Abstract
Background:
After 5 years since the registration of rifaximin-α as a secondary prophylaxis for overt hepatic encephalopathy (HE) in the Netherlands, we aimed to evaluate the use of hospital resources and safety of rifaximin-α treatment in a real-world setting.
Methods:
We carried out prospective identification of all patients using rifaximin-α for overt HE. We assessed hospital resource use, bacterial infections, and adverse events during 6-month episodes before and after rifaximin-α initiation.
Results:
During 26 months we included 127 patients [71.7% male; median age 60.8 years (interquartile range: 56.2–66.1); median model for end-stage liver disease (MELD) score 15.0 (interquartile range: 12.1–20.4); 98% using lactulose treatment]. When comparing the first 6 months after rifaximin-α initiation with the prior 6 months, HE-related hospital admissions decreased (0.86 to 0.41 admissions/patient; p < 0.001), as well as the mean length of stay (8.85 to 3.79 bed days/admission; p < 0.001). No significant differences were found regarding HE-related intensive care unit admissions (0.09 to 0.06 admission/patient; p = 0.253), stay on the intensive care unit (0.43 to 0.57 bed days/admission; p = 0.661), emergency department visits (0.66 to 0.51 visits/patient; p = 0.220), outpatient clinic visits (2.49 to 3.30 bed visits/patient; p = 0.240), or bacterial infections (0.41 to 0.35 infections/patient; p = 0.523). Adverse events were recorded in 2.4% of patients.
Conclusions:
The addition of rifaximin-α to lactulose treatment was associated with a significant reduction in the number and length of HE-related hospitalizations for overt HE. Rifaximin-α treatment was well tolerated.
Keywords: efficacy, end-stage liver disease, healthcare utilization, hepatic encephalopathy, rifaximin-α, safety
Introduction
Hepatic encephalopathy (HE) is a neuropsychiatric complication of advanced liver disease characterized by indiscernible changes (covert HE) to clinically obvious changes (overt HE) in intellect, behaviour, motor function and consciousness.1 Overt HE affects approximately 30–40% of patients with cirrhosis,2 is the most lethal cirrhosis complication with a survival rate between 40–55% at 6 months after diagnosis,3,4 and negatively affects quality of life.5,6
Rifaximin-α is a poorly adsorbed antimicrobial agent and has been registered since 2013 as secondary prophylaxis for overt HE in the Netherlands.7
The pharmacological effect of rifaximin-α has been attributed to a reduction in gut absorption and production of ammonia.8 A meta-analysis of randomized controlled trials of rifaximin-α treatment in HE found that rifaximin-α had a beneficial effect on the secondary prevention of overt HE, increased the proportion of patients who recovered from HE, and reduced mortality.9
At present, the impact of rifaximin-α has not been extensively studied in a real-world setting (i.e. medical data outside controlled research study protocols in a heterogenous patient population). Recently, a cohort study of 114 patients concluded that rifaximin-α significantly reduced hospitalizations, critical care admissions, and accident and emergency (A&E) department attendances in patients using rifaximin-α for at least 6 months.10 However, a potential beneficial effect of rifaximin-α on liver transplantation waiting list mortality or overall mortality has not been clearly established.
The primary aim of this study was to assess the impact of rifaximin-α treatment by evaluating the effect on hospitalizations, A&E department visits, outpatient clinic visits, and bacterial infections in the first 6 months after initiation compared with the prior 6 months. Secondarily, we evaluated the treatment duration and safety profile of rifaximin-α.
Methods
Study design and patients
We aimed to identify all individuals who were treated with rifaximin-α between 1 September 2015 and 1 November 2017 at Erasmus MC, University Medical Center, Rotterdam, the Netherlands. The researchers were immediately informed by the electronic medical record computer software via email when rifaximin-α was prescribed in the Erasmus MC or when a patient using this agent was registered in the hospital. All patients using rifaximin-α as a secondary prophylaxis for overt HE, irrespective of the use of lactulose at that time, were prospectively included in the study. Patients were excluded when rifaximin-α was prescribed in absence of (a history of) HE, clinical data were incomplete, or when nonadherence to rifaximin-α treatment was reported. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the institution’s human research committee (MEC-2015-394) with the determination that written or oral informed consent was not required, considering the design of the study.
Data collection
Data regarding demographics (age, sex), clinical characteristics (aetiology of liver disease; presence of hepatocellular carcinoma; presence of HE; presence of ascites; concomitant lactulose and norfloxacin use; and blood serum values), rifaximin-α use (duration of exposure; dosage; temporary and permanent discontinuation; (serious) adverse events), and clinical outcome (number of HE-related hospital admissions and bed days on a general ward and the intensive care unit; number of liver-related hospitalizations and bed days; number of A&E department and outpatient clinic visits; number and type of infections) were retrospectively collected from electronic patient hospital records. Patients were followed for at least 6 months after rifaximin-α initiation (last data collection on 1 May 2018), or until death, liver transplantation, or permanent discontinuation of rifaximin-α occurred.
Definitions
The model for end-stage liver disease (MELD) and the model for end-stage liver disease including sodium (MELDNa) scores were calculated with formulas used by the Organ Procurement and Transplantation Network and Eurotransplant.11,12 Ascites was classified as diuretic responsive or refractory, and HE was graded according to the West Haven criteria.13,14 The Child–Pugh score and classification were calculated with the HE West Haven grade, severity of ascites, bilirubin level (μmol/l), international normalized ratio (INR) and albumin level (g/l).15 A liver-related hospital admission was defined as a hospitalization with the primary reason of admission being related to the chronic liver disease: HE, variceal bleeding, new-onset or worsening of ascites, infection, hepatorenal syndrome, hepatocellular carcinoma, or general deterioration. Infection diagnosis and determination of infection type were determined following definitions formulated by the Centers for Disease Control.16–19 All liver-related hospital admission comprises of both HE-related and liver-related non-HE hospital admissions.
Statistical analysis
Continuous variables were reported as mean with standard deviation (SD), after visual confirmation of approximate normality. A median and interquartile range (IQR), the range between the 25th and 75th percentile, was computed for continuous variables with a non-normal distribution. Continuous variables were analysed using a paired Student’s t test. Categorical variables were reported as a count with proportion and compared using the Chi-square test, or the McNemar’s test when comparing paired outcomes. A two-sided p value <0.05 was considered significant.
The actuarial probabilities of rifaximin-α use after therapy initiation were estimated using Kaplan–Meier analysis. Death, liver transplantation, and rifaximin-α discontinuation were counted as event in these analyses. All data analyses were performed using IBM SPSS statistics for Windows, version 24.0.
Results
Patient characteristics
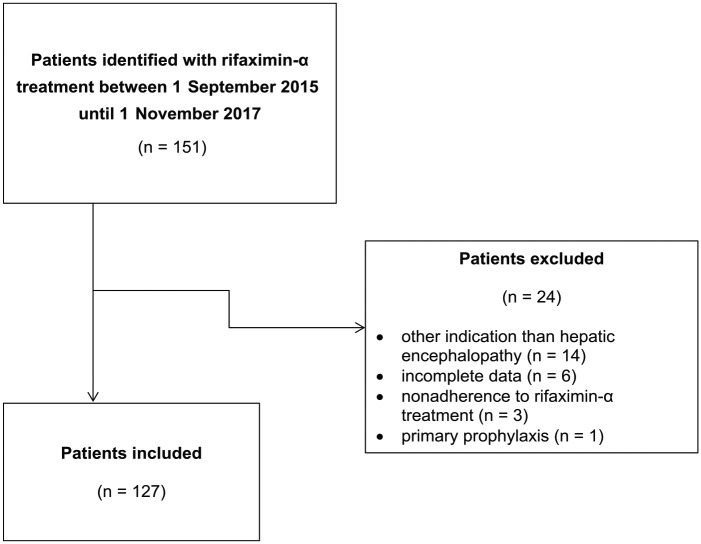
Between 1 September 2015 and 1 November 2017, 151 patients were identified with rifaximin-α treatment in the Erasmus MC. A total of 24 patients were excluded: 14 patients were prescribed rifaximin-α for other indications than HE; data regarding clinical endpoints was incomplete in 6, nonadherence to rifaximin-α was reported in 3 and 1 received rifaximin-α as primary prophylaxis. The remaining 127 patients using rifaximin-α as secondary prophylaxis for overt HE were included in the study analysis (Figure 1). The study cohort included 91 males and 36 females with a median age of 60.8 years (IQR 56.2–66.1). The median MELD score among patients was 15.0 (IQR 12.1–20.4). At time of rifaximin-α initiation, 49.6% of patients were classified as having HE West Haven grade 1, 31.5% with West Haven grade 2, 13.4% with West Haven grade 3, and 5.5% with West Haven grade 4. Lactulose was used by 124 (97.6%) patients and norfloxacin by 33 (26.0%) patients (Table 1).
Figure 1.
Flow chart of study inclusion.
Table 1.
Patient baseline clinical characteristics at the time of rifaximin-α initiation.
| Patients (n = 127) |
|
|---|---|
| Male sex, n (%) | 91 (71.7%) |
| Age in years, median (IQR) | 60.8 (IQR 56.2–66.1) |
| Etiology of liver disease, n (%) | |
| Alcoholic liver disease | 43 (33.9%) |
| Viral hepatitis | 25 (19.7%) |
| NASH | 17 (13.4%) |
| Cryptogenic | 15 (11.8%) |
| PSC/PBC/autoimmune hepatitis | 15 (11.8%) |
| Other | 5 (3.9%) |
| Unknown | 2 (1.6%) |
| HCC, n (%) | 27 (21.3%) |
| Liver disease severity scores | |
| MELD score, median (IQR) | 15.0 (IQR 12.1–20.4) |
| MELDNa score, median (IQR) | 16.8 (IQR 12.4–24.2) |
| Child–Pugh number, median (IQR) | 8.0 (IQR 7.0–10.0) |
| Child–Pugh class, n (%) | |
| A | 20 (15.7%) |
| B | 45 (35.4%) |
| C | 37 (29.1%) |
| HE severity classification, n (%) | |
| West Haven grade 1 | 63 (49.6%) |
| West Haven grade 2 | 40 (31.5%) |
| West Haven grade 3 | 17 (13.4%) |
| West Haven grade 4 | 7 (5.5%) |
| Ascites, n (%) | |
| None | 21 (16.5%) |
| Diuretic responsive | 36 (28.3%) |
| Refractory | 70 (55.1%) |
| Blood serum parameters | |
| Creatinine (mmol/l), median (IQR) | 86.5 (IQR 70.7–126.0) |
| Ammonia (μmol/l), median (IQR)† | 84.0 (IQR 64.0–121.7) |
| Sodium (mmol/l), median (IQR) | 138.5 (IQR 134.0–142.0) |
| Albumin (g/l), median (IQR)‡ | 32.0 (SD 28.0–36.0) |
| CRP (mg/l), median (IQR)§ | 16.0 (IQR 8.0–32.5) |
| ASAT (U/l), median (IQR) | 58.0 (IQR 43.5–87.5) |
| ALAT (U/l), median (IQR) | 40.0 (IQR 26.5–62.0) |
| Gamma-GT (U/l), median (IQR)¶ | 88.0 (IQR 52.5–163.5) |
| Alkaline phosphatase (U/l), median (IQR) | 144.0 (IQR 108.0–210.5) |
| Total bilirubin (μmol/l), median (IQR) | 35.0 (IQR 19.0–69.5) |
| Haemoglobin (mmol/l), median (IQR) | 6.8 (IQR 5.9–8.0) |
| Platelet count (×109/l), median (IQR) | 100.0 (IQR 65.5–146.0) |
| Leukocyte count (×109/l), median (IQR) | 6.0 (IQR 4.2–8.3) |
| INR, median (IQR) | 1.5 (IQR 1.3–1.7) |
| Lactulose use, n (%) | 124 (97.6%) |
| Norfloxacin use, n (%) | |
| None | 94 (74.0%) |
| 400 mg, once daily | 31 (24.4%) |
| 400 mg, twice daily | 2 (1.6%) |
Data were missing for 65 patients; ‡ Data were missing for seven patients; § Data were missing for 39 patients; ¶ Data were missing for 10 patients.
ALAT, alanine transaminase; ASAT, aspartate transaminase; CRP, C-reactive protein; Gamma-GT, gamma-glutamyl transferase; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; INR, international normalized ratio; IQR, interquartile range; MELD, model for end-stage liver disease; MELDNa, model for end-stage liver disease sodium; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Clinical parameters and resource use in the 6 months prior to and after rifaximin-α initiation
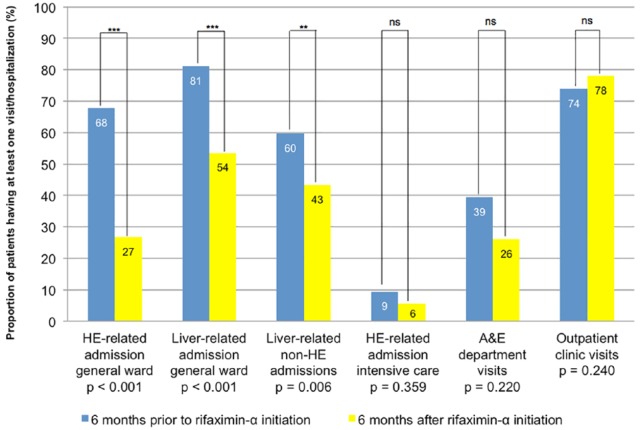
Figure 2 shows the proportion of patients having a hospital admission or visit in the 6 months prior to and after rifaximin-α initiation. The proportion of patients with HE-related hospital admissions to a general ward decreased from 67.7% patients prior to rifaximin-α initiation to 26.8% patients after rifaximin-α initiation (p < 0.001). Similarly, the proportion of patients with liver-related hospital admissions to a general ward decreased (81.1% to 53.5%; p < 0.001), as well as all liver-related non-HE hospital admissions to a general ward (59.8% to 43.3%; p = 0.006). There were no significant changes in HE-related intensive care unit admissions (9.4% to 5.5%; p = 0.359), A&E department visits (39.4% to 26.0%; p = 0.220), or outpatient clinic visits (74.0% to 78.0%; p = 0.240) between the 6 months prior to and after rifaximin-α initiation.
Figure 2.
Differences in proportion of patients with at least one hospital visit or hospitalization during 6-month episodes before and after initiation of rifaximin-α treatment.
A&E, accident and emergency; HE, hepatic encephalopathy.
The total mean number of HE-related hospital admission to the general ward decreased from 0.86 admissions/patient (SD 0.81) to 0.41 (SD 0.80; p < 0.001). Also, the mean length of stay shortened from 8.85 bed days/admission (SD 11.20) to 3.79 (SD 9.37; p < 0.001). The total mean number bed days during liver-related admissions decreased from 17.18 bed days/patient (SD 18.68) to 10.16 (SD 14.81; p = 0.021) and the total mean number of bed days during nonliver-related hospital admissions did not differ with 0.55 bed days/patient (SD 2.27) to 0.40 (SD 1.44; p = 0.585).
No significant differences were found in the mean number of HE-related intensive care unit admissions (0.09 to 0.06 admissions/patient; p = 0.253), or the mean length of stay on the intensive care unit (0.43 to 0.57 bed days/admission; p = 0.661; Table 2).
Table 2.
Hospital visits, admissions and length of stay during 6-month episodes before and after rifaximin-α initiation.
| 6 months prior to rifaximin-α initiation | 6 months after rifaximin-α initiation | p value | |
|---|---|---|---|
| HE-related admissions on the general ward per patient in 6 months, mean (SD) | 0.86 (0.81) | 0.41 (0.80) | <0.001 |
| HE-related hospital bed days on the general ward per admission in 6 months, mean (SD) | 8.85 (11.20) | 3.79 (9.37) | <0.001 |
| HE-related admissions on the intensive care unit per patient in 6 months, mean (SD) | 0.09 (0.29) | 0.06 (0.23) | 0.253 |
| HE-related hospital bed days on the intensive care unit per admission in 6 months, mean (SD) | 0.43 (1.64) | 0.57 (3.17) | 0.661 |
| Liver-related hospital bed days in 6 months, mean (SD) | 17.18 (18.68) | 10.15 (14.81) | 0.021 |
| Nonliver-related hospital bed days in 6 months, mean (SD) | 0.55 (2.27) | 0.40 (1.44) | 0.585 |
| A&E department visits per patient in 6 months, mean (SD) | 0.66 (1.06) | 0.51 (1.11) | 0.220 |
| Outpatient clinic visits per patient in 6 months, mean (SD) | 2.94 (2.64) | 3.30 (3.21) | 0.240 |
A&E, accident and emergency; HE, hepatic encephalopathy; SD, standard deviation.
There were no significant changes in the proportion of patients having a bacterial infection in the 6 months before or after the initiation of rifaximin-α for patients without systemic antibiotic use (25.5% to 22.3%; p = 0.690) or patients using norfloxacin prophylaxis (39.4% to 30.3%; p = 0.629; Table 3).
Table 3.
Bacterial infections during 6-month episodes before and after rifaximin-α initiation.
| Patients in analysis | Bacterial infections in 6 months prior to rifaximin-α initiation | Bacterial infections in 6 months after rifaximin-α-initiation | p value | |
|---|---|---|---|---|
| All study patients | 127 | |||
| Number of infections per patient in 6 months, mean (SD) | 0.41 (0.75) | 0.35 (0.76) | 0.523 | |
| Patients not using norfloxacin | 94 | |||
| Number of infections per patient in 6 months, mean (SD) | 0.41 (0.75) | 0.35 (0.76) | 0.751 | |
| Number of infections, n (%) | 24 (25.5%) | 21 (22.3%) | 0.690 | |
| Bacteremia, n (%) | 9 (9.6%) | 8 (8.5%) | ||
| SBP, n (%) | 6 (6.4%) | 6 (6.4%) | ||
| Respiratory, n (%) | 3 (3.2%) | 4 (4.3%) | ||
| Urogenital, n (%) | 9 (9.6%) | 4 (4.3%) | ||
| Patients using norfloxacin | 33 | |||
| Number of infections per patient in 6 months, mean (SD) | 0.39 (0.79) | 0.30 (0.70) | 0.320 | |
| Number of infections, n (%) | 13 (39.4%) | 10 (30.3%) | 0.629 | |
| Bacteremia, n (%) | 1 (3.0%) | 2 (6.1%) | ||
| SBP, n (%) | 12 (36.4%) | 7 (21.2%) | ||
| Respiratory, n (%) | - | 1 (3.0%) | ||
| Urogenital, n (%) | 1 (3.0%) | - |
SBP, spontaneous bacterial peritonitis; SD, standard deviation.
Rifaximin-α treatment duration and safety profile
The median treatment duration of rifaximin-α was 232 days (IQR 65.0–579.0). Figure 3 shows the estimated rifaximin-α users’ rate until discontinuation. The rifaximin-α users’ rate after initiation was 74% at 3 months, 63% at 6 months, 55% at 1 year, and 44% at 18 months. The reasons for stopping rifaximin-α treatment in the first 6 months were: death in 24 (18.9%) patients, liver transplantation in 16 (12.6%) patients, and temporarily or permanently discontinuation in 8 (6.3%) patients for other reasons.
Figure 3.
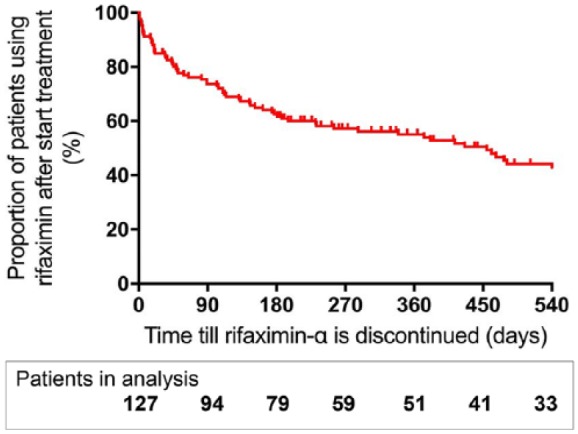
Kaplan–Meier curve showing the proportion (red line) of patients using rifaximin-α after initiation of treatment.
In the long-term follow up (until end of study observation, death, liver transplantation, or rifaximin-α discontinuation), rifaximin-α was temporarily discontinued in seven (5.5%) patients: due to long-term HE resolution in five patients, adverse events in one patient and without any documented reason in one patient, but reinitiated after recurrence of overt HE. Rifaximin-α treatment was permanently discontinued in eight (6.3%) patients: in three patients, prescription was discontinued without a documented reason, two patients had adverse events, in two patients, treatment was withdrawn in the terminal phase of the underlying disease and in one case due to nonadherence. In total, three patients reported an adverse event: nausea assumed to be related to rifaximin-α, rash assumed to be related to rifaximin-α, and polyneuropathy assumed to be nonrelated to rifaximin-α. Rifaximin-α dosage was raised to 1650 mg per day in 11 (8.7%) patients due to recurrence of overt HE while on 1100 mg per day.
Discussion
The present study shows that treatment with rifaximin-α was associated with a reduction in the number of HE- and liver-related hospitalizations on the general ward and the median length of hospitalization. No evidence was found for a significant impact on intensive care unit hospitalizations, A&E department and outpatient clinic visits, or bacterial infections in the first 6 months after initiation compared with the prior 6 months. Treatment with rifaximin-α was well tolerated and rarely discontinued for other reasons than liver transplantation or death.
This study confirms earlier reports that rifaximin-α can reduce the number of HE- and liver-related hospitalizations and bed days.10,20 However, the finding that this treatment was associated with a significant reduction in intensive care unit hospitalizations or bed days, or A&E department visits could not be confirmed in the present study.10 Factors that could potentially explain these contrasting results may include differences in local treatment protocols, varying criteria for intensive care unit admissions and differences in study population characteristics, especially with respect to liver disease aetiology and severity.10
We found no evidence for an effect of rifaximin-α treatment on the incidence of bacterial infections, neither in patients not receiving antibiotic treatment nor in patients using continuous antibiotic treatment for the prophylaxis of spontaneous bacterial peritonitis (SBP). Previous studies have shown that rifaximin-α is an effective antibiotic prophylaxis for SBP.21 This infection is the most common precipitating factor for overt HE.22 Although there was a nonsignificant decrease in SBP in our population, the power of the data might not be sufficient to draw conclusions regarding bacterial infections.
The safety profile of rifaximin-α was considered to be excellent with only 2.4% patients experiencing an adverse event, of which none was considered to be serious. This is comparable to other observational cohort studies reporting adverse events in 4% of rifaximin-α users; however, these were mainly Clostridium difficile infections, an important clinical problem.10,23,24
This is the first study evaluating the efficacy of rifaximin-α with a pre–post study design that did not select solely patients that were alive and without a liver transplantation at 6 months. Approximately one-third of the patients dies or undergoes liver transplantation in the first 6 months after rifaximin-α initiation. Therefore, this study better reflects the efficacy of rifaximin-α in general practice. However, the pre–post observational study design has several limitations, as it is not possible to control all elements in the clinical course, such as the natural progression of the underlying liver disease or for instance a change in diuretic treatment. This is a general difficulty when evaluating the efficacy of treatment for overt HE, as the disease has often an episodic character and does not always present in the same severity. Therefore, hard endpoints as hospitalizations, bed days, and hospital visits were chosen.
Future studies in overt HE management are necessary to individualize treatment strategy. For example, it has not been determined which factors influence rifaximin-α treatment success, the effectiveness of high dose rifaximin-α treatment as previous shown for acute HE, and in which patient treatment can be safely withdrawn.25
In conclusion, this study found an association between a reduction in the number and length of HE and liver-related hospitalizations and the initiation of rifaximin-α treatment. The benefit of rifaximin-α on other types of hospital resources was less clear. Our data support the additional use of rifaximin-α in patients with recurrent overt HE already receiving standard (lactulose) treatment. No evidence was found for an adverse effect on the risk of bacterial infections and treatment was very well tolerated.
Acknowledgments
Rosalie C. Oey and Lennart E.M. Buck contributed equally to this work. The following author contributions are noted:
R.C. Oey: study concept and design, acquisition of data, statistical analysis, interpretation of data, drafting of the manuscript, and final approval of the article.
L.E.M. Buck: acquisition of data, statistical analysis, interpretation of data, drafting of the manuscript, and final approval of the article.
N.S. Erler: statistical analysis, drafting of the manuscript, and final approval of the article.
H. R. van Buuren: study concept and design, interpretation of data, drafting of the manuscript, and final approval of the article.
R. A. de Man: study concept and design, interpretation of data, drafting of the manuscript, and final approval of the article.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was sponsored by the Foundation for Liver and Gastrointestinal Research Rotterdam (SLO) to which an educational grant was provided by Norgine B.V., Amsterdam, the Netherlands.
Conflict of interest statement: The authors have no conflict of interest. Sponsors did not actively participate in content development but reviewed the manuscript for scientific accuracy.
ORCID iD: Rosalie C. Oey  https://orcid.org/0000-0003-1032-495X
https://orcid.org/0000-0003-1032-495X
Contributor Information
Rosalie C. Oey, Department of Gastroenterology and Hepatology, Erasmus MC University Hospital, Room Na-606, 3000 CA Rotterdam, The Netherlands.
Lennart E.M. Buck, Department of Gastroenterology and Hepatology, Erasmus MC University Hospital, Rotterdam, The Netherlands
Nicole S. Erler, Department of Biostatistics, Erasmus MC University Hospital, Rotterdam, The Netherlands
Henk R. van Buuren, Department of Gastroenterology and Hepatology, Erasmus MC University Hospital, Rotterdam, The Netherlands
Robert A. de Man, Department of Gastroenterology and Hepatology, Erasmus MC University Hospital, Rotterdam, The Netherlands
References
- 1. Sherlock S, Dooley JS, Lok ASF, et al. Sherlock’s diseases of the liver and biliary system. Hoboken, NJ, USA: Wiley-Blackwell, 2002. [Google Scholar]
- 2. Amodio P, Del Piccolo F, Petteno E, et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 2001; 35: 37–45. [DOI] [PubMed] [Google Scholar]
- 3. Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30: 890–895. [DOI] [PubMed] [Google Scholar]
- 4. Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010; 51: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 5. Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003; 48: 1622–1626. [DOI] [PubMed] [Google Scholar]
- 6. Bianchi G, Giovagnoli M, Sasdelli AS, et al. Hepatic encephalopathy and health-related quality of life. Clin Liver Dis 2012; 16: 159–170. [DOI] [PubMed] [Google Scholar]
- 7. College ter Beoordeling van Geneesmiddelen. SPC: XIFAXAN 550 mg. Volume access: 17 March 2019: CBG Geneesmiddeleninformatiebank, 2018.
- 8. DuPont HL. Biologic properties and clinical uses of rifaximin. Expert Opin Pharmacother 2011; 12: 293–302. [DOI] [PubMed] [Google Scholar]
- 9. Kimer N, Krag A, Moller S, et al. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther 2014; 40: 123–132. [DOI] [PubMed] [Google Scholar]
- 10. Hudson M, Radwan A, Di Maggio P, et al. The impact of rifaximin-α on the hospital resource use associated with the management of patients with hepatic encephalopathy: a retrospective observational study (IMPRESS). Frontline Gastroenterol 2017; 8: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Network OPaT. Allocation of livers and liver-intestines policy, https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (2018, accessed 12 September 2018).
- 12. Eurotransplant. ET liver allocation system (ELAS), http://www.eurotransplant.org/cms/mediaobject.php?file=chapter5_elas3.pdf (2012, accessed 12 September 2018).
- 13. Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis 1978; 23: 398–406. [DOI] [PubMed] [Google Scholar]
- 14. Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol 2015; 13: 2048–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 16. CDC/NHSN. Surveillance definitions for specific types of infections. Centers for Disease Control. Volume 2018, http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed 06 July 2018).
- 17. CDC/NHSN. Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection). Centers for Disease Control. Volume 2018, http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed 6 July 2016).
- 18. CDC/NHSN. Pneumonia (ventilator-associated [VAP] and nonventilator-associated pneumonia [PNEU]) event. Centers for Disease Control. Volume 2018, http://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed 6 July 2016).
- 19. CDC/NHSN. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. Centers for Disease Control. Volume 2018, http://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf (accessed 5 April 2018).
- 20. Orr JG, Currie CJ, Berni E, et al. The impact on hospital resource utilisation of treatment of hepatic encephalopathy with rifaximin-alpha. Liver Int 2016; 36: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 21. Goel A, Rahim U, Nguyen LH, et al. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther 2017; 46: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 22. American Association for the Study of Liver Diseases and European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014; 61: 642–659. [DOI] [PubMed] [Google Scholar]
- 23. Sharma BC, Sharma P, Lunia MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol 2013; 108: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 24. Mantry PS, Munsaf S. Rifaximin for the treatment of hepatic encephalopathy. Transplant Proc 2010; 42: 4543–4547. [DOI] [PubMed] [Google Scholar]
- 25. Crisafulli E, Demma S, Rigano G, et al. Treatment with rifaximin high dose plus lactulose vs rifaximin standard dose plus lactulose for acute hepatic encephalopathy in ED. ILC 2016. J Hepatol 2016; 64 (Suppl.): S258. [Google Scholar]