Abstract
Background:
The burden of adverse drug event (ADE)-related emergency department (ED) visits is increasing despite several preventive measures. The objective of this paper was to develop and validate a conceptual model for a better understanding of ADE-related ED visits and to guide the design and implementation of effective interventions.
Methods:
The development of the model involved a systematic review of the literature using PubMed and Embase databases. Studies reporting the risk factors associated with ADE-related ED visits were included. The methodological qualities of the included studies were assessed using the Mixed Methods Appraisal Tool (MMAT). The model was mapped and validated using face and content validity by an expert panel. Deficiencies and targeted interventions were identified, and steps for the design and implementation were recommended.
Results:
The literature search generated 1361 articles, of which 38 were included in the review; 41 risk factors associated with ADE-related ED visits were identified. All factors were mapped, and the model was validated through face and content validity. The model consisted of six concepts related to sociodemographic factors, clinical factors, ADE-related to ED visits, ADE while in the ED, outcomes, and consequences. Interventions could be targeted at the factors identified in each concept to prevent ADE-related ED burden.
Conclusion:
A conceptual model to guide the successful design and implementation of strategies to prevent ADE-related ED visits and the occurrence of ADE at ED was developed. Clinicians should take these factors into consideration to prevent untoward events, especially when treating high-risk patients.
Keywords: adverse drug events, drug-related problem, emergency department, pharmacoepidemiology
Background
The trend for the use of medications in the treatment and prevention of acute and chronic disease conditions is increasing among the general population globally.1 This may be partly related to the continuous introduction of new drugs, an ageing population, and overall population growth. In the United States alone, 81% of adults >18 years had used at least one medication during the previous week, and 50% take at least one prescription drug.2 However, according to the World Health Organization’s world medicines situation report, it was estimated that approximately 50% of all medicines were inappropriately prescribed, dispensed, or sold, and half of all patients receiving medications were unable to take their medicines properly.1 Thus, these circumstances may lead to many adverse drug events (ADEs) that may result in hospitalization and an increase in healthcare costs.
Recently, the increasing ADE-related healthcare burden has emerged as a public health concern. It is estimated to be responsible for over 100,000 deaths annually, and represents an estimated increase in healthcare costs of US $201.4 billion.3 ADEs are responsible for many hospital emergency department (ED) visits and admissions. ADEs account for 2–3% hospital admissions in Australia,4 and 30.6% contributed to ED visits in Malaysia.5 ADE-related hospitalization continues to increase despite interventions to minimize the occurrence of ADEs. A fundamental step toward prevention of the increasing ADE-related healthcare burden is continuous identification and investigation of the contributions of ADE-related hospitalizations, including the associated risk factors for ADE-related events, within the general population. This is a sequel to the published report ‘To err is human: Building a Safer Health System’ by the Institute of Medicine in 2000.6 Since then, many studies have been conducted in clinical care settings such as hospital wards and EDs in order to determine the contribution of ADEs in these settings.5,7
A previous study has shown that 3 out of 10 ED visits were related to ADE.5 It has been reported that patients presenting to the ED due to an ADE are more likely to have a longer hospital stay and additional healthcare costs compared to patients with non-ADE visits.8 Patients with ADE-related ED visits may be discharged directly after seeing the ED physician, admitted to the ED ward, or, in many cases, transferred to an intensive care unit (ICU) or hospital ward.5 In addition, ADEs can be moderate or severe and often lead to death or disability.9,10 Moreover, an ADE can also occur in the ED while the patient is receiving care.11 A study reported an incidence rate of 13% for ADE among patients admitted to ED.11 However, ADE-related ED visits are potentially preventable with appropriate interventional measures.12 Factors associated with ADE-related ED visits and ADE occurring in the ED setting can be identified and targeted with interventions that could prevent future occurrences. While these preventive interventions are of public health significance, their successful implementation depends largely on robust theoretical and evidence-based conceptual frameworks that will identify gaps in the targeted interventions.13 The United Kingdom (UK) Medical Research Council guidelines recommend that appropriate existing evidence, theories, modelling processes, and outcomes should be identified in order to facilitate the development of an intervention.13 To prevent ADE-related ED visits, public health interventions based on sound theoretical evidence are therefore needed to address this growing problem.
To our knowledge, there is no available conceptual model concerning ADE-related ED visits in the published literature. Therefore, the aim of the current study was to develop and validate a conceptual model of ADE-related ED visits that can be applied in the identification of ADE-related healthcare burdens in the ED, and to guide the design of preventative interventional measures.
Methods
The design of the model involved the identification of factors associated with ADE-related ED visits through a systematic review of the literature followed by mapping and validation of the identified factors in a conceptual model, and, finally, subjecting the model to a face validity test by an independent expert panel.
Operational definitions
Development of the model
Systematic review
Literature search: A systematic literature search regarding the factors associated with ADE-related ED visits was performed using PubMed and Embase databases for articles published from January 2000 to March 2018. The two databases were selected based on their relevance in biomedical research. A search strategy using pertinent search terms such as medical subject heading (MeSH) and free text as title abstract (tiab) was developed. The search terms include ‘risk factors (MeSH)’ OR risk factor (tiab)’ ‘factor (tiab)’ AND ‘adverse drug event (MeSH)’ OR ‘drug-related problem (tiab)’ AND ‘drug-related visits (tiab)’ AND ‘emergency department (tiab)’. Only original articles published in English were included in the review. Relevant studies were also identified manually from the reference lists of the included articles. Additional information was also retrieved from Google Scholar and ED experts were contacted for relevant unpublished work. Google Scholar was searched using the following term ‘factors associated with adverse drug-related emergency department visit’. Based on the previous recommendations, the first 200 search results from Google scholar were considered for study selection.14
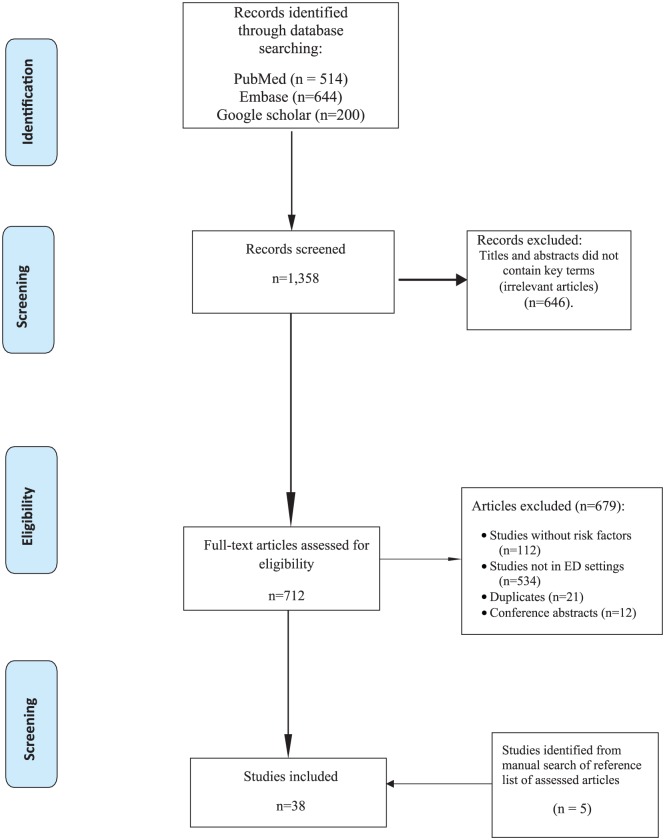
Study selection: The inclusion criteria included article with the following characteristics: reporting factors associated with drug (ADE)-related ED visits; prevalence of ADE studies that reported ADE-related risk factors; and evaluating risk factors associated with a specific category of ADE (e.g. adverse drug reactions, therapeutic failures). Studies were excluded if they examined only ADE-related ED visit incidences or prevalence; investigated ADE-related admissions to other hospital settings such as wards, ambulatory units, and intensive care units; are review articles, editorials, letter to the editor, or conference abstracts. Figure 1 shows the study selection process for the systematic review.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow: study selection.
Quality assessment of the included studies
The methodological qualities of the included studies were assessed using the mixed-methods appraisal tool (MMAT), version 2018.15 Studies were ranked from one to five stars based on meeting the five-item MMAT criteria. Similarly, included studies were also rated based on the National Health Medical Research Council (NHMRC) hierarchy of evidence.16 The quality assessment of the studies was undertaken by two reviewers and all disagreements were resolved through consensus.
Mapping of identified factors into the concepts
Factors associated with ADE-related ED visits identified from the literature were mapped into two concept groups: sociodemographic and clinical factors. The other subgroups in the clinical factor group represented ADEs encountered while in EDs, outcomes of ADE-related ED visits, and the consequences of these visits.
Validation
A table of the mapped variables was presented to an independent expert panel consisting of pharmacists and physicians with specialization and or research experience in pharmacoepidemiology research in emergency medicine. The panel reviewed the relevance of each of the identified factors and checked that each factor was appropriately mapped into each concept group, and included a review of the relationships among the concept groups/subgroups in the model. The model was revised based on feedback from the expert panel. Discrepancies were resolved through consensus by panel members. The final model was presented to the same expert panel for face validity. The panel was asked to give a judgement regarding the appropriateness, and whether the model made any sense, as well as to the relevance of the recommended interventions.
Results
The literature search from the electronic databases generated 1361 articles. Out these, 647 articles were excluded during the title and abstract screening, while 679 were excluded for reasons stated in Figure 1. Five articles were identified from a manual search of articles that were electronically retrieved. A total of 38 articles were included in the review for identifying factors associated with ADE-related ED visits. From the reviewed studies, 41 risk factors were reported to be associated with ADE-related ED visits. The factors were mapped as falling into one of the two concept groups: sociodemographic or clinical.
Quality assessment of the included studies
Of the 38 included studies, 8 met all five MMAT criteria of methodological quality; 16 studies were rated as four-star, 13 as three-star, and 1 study as a two-star rating of methodical quality. In terms of NHMRC hierarchy level of evidence, 10 of the studies were prospective cohorts with level II evidence, 14 were retrospective cohorts (III-2), 4 were case-control, and 10 were cross-sectional studies with level IV evidence (Table 1).
Table 1.
Summary of the included studies and quality assessment results.
| Authors | Settings | Study period | Sample size | Study design | Risk factors identified | NHMRC Level of evidencea | MMAT Scoring b |
|---|---|---|---|---|---|---|---|
| Ab Fatah et al, 201744 | Teaching hospital, Malaysia |
7 weeks | 144 | Case-control | Female sex, currently taking medication, comorbidity, a history of drug allergy and recent hospital admission | III-3 | 4 |
| Salvi et al., 201717 | Geriatric hospital, Italy |
6 months | 4042 | Observational cohort study | Polypharmacy | IV | 5 |
| Chen et al., 201518 | General Hospital, Taiwan |
12 months | 452 | Prospective cohort study | Older population ⩾65 years | II | 5 |
| De Pepe et al., 201319 | University hospital, Belgium |
3 weeks | 87 | Prospective cohort study | Number of medications and age | II | 3 |
| Perrone et al., 201420 | 16 General hospitals, Italy |
24 months | 8862 | Retrospective cohort study | Older age, Yellow and Red triage, number of medications, previous ED visit for the same ADE | III-2 | 3 |
| Chen et al., 201421 | Tertiary medical center, Taiwan |
12 months | 20,628 | Case-control | Number of medications and increased serum creatinine. | III-3 | 4 |
| Asseray et al., 201322 | 11 French academic hospitals, France |
8-week period | 3027 | Prospective observational study | Age, gender, use of nervous system drugs, polypharmacy | II | 4 |
| Roulet et al., 201323 | Tertiary care hospital, France |
2 months | 433 | Cross sectional | Involuntary intoxication, hospitalized patients, poly-pathological condition, endocrine pathology and daily prescription of CVS drugs | IV | 5 |
| Pedros et al., 201351 | Tertiary medical center, Spain |
120 days | 4098 | Cross sectional | Old age and number of medications | IV | 4 |
| Nickel et al., 201324 | University hospital, Basel, Switzerland |
24 months | 633 | Cross-sectional | Comorbidities and number of medications | IV | 4 |
| Castro et al., 2013 52 | University hospital, Barcelona, Spain |
3 months | 652 | Cross-sectional study | Number of medications taken | IV | 3 |
| Heaton et al., 201225 | NHAMCS, US |
3 years | 456,209 | Retrospective cohort study | Mental illness, type II diabetes, nondependent abuse of drug and essential hypertension | III-2 | 4 |
| Jayarama et al., 201226 | Tertiary hospital, Kola, India |
12 months | 133 | Prospective observational studies | Comorbidity, multiple prescribers, visiting many pharmacies and number of medications | II | 3 |
| Chen et al., 201275 | Academic hospital, Taiwan |
12 months | 452 | Prospective cohort study | Elderly age, severity of ADE, higher Charlson comorbidity index scores | II | 4 |
| Wu et al., 201227 | Ontario, Canada | 5 years | Retrospective cohort study | Female gender, old age, comorbidity, Number of medications, newly prescribed drugs, recent ED visit, multiple-pharmacies, recent -admission, and long-term care | III-2 | 4 | |
| Marcum et al., 201253 | 152 Veterans Affairs Medical Centers US |
NR | 778 | Retrospective cohort study | Polypharmacy | III-2 | 4 |
| Hohl et al., 20118 | 2 Tertiary hospitals. Vancouver, Canada |
6 months | 1591 | Prospective observational studies | Comorbidity, antibiotic use within 7 days, medication changes within 28 days, age 80 years, arrival by ambulance, triage acuity, recent hospital admission, renal failure, and use of three or more prescription medications | II | 5 |
| Harduar-Morano, et al., 201128 |
Florida AHCA | NR | 3024 | Retrospective cohort study | Old age, white patients, and female gender | III-2 | 3 |
| Vila-de-Muga et al, 201129 | Academic tertiary care children’s hospital | 1 week | 1906 | Retrospective cohort study | ED shift on weekends, holidays and between 0000 and 0800 hours | III-2 | 3 |
| Perron et al., 201130 | NESARC, US |
NR | 43,093 | Retrospective cohort study of survey database | Heroine, inhalant and marijuana dependence. Pyschopathological factors: personality and mood disorder, socially connected has a protective factor | III-2 | 3 |
| Budnitz et al., 201131 | NEISS-CADES US |
2 years | 177,504 | Retrospective cohort study | Drugs with narrow therapeutic index; digoxin, insulin and warfarin | III-2 | 4 |
| Braden et al., 200232 | HealthCore and Arkansas Medicaid, US |
NR | 48,650 | Retrospective cohort study | Use of short acting Drug Enforcement Agency Schedule II opioids | III-2 | 3 |
| Sikdar et al., 201133 | Two tertiary hospitals, Canada |
12 months | 1458 | Retrospective chart review | Comorbidities and number of medications. | III-2 | 5 |
| Ramos et al., 201034 | University hospital, Spain |
3 months | 888 | Cross sectional study | Number of drugs, female gender and health practice index | IV | 4 |
| Maria et al., 201035 | 3 hospitals, Italy |
3 years | 2644 | Cross-sectional | Comorbidity, multiple drug regimens | IV | 3 |
| Backmund et al., 200936 | Tertiary care hospital, Germany |
NR | 1049 | Retrospective cohort study | Not living with a significant other, drug user, history of suicide attempt, daily use of barbiturates and cannabis | III-2 | 4 |
| Olivier et al., 200937 | University Hospital Toulouse, France |
4 nonconsecutive weeks | 789 | Prospective cohort study | Number of medications taken, self-medication, use of antithrombotic and antibacterial drugs | II | 4 |
| Capuano et al., 200938 | 10 general hospitals in Regione Campania, Italy |
10 days/ED in two study period | 7861 | Prospective cohort and nested case control study | Female gender and age category (30–39 and 60–69), patients taking RAS, NSAIDS, antibiotics, β-adrenoceptors agonist and β-lactam antibiotics | II | 5 |
| Zed et al., 200812 | General hospital, Vancouver Canada |
12 weeks | 1017 | Prospective observational study | Comorbidities, number of medications and multiple prescribers | II | 5 |
| Sauer et al., 200739 | Administrative data, Florida, US |
24 months | 37,063 | Retrospective cohort study | Age, male sex, number of medical conditions, and number of medications | III-2 | 2 |
| Baena et al., 200640 | University hospital, Granada, Spain |
12 months | 2261 | Two-stage probabilistic sampling | Age, number of medications, and combined effect of the two | III-3 | 4 |
| Tipping et al., 200641 | Tertiary hospital, Cape Town, South Africa |
4 months | 517 | Cross-sectional | Number of medications, patients taking NSAIDS, ACE- inhibitor, and warfarin | IV | 3 |
| Budnitz et al., 200676 | 9 NEISS, US | 77 days | 598 | Retrospective cohort study | Use of warfarin and insulin | II-2 | 5 |
| Trifiro et al., 200545 | 22 hospitals, Italy |
10 days at intervals of 3 months | 629 | Prospective cohort study | Older age, male gender | II | 4 |
| Caterino et al., 200477 | EDs of uninstitutionalised general hospital, US |
NR | 16.1 million | Retrospective cohort study | Number of medications at ED | III-2 | 4 |
| Franceschi, et al., 200478 | University hospital, Italy |
10 days | 607 | Cross-sectional | Age and number of medications consumed | IV | 3 |
| Hafner et al., 200242 | Teaching hospital, US |
3 months | 13,004 | Case control | Old age, female gender, and polypharmacy | III-3 | 3 |
| Malhotra et al., 20119 | Referral hospital, India |
7 months | 578 | Cross-sectional | Diabetes, patient living alone, poor recall of medication regimen, seeing multiple physicians, female gender, polypharmacy |
IV | 3 |
ADE: adverse drug event; ED: emergency department; NHAMCS: National Hospital Ambulatory Medical Care Survey; NESARC: National Epidemiology Survey on Alcohol and Related Conditions; NEISS-CADES: National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance project; CVS: cardiovascular system; AHCA: Agency for Health Care Administration; RAS: renin-angiotensin system; NSAIDS: nonsteroidal anti-inflammatory drugs; ACE: angiotensin-converting-enzyme; NEISS: National Electronic Injury Surveillance System; US United States; NR: not reported.
National Health and Medical Research Council level of evidence.
Mixed Methods Appraisal Tool score.
Mapping of the factors
Six concepts were developed, and factors identified from the studies were mapped to one of two concept groups: sociodemographic or clinical concept groups (Table 2). The remaining factors fell under one of four other subgroups: ADE-related ED visits, ADEs occurring while in ED, outcomes of ADE-related ED visits, and consequences from these visits. From the face validity, five factors initially mapped under the sociodemographic concept group were later moved to clinical factors, and two boxes were added to indicate ‘general population’ and ‘ED’ based on the expert panel’s consensus.
Table 2.
Concepts, mapped factors, gaps identified, and targeted interventions.
| Concepts | Factors/ADEs | Gaps in drug-related knowledge | Targeted intervention |
|---|---|---|---|
| Sociodemographic characteristics | Old age, female, being white, low health practice index, social disconnection, nondependent drug abuse, Involuntary intoxication, Long-term care, Self-medication, Level of education. |
Inadequate awareness of ADEs in the public.55
Inappropriate of use medications among elderly. Inappropriate use of medications among pregnant women. Inadequate patient’s knowledge on appropriate medication use. Lack of ADE screening tool in the public56 high rate of drug abuse, self-medication, inadequate of patient information. |
Use Beer’s list at inappropriate medications for elderly63
Improved awareness on rational medicines use in the society Availability of screening tool for detecting ADE in the community.64 |
| Clinical characteristics | Drug allergy, comorbidity, chronic disease, consulting many
prescribers, recent hospital admission, current medication, use of CAM, visiting many pharmacies, Use of multiple medications, Yellow and Red triage ,Drugs with narrow therapeutic index; mental illness, personality and mood disorder, medication nonadherence. |
Inadequate pharmacogenetic studies on drug
effects. Inadequate prospective cohort studies on drugs use in chronic diseases and drug-related ED visits and ED readmission. Paucity of information on CAM use among ED patients (including CAM occurring at ED).57 |
More studies on pharmacogenetic to identify the genetic
variations in drug effects.66 More studies on drug including CAM-related ED visits particularly in developing countries.57 Implementation Beer’s list of appropriate when prescribing drugs to elderly.63 Improve patient education and counselling at healthcare settings. More intervention to improve patient’s medication adherence. More seminars and lectures to HCP on appropriate use of medication in the ED. |
| ADE leading to ED visits | ADEs (1) Adverse drug reaction (2) Medication nonadherence (3) Drug treatment failure (4) Medication error (5) Drug over dosage (6) Drug under dose (7) Untreated indication (8) Treatment without indication |
Inadequate studies on ADE-related ED visits and ADE
occurring at ED58
Lack of valid causality assessment of adverse events related to drug treatment failure, medication errors and drug abuse/misuse59 |
More studies to be conducted on ADE-related ED
visits More studies on contribution of CAM at ED ED-based brief interventions69 Provision of validated ADE screening tool at ED56 |
| ADE encountered at the ED | (1) Adverse drug reaction (2) Medication error (3) Drug treatment failure |
Inadequate studies on ADE occurring at ED58
Inadequate patient-HCP communication,60 inadequate counselling time, busy and overcrowded and multitasking nature of ED environment61 Lack of decision and screening tools to guide the HCP on ADE in the ED56 Lack of clinical pharmacy unit in some ED settings62 |
More studies should be conducted to identify ADE occurring
at ED58
Strategies to improved patient-HCP communication for adequate patient education and shared decision making71 Training and improved collaborative communication among HCP in ED setting72 Presence of a clinical pharmacy at ED for adequate ADE surveillance73 Strategies to prevent to reduce ADEs in the ED such as computerized provider-order entry systems, automated dispensing cabinets, bar-coding systems61 Implementation of Screening tool of Older People’s Prescriptions and Screening Tool to Alert to Right Treatment criteria to detect ADE-related ED visits67Nauta et al., 2017) |
ADE: adverse drug event; ED: emergency department; CAM: complementary and alternative medicine; HCP: health care professionals.
Analysis of the conceptual model for understanding ADE-related ED visits and ADEs encountered at the ED
An ADE-related ED visit can be best explained using pharmacoepidemiological concepts. Pharmacoepidemiology is the study of the clinical use of drugs and ADEs in large numbers of people, and thus, provides an estimate of the probability of beneficial drug effects in a general population in addition to ADEs.43 People use drugs for either therapeutic purposes such as disease management and prevention, or for illicit reasons, including ecstasy, recreational, to fit in with their peers, or for performance-enhancement such as in athletics. ADEs occur as a result of the use of drugs for all these purposes, leading to hospitalization, including unplanned visits to an ED. Empirical evidence from the reviewed studies reveals several factors as predictors of ED visits following drug use. Interventions can be targeted to these factors to prevent the increased healthcare burden of ADE-related ED visits.
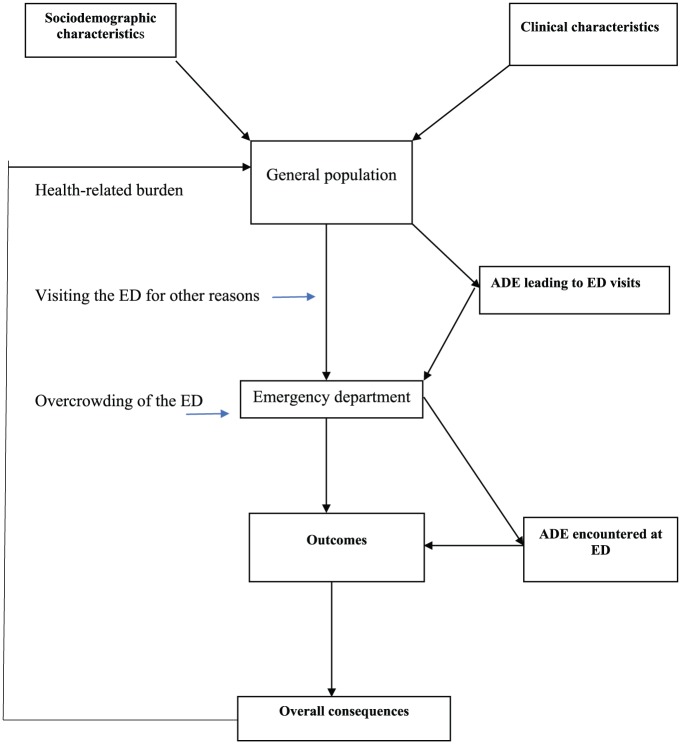
The model starts with the general population. People in the community represent different socio-demographic characteristics. Some of these characteristics, such as old age,18,19,40 female gender,19,44 ethnic disparity (white),28 low health practice index,34 social disconnection (living alone),30 long-term care,27 and history of suicidal attempt, were all found to be associated with ADE-related ED visits. In addition, some individuals in the community will be involved in other use of drugs associated with ADE-related visits to the ED, such as nondependent drug abuse,45 involuntary intoxication (e.g., unintentional poisoning),23 self-medication,37 use of short-acting Drug Enforcement Agency Schedule II opioids,32 and use of cannabis and barbiturates36 – all of which were found to be associated with ADE-related ED visits (see box on left side of general population in Figure 2).
Figure 2.
Conceptual framework for understanding drug-related emergency department (ED) visits.
People in the community also develop illnesses and require medications; thus, being exposed to many risk factors (termed clinical factors; see box on right side of general population in Figure 2). These factors increase the likelihood of visiting an ED due to an ADE from medication use, and include a history of drug allergies,44 chronic illness,25 type II diabetes, essential hypertension and other comorbid conditions,24,39, 45–47 psychopathology (personality and mood disorder),30,48 mental illness,25 recent hospital admission,27 consulting multiple prescribers,12 and pharmacies.26 Other clinical factors include failure to correctly use, or not use, prescription medicines after being prescribed by a physician,49 use of complementary and alternative medicine (CAM),50 current medication use,44 use of multiple medications,17,21,33,35,51–53 yellow and red triage,20 and use of drugs with narrow therapeutic indices.31 Drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) used in the management of chronic diseases,41 antihypertensive medications,38 antidiabetics,45 antibiotics,38 benzodiazepines, antidepressants, anticonvulsants,54 and use of nervous system drugs22 were also identified as factors contributing to ED visits due to an ADE. People with an increased serum creatinine level were also found to be at a higher risk of ED visits due to an ADE.21
Socio-demographic and clinical factors, such as drug abuse/misuse, medication errors, medication nonadherence, and medication under/overdose, are also known as exposure variables, and these predispose an individual to many types of ADEs. The manifestation of these events results in acute clinical conditions leading to an unplanned ED visit (Figure 2). Different outcomes (box in Figure 2) may arise from these visits: the patient may be discharged immediately after seeing an ED physician; admission to the ED observation ward; transfer to the hospital ward or ICU; permanent disability; death.
In some instances, an individual may visit an ED with other nondrug related conditions. Due to the busy nature of the ED environment, many ADEs occur in the ED, leading to complications of pre-existing disease conditions (Figure 2). Commonly encountered ADEs while in the ED environment includes adverse drug reactions, medication errors, drug overdoses, and therapeutic failures.42 Similarly, working hours and day in the ED have been identified by ED healthcare personnel to be independent predictors of an ADE in the ED setting. Muga and colleagues reported working at an ED between 0000 to 0800 hours, and on weekends and holidays as predictors of medication error occurring in ED settings.29
Some consequences of ADE-related ED visits and ADEs encountered while in the ED are an increase in drug-related morbidity, mortality, and healthcare costs, prolonged hospital stay, decreased productivity and lost work hours (overall consequences box in Figure 2).8 These consequences have negative effects on the general population (Figure 2) by increasing the socio-economic burden and ED overcrowding. This will directly or indirectly influence the occurrence of exposure variables, and increase the likelihood of ED visits due to drug use and its continuous cycle. Gaps in knowledge for targeted interventions can thus be identified and applied to any of these concepts in order to prevent or minimize future occurrences of ADE-related ED visits.
Identified gaps for intervention
Table 2 shows the gaps identified in the different concepts, including sociodemographic, clinical factors, ADE, and ADEs encountered while in the ED.
Sociodemographic factors
Previous studies have identified sociodemographic factors associated with people experiencing ADEs in the general population. These included inadequate awareness of ADE by the public,56 high use of inappropriate medications among elderly people,46 and absence of ADE screening tools in the community.56 There are also a high rate of drug abuse, self-medication, and inadequate patient education concerning drug use.
Clinical factors
Identified gaps under clinical factors include inadequate pharmacogenetic and prospective cohort studies on drug use in chronic diseases. There is a minimal number of published studies concerning ADE-related ED visits and readmissions. Information on CAM use among ED patients (including CAM occurring while in the ED) has not been adequately studied or reported.57
ADE leading to ED visits
Published information on ADE-associated ED visits is limited. There are no adequate studies concerning ADE-related ED visits and ADE occurring while in ED.58 Furthermore, there is a lack of validated causality ADE assessment tools such as objective tools or algorithms for the causality assessment of drug treatment failure, medication errors, and drug abuse/misuse.59
ADE occurring while in the ED
There are no adequate studies concerning ADE occurring while in ED.58 Inadequate patient–healthcare provider (HCP) communication was identified as one cause of this problem.60 The busy, overcrowded nature of the ED environment, coupled with inadequate counselling time with a patient, are some of the identified gaps in the ED-associated ADEs.61 Furthermore, there is a lack of decision support tools such as computerized physician order entry systems (CPOE), barcodes, and/or screening tools to guide the HCP at ED.56 ADEs are prevalent due to lack of clinical pharmacy units to oversee the pharmacotherapy in some ED settings.62
Targeted interventions (population and patient-centered)
A fundamental step in preventing drug-related ED visits is to continue identifying the prevalence/incidence of healthcare burden in the ED. More studies are needed to determine the contribution of drugs in ADE-related ED visits, including those ADEs that occur while in the ED. Unfortunately, information regarding this occurrence is limited in the published literature. More published studies are needed to provide comprehensive knowledge of the healthcare burden in order to design and recommend appropriate interventions.
The developed model has identified some areas for targeted interventions. Preventive measures can be targeted from the identified concepts:
Sociodemographic factors: Improving the level of awareness among the population with respect to the rational use of medicines will assist in reducing the occurrence of ADE-related ED visits. The use of Beer’s list of inappropriate medications for older patients in healthcare settings will reduce ADEs among elderly population.63 Availability of ADE screening tools in community pharmacy and primary healthcare settings will detect people at high-risk of experiencing ADEs that may lead to ED visits.64 Thus, one of the most fundamental issues for addressing sociodemographic disparities that contribute to ED visits is to improve primary healthcare systems to allow more access to general practitioners. Therefore, providing appropriate information to patients regarding their medications and improved awareness of drug and substances abuse-associated dangers, especially illicit drugs, indiscriminate smoking, and alcohol consumption, will go a long way towards curbing drug-related ED visits.65
Clinical factors: Risk factors related to the clinical use of medications and disease conditions can be targeted for interventions and other strategies to prevent ADE-related ED visits. To effectively intervene with respect to patients’ clinical characteristics, further studies are required on pharmacogenetic factors as this will help to identify patients’ genetic variations that contribute to drug effects and the possibility of personalized medicines use.66 Furthermore, more studies are required with respect to the use of CAMs among patients in the ED, including ED visits related to CAM toxicities and CAM-related ADEs occurring while a patient is in ED.57 Such studies should be stressed in developing countries. More interventions such as implementation of Beer’s list of inappropriate medications for the elderly,63 Screening Tool of Older People’s Prescriptions, the Screening Tool to Alert to Right Treatment (STOPP/START) criteria to detect ADE-related ED visits,67 and provision for CPOEs will drastically reduce the occurrences of ADE-related ED visits. Telemedicine enables HCPs to prioritize their workloads and support people with long-term conditions in order to play a key role in managing healthcare.68 Telemedicine is another healthcare technology relevant to elderly and physically challenged patients. It promotes safety and security, using at-home sensor monitoring devices that provide alerts for prompt action.68
ADEs leading to ED visits: To reduce the rate of ADEs, more studies are needed to evaluated the burden of ED visits related to ADE, including those associated with CAM use.69 Provisions of interventions such as for validated ADE screening tools in the ED could assist in detecting more ADE-related cases.56 Adequate pharmacovigilance surveillance ADE-related ED visits. The advent of personalized therapy, tailored to an individual patient based on the patient’s diagnosis, medical history, and genetic information, for the purpose of improving therapeutic outcomes minimizing and ADEs could go a long way in preventing ED visits associated with drug use.70
ADEs occurring in the ED: To provide a clear view of the event, more studies need to be conducted on ADEs occurring in the ED setting. ADEs occurring in the ED can be reduced by implementing strategies to improved patient–HCP communication for adequate patient education and shared decision-making.71 Training and improvement of effective communication among HCPs in ED settings will improve patient safety.72 The presence of a dedicated pharmacy unit in the ED that renders full clinical pharmacy services will help in ADE surveillance and provide more patient counselling, and other pharmaceutical care activities.73 Other strategies such as informatics-based hospital interventions in the ED, including CPOE systems, automated dispensing cabinets, and bar-coding systems, have the potential to detect and prevent ADEs in ED settings.61
Role of clinical pharmacists in preventing ADE in the ED: Clinical pharmacists remain the professionals best entrusted with all aspects of pharmacotherapy. The success of therapeutic interventions depends largely on the clinical pharmacist’s commitment to preventing ADEs, particularly in ED.70 Most importantly, the pharmacist must ensure appropriate medication storage conditions in the ED pharmacy unit. Another critical role for a pharmacist is that of screening and scrutinizing prescriptions prior to dispensing them in order to identify any potential drug–drug interaction; drug-disease interaction; inappropriate dosing; or inappropriate dosing frequencies, errors, and ADE reporting. Other roles include identification of patients for enrollment of investigational drug study participants while these potential participants are in the ED, participation on interdisciplinary research committees that review ED-related research protocols, patient counselling and education, toxicology investigation recommendations, and targeted disease state counselling such as anticoagulation, anaphylaxis reactions, medication therapy updates, and education on optimal medical therapy for ED team members.74
Implications of the conceptual model in public health and clinical practice
To our knowledge, this conceptual model is the first to provide an in-depth understanding of ADE-related ED visit by identifying gaps in knowledge and suggesting interventions for preventative measures. The model could guide interventionists, and public health and clinical practice policymakers in identifying areas that need intervention, in addition to planning and implementation of intervention strategies.
Limitations
The current study may be limited to the inclusion only of studies published in the English language; thus, relevant information from studies published in other languages may have been excluded.
Conclusion
A validated conceptual model for better understanding of ADE-related ED visits was developed. We identified gaps in knowledge and clinical practice as well as targeted interventions that can be used to guide implementation of strategies for preventing ADE-related ED visits, including ADEs that occur while in an ED setting. This study underscores the need for the proactive role of clinical pharmacists to ensure optimal use of medicines and minimization of ADE-related ED visits. In elderly patients, consideration of the Beers Criteria for Potentially Inappropriate Medications and Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions/Screening Tool to Alert to Right Treatment Criteria would play a critical role in the prevention of ADE-related ED visits.
Highlights
(1) Drug use in the general population may lead to an ED visit with chief presenting complaints related to an ADE.
(2) An ADE may occur while in the ED from non-ADE related visits, leading to increased morbidity, mortality, and healthcare costs.
(3) The absence of an evidence-based model may lead to an intervention being less successful than anticipated.
(4) A conceptual model can guide the successful design of interventions to prevent ADE-related ED visits.
(5) A successful intervention based on a conceptual model will reduce morbidity, mortality, and healthcare costs.
Acknowledgments
The authors would like to thank and appreciate all members of Young Pharmacists Scholars (YPS) mentoring forum for their support, guidance, and data collection. YPS is a mentoring platform supporting research among young pharmacists. The current study is part of the YPS-mentoring program to mentor the young pharmacists on conducting research and publication.
Footnotes
Authors contribution: We declared that this work was conducted by the authors named in this article and all liabilities relating to the content of this article will be borne by them. All authors meet the criteria for authorship outlined by the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Abubakar Ibrahim Jatau  https://orcid.org/0000-0001-9336-6877
https://orcid.org/0000-0001-9336-6877
Garba Mohammed Khalid  https://orcid.org/0000-0003-4045-7702
https://orcid.org/0000-0003-4045-7702
Contributor Information
Abubakar Ibrahim Jatau, Division of Pharmacy, School of Medicine, University of Tasmania, Australia.
Zayyanu Shitu, Faculty of Health Science, Univeristi Sultan Zainal Abidin, Terengganu, Malaysia.
Garba Mohammed Khalid, Department of Pharmaceutical Sciences, Università degli Studi di Milano, Milan, Italy.
Ismaeel Yunusa, School of Pharmacy, Massachusetts College of Pharmacy and Health Sciences, Boston, USA.
Ahmed Awaisu, College of Pharmacy, Qatar University, Doha, Qatar.
References
- 1. World Health Organization. The world medicines situation, http://apps.who.int/medicinedocs/pdf/s6160e/s6160e.pdf (2004, accessed 12 March 2017).
- 2. Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone Survey. JAMA 2002; 287: 337–344. [DOI] [PubMed] [Google Scholar]
- 3. Ombengi D, Ndemo F, Noreddin AM, et al. The disease burden and the extent of drug therapy problems in an underserved minority population receiving medication therapy management at an ambulatory care free clinic. J Pharm Care Health Syst 2016; 157. [Google Scholar]
- 4. Phillips AL, Nigro O, Macolino KA, et al. Hospital admissions caused by adverse drug events: an Australian prospective study. Aust Health Rev 2014; 38: 51–57. [DOI] [PubMed] [Google Scholar]
- 5. Jatau AI, Aung MMT, Kamauzaman THT, et al. Prevalence of drug-related emergency department visits at a teaching hospital in Malaysia. Drugs Real World Outcomes 2015; 2: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS. (eds) To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press, 2000. [PubMed] [Google Scholar]
- 7. Al Hamid A, Ghaleb M, Aljadhey H, et al. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol 2014; 78: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hohl CM, Nosyk B, Kuramoto L, et al. Outcomes of emergency department patients presenting with adverse drug events. Ann Emerg Med 2011; 58: 270-279. [DOI] [PubMed] [Google Scholar]
- 9. Malhotra S, Karan RS, Pandhi P, et al. Drug related medical emergencies in the elderly: role of adverse drug reactions and non-compliance. Postgrad Med J 2001; 77: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zed PJ, Black KJ, Fitzpatrick EA, et al. Medication-related emergency department visits in pediatrics: a prospective observational study. Pediatrics 2015; 135: 435–443. [DOI] [PubMed] [Google Scholar]
- 11. Lázaro AMDA, Sánchez DS, Romero MO, et al. Evaluation of adverse drug reactions in emergency department practice. Emergencias 2013; 25: 361–367. [Google Scholar]
- 12. Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. Can Med Assoc J 2008; 178: 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bramer WM, Rethlefsen ML, Kleijnen J, et al. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev 2017; 6: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quan Nha H, Pierre P, Sergi F, et al. Mixed methods appraisal tool (MMAT) Version 2018-User guide. Department of Family Medicine; http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf (2018, accessed 6 April 2019). [Google Scholar]
- 16. National Health and Medical Research Council, https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf (2009, accessed).
- 17. Salvi F, Rossi L, Lattanzio F, et al. Is polypharmacy an independent risk factor for adverse outcomes after an emergency department visit? Intern Emerg Med 2017; 12: 213–220. [DOI] [PubMed] [Google Scholar]
- 18. Chen YC, Huang HH, Fan JS, et al. Comparing characteristics of adverse drug events between older and younger adults presenting to a Taiwan emergency department. Medicine (Baltimore) 2015; 94: e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Paepe MP, Outtier L, Van Maele G, et al. Drug interactions and adverse drug reactions in the older patients admitted to the emergency department. Acta Clinica Belgica 2013; 68: 15–21. [DOI] [PubMed] [Google Scholar]
- 20. Perrone V, Conti V, Venegoni M, et al. Seriousness, preventability, and burden impact of reported adverse drug reactions in Lombardy emergency departments: a retrospective 2-year characterization. Clinicoecon Outcomes Res 2014; 6: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YC, Fan JS, Chen MH, et al. Risk factors associated with adverse drug events among older adults in emergency department. Eur J Intern Med 2014; 25: 49–55. [DOI] [PubMed] [Google Scholar]
- 22. Asseray N, Ballereau F, Trombert-Paviot B, et al. Frequency and severity of adverse drug reactions due to self-medication: a cross-sectional multicentre survey in emergency departments. Drug Safety 2013; 36: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roulet L, Ballereau F, Hardouin JB, et al. Assessment of adverse drug event recognition by emergency physicians in a French teaching hospital. Emerg Med J 2013; 30: 63–67. [DOI] [PubMed] [Google Scholar]
- 24. Nickel CH, Ruedinger JM, Messmer AS, et al. Drug - related emergency department visits by elderly patients presenting with non-specific complaints. Scand J Trauma Resusc Emerg Med 2013; 21: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heaton PC, Tundia NL, Luder HR. US emergency departments visits resulting from poor medication adherence: 2005–07. J Am Pharm Assoc 2013; 53: 513–519. [DOI] [PubMed] [Google Scholar]
- 26. Jayarama N, Shiju K, Prabahakar K. Adverse drug reactions in adults leading to emergency department visits. Int J Pharm Pharm Sci 2012; 4: 642–646. [Google Scholar]
- 27. Wu C, Bell CM, Wodchis WP. Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments. Drug Saf 2012; 35: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harduar-Morano L, Simon MR, Watkins S, et al. A population-based epidemiologic study of emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol 2011; 128: 594–600.e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vilà-de-Muga M, Colom-Ferrer L, Gonzàlez-Herrero M, et al. Factors associated with medication errors in the pediatric emergency department. Pediatr Emerg Care 2011; 27: 290–294. [DOI] [PubMed] [Google Scholar]
- 30. Perron BE, Bohnert AS, Monsell SE, et al. Patterns and correlates of drug-related ED visits: results from a national survey. Am J Emerg Med 2011; 29: 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 32. Braden JB, Russo J, Fan M-Y, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med 2010; 170: 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sikdar KC, Alaghehbandan R, Macdonald D, et al. Adverse drug events in adult patients leading to emergency department visits. Ann Pharmacother 2010; 44: 641–649. [DOI] [PubMed] [Google Scholar]
- 34. Ramos Linares S, Díaz Ruiz P, Mesa Fumero J, et al. Incidence rate of adverse drug effects in a hospital emergency unit and its associated factors. J Farma 2010; 34: 271–278. [DOI] [PubMed] [Google Scholar]
- 35. Maria Teresa Ventura RL, Pierfranco C, Piervito P, et al. Adverse drug reactions as the cause of emergency department admission: focus on the elderly. Immunopharmacol Immunotoxicol 2010; 32: 426–429. [DOI] [PubMed] [Google Scholar]
- 36. Backmund M, Schuetz C, Meyer K, et al. The risk of emergency room treatment due to overdose in injection drug users. J Addict Dis 2009; 28: 68–73. [DOI] [PubMed] [Google Scholar]
- 37. Olivier P, Bertrand L, Tubery M, et al. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department. Drugs Aging 2009; 26: 475–482. [DOI] [PubMed] [Google Scholar]
- 38. Capuano A, Irpino A, Gallo M, et al. Regional surveillance of emergency-department visits for outpatient adverse drug events. Eur J Clin Pharmacol 2009; 65: 721. [DOI] [PubMed] [Google Scholar]
- 39. Sauer BC, Hepler CD, Cherney B, et al. Computerized indicators of potential drug-related emergency department and hospital admissions. Am J Manag Care 2007; 13: 29–35. [PubMed] [Google Scholar]
- 40. Baena MI, Faus MJ, Fajardo PC, et al. Medicine-related problems resulting in emergency department visits. Eur J Clin Pharmacol 2006; 62: 387–393. [DOI] [PubMed] [Google Scholar]
- 41. Tipping B, Kalula S, Badri M. The burden and risk factors for adverse drug events in older patients-a prospective cross-sectional study. S Afr Med J 2006; 96: 1255–1259. [PubMed] [Google Scholar]
- 42. Hafner JW, Jr, Belknap SM, Squillante MD, et al. Adverse drug events in emergency department patients. Ann Emerg Med 2002; 39: 258–267. [DOI] [PubMed] [Google Scholar]
- 43. Hopking J. Pharmacoepidemiology research. https://www.hopkinsmedicine.org/gim/research/content/pharmacoepi.html (2018, accessed 12 March 2018).
- 44. Ab Rahman AF, Jatau AI, Aung MMT, et al. Factors associated with drug-related emergency department visits at a teaching hospital in Malaysia. Pharm Med 2017; 31: 175–181. [Google Scholar]
- 45. Trifirò G, Calogero G, Ippolito FM, et al. Adverse drug events in emergency department population: a prospective Italian study. Pharmacoepidemiol Drug Saf. 2005; 14(5): 333–340. [DOI] [PubMed] [Google Scholar]
- 46. Chang CB, Chen JH, Wen CJ, et al. Potentially inappropriate medications in geriatric outpatients with polypharmacy: application of six sets of published explicit criteria. Br J Clin Pharmacol 2011; 72: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roulet L, Ballereau F, Hardouin J-B, et al. Assessment of adverse drug event recognition by emergency physicians in a French teaching hospital. Emerg Med J 2013; 30: 63–67. [DOI] [PubMed] [Google Scholar]
- 48. Perron BE, Bohnert AS, Monsell SE, et al. Patterns and correlates of drug-related ED visits: results from a national survey. Am J Emerg Med 2011; 29: 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hohl CM, Yu E, Hunte GS, et al. Clinical decision rules to improve the detection of adverse drug events in emergency department patients. Acad Emerg Med 2012; 19: 640–649. [DOI] [PubMed] [Google Scholar]
- 50. Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med 2015; 373: 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pedrós C, Quintana B, Rebolledo M, et al. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Euro J Clin Pharm 2014; 70: 361–367. [DOI] [PubMed] [Google Scholar]
- 52. Castro I, Guardiola JM, Tuneu L, et al. Drug-related visits to the emergency department in a Spanish university hospital. Intern J Clin Pharm 2013; 35: 727–735. [DOI] [PubMed] [Google Scholar]
- 53. Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012; 60: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nickel CH, Ruedinger JM, Messmer AS, et al. Drug-related emergency department visits by elderly patients presenting with non-specific complaints. Scand J Trauma Resusc Emerg Med 2013; 21: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Generali JA. Adverse drug event reporting: awareness is not enough. Hosp Pharm 2014; 49: 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karpov A, Parcero C, Mok CP, et al. Performance of trigger tools in identifying adverse drug events in emergency department patients: a validation study. Br J Clin Pharmacol. 2016; 82(4): 1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jatau AI, Aung MMT, Kamauzaman THT, et al. Use and toxicity of complementary and alternative medicines among patients visiting emergency department: systematic review. J Intercult Ethnopharmacol 2016; 5: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel P, Zed PJ. Drug-related visits to the emergency department: how big is the problem? Pharmacotherapy 2002; 22: 915–923. [DOI] [PubMed] [Google Scholar]
- 59. Mascolo A, Scavone C, Sessa M, et al. Can causality assessment fulfill the new European definition of adverse drug reaction? A review of methods used in spontaneous reporting. Pharmacol Res 2017; 123: 122–129. [DOI] [PubMed] [Google Scholar]
- 60. Stevenson FA, Cox K, Britten N, et al. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect 2004; 7: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med 2014; 6: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thomasset KB, Faris R. Survey of pharmacy services provision in the emergency department. Am J Health Syst Pharm 2003; 60: 1561–1564. [DOI] [PubMed] [Google Scholar]
- 63. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 64. Lukazewski A, Martin B, Sokhal D, et al. Screening for adverse drug events in older adults: the impact of interventions. Consult Pharm 2014; 29: 689–697. [DOI] [PubMed] [Google Scholar]
- 65. Bernstein J, Bernstein E, Belanoff C, et al. The association of injury with substance use disorder among women of reproductive age: an opportunity to address a major contributor to recurrent preventable emergency department visits? Acad Emerg Med 2014; 21: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chismark E, Evans DD. Adverse drug events in the emergency department: why genetics matters in practice. Adv Emerg Nurs J 2012; 34: 3–9. [DOI] [PubMed] [Google Scholar]
- 67. Brown JD, Hutchison LC, Li C, et al. Predictive validity of the beers and screening tool of older persons’ potentially inappropriate prescriptions (STOPP) criteria to detect adverse drug events, hospitalizations, and emergency department visits in the United States. J Am Geriatr Soc 2016; 64: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mason S, Mountain G, Turner J, et al. Innovations to reduce demand and crowding in emergency care; a review study. Scand J Trauma Resusc Emerg Med 2014; 22: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. European Monitoring Centre for Drugs and Drug Addiction. Emergency department-based brief interventions for individuals with substancerelated problems: a review of effectiveness. EMCDDA Papers, Publications Office of the European Union, Luxembourg 2016; 61: 1–20. [Google Scholar]
- 70. Abubakar AR, Chedi BAZ, Mohammed KG, et al. Drug interaction and its implication in clinical practice and personalized medicine. Natl J Physiol Pharm Pharmacol 2015; 5: 343–349. [Google Scholar]
- 71. Dingley C, Daugherty K, Derieg MK, et al. Improving patient safety through provider communication strategy enhancements. In: Henriksen K, Battles JB, Keyes MA, et al. (eds) Advances in patient safety: new directions and alternative approaches. Vol. 3: Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality, 2008. [PubMed] [Google Scholar]
- 72. Zayyanu S, Isyaku H, Myat Moe TA, et al. Avoiding medication errors through effective communication in a healthcare environment Movement Health Exercise 2018; 7: 115–128. [Google Scholar]
- 73. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006; 166: 565–571. [DOI] [PubMed] [Google Scholar]
- 74. Morgan SR, Acquisto NM, Coralic Z, et al. Clinical pharmacy services in the emergency department. Am J Emerg Med 2018; 36: 1727–1732. [DOI] [PubMed] [Google Scholar]
- 75. Chen YC, Fan JS, Hsu TF, et al. Detection of patients presenting with adverse drug events in the emergency department. Intern Med J 2012; 42: 651–657. [DOI] [PubMed] [Google Scholar]
- 76. Budnitz DS, Pollock DA, Weidenbach KN, et al. National Surveillance of Emergency department visits for outpatient adverse drug events. JAMA 2006; 296: 1858–1866. [DOI] [PubMed] [Google Scholar]
- 77. Caterino JM, Emond JA, Camargo CA, Jr., et al. Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992-2000. J Am Geriatr Soc 2004; 52: 1847–1855. [DOI] [PubMed] [Google Scholar]
- 78. Franceschi A, Tuccori M, Bocci G, et al. Drug therapeutic failures in emergency department patients: a university hospital experience. Pharmacol Res 2004; 49: 85–91. [DOI] [PubMed] [Google Scholar]