The recent emergence and diffusion in the community of Escherichia coli isolates belonging to the multidrug-resistant and CTX-M-27-producing sequence type 131 (ST131) C1-M27 cluster makes this cluster potentially as epidemic as the worldwide E. coli ST131 subclade C2 composed of multidrug-resistant isolates producing CTX-M-15. Thirty-five extended-spectrum beta-lactamase (ESBL)-producing ST131 isolates were identified in a cohort of 1,885 French children over a 5-year period.
KEYWORDS: C1-M27, CTX-M-27, E. coli, ESBL, risk factors, ST131, children, fecal carriage
ABSTRACT
The recent emergence and diffusion in the community of Escherichia coli isolates belonging to the multidrug-resistant and CTX-M-27-producing sequence type 131 (ST131) C1-M27 cluster makes this cluster potentially as epidemic as the worldwide E. coli ST131 subclade C2 composed of multidrug-resistant isolates producing CTX-M-15. Thirty-five extended-spectrum beta-lactamase (ESBL)-producing ST131 isolates were identified in a cohort of 1,885 French children over a 5-year period. They were sequenced to characterize the ST131 E. coli isolates producing CTX-M-27 recently emerging in France. ST131 isolates producing CTX-M-27 (n = 17), and particularly those belonging to the C1-M27 cluster (n = 14), carried many resistance-encoding genes and predominantly an F1:A2:B20 plasmid type. In multivariate analysis, having been hospitalized since birth (odds ratio [OR], 10.9; 95% confidence interval [CI], 2.4 to 48.8; P = 0.002) and being cared for in a day care center (OR, 9.4; 95% CI, 1.5 to 59.0; P = 0.017) were independent risk factors for ST131 CTX-M-27 fecal carriage compared with ESBL-producing non-ST131 isolates. No independent risk factor was found when comparing CTX-M-15 (n = 11)- and CTX-M-1/14 (n = 7)-producing ST131 isolates with ESBL-producing non-ST131 isolates or with non-ESBL-producing isolates. Several factors may contribute to the increase in fecal carriage of CTX-M-27-producing E. coli isolates, namely, resistance to multiple antibiotics, capacity of the CTX-M-27 enzyme to hydrolyze both cefotaxime and ceftazidime, carriage of a peculiar F-type plasmid, and/or capacity to colonize children who have been hospitalized since birth or who attend day care centers.
INTRODUCTION
Escherichia coli sequence type 131 (ST131) is an emerging pathogen that was first described in 2008 (1). The spread of CTX-M-15 extended-spectrum β-lactamase (ESBL), the most common ESBL variant in the world (2), is mainly driven by CTX-M-15-producing E. coli ST131, explaining the numerous medical researches on this clone.
Moreover, the resistance to extended-spectrum cephalosporins due to CTX-M-15 production is associated with resistance to many other antibiotics in ST131 E. coli. As this clone is frequently responsible for extraintestinal infections, its resistance highlights the important challenges in the management of patients (3, 4).
Within ST131, different sublineages have been identified and organized according to their phylogenetic clade (A, B, and C), fimH allele (type 1 fimbriae adhesin-encoding gene), and antibiotic-resistance profile (5).
Clade A is associated with fimH41, clade B with fimH22, and clade C with fimH30. The variations in fimH alleles are suspected to reflect the capacity of these clades to colonize different sites (6). Within clade C, which has disseminated worldwide, two subclades have been identified. C1 subclade, also called ST131 H30R, comprises isolates with mutations in the chromosomal gyrA and parC genes, which confer resistance to fluoroquinolone. The C2 subclade, also called ST131 H30-Rx, groups isolates with the same gyrA and parC mutations and often the blaCTX-M-15 gene. These two features are suspected to contribute to the epidemiological success of E. coli ST131 (2, 7). C1/H30R and C2/H30-Rx, come from clade B/H22 that is classically cephalosporin and fluoroquinolone susceptible (8, 9). Recently, Matsumura et al. have described the emergence of a distinct cluster (C1-M27) within the C1 subclade (10), belonging to previously reported strains in Asia, in North America, and in Europe (2, 4, 11, 12). These isolates carry the blaCTX-M-27 gene and possess a similar region to a prophage-like genomic island of E. coli PCN033 (10).
Genomic sequence analysis of a number of ST131 isolates has allowed tracing the evolutionary history and the characterization of the main determinants of ST131 (5–8, 13, 14). One of the hypotheses is that plasmids, which use postsegregation killing and addiction systems to persist in strains over time, could play a preponderant role in the evolution of this clone (15). Plasmids belonging to incompatibility (Inc) groups with F replicons (IncF) can acquire genes, including genes encoding antibiotic resistance, and rapidly disseminate. This capacity is suspected to be dependent on the type of IncF plasmid. Indeed, the antibiotic-susceptible isolates of B subclade that is the progenitor of C1 and C2 subclade isolates carry an IncF plasmid with a plasmid multilocus sequence typing (pMLST) type F+:A-:B+ whereas the multidrug-resistant C1 and C2 subclade isolates carry plasmids with pMLST types F+:A+:B+, notably F1:A2:B20 without CTX-M-15, and F+:A+:B-, notably F2:A1:B- with CTX-M-15 (15, 16). Similarly, the C1-M27 isolates, belonging to the C1 subclade, are frequently associated with F1:A2:B20 replicons (12, 17, 18). Although C1-M27 and C1/H30R harboring the blaCTX-M-14 gene have an identical plasmid type (F1:A2:B20), C1-M27 has emerged in human fecal carriage and has a greater epidemiological impact than CTX-M-14-producing C1/H30R (10).
Genomic analyses highlighted geographical and temporal disparities in the evolution of the subclades. Thus, in the French population, we have recently shown the increasing carriage of CTX-M-27-producing E. coli in infants from no CTX-M-27 in 2010 (n = 399) and 2011 (n = 258) to 2.55% (11/431) in 2015. Within the ST131 clonal group, this corresponds to an increase from no CTX-M-27 in 2010% to 65% in 2015 (4, 11).
In this work, we attempted to search for factors explaining the recent emergence of the C1-M27 cluster. We studied lifestyle- and medical history-related factors of children associated with ST131 carriage, considering the CTX-M variants. We studied a cohort of 1,885 children. We used whole-genome sequencing of the ST131 isolates found in this cohort to study the genetic content of these isolates in terms of antibiotic resistance, virulence-factor-encoding genes, and ST131 evolutionary history over a period of 5 years according to the CTX-M variants produced by these ST131 isolates, namely, CTX-M-15, CTX-M-27, and others, including CTX-M-1 and CTX-M-14.
RESULTS
Sequencing data quality.
We used standard metrics to estimate the quality of the de novo assemblies (see Table S1 in the supplemental material). An average of 123 contigs was obtained, the mean N50 value was 163,294 bp, and the mean coverage was 65×.
All the genomic analysis results are presented in Table S1.
Clade, fimH allele, and ESBL types.
Among the 35 ST131 E. coli-colonizing infants, clade typing and fimH allele typing showed that 9 (26%) of the isolates belonged to clade A with fimH41 or single nucleotide variant and 26 (74%) belonged to clade C with fimH30 or single nucleotide variant. Clade A comprised isolates producing CTX-M-1 (n = 3), CTX-M-14 (n = 2), CTX-M-15 (n = 1), and CTX-M-27 (n = 3). Within the C1 subclade, we distinguished the C1-M27 cluster (n = 14) and C1-H30R subclade (n = 2). All C1-M27 isolates had the blaCTX-M-27 gene and possessed the insertion site of the structure (M27PP1), including the direct repeat and the prophage-like genomic island of E. coli PCN033. The two C1-H30R isolates produced CTX-M-14, and the 10 C2-H30Rx subclades comprised isolates with the blaCTX-M-15 gene. Overall, the blaCTX-M-27 gene (n = 17) was the most prevalent blaCTX-M gene representing 48% of the 35 ST131 isolates (Table 1).
TABLE 1.
Distribution of genetic features according to CTX-M variants or clades
| Genetic feature | No. of all isolates (n = 35) | No. of isolates (%) according to CTX-M variant and clade |
P value of: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX-M-27 |
CTX-M-15 |
CTX-M-14/CTX-M-1 |
|||||||||||
| A (n = 3) | C1-H30R (=C1-M27) (n = 14) | Total (n = 17) | A (n = 1) | C2-H30Rx (n = 10) | Total (n = 11) | A (n = 5) | C1-H30R (n = 2) | Total (n = 7) | CTX-M-27 vs CTX-M-15 | CTX-M-27 vs CTX-M14/M1 | CTX-M-15 vs CTX-M14/M1 | ||
| fimH allele and O-type | |||||||||||||
| fimH30 or SNV | 26 | 14 (100) | 14 (82) | 10 (100) | 10 (91) | 2 (100) | 2 (29) | ||||||
| fimH41 or SNV | 9 | 3 (100) | 3 (18) | 1 (100) | 1 (9) | 5 (100) | 5 (71) | ||||||
| O25b-H4 | 26 | 14 (100) | 14 (82) | 10 (100) | 10 (91) | 2 (100) | 2 (29) | ||||||
| O16-H5 | 9 | 3 (100) | 3 (18) | 1 (100) | 1 (9) | 5 (100) | 5 (71) | ||||||
| Resistance and bacteriocin | |||||||||||||
| Mean resistance scorea | 5.8 | 4.9 | 3 | ||||||||||
| Mean acquired resistance genes | 7.8 | 6.2 | 3.5 | ||||||||||
| GyrA-S83L-D87N | 26 | 14 (100) | 14 (82) | 10 (100) | 10 (91) | 2 (100) | 2 (29) | 0.02 | 0.012 | ||||
| ParC-S80I-E84V | 22 | 11 (78) | 11 (65) | 9 (90) | 9 (82) | 2 (100) | 2 (29) | 0.05 | |||||
| ParC-S80I | 9 | 3 (21) | 3 (18) | 1 (10) | 1 (9) | 0.01 | 0.01 | ||||||
| ParE-I529L | 35 | 3 (100) | 14 (100) | 17 (100) | 1 (100) | 10 (100) | 11 (100) | 5 (100) | 5 (71) | ||||
| GyrA-S83L | 3 | 3 (100) | 3 (18) | 5 (100) | 2 (100) | 7 (100) | |||||||
| mphA | 18 | 2 (67) | 10 (71) | 12 (70) | 5 (50) | 5 (45) | 1 (20) | 1 (50) | 2 (29) | ||||
| tet(A) | 18 | 2 (67) | 12 (85) | 14 (82) | 5 (50) | 5 (45) | 1 (20) | 1 (14) | 0.003 | ||||
| tet(B) | 1 | 1 (100) | 1 (9) | ||||||||||
| catB3-like | 3 | 3 (30) | 3 (27) | ||||||||||
| aac(6')Ib-cr | 3 | 3 (30) | 3 (27) | ||||||||||
| blaTEM-1 | 11 | 1 (100) | 4 (40) | 5 (45) | 5 (100) | 1 (50) | 6 (86) | <0.001 | |||||
| blaOXA-1 | 3 | 3 (30) | 3 (27) | ||||||||||
| blaTEM-30 | 1 | 1 (7) | 1 (6) | ||||||||||
| aadA5/A2 | 18 | 2 (67) | 10 (71) | 12 (70) | 5 (50) | 5 (45) | 1 (20) | 1 (14) | 0.02 | ||||
| strA | 16 | 2 (67) | 12 (85) | 14 (82) | 2 (20) | 2 (18) | 1 (20) | 1 (14) | <0.001 | 0.001 | |||
| strB-like | 16 | 2 (67) | 12 (85) | 14 (82) | 2 (20) | 2 (18) | 1 (20) | 1 (14) | <0.001 | 0.001 | |||
| aac(3)-IIa/d-like | 5 | 4 (40) | 4 (36) | 1 (50) | 1 (14) | ||||||||
| fosA3 | 1 | 1 (100) | 1 (9) | ||||||||||
| sul1 | 17 | 2 (67) | 10 (71) | 12 (70) | 5 (50) | 5 (45) | 1 (20) | 1 (14) | |||||
| sul2 | 16 | 2 (67) | 12 (85) | 14 (82) | 2 (20) | 2 (18) | 1 (20) | 1 (14) | 0.001 | 0.003 | |||
| dfrA17 | 17 | 2 (67) | 9 (64) | 11 (65) | 5 (50) | 5 (45) | 1 (20) | 1 (14) | |||||
| sul1/sul2/dfrA17 | 14 | 2 (67) | 9 (64) | 11 (65) | 2 (20) | 2 (18) | 1 (20) | 1 (14) | |||||
| imm | 24 | 1 (33) | 12 (85) | 13 (76) | 1 (100) | 4 (40) | 5 (45) | 5 (100) | 1 (50) | 6 (86) | |||
| colicin Ia | 4 (28) | 4 (23) | 1 (10) | 1 (9) | |||||||||
| Plasmid (pMLST) | |||||||||||||
| F1:A2:B20 | 18 | 14 (100) | 14 (82) | 1 (10) | 1 (9) | 2 (40) | 1 (50) | 3 (43) | <0.001 | <0.001 | |||
| F2:A-:B10 | 3 | 3 (100) | 3 (18) | ||||||||||
| F2:A1:B- | 4 | 4 (40) | 4 (36) | ||||||||||
| F10:A1:B- | 1 | 1 (10) | 1 (9) | ||||||||||
| F36:A-:B1 | 1 | 1 (10) | 1 (9) | ||||||||||
| F2:A-:B- | 1 | 1 (10) | 1 (9) | ||||||||||
| F1:A1:B23 | 1 | 1 (100) | 1 (9) | ||||||||||
| F1:A1:B16 | 1 | 1 (10) | 1 (9) | ||||||||||
| F36:A1:B20 | 1 | 1 (10) | 1 (9) | ||||||||||
| F29:A-:B10 | 3 | 3 (60) | 3 (43) | ||||||||||
| F2:A2:B20 | 1 | 1 (50) | 1 (14) | ||||||||||
| Addiction system | |||||||||||||
| CcdAB | 33 | 3 (100) | 14 (100) | 17 (100) | 1 (100) | 8 (80) | 9 (82) | 5 (100) | 2 (100) | 7 (100) | |||
| Pemk | 35 | 3 (100) | 14 (100) | 17 (100) | 1 (100) | 10 (100) | 11 (100) | 5 (100) | 2 (100) | 7 (100) | |||
| SrnCB | 23 | 14 (100) | 14 (82) | 1 (100) | 5 (50) | 6 (54) | 2 (40) | 2 (100) | 4 (57) | 0.008 | 0.03 | ||
| HokSok | 23 | 3 (100) | 13 (93) | 16 (94) | 1 (100) | 10 (100) | 11 (100) | 4 (80) | 2 (100) | 6 (86) | |||
| PndAC | 4 | 2 (14) | 2 (12) | 2 (20) | 2 (18) | ||||||||
| VagCD | 7 | 1 (100) | 6 (60) | 7 (63) | <0.001 | 0.01 | |||||||
| Virotypeb | |||||||||||||
| C | 24 | 1 (33) | 14 (100) | 15 (88) | 4 (40) | 4 (36) | 3 (60) | 2 (100) | 5 (71) | 0.01 | |||
| A | 6 | 1 (33) | 1 (6) | 3 (30) | 3 (27) | 2 (40) | 2 (29) | ||||||
| other | 3 | 1 (33) | 1 (6) | 1 (100) | 2 (20) | 3 (27) | |||||||
| E | 1 | 1 (10) | 1 (9) | ||||||||||
Resistance score corresponds to the number of antibiotic families affected by resistance.
C, sat; A, nfaE and sat; E, cnf1, hlyC, papGII, papGIII, and sat.
Genes encoding antibiotic resistance and bacteriocin.
Repartition of antibiotic-resistance genes according to CTX-M variant and cluster/subclade type are presented in Table 1. Isolates producing CTX-M-27 were more resistant than the others (this difference was not statistically significant with the Kruskal-Wallis test). Resistance score was 5.8, 4.9, and 3 for the isolates producing CTX-M-27, CTX-M-15, and CTX-M-1/M-14, respectively, and their mean number of acquired antibiotic resistance genes was 7.8, 6.2, and 3.5 genes, respectively. Using the t test, we analyzed the preferential associations and showed that the GyrAS83L/D87N, the ParCS80I, and the tet(A) genes were significantly more frequently associated with the blaCTX-M-27 gene than with the blaCTX-M-14/blaCTX-M-1 genes (Table 1). Furthermore, the strA/strB and sul2 genes were significantly associated with the blaCTX-M-27 gene compared with all other blaCTX-M variants (Table 1). In contrast, the blaTEM-1 gene was significantly associated with the blaCTX-M-1/blaCTX-M-14 genes compared with the blaCTX-M-27 gene, which is never associated with blaTEM-1. Although the distribution of the mphA gene conferring resistance to macrolides, according to the CTX-M type, was not significant, this gene appeared to be more frequent among the CTX-M-27-producing isolates (70%) than among those producing CTX-M-15 (45%) and CTX-M-1/14 (29%).
Concerning immunity protein, we found 24 isolates (68.5%) with the imm gene conferring resistance to colicin Ia, including 6 of the 7 (86%) isolates carrying the blaCTX-M-1/14 genes, 13 of the 17 (76.5%) isolates carrying the blaCTX-M-27 gene, and 5 of the 11 (45%) isolates carrying the blaCTX-M-15 gene. Four isolates (11.5%) carried the cia gene encoding colicin Ia among which 3/4 belonged to C1-M27 cluster.
Plasmids and plasmid maintenance systems.
Different F-type plasmids were detected. The F1:A2:B20 pMLST was present in 18 isolates, including all C1-M27 (P < 0.001) (Table 1).
Among the eight plasmid maintenance systems screened, a homogenous distribution was observed except for the SrnCB system. This system was more frequently detected in the CTX-M-27 isolates than in the CTX-M-15 isolates (P = 0.008) or than in the CTX-M-1/14 isolates (P = 0.03), and the virulence-associated protein (VagCD) was only detected in isolates carrying the blaCTX-M-15 gene. Promotion of nucleic acid (pndAC) was found in only 4 isolates.
Virotypes.
We determined the virotypes according to the combination of virulence factors described by Mora et al. (19) (Table 1). Virotype A (nfaE and sat), virotype C (sat), and virotype E (cnf1, hlyC, papGII, papGIII, and sat) were found in 6, 24, and 1 isolates, respectively. We found a combination of virulence factor-encoding genes (including papGII, papGIII, and sat) in two isolates, which was not previously described by Mora et al. Virotype C was more frequently associated with the blaCTX-M-27 gene than with the blaCTX-M-15 gene (P = 0.01). All C1 subclade (C1-M27 and C1-H30R) isolates displayed only one virotype (virotype C) compared with the virotype heterogeneity observed in subclade C2-H30-Rx and clade A.
Phylogenetic analysis of the 35 E. coli ST131 isolates.
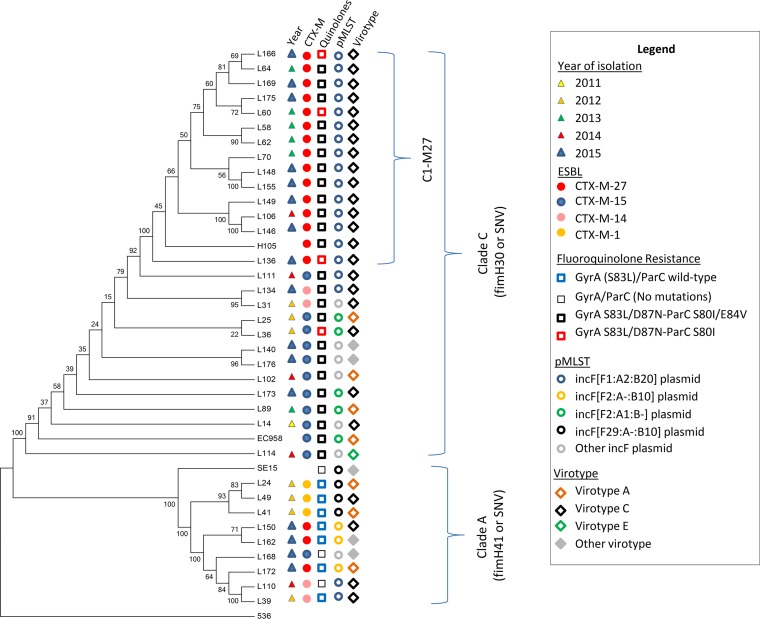
We used CSI phylogeny (40) to build a phylogenetic tree (Fig. 1) from single nucleotide polymorphisms (SNPs) illustrating the isolates’ distribution into two clusters comprising the isolates of fimH41/clade A and the isolates of fimH30/clade C, respectively. In majority, CTX-M-27-producing isolates were clustered in clade C. Core genome phylogenetic comparisons of all C2-H30-Rx isolates based on alignment with the genome of reference E. coli ST131 lineage EC958, revealed an average difference of 442 SNPs. In contrast, the same comparison between isolates within clade A aligned with E. coli ST131 reference strain SE15 revealed a SNP average of 282. For all the isolates belonging to C1-M27 cluster and aligned with H105 reference strain, the average SNP difference was of 68 SNPs.
FIG 1.
Phylogenetic tree of 35 ST131 E. coli isolates, collected from 2010 to 2015 in fecal carriage of French children, and 3 reference strains, including EC958, H105, and SE15, to represent the C2-H30Rx subclade, the C1-M27 clade, and the clade A, respectively. The tree is rooted on E. coli reference isolate 536. The phylogeny is based on the concatenated alignment of the high-quality SNPs using CSI phylogeny 1.4 tool. Year of isolation, fimH allele, belonging to C1-M27 subclade, ESBL variant, chromosomal mutations (GyrA and ParC) conferring resistance to fluoroquinolones, plasmidic pMLST, and virotype are shown using different color symbols.
Risk factor analysis.
The main results of univariate and multivariate analyses comparing CTX-M-27-producing ST131 isolates with ESBL-producing non-ST131 isolates or non-ESBL-producing isolates are presented in Table S2 in the supplemental material. Briefly, multivariate analysis found two independent risk factors associated with fecal carriage of CTX-M-27-producing ST131 isolates, namely, history of hospitalization (OR, 10.9; 95% CI, 2.4 to 48.8; P = 0.002) and being cared for in a day care center (OR, 9.4; 95% CI, 1.5 to 59.0; P = 0.017). Multivariate analysis comparing CTX-M-27-producing ST131 isolates with non-ESBL-producing isolates found history of hospitalization as an independent risk factor associated with carriage of CTX-M-27-producing ST131 isolates (OR, 3.5; 95% CI, 1.3 to 9.6); P = 0.015).
When CTX-M-15- or CTX-M-1/14-producing ST131 isolates were compared with ESBL-producing non-ST131 isolates and with non-ESBL-producing isolates, no independent risk factor was found.
DISCUSSION
We previously described E. coli ST131 carriage in a pediatric community between 2010 and 2015 (4) and observed not only the emergence of the CTX-M-27 enzyme but also its increase in frequency over the study period. CTX-M-27-producing ST131 isolates had been described for the first time in France in 2012 as colonizing children cared for in day care center (12). Since then, emergence of CTX-M-27-producing ST131 isolates has been described in many countries (10, 11, 20–25).
The C1-M27 cluster, which was first observed in our children cohort in 2012, seems to have been selected recently. This is suggested by the following three features: (i) the lower SNP difference between the isolates of this cluster compared with the SNP difference between the isolates of subclade C2 and clade A; (ii) the great homogeneity of the C1-M27 isolates regarding their plasmid content, i.e., IncF plasmid with pMLST F1:A2:B20 as previously described (12, 22, 26); and (iii) the virotype (100% of virotype C).
The switch of CTX-M variants over time leads one to wonder about the reasons for the emergence of certain variants or the selective sweeps of some others. This question remains unsolved. The whole-genome sequencing of our 35 ST131 E. coli isolates revealed several striking features. First, isolates expressing the blaCTX-M-27 gene and particularly those belonging to the C1-M27 cluster tend to be more broadly resistant to antibiotics than the isolates producing other CTX-M variants. Over 70% of the C1-M27 isolates were resistant to fluoroquinolones, macrolides, tetracyclines, aminoglycosides, and sulfonamides. These antibiotic families are rarely used for treating infections in children, except for macrolides, such as azithromycin that is frequently prescribed in infectious diarrhea or respiratory pediatric infections. This use could explain the common presence of the macrolide-resistance-encoding mphA gene in the fecal ST131 isolates, notably in the C1-M27 isolates (71%), detected in children. We also observed that the imm gene encoding immunity against colicin Ia was common in the C1-M27 isolates (85%). The second striking feature is that the CTX-M-27 enzyme, belonging to CTX-M group 9, mimics the historical CTX-M group 1 variant carried by ST131 E. coli, namely CTX-M-15, with regard to the extended-spectrum cephalosporin-resistance phenotype. Indeed, these two enzymes have an excellent hydrolytic trade-off activity on the two extended-spectrum cephalosporins predominantly used in hospitals, namely, cefotaxime and ceftazidime. This is due to the D240G substitution in the two enzymes responsible for a decreased Km for ceftazidime, probably through the modification of the β3 strand-residue position during the hydrolytic process (27). Overall, the multidrug resistance, the ability to hydrolyze the extended-spectrum cephalosporins, and the immunity against colicin Ia, displayed by the CTX-M-27-producing isolates, may have contributed to their emergence and their nascent epidemiological success.
An analysis of the association between the children’s lifestyle, their medical history, and the carriage of CTX-M variant showed that prior hospitalization (since birth) and being cared for in a day care center are independent risk factors for CTX-M-27-producing ST131 carriage compared with non-ST131 ESBL-producing isolates. These risk factors were found neither for the CTX-M-15 variant nor for the CTX-M-1/CTX-M-14 variants.
It is interesting to note that the first CTX-M-27-producing ST131 isolates described in France in 2012 were found in a pediatric population consisting of children who attended day care centers. This observation and our findings suggest that children could be a special reservoir for this cluster and may participate in its dissemination. This contrasts with the sole risk factor independently associated with CTX-M-15-producing ST131 isolates, i.e., prior residence in long-term care facilities (28). Day care centers and long-term-care facilities are environments where human promiscuity might facilitate the CTX-M-27- and CTX-M-15-producing isolate transmission, respectively. The second risk factor found in our study, “hospitalization since birth,” refers to hospital, another environment where multidrug-resistant bacteria are easily transmitted between patients due to health care (29). CTX-M-27-producing ST131 isolates are well-known for their transmission and colonization capacity in rehabilitation wards in Israel, illustrating their diffusion capacity (30).
The most plausible hypothesis regarding our success in finding medical history- and lifestyle-related risk factors associated with CTX-M-27-producing ST131 carriage in children lies in the fact that the CTX-M-27 diffusion is recent, especially since we did not find risk factors associated with isolates producing CTX-M-15. CTX-M-15 is the historical CTX-M enzyme carried by E. coli ST131. Its global diffusion in the community makes it difficult to find specific risk factors (3, 6, 31).
Despite the small number of E. coli bacteria analyzed and sequenced, which is one of the limitations of the study, it appears that hospitalization since birth and attending a day care center allow the diffusion of the ST131 E. coli of variant CTX-M-27. However, the reasons for the recent emergence remain unclear and seem to be multifactorial. The C1-M27 cluster isolates and the C2 subclade isolates share two characteristics; first, the production of a CTX-M variant able to hydrolyze efficiently not only cefotaxime, as any CTX-M enzyme, but also ceftazidime; second, the carriage of type F plasmid encoding multiple resistance genes other than the blaCTX-M genes. If these two characters have impacted the epidemiological success of the C2 subclade isolates, they might also influence the epidemiological success of the C1-M27 cluster isolates. Even though it is not yet known whether this variant will replace the other existing ones and the extent of its diffusion, we consider this epidemiological finding should be closely monitored in the future.
MATERIALS AND METHODS
Patients, isolates, and microbiological investigations.
As previously described (4), a rectal sample was obtained from 1,885 children between October 2010 and June 2015 by 18 pediatricians from three French regions. Children aged from 6 to 24 months were enrolled during routine checkups with normal findings or when they presented with acute otitis media. We collected data potentially linked to the carriage of antibiotic-resistant bacteria, including antibiotic prescription during the 3 months before enrollment, day care attendance modality, premature birth or caesarean birth, siblings, previous hospitalization (during the previous 6 months and since birth), and travel history in the last 6 months specifying the geographical area visited. The exclusion criteria were antibiotic treatment within the 7 days before enrollment and severe underlying disease. As previously described, clonal group ST131 was found in 25.3% (38/150) of ESBL-producing E. coli isolates. Among them, 35, all from different patients, were available for this study.
Whole-genome sequencing.
The library preparation and sequencing were carried out as previously described (32). Briefly, DNA was extracted using the MoBio kit (Qiagen). Library preparation was performed using the Nextera kit (Illumina, San Diego, CA, USA). Sequencing was performed either on a MiSeq instrument with 2 × 300 cycles or on a MiniSeq instrument with 2 × 150 cycles (Illumina technology) for some isolates that required resequencing for quality reasons (n = 7).
Assembly was performed using SPAdes genome assembler (33). We used the Centre for Genomic Epidemiology (CGE) website (http://www.genomicepidemiology.org) to characterize the strains (17, 34–36). A resistance score was defined as the number of antibiotic families affected by resistance and the score of acquired antibiotic resistance genes as the total number of acquired resistance genes. Virulence factor genes were identified using the Virulence Finder tool (37), and we also used the NCBI BLAST tool (BLAST+ version 2.2.31) to search for 166 genes (38). Virotypes were determined as described by Mora et al. (19).
Eight plasmid addiction or toxin/antitoxin systems were sought by in silico PCR, including PemK-PemI (type II toxin-antitoxin system that is a plasmid emergency maintenance), CcdA-CcdB (coupled cell division locus that is a type II toxin-antitoxin system), RelB-RelE (relaxed control of stable RNA synthesis), SrnBC (the SrnB-SrnC toxin-antitoxin system that performs a postsegregation killing function using mRNA stability), ParD-ParE (DNA replication), VagC-VagD (virulence-associated protein that is a toxin-antitoxin system), Hok-Sok (host killing that encodes a type I toxin-antitoxin system), and PndA-PndC (promotion of nucleic acid degradation) (39). Finally, the CSI phylogeny tool (40) was used to identify single nucleotide polymorphisms (SNPs) between isolates, and the neighbor-joining tree was built using Mega software version 3.1 using bootstraps calculated on 100 replicates.
Ethics.
The study was approved by the Saint-Germain-en-Laye Hospital Ethics Committee (number 10/10/2010-CPP06063). Written informed consents from parents or guardians were obtained.
Statistical analysis.
Demographic lifestyle and medical history data were double-entered by using 4D software version 12 and analyzed by using Stata/SE 13.1 (StataCorp., College Station, TX, USA). Microbiological data were analyzed using Fisher exact test or Kruskal-Wallis test for the 35 E. coli ST131 isolates. The cohort study was conducted on 1,885 children, and analyses of variables associated with a carriage of a CTX-M-producing isolate were carried out between each child group colonized with a ST131 isolate producing a specific CTX-M variant (CTX-M-27, CTX-M-15, and CTX-M-1/CTX-M-14) and children colonized with an ESBL-producing non-ST131 isolate (n = 108) and children colonized with a non-ESBL-producing Enterobacteriaceae isolate (n = 1,742). The chi-square test or Fisher’s exact test were used in univariate analysis to identify potential variables associated with carriage of a CTX-M-producing isolate and those with a P value of < 0.2 were included into multivariate analysis. Then, we used a logistic regression model, computing odds ratios (ORs), and 95% confidence intervals (95% CIs) to identify independent variables associated with carriage of ST131-producing CTX-M-27, ST131-producing CTX-M-15, and ST131-producing other CTX-M.
Accession numbers.
Raw sequences of the 35 isolates were deposited in GenBank under BioProject accession number PRJNA522367.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by internal fundings.
This study was partially supported by a grant from the “Fondation pour la Recherche Médicale” to Erick Denamur (Equipe FRM 2016, grant number DEQ20161136698).
We thank Jérémy Chatel and Elsa Sobral for their technical assistance in this work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00382-19.
REFERENCES
- 1.Nicolas-Chanoine M-H, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, Park Y-J, Lavigne J-P, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61:273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 2.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 3.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 4.Birgy A, Levy C, Bidet P, Thollot F, Derkx V, Béchet S, Mariani-Kurkdjian P, Cohen R, Bonacorsi S. 2016. ESBL-producing Escherichia coli ST131 versus non-ST131: evolution and risk factors of carriage among French children in the community between 2010 and 2015. J Antimicrob Chemother 71:2949–2956. doi: 10.1093/jac/dkw219. [DOI] [PubMed] [Google Scholar]
- 5.Schembri MA, Zakour NLB, Phan M-D, Forde BM, Stanton-Cook M, Beatson SA. 2015. Molecular characterization of the multidrug resistant Escherichia coli ST131 clone. Pathogens 4:422–430. doi: 10.3390/pathogens4030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan M-D, Gomes Moriel D, Peters KM, Davies M, Rogers BA, Dougan G, Rodriguez-Baño J, Pascual A, Pitout JDD, Upton M, Paterson DL, Walsh TR, Schembri MA, Beatson SA. 2014. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4:e00377. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, Turner P, Anson LW, Kasarskis A, Batty EM, Kos V, Wilson DJ, Phetsouvanh R, Wyllie D, Sokurenko E, Manges AR, Johnson TJ, Price LB, Peto TEA, Johnson JR, Didelot X, Walker AS, Crook DW, Modernizing Medical Microbiology Informatics Group (M). 2016. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio 7:e02162. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Zakour NL, Alsheikh-Hussain AS, Ashcroft MM, Khanh Nhu NT, Roberts LW, Stanton-Cook M, Schembri MA, Beatson SA. 2016. Sequential acquisition of virulence and fluoroquinolone resistance has shaped the evolution of Escherichia coli ST131. mBio 7:e00347. doi: 10.1128/mBio.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Pitout JDD, Gomi R, Matsuda T, Noguchi T, Yamamoto M, Peirano G, DeVinney R, Bradford PA, Motyl MR, Tanaka M, Nagao M, Takakura S, Ichiyama S. 2016. Global Escherichia coli sequence type 131 clade with blaCTX-M-27 gene. Emerg Infect Dis 22:1900–1907. doi: 10.3201/eid2211.160519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birgy A, Bidet P, Levy C, Sobral E, Cohen R, Bonacorsi S. 2017. CTX-M-27-producing Escherichia coli of sequence type 131 and clade C1-M27, France. Emerg Infect Dis 23:885. doi: 10.3201/eid2305.161865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc V, Leflon-Guibout V, Blanco J, Haenni M, Madec J-Y, Rafignon G, Bruno P, Mora A, Lopez C, Dahbi G, Dunais B, Anastay M, Branger C, Moreau R, Pradier C, Nicolas-Chanoine M-H. 2014. Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising O25b:H4 and O16:H5 ST131 strains. J Antimicrob Chemother 69:1231–1237. doi: 10.1093/jac/dkt519. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi-Akiyama T, Sherchan JB, Doi Y, Nagamatsu M, Sherchand JB, Tandukar S, Ohmagari N, Kirikae T, Ohara H, Hayakawa K. 2016. Comparative genome analysis of extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131 strains from Nepal and Japan. mSphere 5:289–216. doi: 10.1128/mSphere.00289-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallonen T, Brodrick HJ, Harris SR, Corander J, Brown NM, Martin V, Peacock SJ, Parkhill J. 2017. Systematic longitudinal survey of invasive Escherichia coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res 27:1437–1449. doi: 10.1101/gr.216606.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TJ, Danzeisen JL, Youmans B, Case K, Llop K, Munoz-Aguayo J, Flores-Figueroa C, Aziz M, Stoesser N, Sokurenko E, Price LB, Johnson JR. 2016. Separate F-Type plasmids have shaped the evolution of the H30 subclone of Escherichia coli sequence type 131. mSphere 4:121–116. doi: 10.1128/mSphere.00121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitout JDD, DeVinney R. 2017. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000Research 6:195. doi: 10.12688/f1000research.10609.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joensen KG, Tetzschner AMM, Iguchi A, Aarestrup FM, Scheutz F. 2015. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamborova I, Johnston BD, Papousek I, Kachlikova K, Micenkova L, Clabots C, Skalova A, Chudejova K, Dolejska M, Literak I, Johnson JR. 2018. Extensive genetic commonality among wildlife, wastewater, community, and nosocomial isolates of Escherichia coli sequence type 131 (H30R1 and H30Rx Subclones) that carry blaCTX-M-27 or blaCTX-M-15. Antimicrob Agents Chemother 62:e00519-18. doi: 10.1128/AAC.00519-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora A, Dahbi G, López C, Mamani R, Marzoa J, Dion S, Picard B, Blanco M, Alonso MP, Denamur E, Blanco J. 2014. Virulence patterns in a murine sepsis model of ST131 Escherichia coli clinical isolates belonging to serotypes O25b:H4 and O16:H5 are associated to specific virotypes. PLoS One 9:e87025. doi: 10.1371/journal.pone.0087025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micenková L, Šišková P, Bosák J, Jamborová I, Černohorská L, Šmajs D. 2014. Characterization of human uropathogenic ESBL-producing Escherichia coli in the Czech Republic: spread of CTX-M-27-producing strains in a university hospital. Microb Drug Resist 20:610–617. doi: 10.1089/mdr.2014.0013. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura Y, Johnson JR, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S, Kyoto–Shiga Clinical Microbiology Study Group, Kyoto-Shiga Clinical Microbiology Study Group. 2015. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J Antimicrob Chemother 70:1639–1649. doi: 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues C, Machado E, Fernandes S, Peixe L, Novais Â. 2016. An update on faecal carriage of ESBL-producing Enterobacteriaceae by Portuguese healthy humans: detection of the H30 subclone of B2-ST131 Escherichia coli producing CTX-M-27. J Antimicrob Chemother 71:1120–1122. doi: 10.1093/jac/dkv443. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh H, Doijad S, Falgenhauer L, Fritzenwanker M, Imirzalioglu C, Chakraborty T. 2017. blaCTX-M-27-encoding Escherichia coli sequence type 131 lineage C1-M27 clone in clinical isolates, Germany. Emerg Infect Dis 23:1754–1756. doi: 10.3201/eid2310.170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino I, Hernández-García M, Turrientes M-C, Pérez-Viso B, López-Fresneña N, Diaz-Agero C, Maechler F, Fankhauser-Rodriguez C, Kola A, Schrenzel J, Harbarth S, Bonten M, Gastmeier P, Canton R, Ruiz-Garbajosa P, Desilets M, Dul S, Scherrer-Muller F, Huttner B, Uçkay I, Prendki V, Renzi G, R-GNOSIS Study Group. 2018. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother 73:2973–2980. doi: 10.1093/jac/dky296. [DOI] [PubMed] [Google Scholar]
- 25.Harris PNA, Ben Zakour NL, Roberts LW, Wailan AM, Zowawi HM, Tambyah PA, Lye DC, Jureen R, Lee TH, Yin M, Izharuddin E, Looke D, Runnegar N, Rogers B, Bhally H, Crowe A, Schembri MA, Beatson SA, Paterson DL, MERINO Trial investigators. 2017. Whole genome analysis of cephalosporin-resistant Escherichia coli from bloodstream infections in Australia, New Zealand and Singapore: high prevalence of CMY-2 producers and ST131 carrying blaCTX-M-15 and blaCTX-M-27. J Antimicrob Chemother 73:634–642. doi: 10.1093/jac/dkx466. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet R, Recule C, Baraduc R, Chanal C, Sirot D, De Champs C, Sirot J. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J Antimicrob Chemother 52:29–35. doi: 10.1093/jac/dkg256. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh H, Bunk B, Doijad S, Schmiedel J, Falgenhauer L, Spröer C, Imirzalioglu C, Overmann J, Chakraborty T. 2017. Complete genome sequence of blaCTX-M-27-encoding Escherichia coli strain H105 of sequence type 131 lineage C1/H30R. Genome Announc 5:e00736-17. doi: 10.1128/genomeA.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolas-Chanoine M-H, Robert J, Vigan M, Laouénan C, Brisse S, Mentré F, Jarlier V, Coli β study group. 2013. Different factors associated with CTX-M-producing ST131 and non-ST131 Escherichia coli clinical isolates. PLoS One 8:e72191. doi: 10.1371/journal.pone.0072191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluytmans-van den Bergh MFQ, van Mens SP, Haverkate MR, Bootsma MCJ, Kluytmans JAJW, Bonten MJM, SoM Study Group and the R-GNOSIS Study Group. 2018. Quantifying hospital-acquired carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae among patients in Dutch hospitals. Infect Control Hosp Epidemiol 39:32–39. doi: 10.1017/ice.2017.241. [DOI] [PubMed] [Google Scholar]
- 30.Adler A, Gniadkowski M, Baraniak A, Izdebski R, Fiett J, Hryniewicz W, Malhotra-Kumar S, Goossens H, Lammens C, Lerman Y, Kazma M, Kotlovsky T, Carmeli Y, MOSAR WP5 and WP2 study groups. 2012. Transmission dynamics of ESBL-producing Escherichia coli clones in rehabilitation wards at a tertiary care centre. Clin Microbiol Infect 18:E497–505. doi: 10.1111/j.1469-0691.2012.03999.x. [DOI] [PubMed] [Google Scholar]
- 31.Rooney PJ, O'Leary MC, Loughrey AC, McCalmont M, Smyth B, Donaghy P, Badri M, Woodford N, Karisik E, Livermore DM. 2009. Nursing homes as a reservoir of extended-spectrum beta-lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J Antimicrob Chemother 64:635–641. doi: 10.1093/jac/dkp220. [DOI] [PubMed] [Google Scholar]
- 32.Cointe A, Birgy A, Mariani-Kurkdjian P, Liguori S, Courroux C, Blanco J, Delannoy S, Fach P, Loukiadis E, Bidet P, Bonacorsi S. 2018. Emerging multidrug-resistant hybrid pathotype Shiga toxin-producing Escherichia coli O80 and related strains of clonal complex 165, Europe. Emerg Infect Dis 24:2262–2269. doi: 10.3201/eid2412.180272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokurenko EV, Chesnokova V, Dykhuizen DE, Ofek I, Wu XR, Krogfelt KA, Struve C, Schembri MA, Hasty DL. 1998. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A 95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, Aarestrup FM. 2014. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol 52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geslain G, Birgy A, Adiba S, Magnan M, Courroux C, Levy C, Cohen R, Bidet P, Bonacorsi S. 2019. Genome sequencing of strains of the most prevalent clonal group of O1:K1:H7 Escherichia coli that causes neonatal meningitis in France. BMC Microbiol 19:17. doi: 10.1186/s12866-018-1376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mnif B, Vimont S, Boyd A, Bourit E, Picard B, Branger C, Denamur E, Arlet G. 2010. Molecular characterization of addiction systems of plasmids encoding extended-spectrum beta-lactamases in Escherichia coli. J Antimicrob Chemother 65:1599–1603. doi: 10.1093/jac/dkq181. [DOI] [PubMed] [Google Scholar]
- 40.Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. 2014. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One 9:e104984. doi: 10.1371/journal.pone.0104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.