TBI-166, derived from riminophenazine analogues, is under development in a phase I clinical trial in China. TBI-166 showed more potent anti-tuberculosis (anti-TB) activity than did clofazimine in in vitro and animal experiments.
KEYWORDS: BALB/c mice, C3HeB/FeJNju mice, TBI-166, murine model, tuberculosis
ABSTRACT
TBI-166, derived from riminophenazine analogues, is under development in a phase I clinical trial in China. TBI-166 showed more potent anti-tuberculosis (anti-TB) activity than did clofazimine in in vitro and animal experiments. To identify potent regimens containing TBI-166 in TB chemotherapy, TBI-166 was assessed for pharmacological interactions in vitro and in vivo with several anti-TB drugs, including isoniazid (INH), rifampin (RFP), bedaquiline (BDQ), pretomanid (PMD), linezolid (LZD), and pyrazinamide (PZA). Using an in vitro checkerboard method, we found that TBI-166 did not show antagonism or synergy with the tested drugs. The interaction relationship between TBI-166 and each drug was indifferent. In in vivo experiments, aerosol infection models with BALB/c and C3HeB/FeJNju mice were established, testing drugs were administered either individually or combined in treatments containing TBI-166 and one, two, or three other drugs, and the bactericidal activities were determined after 4- and 8-week therapeutic treatments. In BALB/c mice, five TBI-166-containing regimens—TBI-166+BDQ, TBI-166+PZA, TBI-166+BDQ+LZD, TBI-166+BDQ+PMD, and TBI-166+BDQ+PMD+LZD—showed significantly more potent efficacy after 4 weeks of treatment compared to the control regimen, INH+RFP+PZA. At the end of an 8-week treatment, lung log CFU counts decreased to undetectable levels in mice treated with each of the five regimens. The rank order of the potency of the five regimens was as follows: TBI-166+BDQ+LZD > TBI-166+BDQ > TBI-166+PZA > TBI-166+BDQ+PMD+LZD > TBI-166+BDQ+PMD. In C3HeB/FeJNju mice, TBI-166+BDQ+LZD was also the most effective of the TBI-166-containing regimens. In conclusion, five potent chemotherapy regimens that included TBI-166 were identified. The TBI-166+BDQ+LZD regimen is recommended for further testing in a TBI-166 phase IIb clinical trial.
INTRODUCTION
Tuberculosis (TB), multidrug-resistant TB (MDR-TB) in particular, requires a complex and long-term treatment process; even the shorter MDR-TB regimens, according to the most recent World Health Organization (WHO) recommendations, require 9 to 12 months (1). Due to the complexity and long duration of TB treatment, it is difficult for patients to comply with the treatment schedules. Irregular or inadequate treatment courses conversely lead to the selection and spread of drug-resistant Mycobacterium tuberculosis. Therefore, there is an urgent need to accelerate the development of new anti-TB drugs and regimens to simplify and shorten the treatment course for TB.
Clofazimine (CFZ), which has been used for leprosy treatment for decades, has demonstrated effectiveness in TB treatment (2) and is recommended by the WHO as the core second-line medicine in an MDR-TB regimen (3). The addition of CFZ in the original anti-TB scheme could improve the cavity healing, the conversion rate of sputum culture, and the success rate of treatment (4–6). In addition, studies have shown that the addition of CFZ to first-line (7) or second-line (8) regimens could significantly shorten the treatment course of tuberculosis. Unfortunately, CFZ use is hampered because of unwelcome associated skin discoloration.
TBI-166 is a new drug candidate derived from the optimization of riminophenazine analogues. TBI-166 causes less skin discoloration than does CFZ despite its higher tissue accumulation (9). In previous studies, TBI-166 showed higher bactericidal activity than CFZ in vitro against drug-sensitive and drug-resistant M. tuberculosis and against non-TB mycobacteria (10). TBI-166 showed potent anti-TB effects in a murine model tested in which the lung bacterial burden in mice administered TBI-166 was ∼1% that of the bacterial burden of mice given the same dose of CFZ (10). TBI-166 has been approved for clinical trial by the China Food and Drug Administration and is under phase I clinical trials in China (ChiCTR1800018780).
Regimens containing TBI-166 have not been evaluated for the treatment of TB, including combinations with newly approved anti-TB drugs and WHO-recommended first-line drugs. In the present study, we evaluated, using the checkerboard method in vitro and murine models of TB in vivo, the pharmacodynamic interactions between TBI-166 and several anti-TB drugs. Our study results support the performance of future TBI-166 clinical phase II studies for regimen selections and trials.
RESULTS
In vitro interaction profile to evaluate the effects of drug combinations on M. tuberculosis.
The MICs of each individual drug alone were determined by a microplate alamarBlue assay (MABA) to compare to the MICs of two-drug combinations. The individual drug MICs for M. tuberculosis H37Rv were consistent with the MICs observed in our previous studies. The MICs were 0.05 μg/ml for INH and RFP, 0.04 μg/ml for BDQ, 0.07 μg/ml for PMD, 0.3 μg/ml for LZD, and 0.06 μg/ml for TBI-166 (Table 1).
TABLE 1.
MICs of selected anti-TB compounds against M. tuberculosis H37Rv and corresponding interaction profiles with TBI-166 assessed by checkerboard methoda
| Compound A | MIC (μg/ml), alone/in combination |
Interaction profile with TBI-166 |
||
|---|---|---|---|---|
| MICA | MICTBI-166 | FIC index | Outcome | |
| INH | 0.05/0.0125 | 0.06/0.06 | 1.25 | Indifferent |
| RFP | 0.05/0.05 | 0.06/0.015 | 1.25 | Indifferent |
| BDQ | 0.04/0.01 | 0.06/0.03 | 0.75 | Indifferent |
| PMD | 0.07/0.0044 | 0.06/0.03 | 0.56 | Indifferent |
| LZD | 0.30/0.075 | 0.06/0.03 | 0.75 | Indifferent |
Isoniazid, INH; rifampin, RFP; bedaquiline, BDQ; pretomanid, PMD; linezolid, LZD.
As shown in Table 1, the interactions between TBI-166 and all five drugs (INH, RFP, BDQ, PMD, and LZD) were considered purely indifferent,with a fractional inhibitory concentration (FIC) index of >1 but <4. No synergy (FIC ≤ 0.5) or antagonism (FIC ≥ 4) was observed when TBI-166 was combined with one of the drugs listed in Table 1. The checkerboard assay was performed twice, and the results were consistent.
Effect on lung CFU reduction in BALB/c mice.
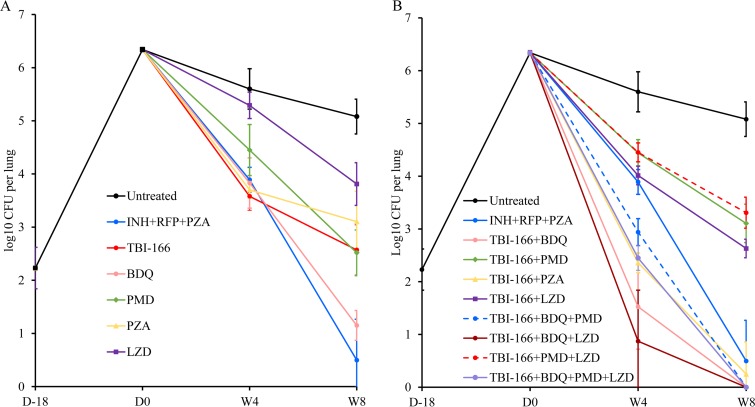
The results of lung CFU counts at the indicated time points are presented in Table S2 in the supplemental material. Ten days after aerosol infection, the mean CFU count was 2.23 log10 CFU in the lungs. The lung CFU count was increased to 6.34 log10 CFU at the initiation of treatment, 4 weeks after infection (Fig. 1 and Table S2). The mean bacterial burden of the untreated mice remained at a relatively high level throughout the trial, whereas the mean bacterial burden in positive-control mice treated with INH+RFP+PZA decreased to 3.89 and 0.50 log10 CFU after 4 and 8 weeks of treatment, respectively.
FIG 1.
Changes in lung CFU counts in BALB/c mice treated with single drugs (A) or TBI-166-containing regimens (B). Isoniazid, INH; rifampin, RFP; pyrazinamide, PZA; bedaquiline, BDQ; pretomanid, PMD; linezolid, LZD. D, day; W, week.
After a 4-week administration of monotherapy, TBI-166 showed the most potent efficacy among the five drugs tested, with a 2.02 log10 CFU reduction compared to the vehicle group. The potency rank order was as follows: TBI-166 > PZA > BDQ > PMD > LZD. After 8 weeks of monotherapy, BDQ showed the most potent efficacy, with a bacterial reduction of 3.93 log10 CFU compared to the vehicle group. The potency rank order after 8 weeks of treatment of monotherapy was as follows: BDQ > PMD > TBI-166 > PZA > LZD. LZD showed the least activity of the five drugs tested (Fig. 1A and Table S2).
TBI-166, BDQ, and PZA showed strong efficacy; the mean CFU counts for mice receiving these drugs alone were even lower than those for mice receiving INH+RFP+PZA after 4 weeks of treatment, although there was no statistically significant difference (P > 0.05). Among the treatment groups, the mean lung CFU count for mice receiving TBI-166 was the lowest, which was 2.02 log10 CFU lower than that for untreated mice. After 8 weeks of monotherapy, the superior efficacy of BDQ was obvious, and the mean CFU count for mice receiving BDQ decreased to 1.15 log10 CFU (standard deviation [SD], 0.92 log10 CFU); two of six mice in this group even had culture-negative lungs (Table S3).
In mice receiving combination therapy, five of eight regimens containing TBI-166 showed remarkable anti-TB activity. Mice treated with these five regimens showed significantly lower lung CFU counts than did positive-control mice (P < 0.001) after 4 weeks of administration. All mice (except for one lung in a mouse receiving TBI-166+PZA that formed a single colony) in these groups were culture negative at the end of 8 weeks of administration (Table S3). Four of the five regimens contained TBI-166 and BDQ, and the other regimen consists of TBI-166 and PZA. The combination TBI-166+BDQ+PMD had the lowest efficacy of the five regimens; the CFU reduction was lower than that observed for TBI-166+BDQ.
Considering that the CFU counts of mice in these five groups were essentially the same after 8 weeks of administration, the efficacies of these five regimens were compared at the end of 4 weeks of treatment. Of the five regimens, TBI-166+BDQ+LZD showed the most potent activity, and the mean lung CFU count was 0.87 log10 CFU (SD, 0.97 log10 CFU) after 4 weeks of administration, and three of six mice in this group had culture-negative lungs (Table S3). The data obtained after 4 weeks of treatment showed that the overall rank order of the bactericidal activity of each combination regimens was as follows: TBI-166+BDQ+LZD > TBI-166+BDQ > TBI-166+PZA > TBI-166+BDQ+PMD+LZD > TBI-166+BDQ+PMD (Fig. 1B and Table S2).
Combination with TBI-166 significantly increased the bactericidal activity of BDQ, PZA, and LZD. One of the most noteworthy results was the prominent effect of TBI-166 on the bactericidal activity of BDQ. Compared to the BDQ-alone group, the mean lung CFU count of mice receiving TBI-166+BDQ decreased by 2.30 and 1.15 log10 CFU after 4 and 8 weeks of treatment, respectively (Table S2). After 8 weeks of administration, two mice in BDQ-alone group had culture-negative lungs, whereas the lungs of all mice receiving TBI-166+BDQ were culture negative (Tables S2 and S3). TBI-166 also significantly enhanced the activity of PZA. The mean CFU count for mice receiving TBI-166+PZA, compared to that for the PZA-alone treatment group, decreased by 1.34 and 2.85 log10 CFU by the end of 4 and 8 weeks of administration, respectively (Table S2). After 8 weeks of administration, all mice in the PZA-alone group had a high level of lung bacterial burden, with a mean value of 3.1 log10 CFU, whereas five of six mice received TBI-166+PZA had no CFU detected in the lungs except that one mouse lung homogenate formed one colony (Tables S2 and S3). Furthermore, after 4 and 8 weeks of treatment, mice that received TBI-166+BDQ or TBI-166+PZA had statistically significantly lower mean lung CFU counts than mice that received TBI-166 alone (P < 0.001), suggesting that BDQ and PZA could also improve the activity of TBI-166.
The efficacy of PMD was not improved after the combination with TBI-166. The mean lung CFU count of mice in TBI-166+PMD group was similar to that in the PMD-alone group after 4 weeks of administration (P > 0.05), while the CFU count for TBI-166+PMD mice was even higher than for mice receiving PMD after 8 weeks of treatment (P < 0.05). In addition, the efficacies of regimens adding PMD were all weaker than those of regimens without PMD, including TBI-166+BDQ+PMD versus TBI-166+BDQ, TBI-166+PMD+LZD versus TBI-166+LZD, and TBI-166+BDQ+PMD+LZD versus TBI-166+BDQ+LZD (Fig. 1 and Table S2). Moreover, mice receiving TBI-166+PMD had higher mean lung CFU counts than mice receiving TBI-166 alone after 4 and 8 weeks of administration (P < 0.05), suggesting that there might be an antagonism effect between TBI-166 and PMD in the murine model of TB.
Although treatment with TBI-166+LZD yielded lung CFU counts that were significantly lower than those obtained using treatment with LZD alone (P < 0.001), the mean lung CFU counts were similar to those for the TBI-166-alone group after treatment for 4 and 8 weeks (P > 0.05), suggesting that LZD did not improve the bactericidal activity of TBI-166 (Fig. S1 and Table S2). However, mice that received TBI-166+BDQ+LZD had significantly lower CFU counts than mice that received TBI-166+BDQ at week 4 (P = 0.011), indicating that LZD plays an important role in the TBI-166+BDQ+LZD combination (Fig. 1 and Table S2).
Efficacy assessment on C3HeB/FeJNju mice model.
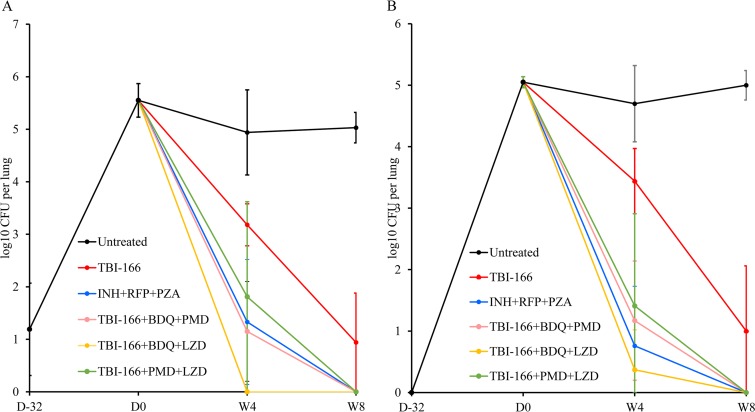
The results of lung and spleen CFU counts at the indicated time points are presented in Fig. 2 and Table S4. After 6 weeks of infection, the lung and spleen CFU counts were 5.55 and 5.05 log10 CFU, respectively (Table S4). After 8 weeks of administration, the log10 CFU values for the lungs and spleens decreased to 0.94 and 1.00, respectively, in the TBI-166-alone group, and the lungs and spleens of all mice in the four combined groups were culture negative (Table S4 and S5).
FIG 2.
Changes in CFU counts in C3HeB/FeJNju mice. CFU counts were determined in the mouse lungs (A) and spleens (B) at the indicated time points. Isoniazid, INH; rifampin, RFP; pyrazinamide, PZA; bedaquiline, BDQ; pretomanid, PMD; linezolid, LZD. D, day; W, week.
As observed in study 1, the rank order of the bactericidal activities of the TBI-166-containing regimens, based on data obtained after 4 weeks of treatment using the C3HeB/FeJNju mice model, was as follows: TBI-166+BDQ+LZD > TBI-166+BDQ+PMD > TBI-166+PMD+LZD (Fig. 2 and Table S4). TBI-166+BDQ+LZD was the most effective of the four combination regimens, including also the INH+RFP+PZA group. After 4 weeks of administration, all lungs and spleens of mice in the TBI-166+BDQ+LZD group were culture negative, except for one mouse with a single detectable CFU in the spleen (Tables S4 and S5).
Assessment of sterilizing activity.
In study 2, five mice were taken from each combination regimen group, and treatment was discontinued for 8 weeks after 8 weeks of administration. Lung and spleen homogenates of mice were cultured on 7H10 plates containing activated carbon. Based on the culture results, culture-positive relapse was detected in two mice in the INH+RFP+PZA group and in three mice in TBI-166+PMD+LZD group. No culture-positive relapses occurred in mice treated with TBI-166+BDQ+LZD or TBI-166+BDQ+PMD.
DISCUSSION
Worldwide TB epidemics remain severe, and researchers have been working on new anti-TB drugs, especially ones that are effective against drug-resistant TB. Considering the difficulty of new drug development and in order to avoid the rapid emergence of new drug resistance, how to reasonably use new drugs to form new and effective regimens is especially important. Moreover, it is also very important to identify drug interactions and find the right combinations to improve the treatment of diseases, especially tuberculosis, a disease that must be treated with a combination of drugs. A reasonable combination can enhance the efficacies and reduce the adverse reactions of drugs to some extent. However, due to the long clinical course and complicated patient conditions, new drug combinations are more suitable for clinical verification rather than for clinical screening.
Based on the good performance of TBI-166 in riminophenazine analogue screening, we studied its anti-TB activity further. The systematic monotherapy activity evaluation has been completed; TBI-166 showed more potent anti-TB activity than clofazimine against both drug-sensitive and drug-resistant M. tuberculosis in vitro and demonstrates at least equivalent activity compared to CFZ in acute and chronic murine models of TB caused by low-dose aerosol infection (9). In addition, TBI-166 causes less skin discoloration than CFZ, which contributes to its more widespread application. In the present experiment, we systematically evaluated the interaction profiles between TBI-166 and several anti-TB drugs or candidates in vitro and in vivo. Five TBI-166-containing combinations identified showed significantly greater bactericidal activity than did the standard first-line regimen at 4 and 8 weeks. The lungs of all mice under these combinations were culture negative after 8 weeks of treatment, except that one lung formed a colony in a mouse receiving the TBI-166+PZA regimen. The most effective combination is the TBI-166+BDQ+LZD regimen; half of the mice in this group had culture-negative lungs after 4 weeks of administration. In the present study, we focused on the interactions between TBI-166 and four drugs, among which BDQ, LZD, and PMD are all quite advanced in the anti-TB drug development pipeline. PZA also plays an important role in multiple regimens of the TB drug development pipeline based on its powerful anti-TB activity, and all four drugs have been shown to be able to shorten TB treatment duration in murine models and even clinical trials (11–13). Our results revealed that TBI-166 is suitable for combination with BDQ, PZA, and LZD; the anti-TB activity of the combined regimen was stronger than that of the single drug. In addition, TBI-166 exhibited synergy with BDQ and PZA and is likely to further shorten the duration of TB treatment, which is very important for the reasonable application of TBI-166 and the prevention and treatment of tuberculosis.
In addition to the commonly used BALB/c mouse evaluation model, we established the C3HeB/FeJNju mouse infection model, which is significant for the demonstration of the conclusions presented in this study. On the one hand, the lung tissues of C3HeB/FeJNju mice could produce hypoxic, well-defined granulomas and exhibit caseous necrosis (14), and the microenvironmental conditions within granulomas might negatively impact drug efficacy, which may not be reflected within more conventional BALB/c mouse models. A previous study suggested that the effect of CFZ may be overestimated in BALB/c mice (15). Although CFZ was highly effective in the lungs of BALB/c mice, its activity is less pronounced in C3HeB/FeJNju mice (15). In our study, we compared the activities of all three-drug combinations in C3HeB/FeJNju mice, and the consistency of the therapeutic effect order in two mouse models could be observed. On the other hand, the relapse assessment results in study 2 reconfirmed the remarkable anti-TB effect of TBI-166+BDQ+LZD. Although the TB bacterium was not detected in mice treated with all combination regimens after 8 weeks of administration, the relapse rate of each group was different, and the TBI-166+BDQ+LZD treatment group had the lowest relapse rate.
Remarkably, the addition of TBI-166 to BDQ and PZA reduced the CFU counts much more than did either drug when used alone (P < 0.001), and the efficacy of TBI-166 could also be significantly increased when combined with BDQ or PZA (P < 0.001) (Table 1 and Fig. S1). Recently, PZA was seen as the “partner” of BDQ; studies showed that the combination of BDQ and PZA could significantly increase the activities of multiple regimens, even the efficacy of BDQ+PZA given for 1 month was superior to a 2-month standard regimen (13). Our findings showed that TBI-166 could not only significantly enhance the curative effect of BDQ but also significantly enhance the curative effect of its “partner,” signifying that it is very appropriate and more effective to add TBI-166 to BDQ+PZA. Studies have reported that the addition of CFZ, which is the congener of TBI-166, increased the activity of BDQ+PZA significantly; none of the mice in this group relapsed after 8 weeks of treatment (16). In view of the higher activity of TBI-166, we believe that the combination of TBI-166 and BDQ+PZA would demonstrate similar or stronger sterilization activity. Moreover, since TBI-166 is not only effective for drug-sensitive M. tuberculosis strains but also for the vast majority of drug-resistant M. tuberculosis strains, the addition of TBI-166 to a regimen containing BDQ is highly significant for the treatment of MDR-TB using this regimen.
In addition, we believe that the interaction between TBI-166 and PMD requires further research and verification. When studying the bactericidal activity of each regimen, we observed that the addition of PMD attenuated the activity of TBI-166 in the mouse model. When PMD was combined with TBI-166+BDQ, TBI-166+LZD, or TBI-166+BDQ+LZD, its potency was reduced compared to regimens without PMD. However, we cannot completely dismiss the combination of TBI-166 and PMD because the antagonistic effect between TBI-166 and PMD was not confirmed in the recurrence assessment in study 2. In fact, a similar problem exists in studies on the interaction between BDQ and PMD. In previous studies of bactericidal activity in mice, antagonism between PMD and BDQ was observed, but this was not confirmed in subsequent recurrence studies (16, 17).
For the combination of LZD and TBI-166, considering that the addition of LZD cannot improve the bactericidal activity of TBI-166, we believe that although the activity of the TBI-166+LZD combination is higher than that of LZD, the efficacy of the combination was mainly due to TBI-166. Moreover, the activity of TBI-166+LZD was lower than that of TBI-166 alone, and the activity of TBI-166+PMD+LZD was lower than that of TBI-166+PMD, although statistically significant differences were not found. The significantly higher activity of TBI-166+BDQ+LZD compared to TBI-166+BDQ might be because LZD could improve the activity of BDQ or the TBI-166+BDQ combination.
In animal experiments, multiple combinations containing TBI-166 presented potent activity, especially when both TBI-166 and BDQ were present; several possible reasons are listed below. (i) First, TBI-166 showed strong anti-TB activity in our study and in previous studies (10). In BALB/c mice, after 8 weeks of administration, the mean lung burden for mice treated with TBI-166 was 2.51 log10 CFU lower than for untreated mice. In C3HeB/FeJNju mice, the mean lung burden for mice treated with TBI-166 was 4.09 log10 CFU lower than that for untreated mice at the end of 8 weeks of administration. (ii) Second, TBI-166 is active against both quiescent bacteria and intracellular bacteria, which can compensate for the inadequate anti-TB capability of other drugs in the combination (10). (iii) Third, as a structural modification of CFZ, TBI-166 may be a CYP3A4/5 inhibitor as well. Thus, TBI-166 may lead to the increased blood exposure and enhanced efficacy of BDQ, since BDQ is a substrate of CYP3A4 (18). (iv) Finally, although the mechanism of action is not fully understood, existing data indicate that BDQ, PZA, and TBI-166 might be all involved in the oxidative phosphorylation pathway and ATP synthesis of M. tuberculosis, which might lead to the synergistic effects between them.
TBI-166 is an analogue of CFZ, but CFZ was not used as a control in our study. There are two main reasons for this. One is that we made a detailed comparison between the single-drug activities of CFZ and TBI-166 in previous experiments (9). The second reason is that when the present study was designed, we knew nothing about the interaction profiles of TBI-166 or which drugs should be used in combination with TBI-166. Therefore, we feel it is not necessary to use CFZ as a control in the preliminary screening of effective regimens. Since so many kinds of CFZ-containing regimens have been studied, it was inappropriate to select one of them, but it was also too complicated to add multiple CFZ-containing regimens into the present study as controls. Therefore, CFZ was not used as a control here.
Our study has several limitations. First of all, the bacterial load was relatively low. In the BALB/c mouse model, the mean lung burden at the beginning of treatment was 6.34 log10 CFU. Thus, all mice in the five TBI-166-containing regimens were at or approaching culture-negative status after 8 weeks of treatment. These results suggested that the anti-TB activity of the five regimens is strong, but it is difficult to distinguish which regimen is more effective. In the C3HeB/FeJNju mouse model, the mean lung burden at the beginning of treatment was 5.55 log10 CFU. After 8 weeks of administration, all of the mice in four regimens were culture negative, making it difficult to distinguish the activity of each regimen. Therefore, the 4-week results were used to rank the potency of each regimen. Moreover, the lower bacterial load also has some potential effects on the recurrence results, so the sterilization activity of regimens in study 2 may be overestimated. However, we could still see from the results that the sterilization activity of TBI-166+BDQ+LZD and TBI-166+BDQ+PMD is higher than that of the standard INH+RFP+PZA scheme.
In addition, since our previous study revealed that no obvious early bactericidal activity (EBA) was observed after 2 weeks of administration of TBI-166 monotherapy, the EBA of each regimen was not evaluated in the present study. However, our results showed that the greatest reduction of CFU counts at 4 weeks appeared in mice receiving TBI-166 alone, suggesting that we could refocus on its EBA. Actually, CFZ also failed to show obvious EBA in single-drug administration, while the EBA of multiple regimens increased after the addition or replacement of CFZ, indicating that an EBA evaluation of TBI-166 should also be conducted in combination regimens. In addition, considering that a single drug cannot be used in the treatment of TB, the EBA evaluation of TBI-166 in combinations can also provide more reliable data for clinical application.
Another limitation is that our study only used one strain of M. tuberculosis. A previous study evaluated the interaction profiles of CFZ with 195 MDR-TB isolates and found that CFZ showed synergistic effects on about 87.5% of MDR-TB strains with EMB and also has synergistic effects with fluoroquinolones and second-line injection drugs on about 15 to 30% of MDR-TB strains (19), suggesting that the study of interactions should take bacterial species into account. A final limitation of our study is that we did not study the interaction between TBI-166 and PZA in in vitro tests. PZA works under acidic conditions, whereas the effects of an acidic environment on the growth of TB bacteria and the efficacy of TBI-166 are likely to bias the results; thus, we did not study this interaction using the checkerboard method.
In conclusion, we have found five highly effective TBI-166-containing regimens with the potential to strengthen and shorten the treatment process for tuberculosis; among these regimens, the activity of TBI-166+BDQ+LZD merits more attention. In addition, our results revealed that TBI-166 might have synergistic effects with BDQ and PZA, whereas its efficacy might be impaired by PMD. Thus, TBI-166 is very suitable for combination with BDQ and PZA, whereas combination with PMD is probably not suitable, and combination with LZD needs to refer to other drugs combined in the regimen. TBI-166 is a promising anti-TB drug, and our findings have provided relevant and informative data for the clinical use of TBI-166. The more effective combinations included TBI-166, and the discovery of interaction mechanisms merits further study.
MATERIALS AND METHODS
All experiments were performed at the Beijing Tuberculosis and Thoracic Tumor Research Institute (Beijing, China). Animal experiments in this study were approved by the Animal Ethics Committee of Beijing Chest Hospital-Affiliate of Capital Medical University, and all animal procedures were performed according to the Animal Care Guidelines of the Institutional Animal Care and Use Committee of Capital Medical University (Beijing, China).
Antimicrobial agents.
Isoniazid (INH), rifampin (RFP), bedaquiline (BDQ), pretomanid (PMD), linezolid (LZD), and pyrazinamide (PZA) were purchased from Sigma-Aldrich. TBI-166 was provided by the Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences (Beijing, China).
Mycobacterial strain.
M. tuberculosis H37Rv strain (ATCC 27294) was grown in 7H9 broth supplemented with 10% Middlebrook acid-albumin-dextrose-catalase (OADC) enrichment medium (Difco), 0.2% glycerol, and 0.05% Tween 80. Log-phase cultures incubated at 37°C with 5% CO2 were used in all studies.
Checkboard experiment in vitro.
A checkerboard method was used to evaluate the interaction profiles of TBI-166 with other drugs. First, the MIC of each drug used alone was determined by MABA, as previously described (20). Then, serial concentrations of two drugs based on these MICs were added in the rows and columns on a 96-well plate by consecutive double dilution. One drug was horizontally diluted (rows B to G), and the other drug was vertically diluted (columns 1 to 6). The top row (row A) contained a positive control and a negative control. A 100-μl portion of a log-phase culture of M. tuberculosis H37Rv (6 × 105 CFU/ml) was added to each well, and the final concentrations of each drug ranged from 2 to 1/16 MIC determined alone (Table S1). The MABA method was also used in the assessment of combination MICs. According to fluorescence and color changes in the incubation medium, the combination MICs were determined based on the minimal drug concentrations without obvious bacterial growth. The FIC index was calculated as follows: [MICA in combination]/[MICA alone] + [MICB in combination]/[MICB alone], where MICA alone and MICB alone are the MICs obtained when each drug was tested alone, and MICA in combination and MICB in combination are the concentrations of each compound at the lowest effective combination. The FIC was calculated to classify the interactions as one of three kinds: synergy (FIC index ≤ 0.5), indifferent (FIC index > 0.5~4), and antagonism (FIC index > 4) (21, 22).
Establishment of infection in mice.
For study 1, 192 female BALB/c mice, aged 6 weeks and weighing 18 to 20 g, were purchased from Beijing Vital River Laboratory Animal Technology Company. The mice were assigned to cages at six mice per cage and allowed to acclimatize the environment for 1 week before the experiment. Housing was equipped for constant temperature and humidity using an air conditioning system. Mice were aerosol infected with mouse-passaged M. tuberculosis H37Rv by using an inhalation exposure system (099C A4224; Glas-Col, Terre Haute, IN). Three untreated mice from each infection run were sacrificed at 10 days and 4 weeks after the infection to determine the counts of bacteria at the beginning of infection and at the start of treatment, respectively. Six mice from each group were sacrificed after 4 or 8 weeks of treatment to assess the bactericidal activity of each regimen. For study 2, female C3HeB/FeJNju mice (n = 86), aged 6 weeks, were purchased from Nanjing Biomedical Research Institute of Nanjing University. The mice were assigned to cages of five mice per cage and allowed to acclimatize the environment for 1 week before the experiment. All 86 mice were also infected with the M. tuberculosis H37Rv strain. Three untreated mice were sacrificed 10 days and 6 weeks after the infection to determine the baseline counts of bacteria implanted in lungs and at the start of treatment, respectively. Five mice from each group were sacrificed after 4 or 8 weeks of treatment to assess the bactericidal activity of each regimen. In addition, five mice treated for 8 weeks in each of the four combined regimens were evaluated for relapse 8 weeks after drug withdrawal.
Chemotherapy regimens.
In study 1, after 4 weeks of infection, BALB/c mice were randomized into fifteen groups (Table 2). In study 2, after 6 weeks of infection, C3HeB/FeJNju mice were randomized into six groups (Table 3). All drugs were prepared in 0.5% sodium carboxymethyl cellulose (CMC) in distilled water for single-drug or multidrug administrations. Drugs were administered at the following doses: INH (10 mg/kg), RFP (10 mg/kg), PZA (150 mg/kg), TBI-166 (20 mg/kg), BDQ (25 mg/kg), PMD (100 mg/kg), and LZD (100 mg/kg). The dosage of TBI-166 was obtained from the previous experiment. In a previous study, we found that TBI-166 exhibited dose-independent bactericidal activity; the anti-TB activity of 20 mg/kg TBI-166 was no different than that of an equivalent dose of CFZ in the acute infection model, and TBI-166 at 20 mg/kg had relatively lower activity (log10 CFU < 1.0) than CFZ at 20 mg/kg in the chronic infection model. Thus, we chose 20 mg/kg as the dosage of TBI-166 in this study (9). All mice were administered orally by gavage five times per week (Monday to Friday) in a single dose of 0.2 ml. Negative- and positive-control groups received 0.2 ml of CMC or INH+RFP+PZA, respectively. RFP was administered before INH+PZA for at least 1 h to avoid the antagonism interaction between INH and RFP (23). RFP and INH+PZA were dissolved in 0.1 ml of distilled water containing 0.5% CMC, respectively.
TABLE 2.
Experimental design used in study 1
| Group | Drug(s)a | No. of mice sacrificed in each groupb
|
Total no. of mice | |||
|---|---|---|---|---|---|---|
| D–18 | D0 | W4 | W8 | |||
| Negative control | Untreated | 6 | 6 | 6 | 6 | 24 |
| Positive control | INH+RFP+PZA | 6 | 6 | 12 | ||
| Single drugs | TBI-166, BDQ, PMD, PZA, LZD | 30 | 30 | 60 | ||
| Two-drug combinations | TBI-166+BDQ, TBI-166+PMD, TBI-166+PZA, TBI-166+LZD | 24 | 24 | 48 | ||
| Three-drug combinations | TBI-166+BDQ+PMD, TBI-166+BDQ+LZD, TBI-166+PMD+LZD | 18 | 18 | 36 | ||
| Four-drug combination | TBI-166+BDQ+PMD+LZD | 6 | 6 | 12 | ||
| Total no. of mice | 6 | 6 | 90 | 90 | 192 | |
Drugs (abbreviations) and doses: isoniazid (INH), 10 mg/kg; rifampin (RFP), 10 mg/kg; pyrazinamide (PZA), 150 mg/kg; TBI-166 (20 mg/kg); bedaquiline (BDQ), 25 mg/kg; pretomanid (PMD), 100 mg/kg; linezolid (LZD), 100 mg/kg.
D, day; W, week.
TABLE 3.
Experimental design used in study 2
| Groupa | No. of mice sacrificed in each groupb
|
Total no. of mice | |||
|---|---|---|---|---|---|
| D–32 | D0 | W4 | W8 | ||
| Untreated | 3 | 3 | 5 | 5 | 16 |
| TBI-166 | 5 | 5 | 10 | ||
| INH+RFP+PZA | 5 | 5 + 5 | 15 | ||
| TBI-166+BDQ+LZD | 5 | 5 + 5 | 15 | ||
| TBI-166+BDQ+PMD | 5 | 5 + 5 | 15 | ||
| TBI-166+LZD+PMD | 5 | 5 + 5 | 15 | ||
| Total no. of mice | 3 | 3 | 30 | 50 | 86 |
Drugs (abbreviations) and doses: isoniazid (INH), 10 mg/kg; rifampin (RFP), 10 mg/kg; pyrazinamide (PZA), 150 mg/kg; TBI-166 (20 mg/kg); bedaquiline (BDQ), 25 mg/kg; pretomanid (PMD), 100 mg/kg; linezolid (LZD), 100 mg/kg.
D, day; W, week.
Assessment of treatment effect.
The treatment effect was assessed based on the lung CFU counts during treatment. Six mice (five mice in study 2) from each group were sacrificed after treated for 4 or 8 weeks. After the mice were killed, the lungs or spleens were dissected and homogenized in 3.0 ml of sterilizing saline. Tissue homogenate was diluted 10, 100, and 1,000 times, and 0.1 ml of undiluted homogenate or each dilution was plated on selective 7H10 agar plates enriched with 10% OADC enrichment medium (Difco) and supplemented with ampicillin (50 μg/ml), polymyxin B (33.3 μg/ml), trimethoprim (20 μg/ml), and cycloheximide (200 μg/ml) to prevent the contamination of other bacteria. To limit the consequence of TBI-166 carryover, lung homogenates from the TBI-166-treated mice were plated on 7H10 selective agar supplemented with 0.4% (wt/vol) activated charcoal, which could absorb the residual TBI-166. All plates were incubated at 37°C with 5% CO2 for 4 weeks before the CFU were enumerated. The same plating scheme was also used for the recurrence assessment.
Statistical analysis.
CFU counts (x) were log transformed as log10(x + 1) before analysis. Comparisons of CFU means between different groups were analyzed by one-way analysis of variance with Dunnett’s posttest to correct for multiple comparisons. The significance level was 0.05. The SPSS program (19.0, version for Windows; SPSS, Chicago, IL) was used for all statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Institute of Materia Medica, Peking Union Medical College & Chinese Academy of Medical Sciences, for providing TBI-166.
This study was supported by the National Science and Technology Project of China (2015ZX09102007-015) and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL2015150).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02496-18.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Ahmad Khan F, Salim MAH, Du Cros P, Casas EC, Khamraev A, Sikhondze W, Benedetti A, Bastos M, Lan Z, Jaramillo E, Falzon D, Menzies D. 2017. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J 50:1700061 Electronic). doi: 10.1183/13993003.00061-2017. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. 2013. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 68:284–293. doi: 10.1093/jac/dks389. [DOI] [PubMed] [Google Scholar]
- 5.Gopal M, Padayatchi N, Metcalfe JZ, O’Donnell MR. 2013. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 17:1001–1007. doi: 10.5588/ijtld.12.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padayatchi N, Gopal M, Naidoo R, Werner L, Naidoo K, Master I, O’Donnell MR. 2014. Clofazimine in the treatment of extensively drug-resistant tuberculosis with HIV coinfection in South Africa: a retrospective cohort study. J Antimicrob Chemother 69:3103–3107. doi: 10.1093/jac/dku235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyagi S, Ammerman NC, Li SY, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li SY, Ammerman NC, Bishai WR, Enarson D, Trebucq A. 2013. Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188:608–612. doi: 10.1164/rccm.201304-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Wang B, Fu L, Zhu H, Guo S, Huang H, Yin D, Zhang Y, Lu Y. 2019. In vitro and in vivo activities of the riminophenazine TBI-166 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 63:e02155-18. doi: 10.1128/aac.02155-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Zheng M, Wang B, Fu L, Zhao W, Li P, Xu J, Zhu H, Jin H, Yin D, Huang H, Upton AM, Ma Z. 2011. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob Agents Chemother 55:5185–5193. doi: 10.1128/AAC.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada M. 2001. Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients. Kekkaku 76:33–43. [PubMed] [Google Scholar]
- 12.Tasneen R, Betoudji F, Tyagi S, Li SY, Williams K, Converse PJ, Dartois V, Yang T, Mendel CM, Mdluli KE, Nuermberger EL. 2016. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 60:270–277. doi: 10.1128/AAC.01691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim M, Andries K, Lounis N, Chauffour A, Truffot-Pernot C, Jarlier V, Veziris N. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother 51:1011–1015. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, Lenaerts AJ. 2012. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin SM, Gruppo V, Brooks E, Gilliland J, Scherman M, Reichlen MJ, Leistikow R, Kramnik I, Nuermberger EL, Voskuil MI, Lenaerts AJ. 2014. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrob Agents Chemother 58:4026–4034. doi: 10.1128/AAC.02565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maartens G, Brill MJE, Pandie M, Svensson EM. 2018. Pharmacokinetic interaction between bedaquiline and clofazimine in patients with drug-resistant tuberculosis. Int J Tuberc Lung Dis 22:26–29. doi: 10.5588/ijtld.17.0615. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Li T, Qu G, Pang Y, Zhao Y. 2015. In vitro synergistic activity of clofazimine and other antituberculous drugs against multidrug-resistant Mycobacterium tuberculosis isolates. Int J Antimicrob Agents 45:71–75. doi: 10.1016/j.ijantimicag.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Wang B, Hu M, Huo F, Guo S, Jing W, Nuermberger E, Lu Y. 2017. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 61:e00239-17. doi: 10.1128/aac.00239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechartier B, Hartkoorn RC, Cole ST. 2012. In vitro combination studies of benzothiazinone lead compound BTZ043 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:5790–5793. doi: 10.1128/AAC.01476-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 23.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.