susceptibility of the wild-type K. pneumoniae Y17 strain, K. pneumoniae Y4 strain, and the K. pneumoniae Y17-ΔtetA, Y17-ΔramR, and Y4-ΔmgrB mutant strains (Table 1) using the broth dilution method; the resistance breakpoints for colistin and tigecycline were both >2 mg/liter according to the 2017 EUCAST breakpoint guideline (available at http://www.eucast.org/clinical_breakpoints/). Pseudomonas aeruginosa ATCC 27853 and Escherichia coli NCTC 13846 served as susceptible (0.5 to 2 mg/liter) and nonsusceptible (4 to 8 mg/liter) quality control strains for colistin, respectively. In addition, we performed a whole spectrum of antibiotic susceptibility tests against the strains (see Table S1 in the supplemental material). Inactivation of the tetA gene significantly increased bacterial susceptibility to tigecycline, while deletion of the ramR gene resulted in a dramatic increase in the MIC, from 8 to 64 mg/liter (Table 1). The deletion of the mgrB gene resulted in a significant decrease in bacterial susceptibility to colistin. These results verified that the genetic status of the tetA and ramR genes were important factors that determine tigecycline susceptibility in the wild-type K. pneumoniae Y17 strain and that the mgrB gene was an important factor that confers colistin resistance in the K. pneumoniae Y4 strain.
TABLE 1.
MIC values of colistin and tigecycline against bacterial strains used in this study
| K. pneumoniae strain | Description | MIC (mg/liter) |
|
|---|---|---|---|
| Colistin | Tigecycline | ||
| Y4 | Wild-type clinically isolated K. pneumoniae strain Y4 | 0.25 | 1 |
| Y4-ΔmgrB | K. pneumoniae Y4 △mgrB | 16 | 1 |
| Y17 | Wild-type clinically isolated K. pneumoniae strain Y17 | 64 | 8 |
| Y17-ΔtetA | K. pneumoniae Y17 tetA W301 mutation to stop codon | 64 | 2 |
| Y17-ΔramR | K. pneumoniae Y17 △ramR | 64 | 64 |
Quantitative real-time PCR (qRT-PCR) was performed to determine the expression levels of the efflux pumps AcrAB and OqxAB and their transcriptional regulators RamA, MarA, RarA, and SoxS. The primers used for real-time PCR and the procedure were described previously (16). The biological replicates and three technical replicates of experiments were carried out in triplicate. The expression level of each target gene was normalized to that of a housekeeping gene (16S rRNA, rrsE), and K. pneumoniae ATCC 13883 was used as the reference strain with a MIC of tigecycline of 0.5 mg/liter. The relative gene expression data were analyzed on the basis of the 2-△△Ct method as previously described (17). Overexpression of ramA (53.3-fold), acrB (6.42-fold), acrA (5.08-fold), and oqxB (1.73-fold) were detected in the K. pneumoniae Y17-ΔramR mutant strain (Table S2 and Fig. S1). No positive effect on the overexpression of the efflux pumps AcrAB and OqxAB in the K. pneumoniae Y17-ΔtetA mutant strain was identified.
Genome editing has been proved to be a powerful tool for the study of multidrug resistance mechanisms in pathogens, including multidrug resistant K. pneumoniae, which posed serious threat to public health. Mutations in chromosomal genes frequently confer resistance to the last line antibiotics colistin and tigecycline in K. pneumoniae, and should therefore be closely monitored (18). The recently developed CRISPR/Cas9-based gene editing method enables precise and rapid genetic manipulation in K. pneumoniae strains (14). In this study, we successfully verified the functions of genes related to tigecycline and colistin resistance in a carbapenem-resistant K. pneumoniae strain isolated from a leukemia patient using the CRISPR/Cas9-based gene editing method (15).
Tigecycline resistance was previously found to be predominantly associated with mutations that cause overexpression of AcrAB-TolC, OqxAB, and RND efflux pumps, including the reported regulator genes acrR, marA, soxS, rarA, rob, and ramA and the effector genes acrA, acrB, tolC, oqxA, and oqxB (19). Mutations in the ramR gene were previously demonstrated to cause overexpression of the AcrAB-TolC efflux pump, thus triggering tigecycline resistance (5). Deletion of the ramR gene resulted in significant overexpression of the ramA gene, which could further enhance the expression level of the AcrAB efflux pump and lead to tigecycline resistance (8). Genomic analysis indicated that the tigecycline resistance in K. pneumoniae Y17 strain was due to the carriage of the tetA variant gene (15). However, a previous study showed that carriage of the tetA variant did not result in variation in the expression level of any efflux pump-related genes (20). Likewise, deletion of the tetA variant also did not result in a change in expression level of the efflux pump genes. The mechanism of tigecycline resistance triggered by tetA variant needs further study.
MgrB was produced upon activation of the PhoPQ signaling system. Inactivation of the mgrB gene leads to the upregulation of the PhoPQ two-component system, consequently upregulating the expression of pmrHFIJKLM (12). Deletion of mgrB via CRISPR/Cas9-based genome editing resulted in a marked increase in MIC of colistin, further confirming the findings in previous studies.
In summary, this study applied the recently developed CRISPR/Cas9-based method to studying the functions of ramR, tetA, and mgrB genes in conferring tigecycline or colistin resistance in carbapenem-resistant K. pneumoniae. The related mutants were constructed efficiently and precisely. Mutations in tetA and ramR genes were found to be associated with tigecycline resistance through different mechanisms, while deletion of the mgrB gene is related to colistin resistance. The CRISPR/Cas9-based genome editing method was proved to be effective in characterization of multidrug resistance genes in K. pneumoniae.
Accession number(s).
All the plasmids in this study are available in Addgene with numbers 117231 (pCasKP-apr), 117234 (pSGKP-spe), and 117236 (pBECKP-spe) and in GenBank under accession numbers MH587687 (pCasKP-apr), MH587684 (pSGKP-spe), and MH587686 (pBECKP-spe). Whole-genome sequencing was further utilized to rule out the off-target effects in all three mutants generated by the CRISPR/Cas9 method. The sequencing data accession numbers of the K. pneumoniae Y17-ΔtetA, Y17-ΔramR, and Y4-ΔmgrB strains are SAMN11053200, SAMN11053202, and SAMN11053203 in BioSample (NCBI), respectively.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants 81772250, 8181101376, and 81601815).
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00113-19.
REFERENCES
- 1.Wyres KL, Holt KE. 2018. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol 45:131–139. doi: 10.1016/j.mib.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ovejero CM, Escudero JA, Thomas-Lopez D, Hoefer A, Moyano G, Montero N, Martin-Espada C, Gonzalez-Zorn B. 2017. Highly tigecycline-resistant Klebsiella pneumoniae sequence type 11 (ST11) and ST147 isolates from companion animals. Antimicrob Agents Chemother 61:e02640-16. doi: 10.1128/AAC.02640-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 5.Villa L, Feudi C, Fortini D, García-Fernández A, Carattoli A. 2014. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob Agents Chemother 58:1707–1712. doi: 10.1128/AAC.01803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hentschke M, Wolters M, Sobottka I, Rohde H, Aepfelbacher M. 2010. ramR mutations in clinical isolates of Klebsiella pneumoniae with reduced susceptibility to tigecycline. Antimicrob Agents Chemother 54:2720–2723. doi: 10.1128/AAC.00085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Datta S, Viswanathan R, Singh AK, Basu S. 2013. Tigecycline susceptibility in Klebsiella pneumoniae and Escherichia coli causing neonatal septicaemia (2007-10) and role of an efflux pump in tigecycline non-susceptibility. J Antimicrob Chemother 68:1036–1042. doi: 10.1093/jac/dks535. [DOI] [PubMed] [Google Scholar]
- 8.Ruzin A, Visalli MA, Keeney D, Bradford PA. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob Agents Chemother 49:1017–1022. doi: 10.1128/AAC.49.3.1017-1022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt ME, Elliott AG, Cao MD, Ganesamoorthy D, Karaiskos I, Giamarellou H, Abboud CS, Blaskovich MAT, Cooper MA, Coin L. 2018. Multifactorial chromosomal variants regulate polymyxin resistance in extensively drug-resistant Klebsiella pneumoniae. Microb Genom 4:e000158. doi: 10.1099/mgen.0.000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright MS, Suzuki Y, Jones MB, Marshall SH, Rudin SD, van Duin D, Kaye K, Jacobs MR, Bonomo RA, Adams MD. 2015. Genomic and transcriptomic analyses of colistin-resistant clinical isolates of Klebsiella pneumoniae reveal multiple pathways of resistance. Antimicrob Agents Chemother 59:536–543. doi: 10.1128/AAC.04037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YH, Lin TL, Lin YT, Wang JT. 2016. Amino acid substitutions of CrrB responsible for resistance to colistin through CrrC in Klebsiella pneumoniae. Antimicrob Agents Chemother 60:3709–3716. doi: 10.1128/AAC.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Jayol A, Bontron S, Villegas MV, Ozdamar M, Turkoglu S, Nordmann P. 2015. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother 70:75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 13.Cannatelli A, Giani T, D'Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini GM. 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 58:5696–5703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wang S, Chen W, Song L, Zhang Y, Shen Z, Yu F, Li M, Ji Q. 2018. CRISPR-Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microbiol 84:e01834-18. doi: 10.1128/AEM.01834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Dong N, Huang Y, Zhou H, Xie M, Chan EW, Hu Y, Cai J, Chen S. 2018. Evolution of tigecycline- and colistin-resistant CRKP (carbapenem-resistant Klebsiella pneumoniae) in vivo and its persistence in the GI tract. Emerg Microbes Infect 7:127. doi: 10.1038/s41426-018-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Han Y, Zhou Y, Du Z, Wu H, Wang J, Chen Y. 2017. Tigecycline susceptibility and molecular resistance mechanisms among clinical Klebsiella pneumoniae strains isolated during non-tigecycline treatment. Microb Drug Resist 23:139–146. doi: 10.1089/mdr.2015.0258. [DOI] [PubMed] [Google Scholar]
- 17.Chum Y, Haas A, Kelley M. 2012. Solution for RT-qPCR: relative gene expression analysis using Thermo Scientific PikoReal real-time PCR system and Solaris gene expression reagents. Thermo Fisher Scientific, Vantaa, Finland. [Google Scholar]
- 18.Navon-Venezia S, Kondratyeva K, Carattoli A. 2017. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 19.Zhong X, Xu H, Chen D, Zhou H, Hu X, Cheng G. 2014. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One 9:e115185. doi: 10.1371/journal.pone.0115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du X, He F, Shi Q, Zhao F, Xu J, Fu Y, Yu Y. 2018. The rapid emergence of tigecycline resistance in blaKPC-2 harboring Klebsiella pneumoniae, as mediated in vivo by mutation in tetA during tigecycline treatment. Front Microbiol 9:648. doi: 10.3389/fmicb.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.