Abstract
Nonalcoholic fatty liver disease (NAFLD) is diagnosed across the age spectrum and contributes to significant morbidity and mortality. The pathophysiology of NAFLD is not entirely understood; however, recent evidence has implicated the intestinal microbiome. Through the effects on host appetite, energy expenditure, digestion, gene expression, intestinal permeability, as well as immune activation, a dysbiotic microbiome can contribute to the development and progression of the hepatocellular steatosis, inflammation and fibrosis seen in the context of NAFLD. As such, intestinal microbiota and products of their metabolism have been targeted as treatment approaches for NAFLD.
Keywords: microbiota, NAFLD, NASH, treatment, probiotics
Background
Nonalcoholic fatty liver disease (NAFLD) is a major public health concern, as it is the most common cause of liver disease in the western world, affecting patients across the age spectrum.1,2 NAFLD refers to a spectrum of liver diseases that range from hepatic steatosis [nonalcoholic fatty liver (NAFL)] to steatosis with hepatocellular injury and possibly fibrosis [nonalcoholic steatohepatitis (NASH)]. NASH is the progressive form of NAFLD and can lead to cirrhosis and hepatocellular carcinoma increasing liver related morbidity and mortality. NAFLD typically occurs in the context of obesity or metabolic dysregulation. Its pathophysiology is not entirely understood; however, it is thought to be the result of multiple dietary, environmental, genetic and, more recently recognized, microbial hits to the liver.3,4
From a steatosis perspective, hepatocellular lipid accumulation occurs when there is an imbalance in the processes that regulate lipid handling within the liver.5 Processes that increase hepatic steatosis include: (1) lipolysis in the adipose tissue in the context of insulin resistance that increases the influx of fatty acids to the liver; (2) hepatocellular de novo lipogenesis; and, (3) exposure of hepatocytes to dietary fat/cholesterol/simple sugars. In contrast, processes that decrease the fat content of the hepatocytes include: (1) fatty acid oxidation in the mitochondria and peroxisomes; and, (2) packaging and export of very low-density lipoproteins (VLDLs) from the hepatocytes to the circulation. Hepatocellular inflammation and injury occur secondary to immune system activation, as well as due to oxidative stress seen in the context of lipotoxicity.6 Fibrosis develops as a result of chronic hepatocellular injury. Interestingly however, not everyone develops fibrosis, and there is significant variability in the severity and rate of fibrosis progression, suggesting that multitude of variables may play a role in liver disease progression depending upon the gene–environment interactions in the host.7 Several preclinical and also some human studies suggest that the intestinal microbiota are involved in the pathogenesis of the steatosis, inflammation and fibrosis seen in the context of NAFLD (Figure 1).
Figure 1.
Intestinal microbiota can contribute to the development of hepatic steatosis through a variety of mechanisms, including effects on appetite regulation and energy extraction from the diet. LPSs released from the cell wall of Gram-negative bacteria increases peripheral insulin resistance leading to an increased influx of FFAs from the adipose tissue to the liver. Ethanol can be used as a substrate for lipogenesis in the liver but can also affect the gut barrier, worsening the endotoxemia seen in this context. In the context of dysbiosis, SCFA synthesis and bile acid homeostasis are perturbed. These leads to upregulation of the metabolic processes that drive hepatic steatosis and a decrease in the processes that attenuate the development of fatty liver disease. The aforementioned molecules (LPSs, ethanol, bile acids and SCFAs) can also contribute to hepatic inflammation or fibrosis development.
DNL, de novo lipogenesis; FAO, fatty acid oxidation; FFA, free fatty acid; IR, insulin resistance; LPS, lipopolysaccharide (endotoxin); SCFA, short-chain fatty acid; TG, triglyceride; VLDL, very low-density lipoprotein.
The intestinal microbiome has been a topic of rigorous scientific research for over a decade now.8 The health of the host depends on the integrity of their microbiome, which is composed of different forms of life including bacteria, viruses, fungi and occasionally archaea. Dysbiosis refers to an imbalance between health and disease-promoting microbiota.9 Dysbiosis is a general term that reflects changes to the microbiome, such as decreased microbial diversity, or fluctuations in the relative abundance of certain microorganisms. Describing the intestinal microbiota composition of patients can be a helpful first step in discerning the role of the microbiome in the pathogenesis of various conditions. However, the literature has underscored a general lack of reproducibility in the results of these descriptive studies. This may be due to the fact that it is the microbial function, and not composition, that ultimately determines the risk of disease development. Since microbial functions are shared among microbiota, simple comparisons of the intestinal microbiota composition between groups may not provide sufficient information and may contribute to erroneous assumptions regarding the role of the microbiome in the development of disease states. More recent literature has addressed this issue by including metabolomic studies of the microbiome. Certain definitions that are specific to the microbiome are included in Table 1.
Table 1.
Definitions of terms related to the microbiota.
| Term | Definition |
|---|---|
| Dysbiosis | Imbalance between health and disease-promoting microorganisms |
| Probiotics | Microorganisms which when consumed in adequate amounts confer a health benefit to the host |
| Prebiotics | Substrates that are selectively used by host microorganisms to produce a health benefit |
| Synbiotics | A combination of a prebiotic and a probiotic |
Dysbiosis in NAFLD
Dysbiosis has been linked to various chronic conditions, including obesity and metabolic syndrome. NAFLD has also been linked to dysbiosis in both adults and children, in an obesity-independent manner. However, a consistent microbiota signature has not been determined in patients with NAFLD, as discussed in detail in a recent systematic review.9 This may be due to the aforementioned limitations of describing the composition but not the function of the intestinal microbiome. It may also arise from certain limitations of the literature on dysbiosis in the context of NAFLD. These include the study of small cohorts (n < 40) of patients, the lack of homogeneity in the diagnosis of NAFLD (e.g. histologic confirmation versus imaging, versus biochemistry-based diagnosis) and the differences in the control groups studied (e.g. lean versus obese, the approach of the investigators to confirming the absence of NAFLD in these patients). Furthermore, differences in the methods to collect, store and analyze the microbiome (e.g. 16S rRNA sequencing, polymerase chain reaction, metagenomic sequencing) may have also contributed to the variable results. In spite of these limitations, certain NAFLD-specific patterns of dysbiosis are still possible to discern.
First, most studies support the notion that the microbiome of patients with NAFLD is different than that of non-NAFLD controls, even after adjusting for obesity. Second, small intestinal bacterial overgrowth is more common in patients with NAFLD than healthy controls.10,11 Third, in spite of differences in the relative abundance of specific microbes, there is some consistency in the metabolomic profiles of these patients [e.g. increased short-chain fatty acids (SCFAs), increased ethanol levels], as discussed in more detail later in this review. Large-scale metabolomic studies are needed to further our understanding of the microbiome’s impact on the host, as it pertains to the development and progression of NAFLD.
In spite of the data on dysbiosis in NAFLD, there is currently no evidence of a direct, causative link between alterations in intestinal microbiota composition or function and NAFLD development. It is also not clear whether the dysbiosis described in this context precedes the development of the liver disease, or whether it results from it. However, there is evidence to suggest an indirect link between intestinal microbiota and the development of hepatic steatosis, inflammation and fibrosis. Bile acids and SCFAs are examples of such indirect links, and as such, their interplay with the microbiome and their metabolic effects are discussed here in more detail.
Intestinal microbiota and bile acids
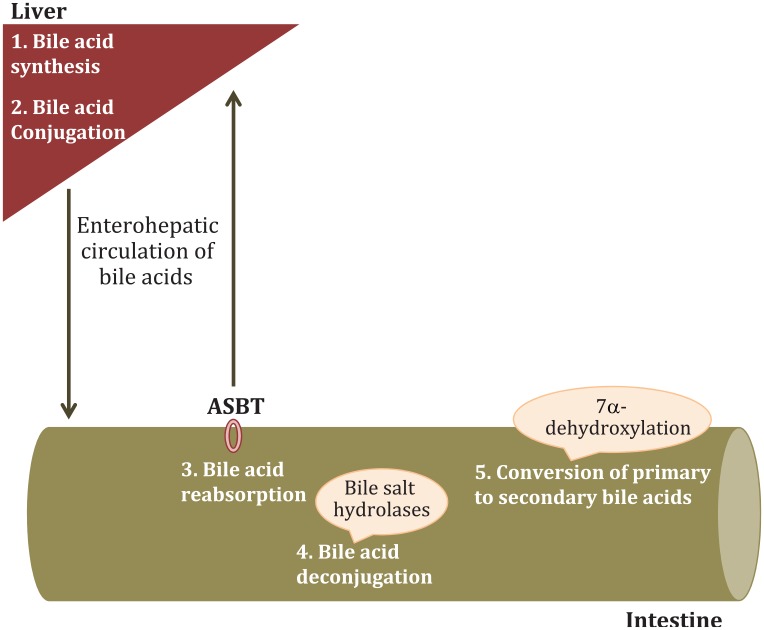
The intestinal microbiota are intimately involved in bile acid homeostasis.12 Primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized in the liver. The expression of certain bile acid synthesis enzymes is regulated by the microbiome (Figure 2). Similarly, bile acid conjugation is regulated by the intestinal microbiota. Conjugated primary bile acids are exported into the biliary tree, through the bile salt export pump. In the small intestine, they assist with digestion and ultimately 95% of bile acids are reabsorbed in the terminal ileum through the apical sodium-dependent bile-acid transporter (ASBT) and return to the liver. Only conjugated bile acids can be reabsorbed by ASBT. Intestinal microbiota regulate ASBT expression. In the small intestine, bile acids can be deconjugated by bile salt hydrolases that are widespread among intestinal microbiota. Hence, the efficacy of the enterohepatic circulation of bile acids is, in part, regulated by the microbiome. Deconjugated bile acids that have escaped absorption in the terminal ileum can be converted to secondary bile acids [lithocholic (LCA) and deoxycholic (DCA)] in the colon, only through the action of microbial enzymes. In summary, every aspect of bile acid homeostasis is regulated by the intestinal microbiome. This is important, as bile acids exert significant metabolic and immune effects than can have an impact on NAFLD development.
Figure 2.
Aspects of bile acid homeostasis that are regulated by the intestinal microbiota. The rate-limiting enzyme for the classical pathway is cholesterol 7α-hydroxylase (CYP7A1) whereas the first enzyme for the alternative pathway is sterol-27 hydroxylase (CYP27A1). The expression of both of these enzymes, along with other enzymes involved in the biosynthesis of bile acids (e.g. oxysterol 7α-hydroxylase; CYP7B1, acting in the alternative pathway), is regulated by the intestinal microbiota. In humans, primary bile acids are conjugated with glycine or taurine in the liver. The synthesis of taurine is regulated by the microbiota and so is the first enzyme of the bile acid conjugation cascade (bile acid acyl-CoA-synthetase). In the terminal ileum, microbiota regulate ASBT expression, ultimately determining the bile acid reabsorption rate. Bile acids can be subsequently deconjugated by bile salt hydrolases and ultimately escape reabsorption by ASBT. Deconjugated primary bile acids can be converted to secondary bile acids in the colon. The enzyme responsible for the 7-dehydroxylation that converts primary to secondary bile acids is expressed in a small number of intestinal microbiota (and not the host) that belong to the Firmicutes phylum (Clostridium clusters XIVa and XI and Eubacterium genera).
CYP7A1, cholesterol 7α-hydroxylase; CYP27A1, sterol-27 hydroxylase; ASBT, apical sodium-dependent bile-acid transporter.
Metabolic effects of bile acids
The outcome of bile acid signaling depends on the receptor that is being activated. The affinity of bile acids to their receptors varies, and as such changes in bile acid composition have a direct impact on bile acid signaling.
From a metabolic perspective, the most important bile acid receptors for NAFLD are farnesoid X receptor (FXR; predominantly activated by primary bile acids: CDCA > CA) and transmembrane G-protein coupled receptor 5 (TGR5; predominantly activated by secondary bile acids).13 FXR activation regulates bile acid synthesis. The latter occurs either through direct, bile acid-dependent, activation of hepatocellular FXR or indirectly, through intestinal fibroblast growth factor (FGF) 19 synthesis. FGF19 is an intestinal hormone released in response to bile acid-dependent activation of FXR found in enterocytes. In addition, FXR is involved in glucose homeostasis [e.g. decreasing gluconeogenesis, regulating the expression of glucose transporter 4, decreasing the intestinal expression of glucagon-like peptide 1 (GLP1), increasing hepatic glycogen synthesis] and lipid metabolism (e.g. inhibition of hepatic de novo lipogenesis, increased fatty acid oxidation, regulation of genes involved in triglyceride homeostasis).13 TGR5 activation also regulates glucose homeostasis (e.g. increasing intestinal expression of GLP1).14 In addition, TGR5 increases energy expenditure through the activation of thyroid hormones.15,16 In animals, TGR5 activation has anti-inflammatory effects. While normal bile acid signaling attenuates steatosis and inflammation, an imbalance between primary and secondary bile acids can be pathogenic. For example, increased enterohepatic circulation of DCA has been shown to contribute to the development of hepatocellular carcinoma in obese mice.17 This is significant, as adults with steatohepatitis (a risk factor for hepatocellular carcinoma development) have higher hepatic DCA levels compared with healthy controls.18 Considering the synergy between microbiota and bile acid homeostasis, as well as the impact of bile acid signaling on the health of the host, the microbiota–bile acid axis is important from an NAFLD pathophysiology and treatment perspective.
Intestinal microbiota and SCFAs
Acetate, propionate and butyrate are the main SCFAs produced in the colon through the microbial fermentation of foods (predominantly complex carbohydrates, and less commonly proteins and peptides) that the host lacks the enzymatic capacity to digest. Most SCFAs are utilized or absorbed by colonocytes leaving only 5–10% to be excreted in the feces. SCFAs act predominantly on the G-protein-coupled receptors GPR41 and GPR43, which are found in the colon, white adipose tissue, skeletal muscle and the liver. Propionate is the primary activator of GPR41 (propionate > butyrate > acetate) while all three SCFAs activate GPR43 equally.
Metabolic effects of SCFAs
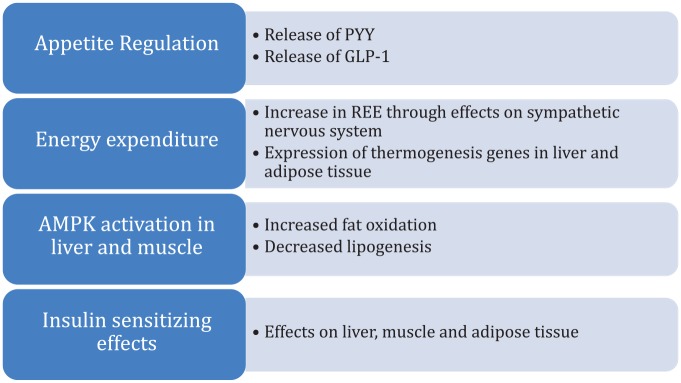
SCFAs have a multitude of metabolic effects that span from appetite regulation to specific effects on different body compartments that can have an impact on NAFLD development, as summarized in Figure 3. Intestinal recognition of SCFAs leads to the release of the anorexigenic and insulin sensitizing peptides peptide tyrosine tyrosine (PYY) and GLP1. Propionate and butyrate increase energy expenditure through the effects on the sympathetic nervous system. In animal studies, acetate and butyrate stimulate the expression of thermogenesis genes involved in the liver and the brown adipose tissue.19,20 Recognition of SCFAs by its receptors in the peripheral adipose tissue has anabolic effects (e.g. decreased lipolysis, increased adipogenesis). In skeletal muscle and the liver, SCFAs activate adenosine monophosphate-activated protein kinase (AMPK), which then leads to increased fat oxidation and decreased lipogenesis. Lastly, the effects of SCFAs on adipose tissue, skeletal muscle and liver promote insulin sensitivity. It should be noted that, while animal data suggest a role for SCFAs in glucose homeostasis, interventional trials in humans have largely failed to show a convincing effect of orally, rectally or intravenously administered SCFAs on serum glucose or insulin levels.21 In terms of inflammation, in vitro and animal data support the notion that SCFAs exert immune regulatory and anti-inflammatory effects that remain to be investigated further in humans.21 In summary, SCFAs are another indirect link between the intestinal microbiome and the development of insulin resistance, obesity and NAFLD.
Figure 3.
Summary of the metabolic effects of short-chain fatty acids that are of relevance to nonalcoholic fatty liver disease.
AMPK, adenosine monophosphate-activated protein kinase; REE, resting energy expenditure.
Microbiome and the development of hepatic steatosis, inflammation and fibrosis
Steatosis
The intestinal microbiota can contribute to the development of hepatic steatosis through a multitude of effects on the host that include appetite regulation, energy extraction from the diet, regulation of energy expenditure and lipid handling within the liver and also through effects on insulin sensitivity.
Appetite regulation
While intestinal microbiota are not the sole regulators of appetite, as shown in experiments using germ-free mice which are able to achieve and maintain their set weight, there is evidence from human studies to suggest that the microbiome does impact host satiety.22 In healthy men, metabolic endotoxemia is associated with food intake.23 Furthermore, exposure of healthy people to prebiotics leads to decreased hunger scores with concurrent increases in anorexigenic signaling molecules (e.g. increased plasma levels of PYY and GLP1).24
From a mechanistic perspective, the intestinal microbiota-driven regulation of appetite can be direct or indirect. Direct signaling occurs when microbial components (e.g. lipopolysaccharides; LPSs) or metabolic byproducts of bacterial metabolism (e.g. SCFAs) activate either enteroendocrine cells or intestinal vagal afferents. Activation of enteroendocrine cells leads to the release of anorexigenic peptides (e.g. PYY). Activation of the vagus nerve transmits cholinergic satiety signals from the intestine to the central nervous system. Ultimately these pathways contribute to appetite regulation.
Indirect appetite signaling can also occur, in part through bacterial quorum sensing.22,25 The latter is a process that regulates the diurnal oscillations in the abundance of the intestinal microbiome. These microbial oscillations have been shown to be associated with feeding behaviors of the host (and vice versa).22,26,27 It needs to be investigated further whether there is a direct causative link between bacterial quorum sensing and appetite regulation.
Energy extraction from the diet
Dysbiosis associated with obesity has been linked to an increased capacity of the microbiome to extract calories from the diet. Turnbaugh and colleagues showed that the microbiome of ob/ob mice is enriched in genes involved in the metabolism of nutrients that are nondigestible by the host.28 These mice have increased concentrations of SCFAs in their cecum and decreased energy remaining in their stool, suggesting increased energy harvest. Human obesity has also been associated with an intestinal microbiome that has an increased capacity to extract calories from the diet (e.g. increased abundance of enzymes metabolizing polysaccharides).29 In spite of these original reports, recent research has challenged the link between obesity, dysbiosis and energy extraction from the diet.30–33 This field remains to be investigated further.
Energy expenditure
The microbiome can impact the host’s energy expenditure, ultimately contributing to their overall predisposition to developing obesity and NAFLD. As already mentioned, the microbiome can modify the energy expenditure of the host through secondary bile acid signaling. TGR5 activation by secondary bile acids leads to increased thermogenesis in skeletal muscle and brown adipose tissue. Human studies have shown that not only is there a correlation between serum bile acid levels and energy expenditure, but also, treatment of healthy people with bile acids increases their energy expenditure.34,35 Energy expenditure can also be modified by SCFAs. Based on animal and human studies, it has been estimated that 7% of the host’s resting energy expenditure is driven by the turnover of SCFAs.36,37 In addition, administration of butyrate leads to increased energy expenditure in obese mice.19 Similarly, the infusion of acetate and propionate in the colons of overweight/obese men can further increase energy expenditure.38
Lipid handling within the liver
The balance between de novo lipogenesis and fatty acid oxidation in the liver determines the severity of hepatic steatosis. Bile acid signaling, particularly through FXR activation, leads to increased fatty acid oxidation and decreased lipogenesis. This ultimately has a beneficial impact on hepatic steatosis, as shown in studies using FXR agonists for the treatment of NAFLD (see ‘Targeting the microbiome for the treatment of NAFLD’ section). Similarly, SCFAs have direct effects on fatty acid oxidation (e.g. increased oxidation secondary to AMPK activation), as well as variable effects on de novo lipogenesis.39 Specifically, acetate is used as a substrate for lipogenesis, whereas propionate downregulates lipogenesis through effects on the enzyme fatty acid synthase.
Effects on insulin sensitivity
Animal and human studies have revealed that the microbiome regulates insulin signaling. The most convincing literature on the role and importance of the microbiome in the pathogenesis of insulin resistance in humans comes from fecal transplantation studies, whereby the transfer of stool from people with insulin resistance to healthy controls (or vice versa) transfers the insulin resistant phenotype.40,41 This is important as insulin resistant-induced adipose tissue lipolysis releases the majority of the fat found in the livers of patients with NAFLD.42
The microbiome can affect insulin signaling in a variety of ways. SCFAs and bile acid signaling leads to the release of hormones, such as GLP-1, that regulate insulin signaling. Endotoxin, released from the cell wall of Gram-negative bacteria, prevents the phosphorylation of insulin receptor substrate 1, which is required for insulin signaling to occur. Lastly, intestinal bacterial synthesis of branched chain amino acid has also been implicated to the development of insulin resistance in the host.43
In summary, the literature to date suggests that the intestinal microbiota may be involved in the development of hepatic steatosis. Research is needed to determine the impact of interventions aimed at treating dysbiosis on the severity and natural history of NAFLD.
Hepatic inflammation
Beyond steatosis, dysbiosis can contribute to hepatic inflammation in the context of NAFLD in a variety of ways, including effects on the gut barrier, interaction with the immune system and through direct hepatotoxic effects of microbial metabolites.
Intestinal permeability
Adults and children with NAFLD have an impaired gut barrier, which is partly affected by the intestinal microbiota. Measuring the urinary excretion of44 Cr-ethylene diamine tetraacetate in a cohort of 37 adults with NAFLD, Miele and colleagues showed evidence of increased intestinal permeability, as well as decreased expression of tight junction zona occludens-1.45 These patients also had small intestinal bacterial overgrowth (SIBO), which along with the increased intestinal permeability, correlated with the severity of steatosis. Volynets and colleagues was unable to replicate the findings of increased SIBO in a cohort of 20 adults with NAFLD; however, they did show that NAFLD was associated with increased intestinal permeability assessed directly using a lactulose/mannitol test, and indirectly measuring serum endotoxin levels.46 Similarly, in a pediatric cohort of 39 patients with NAFLD, a lactulose/mannitol test confirmed the presence of increased intestinal permeability, which correlated with liver disease severity. Serum endotoxin levels were also increased in those with NAFLD and correlated with histologic markers of liver injury.47 Lastly, plasma levels of LPS binding protein were elevated in a different cohort of 40 severely obese patients with NAFLD, and also correlated with liver disease severity.48 Fructose consumption has been linked to endotoxemia and elevation in circulating inflammatory cytokines, which is interesting considering the known association between excess fructose consumption and dysbiosis.49–51 In summary, literature across different NAFLD cohorts suggests that patients with NAFLD have an impaired gut barrier, which allows the translocation of intestinal microbiota or their components (e.g. LPSs) in the circulation. That in turn can contribute to metabolic and inflammatory processes within the liver that lead to NAFLD development and progression.
Cross-talk between intestinal microbiota and the host immune system
Animal models of fatty liver disease have shown that the recognition of intestinal microbiota by the immune system is necessary for the development of severe NAFLD. For example, models whereby certain innate immune system receptors (e.g. Toll-like receptors; TLRs) have been knocked out (TRL4 or TLR9) fail to develop severe NAFLD.44,52,53 TLR-based recognition of bacterial products by the immune system (e.g. TLR4 recognizes LPSs and TLR9 recognizes bacterial DNA) initiates an inflammatory cascade, which is thought to contribute to hepatic inflammation. Consumption of fructose, which has been associated with a more severe NAFLD phenotype and dysbiosis, upregulates the expression of certain TLRs.54 Exposure of healthy people to probiotics upregulates intestinal expression of tight junctions in a TLR2-dependent manner.55 Lastly, adults with NAFLD have evidence of impaired mucosal immunity in the context of dysbiosis, as shown by decreased numbers of CD4 and CD8-positive cells in the lamina propria of their duodenal mucosa.56
The cross-talk between the microbiome and the immune system is bidirectional, such that the composition of the intestinal microbiota is also under the control of the host immune system. This has been shown in a TLR5 knockout model, where mice develop metabolic dysregulation and obesity along with dysbiosis.57 The immune dysregulation-driven dysbiosis that is seen in this context causes the obese phenotype, as proven by fecal transplantation studies. In summary, while immune surveillance and detection of intestinal microbiota in the circulation is important from an infectious perspective, it may also be involved in the development of hepatic inflammation in patients with NAFLD.
Microbial synthesis of ethanol
Adults and children with NAFLD have increased serum ethanol levels, in the absence of alcohol consumption. Ethanol is presumed to originate from the metabolism of the intestinal microbiota. Zhu and colleagues showed that not only do children with NASH have elevated blood ethanol levels compared with their lean or obese non-NASH counterparts, but they also have dysbiosis characterized by an increased relative abundance of ethanol-producing bacteria (e.g. E. coli).58 Similarly, Volynets and colleagues showed that patients with NAFLD have elevated plasma levels of alcohol along with increased intestinal permeability.46 Engstler and colleagues also found that young children with NAFLD have elevated plasma levels of ethanol; however, they challenged the microbial origin of the ethanol.59 Using ob/ob mice, they showed that ethanol concentration in the portal vein was similar to that measured in the intestinal chyme but much lower than that measured in the vena cava. This was associated with a decreased alcohol dehydrogenase activity in the livers of ob/ob mice, which in turn correlated with insulin resistance. Their findings suggest that decreased hepatic metabolism of ethanol in the context of insulin resistance is responsible for the elevated ethanol levels seen in patients with NAFLD, and not increased microbial synthesis of ethanol. This remains to be investigated further.
Ethanol can contribute to NAFLD through the effects on intestinal permeability (decreases tight junction expression, dissolves the lipids found in the mucin layer), lipid handling within the liver (increased de novo lipogenesis, decreased fatty acid oxidation, decreased export of VLDL from the liver), as well as through direct hepatotoxicity, as seen in the context of alcoholic liver disease.60–63
Fibrosis
Beyond the aforementioned dysbiosis-driven immune activation that triggers proinflammatory cascades, the involvement of the intestinal microbiota in the development and progression of the hepatic fibrosis seen in patients NAFLD is not well understood.
The only direct link between microbiota and the development of fibrosis in human NAFLD is the finding that hepatic stellate cells (HSCs) express significant levels of TLR4, even in a quiescent state.64 Considering the endotoxemia observed in patients with NAFLD, it is possible that fibrosis develops as a direct effect of dysbiosis. Fructose consumption may be an example of such as model, as excess fructose consumption is not only associated with dysbiosis and endotoxemia.50,51 but also hepatic fibrosis in patients with NAFLD.65 From a mechanistic perspective, excess fructose consumption was recently postulated to exert its hepatotoxic potential through its conversion to toxic metabolites by colonic microbiota.66
Experimental models of NASH support the role of the microbiome in the development of fibrosis. In a choline-deficient amino acid (CDAA)-induced hepatic fibrosis model in rats, the use of antibiotics decreased HSC activation and the severity of fibrosis.67 In this study, the use of antibiotics led to improved gut barrier function (as shown by increased expression of tight junction proteins and decreased endotoxemia), as well as decreased TLR4 expression in the liver, which subsequently led to attenuation of HSC activation. In a different experimental model of NAFLD in mice, it was shown that the inflammasome, a protein complex responsible for the detection of invading microorganisms, was necessary for the development of severe fibrosis.68 Interestingly, while dysbiosis is associated with the development of hepatic fibrosis, so is the inability to detect the presence of intestinal microbiota. This was shown in an animal model, whereby exposure of germ-free mice to thioacetamide or carbon tetrachloride led to worse liver injury and fibrosis compared with wildtype mice.69
In summary, intriguing research so far has underscored a link between the intestinal microbiota and the development of hepatic fibrosis in the context of NAFLD. The exact pathophysiologic mechanisms leading to the development of fibrosis in children and adults with NAFLD remain to be determined.
Potential clinical applications of microbiome data
Leveraging the intestinal dysbiosis seen in patients with NAFLD, investigators have assessed whether microbiome data can serve as predictors of histologic liver disease severity. Using 16S rRNA sequencing of stool samples, Boursier and colleagues studied the intestinal microbiome of 57 French patients with NAFLD (n = 35 with NASH, n = 30 with fibrosis stage 0–1 and n = 27 with fibrosis stage 2–4).70 Disease severity was associated with different microbiota signatures (increased Bacteroides abundance in those with NASH and increased Ruminococcus abundance in those with fibrosis stages 2–4), as well as distinct metabolomic pathways (inferred using Kyoto Encyclopedia of Genes and Genomes (KEGG) data). In this study, Bacteroides and Ruminococcus abundance were independent predictors of NASH and severe fibrosis, respectively. Interestingly, in another study from Europe that performed 16S rRNA sequencing to determine differences in the intestinal microbiota of a cohort with severe obesity (n = 44), the abundance of Ruminococcaceae was lower in patients with any fibrosis compared with those without fibrosis.71
More recently, Loomba and colleagues used detailed metagenomic sequencing to study the fecal microbiome of a well-characterized cohort of 86 adults with NAFLD (n = 72 with mild/moderate NAFLD, defined as fibrosis stage 0–2, and n = 14 with severe NAFLD, defined as fibrosis stages 3–4) from the United States.72 Metagenomic and metabolomic data were combined with clinical information and random forest modeling was used to determine the optimal combination of clinical, metagenomic and metabolomic data that accurately distinguished patients with mild/moderate from those with severe NAFLD. The combination of age, body mass index (BMI), Shannon diversity index and 37 bacterial species had an area under the curve of 0.936 in predicting the presence of advanced fibrosis. The repeatability and generalizability of these findings remain to be shown.
These preliminary studies suggest that inclusion of information on the intestinal microbiome in models that noninvasively predict liver disease severity can significantly improve accuracy.
Beyond prediction models, data on the microbiome can be used to personalize the approach to treatment. For example, it is known that intestinal microbiota can affect drug metabolism and efficacy.73 As such, it may be that the discrepancies in the treatment response observed to date in the randomized controlled trials (RCTs) performed for the treatment of NAFLD are, in part, due to the differences in the intestinal microbiota composition among patients. This is even more relevant when the interventions studied target the microbiota specifically (e.g. treatment with probiotics or prebiotics). While the available data do suggest that targeting the microbiome may be a helpful approach to treating patients with NAFLD (see section ‘Targeting the microbiome for the treatment of NAFLD’ below) it would be interesting to determine whether a personalized approach to treatment (e.g. using a specific prebiotic or probiotic to treat a patient based on their baseline intestinal microbiota composition) would be more successful.
Targeting the microbiome for the treatment of NAFLD
Intestinal dysbiosis has been investigated as a treatment target for NAFLD. Prebiotics, probiotics, synbiotics and antibiotics have been used for that purpose. In addition, fecal transplantation studies have already been performed in patients at risk for NAFLD (e.g. patients with insulin resistance), and some are underway (ClinicalTrials.gov). Furthermore, novel treatment approaches that target microbiome-related pathways (e.g. bile acid signaling, specific dietary approaches) are also being investigated. These are summarized in Table 2.
Table 2.
Summary of treatments that target the microbiome for the treatment of NAFLD.
| Treatment | Result |
|---|---|
| Prebiotics | • Modest reductions in serum aminotransferases, no histologic
outcomes • Small cohorts studied |
| Probiotics | • Small reductions in serum aminotransferases, no histologic
outcomes • No personalized treatment (i.e. no prior knowledge of microbiota composition) |
| Synbiotics | • No reduction in serum ALT levels |
| Antibiotics | • Short-term, limited studies |
| Fecal Microbial Transplantation | • In process, no study aimed at treating NAFLD has been published yet |
| JKB-121 | • TLR4 receptor antagonist studied in phase II, double-blind,
randomized, placebo-controlled trial × 24 weeks • Not superior to placebo in reducing MRI-PDFF and ALT in adults with NASH |
| Obeticholic acid | • FXR agonist studied in a double-blind, placebo-controlled,
randomized, phase IIb trial × 72 weeks • Decreases NAS by ⩾2 without worsening of fibrosis with a relative risk reduction of 1.9 • Concerns regarding increase in LDL-C |
| NGM282 | • Engineered variant of human FGF19 studied in a phase II,
placebo-controlled, randomized controlled trial ×
12 weeks • Leads to MRI-PDFF of <5% in up to 39% of patients with noncirrhotic NASH • Concerns regarding increase in LDL-C |
ALT, alanine aminotransferase; FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor; LDL-C, low-density lipoprotein cholesterol; MRI-PDFF, magnetic resonance imaging proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; TLR, Toll-like receptor.
Prebiotics for the treatment of NAFLD
Prebiotics are defined as substrates that are ‘selectively used by host microorganisms to produce a health benefit’.74 A variety of different prebiotics have been studied in NAFLD, including psyllium, fructooligosaccharides (FOSs) and xylooligosaccharides (XOSs), fiber extracts and chicory inulin.75 Prebiotic use has not been attempted in a personalized fashion (i.e. to target each patient’s dysbiosis individually with a particular prebiotic). Some of the prebiotic trials have used vitamin E or other antioxidants in conjunction with the prebiotic. All prebiotic trials have been performed in adults and most are of a small sample size. Furthermore, none of the prebiotic trials performed in the context of NAFLD have had a histological outcome, some have not even included patients with histologic confirmation of NAFLD and none have been performed in North America (most have been done in Asia and South America).
A recent meta-analysis showed that prebiotic use was associated with a small reduction in BMI (by 5 kg/m2), as well as modest reductions in serum alanine aminotransferase (ALT; 10 U/l) and aspartate transaminase (AST; 6 U/l) levels.75 Serum markers of inflammation and total cholesterol were not affected by the use of prebiotics. However, prebiotic use led to reductions in low-density lipoprotein cholesterol (LDL-C) and mild increases in high-density lipoprotein (HDL). These results were only seen in patients with histologically confirmed NAFLD.
Considering the ease of use of prebiotics, their safety profile and overall low cost, they are ideal treatment candidates for NAFLD. To determine whether prebiotics are useful for the treatment of NAFLD, future studies should address their impact on hepatic steatosis and fibrosis either using histology or advanced imaging measurements (e.g. magnetic resonance imaging proton density fat fraction and elastography) as primary outcomes. Such trials are currently underway (source: ClinicalTrials.gov).
Probiotics for the treatment of NAFLD
Probiotics, defined as microorganisms which when consumed in adequate amounts confer a health benefit to the host, have also been trialed in the context of NAFLD. The majority of the studies have been performed in adults living in Europe, Asia and North America; the mean age at enrollment in these studies is lower than that of prebiotics trials.75 Various probiotics have been studied, mostly combinations of Lactobacillus and Bifidobacteria species. The mean duration of probiotic use studied is approximately 3 months.
Similar to the prebiotic studies, the impact of probiotics has been assessed using biochemical markers of liver injury or serum markers of metabolic dysregulation. In the aforementioned meta-analysis by Loman and colleagues, the effect of probiotics on BMI was similar to that seen with prebiotics (reduction by 0.5 kg/m2), but the change in ALT and AST was even more modest than that seen with prebiotics (reduction by 7 U/l and 3 U/l, respectively). Probiotic use was not effective in reducing total cholesterol, LDL-C or triglycerides, and it was associated with a decrease in HDL of 1.3 mg/dl.
Synbiotics for the treatment of NAFLD
Synbiotics refer to the combination of pre- and probiotics. Various synbiotics have been used for the treatment of NAFLD, predominantly in adults. On average, synbiotic trials have been of longer duration than pre- or probiotic trails (with the intervention lasting for up to 6 months). The majority of the synbiotic trials reported to date have been done in Iran.75
The use of synbiotics is associated with a clinically nonsignificant decrease in BMI 0.1 kg/m2. Synbiotic use does not affect serum ALT levels, but do decrease AST by 8 U/L. In spite of the lack of impact on BMI, synbiotics lead to an average reduction in total cholesterol of 15 mg/dl but have no effect on serum LDL-C, triglyceride and HDL levels.
Antibiotics
Gangarapu and colleagues studied the impact of a short-term (28 days) course of the nonabsorbable antibiotic rifaximin on markers of liver injury, immune activation and endotoxemia, in a cohort of 42 patients with NAFLD (27 with NASH).76 In this open-label, observational, cohort study, rifaximin was associated with a small decrease in BMI, as well as statistically significant decreases in serum levels of ALT, AST, gamma glutamyl transferase, LPS and ferritin in patients with NASH.
Reijnders and colleagues studied the impact of a 7-day course of antibiotics (amoxicillin versus vancomycin versus placebo) on the metabolism of obese adults in a randomized, double-blind, placebo-controlled trial.33 They showed that vancomycin (but not amoxicillin) changed the intestinal microbiome significantly, a finding that was accompanied by changes in SCFA and bile acid levels. In spite of these changes however, insulin resistance, energy expenditure and energy extraction from the diet remained unaffected and unchanged from baseline. This study revealed that the interplay between the microbiome and the host is complex and the metabolic impact of this interaction can be unpredictable. The role of short- and long-term courses of antibiotics for the treatment of NAFLD remains to be investigated in appropriately designed RCTs.
Fecal transplantation for the treatment of NAFLD
Fecal microbial transplantation (FMT) has been used successfully in the treatment of recurrent infection with Clostridium difficile. FMT leads to increased bacterial diversity,77,78 as such, may be useful in the context of conditions associated with dysbiosis and overall decreased microbial diversity, such as NAFLD. To date, no fecal transplantation studies have reported on the impact of this intervention on serum, imaging or histology outcomes of patients with NAFLD. In patients with metabolic syndrome however, Vrieze and colleagues have shown improved insulin sensitivity following fecal transplantation from lean individuals.40 Studies focusing on the role of FMT in the treatment of NAFLD or associated comorbidities are currently underway (ClinicalTrials.gov). Future aspects of care that will need to be addressed, should FMT be found to be useful in the treatment of NAFLD, include the approach to selecting the donor, the optimal mode of delivery of the fecal material and the dose/frequency necessary to achieve the desired outcome.
Other approaches to target the microbiome
Considering the presence of metabolic endotoxemia in patients with NAFLD, as well as the role of TLR4 signaling in inflammation and fibrosis, this pathway has been targeted for the treatment of NASH. JKB-121, a weak TLR4 receptor antagonist was studied in a recent phase II, double-blind, randomized, placebo-controlled trial.79 After 24 weeks of treatment, JKB-121 was not superior to placebo in reaching the primary endpoint of liver fat content reduction (measured with magnetic resonance imaging proton density fat fraction) or serum ALT reduction in adults with NASH. Interestingly, in this study, the placebo response was significant with 32% of participants in the placebo arm demonstrating a sustained biochemical remission (ALT < 40 U/l on two consecutive measures). The role of TLR4 signaling antagonism for the treatment of NASH remains to be investigated further.
Targeting bile acid homeostasis as a treatment of NAFLD
As already mentioned, intestinal microbiota are tightly linked to bile acid homeostasis.13 Concurrently, bile acids can have a direct impact on the intestinal microbiota composition. Specifically, bile acids act as detergents on microbial cell membranes, signal the release of antimicrobial peptides and can also stimulate the germination of microbial spores.80,81 Given the interplay between intestinal microbiota and bile acids, any intervention targeting bile acid homeostasis has an impact on the microbiota and vice versa.
Bile acid homeostasis has been targeted for the treatment of NAFLD. Obeticholic acid is a partially synthetic derivative of chenodeoxycholic acid and a potent FXR agonist that has been studied specifically for the treatment of NASH. Obeticholic acid increases Gram-positive facultative anaerobic bacteria in the stool of healthy people, likely as a result of an FXR-dependent reduction in small intestinal bile acid levels.82 In addition, in cirrhotic rats it improves dysbiosis, reduces bacterial translocation and ameliorates the intestinal immune cell infiltration.83 FLINT, a double-blind, placebo-controlled, randomized, phase IIb trial assessed the effect of 25 mg of obeticholic acid given daily in adults with NASH over 72 weeks. Obeticholic acid was associated with weight loss (2.3 kg on average) and improved liver histology [decrease in NAFLD activity score (NAS) by ⩾2 without worsening of fibrosis; relative risk (RR) reduction of 1.9, 95% confidence interval (CI) 1.3–2.8]. Fibrosis improved in 35% of patients and NASH resolution was seen in 22% (not statistically different from placebo). Adverse effects associated with the use of obeticholic acid included pruritus, increase in insulin resistance and worsening cholesterol levels (increased total serum cholesterol and LDL levels and decreased HDL). Considering that cardiovascular disease is the primary cause of death in this population, these trends in cholesterol are concerning; however, a combination of obeticholic acid with a statin may prove to be beneficial. The role of obeticholic acid for the treatment of NASH is currently being investigated in a large, phase III trial (REGENERATE).
NGM282 is an engineered variant of human FGF19 that has been studied for the treatment of NASH. In a phase II, placebo-controlled, RCT, daily subcutaneous injections with NGM282 for 12 weeks normalized the hepatic fat content (defined as magnetic resonance imaging proton density fat fraction < 5%) of 26–39% of patients with noncirrhotic NASH (with the 3-mg and 6-mg dosing, respectively).84 Use of the drug was associated with an increase in LDL-C levels. NGM282 was subsequently studied in combination with rosuvastatin, in a phase II, open-label study of adult NASH.85 The results of this 12-week trial revealed a 75% decrease in NAS and reduction in fibrosis score in 42% of patients. The concurrent use of rosuvastatin prevented increases in serum LDL levels noted in the previous trial.86 The most common adverse effects associated with the use of NGM282 were nausea and diarrhea.
Conclusion
The literature to date supports the notion that dysbiosis occurs in patients with NAFLD and that can be of relevance in terms of the pathophysiology and the treatment of this condition. Future studies should focus on the determination of direct links between the microbiome and the development and natural history of NAFLD, as well as the impact of interventions aimed at treating dysbiosis on liver disease severity.
Acknowledgments
Marialena Mouzaki drafted the manuscript, critically revised the manuscript and approved the final submission.
Rohit Loomba was responsible for the study concept and design, critical revision of the manuscript, and approved final submission
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RL receives grant funding support from NIDDK (R01-DK106419) and NIEHS (5P42ES010337). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest statement: Dr R. Loomba serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. His institution receives grant support from Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, Prometheus, and Siemens. He is also cofounder of Liponexus, Inc. The terms of these arrangements have been reviewed and approved by the University of California, San Diego, CA, USA in accordance with its conflict of interest policies.
ORCID iD: Marialena Mouzaki  https://orcid.org/0000-0002-9809-6199
https://orcid.org/0000-0002-9809-6199
Contributor Information
Marialena Mouzaki, Steatohepatitis Center, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH, USA.
Rohit Loomba, NAFLD Research Center, Division of Gastroenterology, Department of Medicine, University of California at San Diego, ACTRI Building, 1W202, 9452 Medical Center Drive, La Jolla, CA 92037, USA.
References
- 1. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 2. Anderson EL, Howe LD, Jones HE, et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015; 10: e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med 2017; 15: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 5. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010; 52: 1836–1846. [DOI] [PubMed] [Google Scholar]
- 6. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018; 68: 280–295. [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643–654 e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 9. Wieland A, Frank DN, Harnke B, et al. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2015; 42: 1051–1063. [DOI] [PubMed] [Google Scholar]
- 10. Guercio Nuzio S, Di Stasi M, Pierri L, et al. Multiple gut-liver axis abnormalities in children with obesity with and without hepatic involvement. Pediatr Obes 2017; 12: 446–452. [DOI] [PubMed] [Google Scholar]
- 11. Belei O, Olariu L, Dobrescu A, et al. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J Pediatr Endocrinol Metab 2017; 30: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 12. Wahlstrom A, Sayin SI, Marschall HU, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016; 24: 41–50. [DOI] [PubMed] [Google Scholar]
- 13. Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017; 65: 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe M, Morimoto K, Houten SM, et al. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 2012; 7: e38286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499: 97–101. [DOI] [PubMed] [Google Scholar]
- 18. Aranha MM, Cortez-Pinto H, Costa A, et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur J Gastroenterol Hepatol 2008; 20: 519–525. [DOI] [PubMed] [Google Scholar]
- 19. Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009; 58: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kondo T, Kishi M, Fushimi T, et al. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem 2009; 57: 5982–5986. [DOI] [PubMed] [Google Scholar]
- 21. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11: 577–591. [DOI] [PubMed] [Google Scholar]
- 22. Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 2017; 13: 11–25. [DOI] [PubMed] [Google Scholar]
- 23. Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008; 87: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 24. Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009; 90: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 25. Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett 2006; 254: 1–11. [DOI] [PubMed] [Google Scholar]
- 26. Zarrinpar A, Chaix A, Yooseph S, et al. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 2014; 20: 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014; 159: 514–529. [DOI] [PubMed] [Google Scholar]
- 28. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 29. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 2010; 59: 1635–1642. [DOI] [PubMed] [Google Scholar]
- 31. Dalby MJ, Ross AW, Walker AW, et al. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep 2017; 21: 1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137: 1716–1724, e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reijnders D, Goossens GH, Hermes GD, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab 2016; 24: 341. [DOI] [PubMed] [Google Scholar]
- 34. Ockenga J, Valentini L, Schuetz T, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab 2012; 97: 535–542. [DOI] [PubMed] [Google Scholar]
- 35. Broeders EP, Nascimento EB, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 2015; 22: 418–426. [DOI] [PubMed] [Google Scholar]
- 36. Pouteau E, Nguyen P, Ballevre O, et al. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc Nutr Soc 2003; 62: 87–93. [DOI] [PubMed] [Google Scholar]
- 37. Bakker GJ, Zhao J, Herrema H, et al. Gut microbiota and energy expenditure in health and obesity. J Clin Gastroenterol 2015; 49(Suppl. 1): S13–S19. [DOI] [PubMed] [Google Scholar]
- 38. Canfora EE, van der Beek CM, Jocken JWE, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep 2017; 7: 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Demigne C, Morand C, Levrat MA, et al. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr 1995; 74: 209–219. [DOI] [PubMed] [Google Scholar]
- 40. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–916, e7. [DOI] [PubMed] [Google Scholar]
- 41. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514: 181–186. [DOI] [PubMed] [Google Scholar]
- 42. Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016; 535: 376–381. [DOI] [PubMed] [Google Scholar]
- 44. Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 2007; 47: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 46. Volynets V, Kuper MA, Strahl S, et al. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig Dis Sci 2012; 57: 1932–1941. [DOI] [PubMed] [Google Scholar]
- 47. Giorgio V, Miele L, Principessa L, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis 2014; 46: 556–560. [DOI] [PubMed] [Google Scholar]
- 48. Ruiz AG, Casafont F, Crespo J, et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg 2007; 17: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 49. Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008; 138: 1452–1455. [DOI] [PubMed] [Google Scholar]
- 50. Jin R, Willment A, Patel SS, et al. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol 2014; 2014: 560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes Rev 2012; 13: 799–809. [DOI] [PubMed] [Google Scholar]
- 52. Miura K, Ishioka M, Minami S, et al. Toll-like receptor 4 on macrophage promotes the development of steatohepatitis-related hepatocellular carcinoma in mice. J Biol Chem 2016; 291: 11504–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 2010; 139: 323–334 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wagnerberger S, Spruss A, Kanuri G, et al. Toll-like receptors 1–9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr 2012; 107: 1727–1738. [DOI] [PubMed] [Google Scholar]
- 55. Karczewski J, Troost FJ, Konings I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 2010; 298: G851–G859. [DOI] [PubMed] [Google Scholar]
- 56. Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 2015; 5: 8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013; 57: 601–609. [DOI] [PubMed] [Google Scholar]
- 59. Engstler AJ, Aumiller T, Degen C, et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut 2016; 65: 1564–1571. [DOI] [PubMed] [Google Scholar]
- 60. You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol 2004; 34: 39–43. [DOI] [PubMed] [Google Scholar]
- 61. Crabb DW, Galli A, Fischer M, et al. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol 2004; 34: 35–38. [DOI] [PubMed] [Google Scholar]
- 62. Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2004; 286: G881–G884. [DOI] [PubMed] [Google Scholar]
- 63. Yi HW, Ma YX, Wang XN, et al. Ethanol promotes saturated fatty acid-induced hepatoxicity through endoplasmic reticulum (ER) stress response. Chin J Nat Med 2015; 13: 250–256. [DOI] [PubMed] [Google Scholar]
- 64. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 2015; 61: 1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010; 51: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jang C, Hui S, Lu W, et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 2018; 27: 351–361 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Douhara A, Moriya K, Yoshiji H, et al. Reduction of endotoxin attenuates liver fibrosis through suppression of hepatic stellate cell activation and remission of intestinal permeability in a rat non-alcoholic steatohepatitis model. Mol Med Rep 2015; 11: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wree A, McGeough MD, Pena CA, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014; 92: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mazagova M, Wang L, Anfora AT, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J 2015; 29: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016; 63: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lelouvier B, Servant F, Paisse S, et al. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 2016; 64: 2015–2027. [DOI] [PubMed] [Google Scholar]
- 72. Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017; 25: 1054–1062 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Spanogiannopoulos P, Bess EN, Carmody RN, et al. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 2016; 14: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14: 491–502. [DOI] [PubMed] [Google Scholar]
- 75. Loman BR, Hernandez-Saavedra D, An R, et al. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev 2018; 76: 822–839. [DOI] [PubMed] [Google Scholar]
- 76. Gangarapu V, Ince AT, Baysal B, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2015; 27: 840–845. [DOI] [PubMed] [Google Scholar]
- 77. Song Y, Garg S, Girotra M, et al. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS One 2013; 8: e81330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Diehl AM. JKB-121 in patients wtih nonalcoholic steatohepatitis: a phase 2 double-blind randomized control study. The International Liver Congress 2018 Abstract Book. J Hepatol 2018; 68 (Suppl. 1): S1–S926. [Google Scholar]
- 80. Kochan TJ, Somers MJ, Kaiser AM, et al. Intestinal calcium and bile salts facilitate germination of Clostridium difficile spores. PLoS Pathog 2017; 13: e1006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tremblay S, Romain G, Roux M, et al. Bile acid administration elicits an intestinal antimicrobial program and reduces the bacterial burden in two mouse models of enteric infection. Infect Immun 2017; 85: e00942–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Friedman ES, Li Y, Shen TD, et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 2018; 155: 1741–1752, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ubeda M, Lario M, Munoz L, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 2016; 64: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 84. Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018; 391: 1174–1185. [DOI] [PubMed] [Google Scholar]
- 85. Harrison SA. NGM282 rapidly improves NAFLD activity score (NAS) and fibrosis in 12 weeks in patients with biopsy confirmed nonalcoholic steatohepatitis (NASH). Results of a phase 2 multi-center dose finding study. In: AASLD, San Francisco, 2018. [Google Scholar]
- 86. Rinella ME, Trotter JF, Abdelmalek MF, et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with nonalcoholic steatohepatitis. J Hepatol. Epub ahead of print 8 December 2018. DOI: 10.1016/j.jhep.2018. [DOI] [PubMed] [Google Scholar]