Abstract
Cardiovascular disease remains one of the top contributors to morbidity and mortality in the United States. Increasing evidence suggests that many processes, pathways, and programs observed during development and organogenesis are recapitulated in adults in the face of disease. Therefore, a heightened understanding of cardiac development and organogenesis will help increase our understanding of developmental defects and cardiovascular diseases in adults. Chicks have long served as a model system in which to study developmental problems. Detailed descriptions of morphogenesis, low cost, accessibility, ease of manipulation, and the optimization of genetic engineering techniques have made chicks a robust model for studying development and make it a powerful platform for cardiovascular research. This review summarizes the cardiac developmental milestones of embryonic chickens, practical considerations when working with chicken embryos, and techniques available for use in chicks (including tissue chimeras, genetic manipulations, and live imaging). In addition, this article highlights examples that accentuate the utility of the embryonic chicken as model system in which to study cardiac development, particularly epicardial development, and that underscore the importance of how studying development informs our understanding of disease.
Abbreviations: CVD, cardiovascular disease; PEO, proepicardial organ
The Burden of Cardiac Disease, the Importance of Cardiovascular Research, and the Embryonic Chicken in Scientific Discovery
In the 2018 Update on Heart Disease and Stroke Statistics released from the American Heart Association, it was noted that the prevalence of total cardiovascular disease (CVD) is estimated at 92,100,000 cases, and CVD accounts for nearly 836,546 deaths in the United States.10 This figure represents approximately 2300 Americans each day, or 1 death every 38 s. Direct and indirect costs are estimated to total more than $329.7 billion, including both health expenditures and lost productivity. The total direct medical costs associated with CVD are estimated to reach $749 billion in the year 2035. With heart disease accounting for 1 of every 7 deaths, CVD remains the leading cause of death in the United States. In addition to the 2018 report from the American Heart Association, a series of additional studies assessed CVD in specific demographic groups including Latin Americans,38 Hispanic populations,137 African Americans,111 and other research investigated cardiovascular health in US and nonUS populations.176
The aforementioned figures and statistics serve to underscore the importance of and highlight necessity for, expanding our understanding of cardiovascular biology and disease. With this knowledge, we can better inform our approach to exploring and discovering innovative and efficacious therapeutic modalities.
With increasing technologies, more and more animal models are becoming available that are amenable to the manipulations necessary to address specific research goals.122 This review highlights the embryonic chicken as an important model for studying cardiovascular biology, focusing specifically on cardiac and epicardial development.
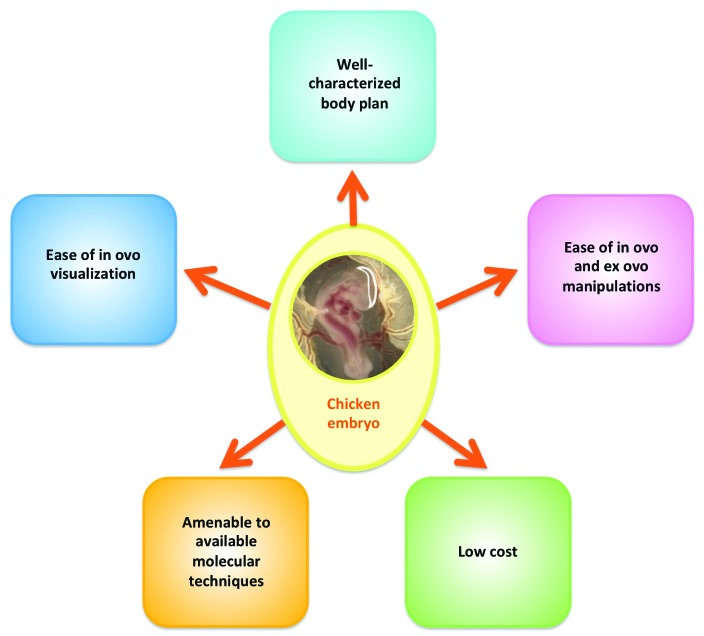
There are numerous benefits to using embryonic chickens as a model system (Figure 1). Embryonic chickens are a widely available model organism and have been used in a variety of research endeavors. Areas in which embryonic chickens have been used range from tissue patterning and symmetry,4,6,98,138,146 cell migration,1,8,16,75,76,96,121,168,174 vasculogenesis,24,86 and specific organ system biology, such as cardiac morphogenesis44,70,88,90,165 (which is the focus of this review). In addition to its wide use in developmental biology studies, embryonic chickens have also been used for studying cancer biology and cancer metastasis,81,172 including cancer biology studies for malignancies affecting veterinary domestic species.166,177 Lastly, chicken embryos have been used to study various aspects of toxicology,73,77 including developmental toxicology, such as fetal alcohol syndrome,139 and infectious disease research.64,153
Figure 1.
Benefits of using domestic chickens for studying developmental biology questions.
Presented in the current review are various components concerning the use of the embryonic chicken (Gallus gallus domesticus) in scientific discovery, with a focus on cardiac development. Included is a timeline of key cardiac developmental events, practical experimental considerations, and examples of experimental techniques available for use in chicks (specifically tissue chimeras, genetic manipulations, and live imaging). This review also addresses how the study of developmental mechanisms can heighten our understanding of disease states. Emphasized is how embryonic chickens have increased our knowledge of epicardial development. This review concludes with a discussion regarding how embryologic investigation can inform the decision-making process in efforts dedicated to the exploration of therapeutic modalities, and why embryonic chickens are ideal candidates for such endeavors.
Cardiac Development of the Chicken Embryo
Roadmap to the entire embryo: the contribution of Hamburger and Hamilton.
Because of the chicken's extensive use as a model organism, detailed observations on the formation of organs of the chicken embryo are readily available. In 1951, Viktor Hamburger and Howard Hamilton published a series describing the normal stages of development of the chicken embryo,52 in which they enumerated and described the features of different stages and the associated timeline in hours or days. The stages described have come to be known as the Hamburger and Hamilton (HH) stages of chick development, and this designation is routinely used in developmental biology studies involving embryonic chickens. In their descriptions, Hamburger and Hamilton go into considerable morphologic detail, including that of the somites, otic and optic vesicles (and at a later time, ears and eyes), heart tube (and at a later time, heart), tissue folds, limb primordia and buds (and at a later time, limbs), location and extent of the amnion, among others. Almost 70 y later, HH stages are still widely used as a roadmap to the progression of development and organogenesis. Moreover, the publication51 serves as a robust reference against which to compare deranged development arising from experimental manipulations.
Cardiac developmental stages in chicken embryos
Using as a template the stages described by Hamburger and Hamilton in 1951,52 Martinsen published a reference guide to the developmental stages of the embryonic chicken heart.93 This guide provides detailed descriptions of the morphogenetic events throughout cardiac development, including fusion of the heart tubes, cardiac looping, septation, coronary vascular development, epicardial development, valvular development, and the development of cardiac innervation. Taken directly from Martinsen's guide, select milestones throughout the progression of cardiac development in the embryonic chicken have been summarized in Table 1.
Table 1.
Cardiac developmental milestones in the chicken according to HH stages
| HH stage | Time of incubation | Major cardiac developmental milestones |
| 1 | First few hours | Identification of cardiac progenitors54 |
| 2 | 6–7 h | Presence of precardiac cells in the epiblast lateral to the primitive streak147 |
| 3 | 12–13 h | Presence of cardiac progenitor cells of 2 distinct lineages in the anterior 2 thirds of primitive streak170 |
| 4–5 | 18–22 h | Precardiac mesoderm is specified to some degree130 |
| Cardiac precursor cells migrate to the lateral plate mesoderm (primary heart fields)84 | ||
| 6–7 | 23–26 h | The first reported markers of terminal myocardial differentiation are detected in the primary heart fields11 |
| Presumptive endocardial cells from the splanchnic mesoderm initiate the formation of the bilateral endocardial heart tubes | ||
| 8 | 26–29 h | Process of endocardial tube fusion starts to give rise to a straight heart tube160 |
| 9 | 29–33 h | Cardiac crest cells begin migrating from the neural tube's neural crest toward the aorticopulmonary septum of the outflow tract12 |
| Linear heart tube starts to loop | ||
| 10–11 | 33–45 h | Tubular heart is completely fused (except the straight portion of the outflow tract) and spontaneously beats (despite cardiac pacemaker and conduction system not being developed yet)34,46 |
| 12–13– | 45–49 h | Dextral looping phase of cardiac looping is completed90 |
| Endocardial cushions are seen as an expansion of the cardiac jelly, preceding the cushion mesenchyme92 | ||
| 13 | 50–52 h | Transformation of the c-shaped heart loop into an S-shaped heart loop90 |
| 14–15 | 50–55 h | Atrioventricular ring has formed (part of the central conduction system)19 |
| Emergence of the proepicardial organ as villous projections from the surface of the sinus venosus that gives rise to the epicardium (which eventually becomes coronary arteries and subepicardial mesenchyme)116 | ||
| 16 | 51–56 h | Atria begin to form as lateral expansions of the primitive atrial region90 |
| Both the atrioventricular and outflow tract cushions emerge92 | ||
| Trabeculation begins in the inner myocardial layers at the level of the greater curvature58 | ||
| 17–18 | 52–69 h | S-shaped heart loop is completed90 |
| The primary atrial septum begins to form91 | ||
| Villous projections of the proepicardial organ reach the dorsal wall of the heart, and the epicardium begins to cover the heart116 | ||
| 19–20 | 68–72 h | Epicardium has moved over the inner curvature of the heart116 |
| Dorsal and ventral cushions of the atrioventricular canal have started to form29 | ||
| 21–23 | 3.5–4 d | Cardiac neural crest cells have reached the outflow tract164 |
| Epicardial cells enter the myocardium for the first time89 | ||
| Leaflet formation in the atrioventricular cushion begins145 | ||
| Trabecular bundles at the level of the ventricular groove are seen (although a true interventricular septum is not present)9 | ||
| 24 | 4 d | Interatrial septum fuses with the dorsal and ventral cushions, and develops numerous secondary communications (allowing shunting of blood)99 |
| Late phase of cardiac looping (septation) begins90 | ||
| 25–26 | 4.5–5 d | Aorticopulmonary septum begins to divide the aortic and pulmonary sides of the aortic sac164 |
| Coronary vasculature begins to develop163 | ||
| 27 | 5 d | Formation of the epicardium is completed89 |
| Thickening of the compact myocardium, and trabeculation of the atrial myocardium131 | ||
| 28 | 5.5 d | Sinoatrial and atrioventricular nodes and the upper part of the atrioventricular bundle start to form3,114 |
| Atrioventricular valves start to form21,173 | ||
| 29 | 6 d | Distinct mitral and tricuspid valve primordia are present85 |
| Interventricular septum has grown toward, and fused with, the atrioventricular cushions29 | ||
| Prominent myocardial proliferation (lasting through day 8), increasing the density in the ventricular myocardial compact zone by 80%113 | ||
| 30 | 6.5 d | Formation of capillary plexi and venous sinusoids165 |
| 31 | 7 d | Septation of the distal 2/3 of the truncus is almost complete164 |
| Developing aortic and pulmonary valve leaflets are offset and angled relative to each other118 | ||
| 32–33 | 7.5–8 d | Multiple vascular channels connect the left and right aortic sinuses, and dominant channels become the left and right coronary arteries165 |
| Epicardium-derived cells are distributed throughout the heart44,89 | ||
| 34 | 8 d | Completion of the outflow tract septation by (1) the aorticopulmonary septum (distal outflow tract) and (2) the joining of the 2 proximal cushions (proximal outflow tract)71,118,164 |
| Coronary arteries are formed, and the medial and adventitial layers start to form89 | ||
| Semilunar valves are completely formed118 | ||
| Ventricular septation is complete164 | ||
| 36 | 10 d | Formation of putative conductive cells (leading to differentiation of Purkinje fibers)47,48 |
| Cardiac neural crest cells differentiate into cardiac ganglion cells156 | ||
| Atrioventricular valves are completely formed21,85 | ||
| 38 | 12 d | Coronary veins are formed, and the medial and adventitial layers begin to form |
| 40 | 14 d | Innervation by the cardiac neural crest cells is complete |
| Differentiation of medial and adventitial layers of coronary arteries and veins is complete by HH43163 | ||
| 46 | 20–21 d | Interatrial septum is closed by 2 d after hatching119 |
| Reduction of the rate of myocardial proliferation, which ceases at hatching, but increases again after hatching61 |
HH, Hamburger and Hamilton
Adapted with permission from reference 93.
Lastly, an elegant atlas of the cardiac developmental stages in chick that includes color plates has been published.2 Refinement of anatomic road mapping techniques is still the subject of contemporary investigations. To this end, various investigator groups have developed novel systems for staging developing embryos and have used the chicken as a species in which to perform validation of such techniques. As an example, one novel system is based on cross-sectional images of organs (rather than 3D structures) by using elliptic Fourier descriptors for quantification.67
Using the Chicken Embryo as a Model Organism
Getting your chicks in a row: getting started.
Investigators using chicken embryos for research purposes should adhere to institutional guidelines for handling of embryos: guidelines are often based on the age and developmental or incubation stage of the embryos. Investigators are always encouraged to verify with their IACUC to ensure adherence to the institution's specific policies.
The necessary laboratory set-up, including the equipment, is dependent on the specific type of research being conducted. However, an egg incubator is a universal necessity. Numerous types of egg incubators are commercially available, and the specific model depends on the volume of the research to be done (and thus the number of eggs needed at any single time), laboratory space availability, and budget. Egg incubators range from small tabletop units that hold a maximum of 7 eggs to large floor units with capacities of more than 200 eggs and equipped with automatic temperature and humidity controls as well as programmable turning functions. Because of the critical importance of maintaining a consistent temperature during incubation (discussed later), a secondary means of temperature monitoring is recommended. Depending on the incubator type, the temperature within various parts of the incubation chamber may differ, especially when incubation batches differ in egg count. Therefore, it is important to become familiar with the specific incubator selected and to perform numerous trials of incubator thermometers to ensure thermal consistency during incubation for experiments.
Acquisition of chicken embryos and preincubation conditions.
There are several sources for the acquisition of fertilized chicken embryos for developmental biology studies. Academic institutions with Animal Science programs may have avian production facilities from which fertilized chicken embryos can be obtained. In addition commercial suppliers of fertilized egg are available, some of which provide SPF fertilized eggs. Depending on the location of the supplier, fertilized eggs may need to be stored; storage of fertilized eggs can alter the progression of embryonic development (addressed later). Therefore, the logistics of when fertilized eggs are ordered, how long delivery will take, and how long before incubation will start must all be considered closely and carefully orchestrated.
Another important component to consider when contemplating developmental biology studies using chicken embryos is the source flock. Whereas younger flocks tend to produce smaller eggs with smaller yolks, older flocks tend to produce larger eggs with thinner shells. Time of the year needs to be considered also, given that increased ambient temperatures can result in decreased eggshell quality.148 Therefore, eggs laid during the summertime, especially when from an older flock, will tend to be fragile and prone to cracking and breaking, in turn rendering the eggs nonusable for incubation and subsequent developmental biology studies. Therefore, the quality of the egg is dependent on various factors, including nutrition, the age of the flock, and time of the year.
Attention should be paid to storage and preincubation conditions of fertilized eggs, because these 2 factors can greatly influence the development of the embryos. Prolonged storage of fertilized eggs reduces hatchability: the longer the storage period, the lower the hatchability. In addition, as the preincubation storage period increases, the development of both chicken and turkey embryos is increasingly delayed after the initiation of incubation.5 For example, at 40 h of incubation (approximately HH10–11), chicken embryos from eggs that had not been stored or had been stored for only 3 d prior to incubation had 10 to 12 somites. However, embryos from chicken eggs that had been stored for 7 d had 7.8 somites on average at 40 h of incubation, and embryos from eggs stored for 21 d prior to incubation had 2.4 somites on average at the same time point.5 A similar phenomenon was observed in turkey embryos. In general, storing fertilized eggs for more than 1 wk reduces the hatchability of embryos, and long-term storage of fertilized eggs leads to delayed incubation times to hatch.36 Upregulation of proapoptotic genes in the chick blastoderm occurs with increasing preincubation storage times, thus suggesting that the overall decrease in embryo quality on stored fertilized eggs is due, at least in part, to apoptosis in the blastoderm.53
Various groups have studied the effect of prestorage incubation on embryonic development and hatchability. Prestorage incubation treatment refers to subjecting fertilized eggs to incubation at 37.5 °C for various amounts of time before storing the eggs. Increased hours of prestorage incubation advanced embryonic development.37 The effects of prestorage incubation are dependent on various factors, including the storage time (storage for 8 d or more greatly reduces overall success), embryonic development at the time of egg collection, and the duration of the prestorage incubation period.120 In summary, storage of fertilized eggs used for developmental biology studies should be minimized, because prolonged storage not only affects the number of viable embryos but also delays the onset and progression of embryonic development once incubation is initiated.
Processing of fertilized eggs and incubation.
Prior to incubation, fertilized chicken eggs should be inspected thoroughly. Any dirt or fecal material that was not removed prior to transport should be removed before incubation. For this purpose, a 70% ethanol solution can be gently wiped on the eggshell to remove debris without detrimental effects on embryonic development. The eggshells should be inspected thoroughly for any cracks and defects; when present, the eggs should be discarded. During incubation, eggs should be placed on their side in cardboard or nonporous plastic trays; nonporous plastic trays can be cleaned and disinfected easily and thus are reusable, thereby reducing waste.
For incubation, fertilized chicken eggs should be placed in a humidified incubator and maintained at 37.5 to 37.8 °C and 50% to 60% relative humidity. The water supply system for maintaining humidified conditions is dependent on the incubator model used. Regardless of the model used, sterile water should be used for humidification. Various biocidal products to prevent the growth of bacteria, fungi, and algae in water incubation reservoirs are commercially available. Between incubation batches, the water in the reservoir should be changed and the reservoir cleaned. Various disinfectant agents are available, but not all are adequate. Quaternary ammonium disinfectants are regarded as perhaps the most appropriate compounds both for cleaning the incubator and as a disinfection additive for the water reservoir. Copper sulfate should not be used long term, and bleach-containing compounds should be avoided.
As mentioned previously, the incubation temperature should be maintained consistently at 37.5 to 37.8 °C during the incubation period. Unnecessary opening of the incubation chamber should be avoided to prevent the escape of warm air, which will lower the temperature within the chamber. It is worth emphasizing that the developmental stage or incubation ‘percentage progress’ of an embryo is not necessarily equivalent to the amount of time that has lapsed since the initiation of incubation, especially if the temperature has not been kept consistently at 37.5 to 37.8 °C. On average, fertilized chicken embryos will hatch at 21 d from the initiation of incubation when maintained at a constant incubation temperature of 37.5 to 37.8 °C. However, if the temperature drops during the incubation period, the developmental process will slow down, leading to incongruence between the expected and actual developmental progress. Minimal (–3 °C) and intermittent (4-h periods for each of embryonic days 16, 17, and 18) decreases in temperature slow down metabolism but do not necessarily alter the growth of the embryo nor the hatchability at day 21.171 However, a decrease in temperature below 27 °C will halt embryonic development,39 and when reincubation at 37.5 to 37.8 °C is reinstated, incubation times are delayed approximately as long as the time of embryonic cooling.144 In the author's experience, incubation times are delayed with cooling at ambient temperature, with the delay approximating the length of cooling as described by Suarez. The author has only exposed embryos to room temperature after 72 h of incubation (stages HH18-19) for 24 h, and then reincubated at 37.5 to 37.8 °C. Development is continued once the embryos are reincubated at 37.5 to 37.8 °C. In summary, maintaining a consistent temperature is critical for developmental biology studies, given that major developmental processes and events can happen in a short time window, making exact timing of the essence.
Euthanasia of chicken embryos.
In 2013, the AVMA published guidelines for the euthanasia of animals.7 Concerning the euthanasia of avian species, especially of eggs, embryos, and neonates, the AMVA guidelines specify:
“Bird embryos that have attained >50% incubation have developed a neural tube sufficient for pain perception; therefore, they should be euthanized by similar methods used in avian neonates such as anesthetic overdose, decapitation, and prolonged (>20 min) exposure to CO2. Eggs at <50% incubation may be destroyed by prolonged exposure (>20 min) to CO2, cooling (<4°C for 4 h), or freezing. Anesthesia can be used prior to euthanasia and is most easily accomplished with exposure to inhaled anesthetics via entry into the air cell at the large end of the egg. Egg addling can also be used to destroy the viability of embryos.”
Resources for developmental biology studies using embryonic chickens
A series of online resources that are useful during developmental biology studies using embryonic chickens is presented in Table 2.
Table 2.
Useful online resources for chick developmental studies
| Resource | Notes | URL |
| Chicken Genome Nomenclature Consortium (CGNC) | Standardized gene nomenclature for chicken genes | http://birdgenenames.org/cgnc/about |
| Gallus Expression in Situ Hybridization Analysis (GEISHA) | Repository for in situ hybridization data for genes expressed in embryonic chickens | http://geisha.arizona.edu/geisha/index.jsp |
| Bird Base | Provides a platform for bird genomes and genomics, and promotes collaboration between scientists using birds as model organisms | http://birdbase.arizona.edu/birdbase/index.jsp |
| eChick Atlas Project | Aims to establish a database of gene expression patterns during chick development | http://www.echickatlas.org/ecap/home.html |
| Ensemble | Genomics browser | https://uswest.ensembl.org/Gallus_gallus/Info/Index |
| NCBI Genome Browser | Genome browser | https://www.ncbi.nlm.nih.gov/genome?term=gallus%20gallus |
| Developmental Studies Hybridoma Bank (DSHB) | Supplier of monoclonal antibodies for research; can search by species or antigen | http://dshb.biology.uiowa.edu/ |
| Addgene | Plasmid repository | https://www.addgene.org/ |
Techniques Available for Use in Embryonic Chickens
The chicken embryo is a robust system that is amenable to a variety of experimental techniques and manipulations (Figure 2). Embryonic chickens are an easily operable system from the standpoint of visualization, access, and physical and surgical manipulation (Figure 3 A). In addition, chicken embryos have evolved as a genetically tractable system that is amenable to a variety of experimental molecular approaches.
Figure 2.
Techniques available for developmental biology studies in chickens.
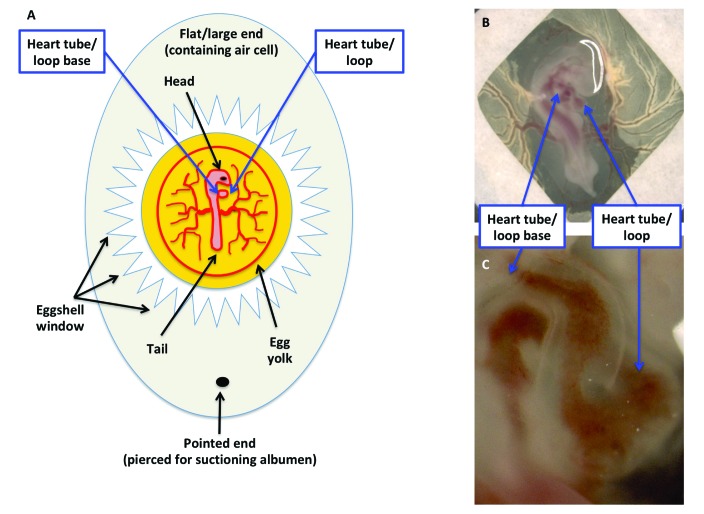
Figure 3.
Ease of visualization and manipulation of the developing chicken embryo. (A) Schematic diagram of a fertilized egg containing a HH16–18 embryo. The end opposite to the air cell has been pierced to remove albumin, and a window in the shell has been created by using jeweler's forceps. (B) Modification of the early chick (EC) culture system, in which chicken embryos within a filter paper frame are transferred to a culture dish. (C) Close-up of panel B.
Tissue chimeras.
Since the 1970s, quail–chick chimeras have been used to answer developmental biology questions.30,51 In 1969, the specific features of the quail heterochromatin that allow it to be differentiated from that of chick cells were described, and in 1980, the use of quail–chick chimeras for following the migration of neural crest cells was proposed.79 This promising platform prompted the development of improved techniques, such as an enhanced histology technique for quail–chick chimeras.57 The assumption made when using tissue chimeras is that the developmental process of interest occurs in the chimera as it would during normal development. In principle, a given territory of a recipient embryo is removed and then replaced (as precisely as possible) with the equivalent region from the donor (Figure 4)149 . Protocols for preparing quail-chick chimeras have been published elsewhere,143,149 including quail–chick chimeras of the epicardial primordium.89 After the chimera is prepared, the embryo (or the portion of interest) can be stained by using the quail-specific QCPN or QH1 antibody. The QCPN antibody binds to antigens on quail nuclei, and QH1 binds to a surface marker of quail endothelial and hematopoietic cells.112,133Because tissue chimeras involve extremely precise surgical manipulation, this technique generally involves a steep learning curve.
Figure 4.
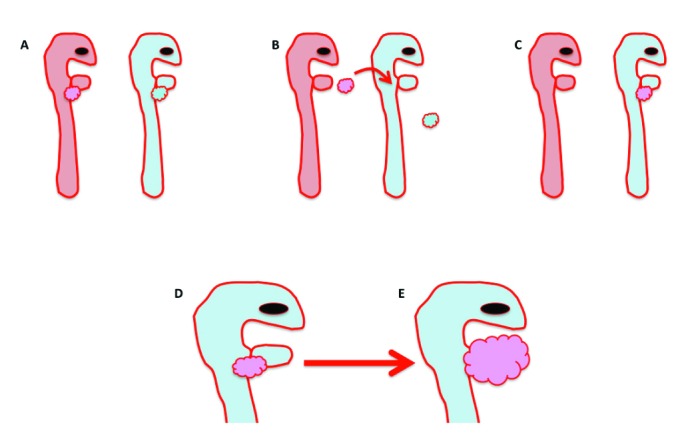
Schematic diagram of proepicarial organ quail–chicken chimera experiments. (A) For these experiments, a quail embryo (pink) serves as the donor, and the chicken embryo (blue) is the recipient. (B) The proepicardial organ from the quail donor (pink bubbles, B) is surgically removed and placed at the site of the recipient's proepicardial organ (blue bubbles). (C, D) The chicken recipient is reincubated with the transplanted quail epicardium (pink bubbles), and (E) the proepicaridal organ develops into the epicardium, which then envelops the entire heart (pink bubbles) as it would during normal development.
Fate mapping and lineage tracing by using vital dyes.
Vital dyes are stains that have the capacity to penetrate living cells or tissues without detrimental effects to those cells or tissues. These dyes can be used to highlight specific organelles, including mitochondria (for example, rhodamine 123, MitoTracker), nuclei (for example, Hoescht stain), Golgi apparatus (for example, CellLight), endoplasmic reticulum (for example, ER-Tracker, BODIPY), and cell membranes (for example, DiI [red-orange], DiO [green], DiR [infra red], and DiD [red]). The use of vital dyes has been a powerful tool for fate mapping and lineage tracing experiments in chicken embryos. The use of this tool has expanded the current understanding of cell contributions and cell fates involving the sinus venosus,69 heart fields17 (during cardiac morphogenesis), epicardial derived cells,115 and most notably the neural tube and neural crest.127,134-136 Due to dilution effect as cells replicate (the same amount of dye is distributed among more cells), the signal obtained from vital dyes may become increasingly dimmer as cell division occurs.
Ex ovo manipulations.
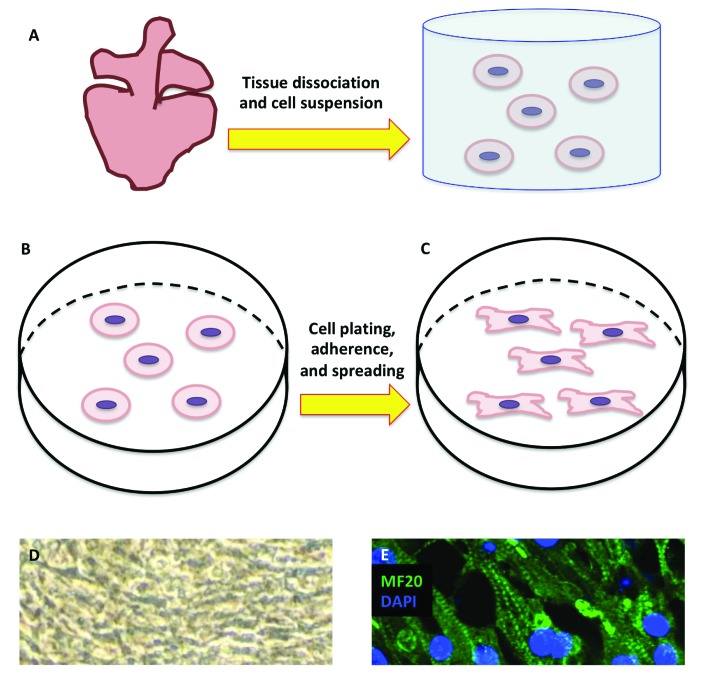
The chicken embryo allows for easy access and visualization in ovo (Figure 3 A). When working in ovo, diluted and sterile India ink solution can be injected into the yolk to contrast with the opaque, semitransparent embryo; this technique may be particularly helpful in embryos younger than stages HH13–14). Numerous authors have developed ex ovo, shell-less set-ups for improved visualization and ease of surgical manipulation. First described in 1955 is an in vitro technique in which the chick blastoderm is explanted into a piece of vitelline membrane stretched across a glass ring; this technique was then used to study chick blastoderm expansion.104 Since then, additional ex ovo techniques have been developed. For example, the early chick (EC) culture system (Figure 3 B and C) is a variation of the technique just described.20 In the EC culture system, a piece of filter paper is used as a carrier to transfer the embryo ex ovo as the membranes are maintained under tension; this tension is a critical requirement for ensuring continued and orderly embryonic morphogenesis. Other techniques described for ex ovo culture of chicken embryos include polyurethane membrane ‘hammocks’, 175 laboratory weighboat humidified chambers,23 and the submerged filter-paper sandwich128 technique, which is a modification of the EC culture system. In addition to the whole-embryo culture systems just described, chicken embryos are amenable to organ slice culture and tissue culture techniques. A previously described technique62 has been modified to yield cardiomyocyte cultures that achieve electromechanical coupling, beat synchronously, and have well-developed myosin heavy chain (MF20)–positive cytoplasmic striations (Figure 5).
Figure 5.
Chicken cardiomyocyte culture for in vitro studies. (A) HH36 (E10) embryonic chicken hearts are mechanically and enzymatically dissociated, after which cardiomyocytes are suspended in medium, centrifuged, and subsequently resuspended. After filtering, (B) the cell suspension is plated, and (C) the plate is left unperturbed to allow myoblasts to attach and spread. (D) Attached myoblasts eventually become confluent, achieve electromechanical coupling, and beat synchronously. (E) Cultured myoblasts express the cardiac contractile protein MF20 (stained with Alexa Fluor 488), highlighting the striated nature of the muscle cells (nuclei stained with DAPI; magnification, 40×).
Genetic manipulations in chicken embryos.
The chicken genome assembly was completed and published in 2004.14,167 This major breakthrough represented the first avian genome to be sequenced and thus reestablished the chicken embryo as a key system in developmental biology and biomedical research endeavors.15 Multiple publications have used gene modification techniques in chicken embryos,126 making chicken a genetically tractable model species.
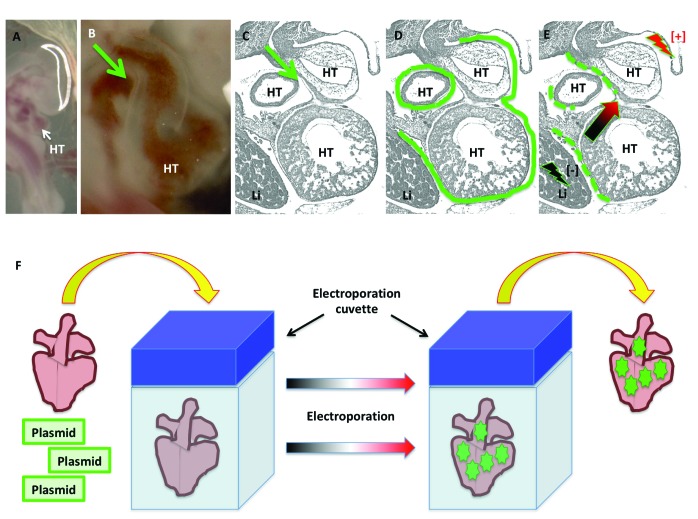
In 1982, the use of electric impulses to enhance the uptake of DNA into mouse cells was described.103 This technique—electroporation—uses electrical fields as a way to facilitate the transfer of genetic material into cells. The electrical currents create transient membrane destabilization resulting in the formation of reversible pores through which nucleic acids and their analogs can be transported readily into the cytosol.126 Electroporation has long been used as a platform for delivering genetic material to chicken embryos,59,107 and numerous applications are reliant on this platform for both gain-of-function and loss-of-function experiments.100,106 Some of the technologies used for genetic manipulation and misexpression experiments in the chick (and that use electroporation for their delivery) include: overexpression constructs (gain-of-function), morpholino antisense oligonucleotides (loss-of-function),105,161 dominant-negative constructs (loss-of-function), and RNAi technology (loss-of-function). Techniques for electroporating epicardial cells in the chick intact heart in ovo (Figure 6 A through E) and for the preparation of vibratome tissue slices for live imaging (Figures 6 F and 7) are available. Electroporation results in mosaicism, which may be a desired feature depending on the application and can facilitate the comparison of targeted and nontargeted cells in the same sample.
Figure 6.
In ovo and ex ovo epicardial electroporation in embryonic chickens. (A through E) In ovo epicardial electroporation for stages HH21–24. For in ovo epicardial electroporation, (A) the embryo is visualized, and (B and C) the pericardial cavity injected (green arrows) with (D) plasmid solution (green continuous line). Immediately after plasmid injection into the pericardial space, (E) the epicardium is electroporated by delivering currents at the indicated sites (lighting symbols), causing the plasmids to enter the epicardial cells. (F) For ex ovo epicardial electroporation, the heart is dissected and placed inside an electroporation cuvette containing a plasmid solution. Delivery of electric current results in the electroporation of epicardial cells. The green dotted lines in panel E and the green stars in panel F represent the successfully electroporated cells. HT, heart tube; Li, liver.
Figure 7.
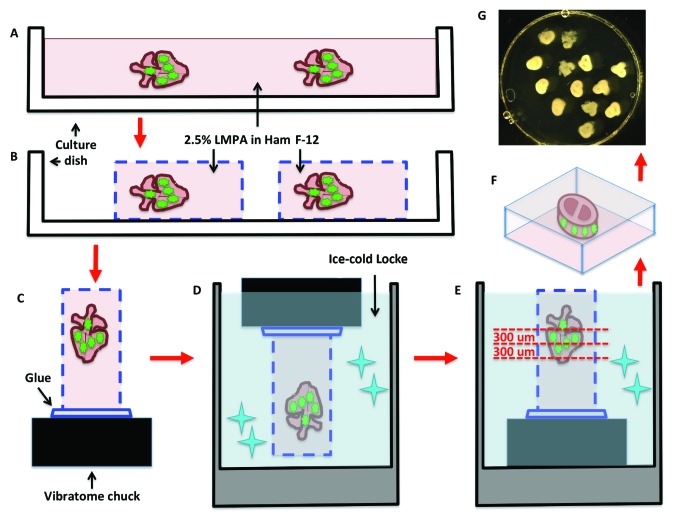
Vibratome sectioning for live tissue culture slices of electroporated embryonic hearts. (A) Electroporated whole hearts are embedded in a plate containing low-melting point agarose (LMPA) suspended in Ham F-12 nutrient medium. Once solidified, (B) the agarose is cut to create agarose blocks containing the electroporated hearts, which are then (C) mounted on a vibratome chuck and (D) immersed in ice-cold Locke solution to solidify the mounting glue. (E) While in ice-cold Locke solution, the agarose blocks containing the hearts are sectioned with a vibratome (F) into 300-μm-thick slices, which then (G) are transferred to a culture dish and covered with culture medium.
Some recent techniques for use in chicken embryos include the use of CRISPR–Cas9 technology for targeted mutagenesis,40,109,157,180 flipase recombinase-mediated cassette exchange,80 and an inducible transgene expression system.41 A 2016 review outlines the advances in genetic engineering in chicken embryos, with historical perspectives of the milestones achieved on this front.31
Regarding vibratome sectioning for live imaging, the most influential factors in successful sectioning are likely the stiffness and size of the tissue. Often softer tissues (such as the trunk of a HH16 embryo) are easier to section than harder tissues (such as the heart of a HH36 embryo). In addition, the smaller the diameter of the tissue, the easier it is to section. Therefore, a limitation of this technique is that it may not yield adequate sections of stiffer and larger tissues. In general, limitations associated with vibratome sectioning are 1) individual sections (as compared with a series of consecutive sections [‘ribbons’] produced by microtomes from formalin-fixed, paraffin-embedded tissues), 2) thicker sections, and 3) fragility of the tissue blocks, which are embedded in agarose (as compared with stiffer paraffin wax). However, in contrast to processing for formalin-fixed, paraffin-embedded tissues, vibratome sectioning allows for obtaining live tissue slices. Vibratome sectioning can be performed at multiple stages depending on the target section, such as whole embryos at the level of the trunk at stages HH12–18 and sections of dissected hearts from embryos of stages HH16–24.
Time-lapse live imaging.
As mentioned previously, chicken embryos allow for ready access and ease of manipulation, affording the ability to perform a variety of techniques not easily performed in other models. Two of these techniques include live tissue imaging and time-lapse video microscopy. Imaging of live tissues, and especially time-lapse imaging provides a firm biologic and cell-based context in which to interpret observations made through other modalities (especially those based on fixed tissues, which only provide a single snapshot in time). In the chick, time-lapse live imaging has been used to study a variety of cell processes including cell behavior of the neuroepithelium,25 neural crest behavior, and migration,1,141 somitogenesis,74 and migration of cardiac progenitor cells.140 In addition, time-lapse imaging has been optimized to study cell migratory behavior during epicardial development in the chick (Figure 8). Other techniques for live imaging of chicken embryos, such as 3D microCT have been described.55
Figure 8.
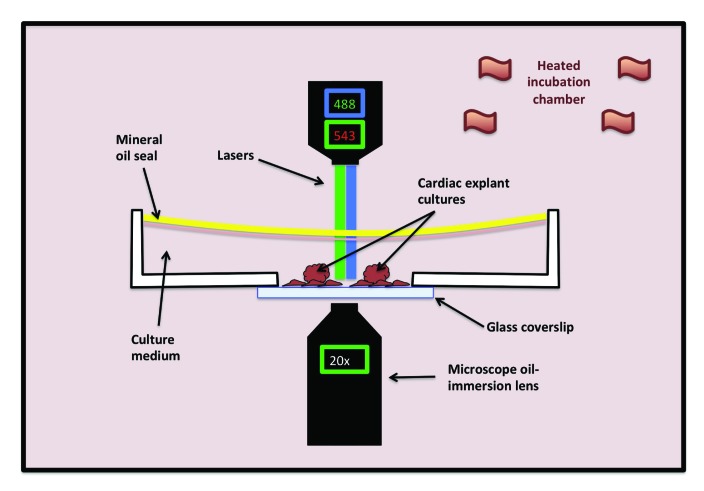
Set-up for fluorescent imaging of live tissue culture cells. A glass-bottom culture dish containing the fluorescently labeled tissues of interest and culture medium and sealed with mineral oil (to prevent medium evaporation) is placed on the stage of an inverted microscope within a heated incubation chamber. Depicted in this diagram is an inverted fluorescent microscope with a 488-nm laser to visualize the green channel and a 543-nm laser to visualize the red channel at 20× magnification.
Limitations associated with confocal imaging are dependent on the intended application. In the case of time-lapse live-cell imaging of cardiac tissues, one of the biggest challenges is the movement associated with the intrinsic beating of cardiac tube, which can make individual cells in the intact heart difficult to follow over time. Cardioplegic agents (such as potassium-containing solutions) can be added to the culture medium to prevent contractions. However, this addition may interfere with normal cell and tissue processes that are dependent, at least to a degree, on mechanical cues. Time-lapse confocal imaging of the intact heart is particularly challenging given that contraction occurs on all tissue planes. This feature makes imaging on different Z planes (especially when using multiple fluorophores) difficult and may result in images that do not yield accurate volumetric reconstructions (that is, ‘stacks’). For this reason, surrogates for the convoluted 3D intact heart (such as beating myoblast and cardiomyocyte flat cultures) afford the ability to image over time yet maintain synchronous beating that resembles the electromechanically coupled heart. Imaging by confocal microscopy is not limited to the embryonic stages described herein, because imaging was performed on either 1) cell monolayers or 2) vibratome tissue slices of a specified thickness (300 μm in this case). The latest stage at which confocal imaging of whole embryos can be done is likely dependent on the species, in light of the size that they attain by any given stage.
From Organogenesis to Disease: The Importance of Developmental Biology Investigation in Translational Research
The understanding of development, morphogenesis, and organogenesis greatly informs our understanding of disease and vice versa. As the exploration of development and disease continue, it becomes increasingly clear that many developmental processes in the embryo are redeployed in the adult in the face of disease and subsequent reparative attempts. Moreover, disease manifestations in the adult may be the expression of dysregulated developmental programs or processes.124 Although attempting to enumerate all possible instances in which these parallels exist is beyond the scope of this review, a few are mentioned to illustrate that strengthening our knowledge of development can greatly inform our understanding of the disease. The discussion presented in this review will focus on those parallels relating to cardiovascular development and CVD.
Congenital heart disease.
Many forms of congenital heart disease are a result of deranged cardiac developmental processes. Therefore better understanding how these arise requires an understanding of cardiac morphogenesis. Cardiac morphogenesis and the implications for congenital heart diseases have been reviewed recently.43 Briefly, the bilateral embryonic cardiac mesoderm unites at the midline to form the cardiac tube,142 which inherently beats even before the formation of blood elements. Part of this cardiac tube—the ‘cardiac jelly’—in turn leads to the formation of the endocardial cushions. At a later stage, and with contributions from the cardiac crest, the endocardial cushions lead to the formation of the cardiac valves and the outflow tract. As development progresses, dextral looping (right-sided) of the heart tube occurs to form the cardiac loop, and after a series of fusions and cell movements, the chambers begin to form and divisions between the various compartments take place. Derangements or accidents in the formation of any of these delicately orchestrated developmental events can result in a variety of malformations, including atrial and ventricular septal defects, Tetralogy of Fallot, valvular abnormalities, and aortic arch malformations, among others.
The epithelial-to-mesenchymal transition: an advantageous developmental process with negative implications in the adult organism.
First described by the laboratory of Elizabeth Hay,49 the phenomenon initially coined as epithelial-to-mesenchymal ‘transformation’ (EMT) is worthy of special recognition. Referred to in the current literature as epithelial-to-mesenchymal ‘transition,’ the EMT phenomenon has been studied extensively given its implications both in development and disease.65 EMT (and the reverse—MET) is widespread and commonplace in the developing embryo, most notably known for its role in gastrulation101,108 and delamination of the neural crest from the neural tube.150,179 In the adult, the EMT plays a crucial role in reparative attempts that result in scarring18,178 and, most notably, in cancer progression and metastasis.27,28,82,110 Interestingly, a body of literature suggests that the EMT is involved in the development of cardiac disease in adults, associated with myocardial scarring after infarction,22,162 whereas others indicate that the EMT may lead to the generation of cardiac progenitor cells.42,94 The EMT is a prime example of how harnessing developmental processes informs our understanding of the nuances underlying numerous disease processes.83,102,151
How Chicken Embryos Have Informed the Understanding of Epicardial Development and Point toward Future Investigation
This section is dedicated to highlighting how the chicken embryo has increased the understanding of epicardial development. All of the experiments featured in this section were performed in embryonic chickens (except when explicitly indicated), underscoring the importance of this model organism as a key player in understanding this important biologic process.
The epicardium is a single mesothelial cell layer that envelops the beating heart during development, providing a smooth gliding surface against the pericardial sac during cardiac contraction and relaxation. For a long time, the epicardium was considered to be a quiescent structure. However, interest has revived in further understanding the biology of this tissue due to the implications it has in the progression of myocardial disease in adults, particularly after myocardial infarction.
In the developing embryo, and at the level of the sinus venosus, the coelomic mesothelium forms a cerebriform cluster of cells, termed the proepicardial organ (PEO). This tissue is largely believed to arise from the splanchnic mesoderm; however, some experiments suggest that at least a portion of the cells comprising the PEO arise from the somatic mesoderm.128 Epicardial cells and myocardial cells from the inflow tract arise from a common precursor. Later on, this precursor separates into 2 lineages as a result of interactions between bone morphogenetic protein and fibroblast growth factor 2; bone morphogenetic protein signaling promotes myocardial formation, whereas fibroblast growth factor 2 signaling promotes epicardial differentiation.155 At HH14, the PEO forms villous-like projections, and by HH17, these villous projections make contact with the dorsal surface of the naked, continuously looping, and already beating myocardial tube88 (Figure 9 A). In agreement with previous experiments,155 other work showed that fibroblast growth factor ligands promote the formation of these villous projections, whereas fibroblast growth factor inhibition leads to their arrest.152 Once this cellular bridge is established, cells from the PEO begin a collective migration toward and over the cardiac tube to completely envelop it, as has been observed in scanning electron microscopy experiments.56 Studies suggest that, in addition to the main contribution of epicardial cells by direct contact through the epicardial villi, there are isolated, free-floating clusters of epicardial cells presumed to emanate as vesicular extrusions from the epicardial villi. These vesicular extrusions attach to—and migrate over—the naked myocardial tube.132
Figure 9.
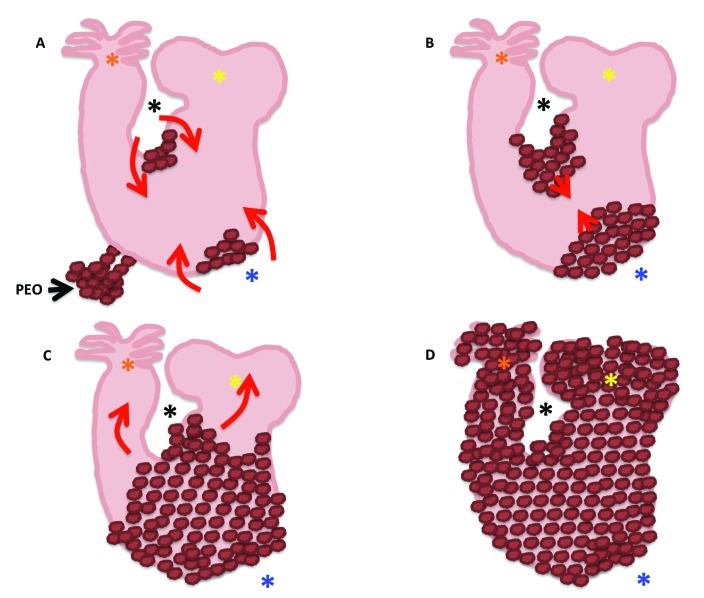
Schematic representation of the geographic course of the epicardial spreading over the myocardial tube. (A) Epicardial villi extend from the proepicardial organ (PEO) at HH17 and start migrating over the previously naked myocardial tube. (B) Epicardial cells (brown bubbles) first cover the concave (black asterisk) and convex (blue asterisk) curvatures of what will become the ventricles, by HH19. (C) Once the epicardial cells have covered the U shape of the cardiac tube (the portion what will become the ventricles), they start migrating distally toward the atria (yellow asterisks), and cranially toward the bulbus cordis and truncus arteriosus (orange asterisks). (D) The myocardial tube is completely enveloped by epicardium by HH24 (D).
As previously mentioned, the epicardial villi arising from the PEO attach to the myocardial tube by HH17. By HH19, the migrating epicardium can be seen along the convex and concave surfaces of the U portion of the cardiac tube (which corresponds to the future ventricles; Figure 9 A and B).56 The leading cells have long, slender filopodia that make contact with the myocardium they are migrating over, as well as ruffles on their free edges. By HH21, the leading edges of the migrating epicardium fuse, and thus the ventricular portion of the myocardial tube is completely enveloped (Figure 9 C and Figure 10). From here, epicardial cells then migrate cranially to envelop the bulbus cordis and distally toward the atria. The once-naked myocardial tube becomes completely enveloped by epicardium by HH24 (Figure 9 D);56 in quail–chick chimeras, the quail epicardium finishes its migration by HH27.89 The epicardium is generally referred to as an epithelium in the scientific literature. How is it that an epithelial sheet can spread such a long distance and over such convoluted topography as that of the developing and beating cardiac tube if the tractional force is being generated at the leading edge? This problem has been investigated by using the chicken embryo as a model system. Specifically, studies have been aimed at investigating the mode of migration of the epicardium, and results are forthcoming.
Figure 10.
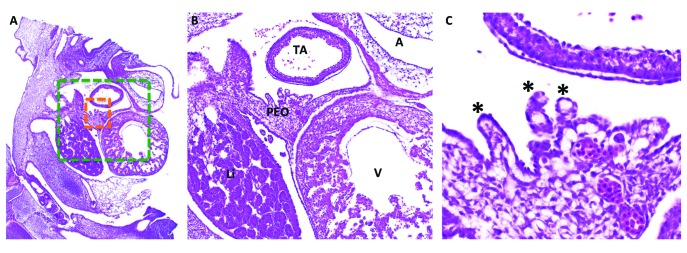
(A) Histology (hematoxylin and eosin stain) of the developing chick embryo at HH21–23. (B) Green-boxed area in panel A at increased magnification. (C) Orange-boxed area in panel A at increased magnification. The proepicardial organ (PEO) has villous projections (black asterisks) that attach to the myocardial tube and begin to spread over the portion of the tube that will become the ventricle (V), followed by the atria (A) and truncus arteriosus (TA). Li, liver.
Once the epicardium has covered the cardiac tube, EMT events take place (Figure 11)116,163, through which epicardial cells leave the epicardial epithelium and migrate deep toward the myocardium, forming a subepicardial compartment (Figure 11 C). These epicardium-derived cells contribute to various cell lineages including coronary endothelium, smooth muscle, and perivascular fibroblasts as well as the fibroblasts that make up the fibrous skeleton of the heart (Figure 11 D). Much of the evidence supporting these claims come from lineage tracing experiments, including labeling of proepicardial cells with vital dyes and quail–chick chimera experiments.44,97,115,116 The ability of the epicardium to contribute to the formation of vascular components has been observed both in vivo and in vitro and is modulated by vascular endothelial growth factor and basic fibroblast growth factor signaling.50 Further evidence of the proepicardial–epicardial contributions toward coronary angioblasts is the observation that these cells express Wilms tumor-associated transcription factor, which is a marker for coelomic epithelium.50 Recent experiments suggest that coronary endothelial cells have a heterogeneous origin, including sinus venosus endothelium-derived cells and surface mesothelium-derived cells.66
Figure 11.
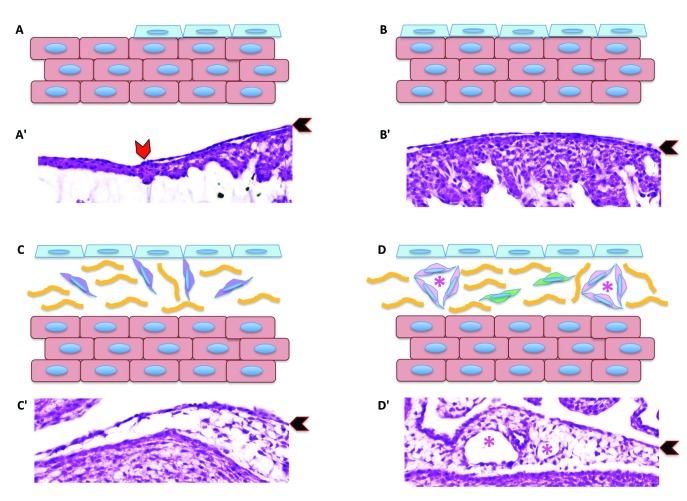
Schematic diagram showing epicardial migration over the myocardium and the subsequent epithelial-to-mesenchymal transition. (A) The epicardial cell layer (pale-blue cells) migrate over the naked cardiomyocytes (salmon-colored cells). (B) Eventually the entire myocardium is covered by a sheet of epicardial cells. (C) Once the the layer of epicardium covers the entire myocardium, epicardial cells begin the epithelial-to-mesenchymal transition, giving rise to epicardium-derived cells (purple cells). (D) In turn, epicardium-derived cells become cardiac fibroblasts (green cells) or cells contributing to the formation of blood vessels (pink cells), including endothelial cells, smooth muscle cells, and perivascular fibroblasts. Panels Aʹ through Dʹ are corresponding hematoxylin and eosin–stained sections of embryonic chick hearts. Red chevron in panel Aʹ indicates the migrating leading edge of the epicardial cells over the myocardium. Black chevrons in panels Aʹ through Dʹ indicate the epicardium. Yellow lines in panels C and D represent indicate subepicardial connective tissue. Pink asterisks in panels D and Dʹ indicate the blood vessel lumen.
Additional studies in chicken embryos suggest that the epicardium may be involved in the autonomic response during early cardiac development,68 correct atrioventricular electrical conduction patterns,158 and appropriate differentiation of Purkinje fibers.33 Numerous studies have investigated the contributions of the epicardium toward the cardiomyocyte pool, but so far the evidence suggests that the epicardium does not contribute (at least in avian species) to myocardial lineages.89 However, some studies that suggest an important role for the epicardium in the proliferation of the developing myocardium through fibroblast growth factor 2 signaling113 and through direct epicardial–myocardial cell–cell contact.169 This possibility is of great interest due to the potential implications for inducing myocardial regeneration through modulation of the epicardium.
Many developmental programs in the embryo are redeployed in the adult in the face of disease. As shown herein, a lot of attention has been directed at improved understanding of epicardial biology due to the implications for generating vascular endothelial cells and vascular smooth muscle cells, which would be of great benefit during postinfarctive reparative processes. At the same time, understanding the underpinnings of fibroblast differentiation may open the door for modulating the onset of cicatricial fibroblast proliferation, in an attempt to halt the detrimental outcome of myocardial scarring. Moreover, understanding the interplay between the epicardium and the myocardium during development may provide great insight into how to promote cardiomyocyte proliferation for therapeutic purposes. All of the experiments presented in this section were performed in chicken embryos, underscoring how much this model has contributed to the field of cardiac biology. With increasing experimental modalities available that allow for further manipulations, the embryonic chicken is reemerging as an important model for cardiovascular research.
Discussion
Supported by reports from the American Heart Association, it is well known that CVD remains one of the biggest drivers of morbidity and mortality in the United States and other developed countries.10 The American Heart Association has published national goals for cardiovascular health promotion and disease reduction,87 and guidelines aligned with the concept of cardiovascular health are described.13 Of course, attaining these goals of disease reduction relies heavily on a clearer understanding of CVD to better inform the path to follow when developing diagnostic and therapeutic approaches.
Our current understanding of the mammalian adult heart is that it has little to no capacity to regenerate after injury. In neonatal mice, the heart can regenerate after surgical resection, but this regenerative ability is lost by 7 d of age.117 However, the heart of other vertebrates (including amphibians and zebrafish) has a regenerative capacity that persists throughout life.95 What mechanisms allow myocardial proliferation in the embryo but not the adult? What mechanisms govern the difference in regenerative capacity between species? How can we harness the regeneration-promoting programs to advance regenerative therapeutic modalities?
In multiple disease states and reparative processes, developmental pathways are redeployed.35,45 After myocardial injury in adult mammals, developmental mechanisms associated with epicardial organogenesis reactivate and may strongly influence the reparative or cicatricial (scarring) outcomes.32,123,125,154 A recent review summarized findings that speak to the plasticity of cells during cardiac development and disease.26 In that review,26 the authors explore how developmental approaches such as lineage tracing experiments have uncovered that there are multiple cell sources for a single cell type and that numerous cells have a varied expansion potential in the face of disease. Therefore, it has become increasingly clear that understanding developmental events is key to understanding a wide range of disease processes.
As mentioned earlier, the epicardium arises from a cluster of mesothelial cells at the level of the sinus venosus. The epicardium spreads over the developing heart tube and purportedly undergoes EMT to give rise to numerous cellular components. These components include endothelial cells, smooth muscle cells, and fibroblasts that form the structural scaffolding of the heart. The epicardium was believed to remain relatively quiescent in adult mammals; however, current evidence shows that it becomes reactivated in the face of disease, especially after myocardial infarction. Because of the mammalian inherent inability to regenerate cardiomyocytes, a stereotypical outcome of myocardial infarction is healing by fibrosis (without cardiomyocyte replenishment). The work by many groups has poised the epicardium as a potential therapeutic target, reigniting interest in the study of this issue which had long been ignored.35,60,72,94,123,159 An obvious step toward unlocking the epicardial potential for therapeutic modalities is to thoroughly understand its biology during development. As described in the current review, much of this knowledge has been learned through studies in the embryonic chicken.
The chicken embryo remains an ideal model system for developmental and cardiac biology research. Key morphogenetic findings first made in this species, a detailed and well-documented body plan, ease of use and manipulation, low cost, and robustness of the model poise the chicken embryo as well suited to these studies.63,66,78 In conclusion, with the continued development and optimization of molecular techniques that can be used in this species, the chicken embryo remains a cornerstone of developmental biology and biomedical research.
Acknowledgments
The author acknowledges Drs SA Felt and S Baker (Stanford University) for their comments on the manuscript. Thanks to Dr CA Erickson (University of California–Davis) for mentorship of the original research and technique optimization mentioned in the manuscript. Funding sources for the original research performed by JV-M at the University of California-Davis include grants NSF IOS1146480 (to CAE), NIH T32OD011147 (to JV-M), and the UCD GSSP Fellowship Program (to JV-M).
References
- 1.Ahlstrom JD, Erickson CA. 2009. The neural crest epithelial–mesenchymal transition in 4D: a ‘tail’ of multiple nonobligatory cellular mechanisms. Development 136:1801–1812. https://doi.org/10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Naieb S, Happel CM, Yelbuz TM. 2013. A detailed atlas of chick heart development in vivo. Ann Anat 195:324–341. https://doi.org/10.1016/j.aanat.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Argüello C, Alanis J, Valenzuela B. 1988. The early development of the atrioventricular node and bundle of His in the embryonic chick heart. An electrophysiological and morphological study. Development 102:623–637. [DOI] [PubMed] [Google Scholar]
- 4.Arias CF, Herrero MA, Stern CD, Bertocchini F. 2017. A molecular mechanism of symmetry breaking in the early chick embryo. Sci Rep 7:1–6. https://doi.org/10.1038/s41598-017-15883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora KL, Kosin IL. 1966. Developmental responses of early turkey and chicken embryos to preincubation holding of eggs: inter- and intra-species differences. Poult Sci 45:958–970. https://doi.org/10.3382/ps.0450958. [DOI] [PubMed] [Google Scholar]
- 6.Arvind V, Huang AH. 2017. Mechanobiology of limb musculoskeletal development. Ann N Y Acad Sci 1409:18–32. https://doi.org/10.1111/nyas.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AVMA. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 05 June 2019]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 8.Bangasser BL, Shamsan GA, Chan CE, Opoku KN, Tüzel E, Schlichtmann BW, Kasim JA, Fuller BJ, McCullough BR, Rosenfeld SS, Odde DJ. 2017. Shifting the optimal stiffness for cell migration. Nat Commun 8:1–10. 10.1038/ncomms15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Shachar G, Arcilla RA, Lucas RV, Manasek FJ. 1985. Ventricular trabeculations in the chick embryo heart and their contribution to ventricular and muscular septal development. Circ Res 57:759–766. 10.1161/01.RES.57.5.759. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. 2018. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 137:e67–e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 11.Bisaha JG, Bader D. 1991. Identification and characterization of a ventricular-specific avian myosin heavy chain, VMHC1: expression in differentiating cardiac and skeletal muscle. Dev Biol 148:355–364. 10.1016/0012-1606(91)90343-2. [DOI] [PubMed] [Google Scholar]
- 12.Boot MJ, Gittenberger-De Groot AC, Van Iperen L, Hierck BP, Poelmann RE. 2003. Spatiotemporally separated cardiac neural crest subpopulations that target the outflow tract septum and pharyngeal arch arteries. Anat Rec A Discov Mol Cell Evol Biol 275A:1009–1018. 10.1002/ar.a.10099. [DOI] [PubMed] [Google Scholar]
- 13.Brant LCC, Ribeiro ALP. 2018. Cardiovascular health: a global primordial need. Heart 104:1232–1233. 10.1136/heartjnl-2017-312562. [DOI] [PubMed] [Google Scholar]
- 14.Burt DW. 2004. The chicken genome and the developmental biologist. Mech Dev 121:1129–1135. 10.1016/j.mod.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Burt DW. 2005. Chicken genome: current status and future opportunities. Genome Res 15:1692–1698. 10.1101/gr.4141805. [DOI] [PubMed] [Google Scholar]
- 16.Busch C, Krochmann J, Drews U. 2013. The chick embryo as an experimental system for melanoma cell invasion. PLoS One 8:1–9. 10.1371/journal.pone.0053970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camp E, Dietrich S, Munsterberg A. 2012. Fate mapping identifies the origin of SHF/AHF progenitors in the chick primitive streak. PLoS One 7:1–13. 10.1371/journal.pone.0051948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carew RM, Wang B, Kantharidis P. 2012. The role of EMT in renal fibrosis. Cell Tissue Res 347:103–116. 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 19.Chan-Thomas PS, Thompson RP, Robert B, Yacoub MH, Barton PJ. 1993. Expression of homeobox genes Msx1 (Hox7) and Msx2 (Hox8) during cardiac development in the chick. Dev Dyn 197:203–216. 10.1002/aja.1001970305. [DOI] [PubMed] [Google Scholar]
- 20.Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. 2001. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 220:284–289. . [DOI] [PubMed] [Google Scholar]
- 21.Chin C, Gandour-Edwards R, Oltjen S, Choy M. 1992. Fate of the atrioventricular endocardial cushions in the developing chick heart. Pediatr Res 32:390–393. 10.1203/00006450-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Chua KN, Poon KL, Lim J, Sim WJ, Huang RY, Thiery JP. 2011. Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev 63:558–567. 10.1016/j.addr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Cloney K, Franz-Odendaal TA. 2015. Optimized ex–ovo culturing of chick embryos to advanced stages of development. J Vis Exp 95:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comşa S, Ceaușu RA, Popescu R, Cîmpean AM, Raica M. 2017. The human mesenchymal stem cells and the chick embryo chorioallantoic membrane: the key and the lock in revealing vasculogenesis. In Vivo 31:1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das RM, Wilcock AC, Swedlow JR, Storey KG. 2012. High-resolution live imaging of cell behavior in the developing neuroepithelium. J Vis Exp 62:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Red-Horse K. 2017. Cellular plasticity in cardiovascular development and disease. Dev Dyn 246:328–335. 10.1002/dvdy.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis FM, Stewart TA, Thompson EW, Monteith GR. 2014. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci 35:479–488. 10.1016/j.tips.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 28.De Craene B, Berx G. 2013. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13:97–110. 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 29.De la Cruz MV, Giménez-Ribotta M, Saravalli O, Cayré R. 1983. The contribution of the inferior endocardial cushion of the atrioventricular canal to cardiac septation and to the development of the atrioventricular valves: study in the chick embryo. Am J Anat 166:63–72. 10.1002/aja.1001660105. [DOI] [PubMed] [Google Scholar]
- 30.Dieterlen-Liévre F. 1974. [[The origin of definitive hematopoetic stem cells in bird embryos: experimental analysis using quail-chicken chimeras]] C R Acad Sci Hebd Seances Acad Sci D 279:915–918. [Article in French]. [PubMed] [Google Scholar]
- 31.Doran TJ, Cooper CA, Jenkins KA, Tizard ML. 2016. Advances in genetic engineering of the avian genome: “Realising the promise”. Transgenic Res 25:307–319. 10.1007/s11248-016-9926-8. [DOI] [PubMed] [Google Scholar]
- 32.Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, Majesky M, Deb A. 2011. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J 31:429–442. 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eralp I, Lie-Venema H, Bax NA, Wijffels MC, Van Der Laarse A, Deruiter MC, Bogers AJ, Van Den Akker NM, Gourdie RG, Schalij MJ, Poelmann RE, Gittenberger-De Groot AC. 2006. Epicardium-derived cells are important for correct development of the Purkinje fibers in the avian heart. Anat Rec A Discov Mol Cell Evol Biol 288:1272–1280. 10.1002/ar.a.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faber JJ, Green TJ, Thornburg KL. 1974. Embryonic stroke volume and cardiac output in the chick. Dev Biol 41:14–21. 10.1016/0012-1606(74)90278-4. [DOI] [PubMed] [Google Scholar]
- 35.Fang M, Xiang FL, Braitsch CM, Yutzey KE. 2016. Epicardium–derived fibroblasts in heart development and disease. J Mol Cell Cardiol 91:23–27. 10.1016/j.yjmcc.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasenko GM. 2007. Egg storage and the embryo. Poult Sci 86:1020–1024. 10.1093/ps/86.5.1020. [DOI] [PubMed] [Google Scholar]
- 37.Fasenko GM, Robinson FE, Whelan AI, Kremeniuk KM, Walker JA. 2001. Prestorage incubation of long-term stored broiler breeder eggs: 1. Effects on hatchability. Poult Sci 80:1406–1411. 10.1093/ps/80.10.1406. [DOI] [PubMed] [Google Scholar]
- 38.Fernando L, Pamela S, Alejandra L. 2014. Cardiovascular disease in Latin America: the growing epidemic. Prog Cardiovasc Dis 57:262–267. 10.1016/j.pcad.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 39.French NA. 2009. The critical importance of incubation temperature. Avian Biol Res 2:55–59. 10.3184/175815509X431812. [DOI] [Google Scholar]
- 40.Gandhi S, Piacentino ML, Vieceli FM, Bronner ME. 2017. Optimization of CRISPR/Cas9 genome editing for loss-of-function in the early chick embryo. Dev Biol 432:86–97. 10.1016/j.ydbio.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerety SS, Breau MA, Sasai N, Xu Q, Briscoe J, Wilkinson DG. 2013. An inducible transgene expression system for zebrafish and chick. Development 140:2235–2243. 10.1242/dev.091520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Germani A, Foglio E, Capogrossi MC, Russo MA, Limana F. 2015. Generation of cardiac progenitor cells through epicardial to mesenchymal transition. J Mol Med (Berl) 93:735–748. 10.1007/s00109-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 43.Gittenberger-de Groot AC, Bartelings MM, Poelmann RE, Haak MC, Jongbloed MR. 2013. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Semin Fetal Neonatal Med 18:237–244. 10.1016/j.siny.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. 1998. Epicardium–derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 82:1043–1052. 10.1161/01.RES.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 45.Gittenberger-de Groot AC, Winter EM, Bartelings MM, Goumans MJ, DeRuiter MC, Poelmann RE. 2012. The arterial and cardiac epicardium in development, disease and repair. Differentiation 84:41–53. 10.1016/j.diff.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 46.Goenezen S, Rennie MY, Rugonyi S. 2012. Biomechanics of early cardiac development. Biomech Model Mechanobiol 11:1187–1204. 10.1007/s10237-012-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. 1993. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res 72:278–289. 10.1161/01.RES.72.2.278. [DOI] [PubMed] [Google Scholar]
- 48.Gourdie RG, Mima T, Thompson RP, Mikawa T. 1995. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 121:1423–1431. [DOI] [PubMed] [Google Scholar]
- 49.Greenburg G, Hay ED. 1986. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol 115:363–379. 10.1016/0012-1606(86)90256-3. [DOI] [PubMed] [Google Scholar]
- 50.Guadix JA, Carmona R, Muñoz-Chápuli R, Pérez-Pomares JM. 2006. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev Dyn 235:1014–1026. 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 51.Gumpel-Pinot M, Martin C, Croisille Y. 1971. [[Organogenesis of the mesonephros in birds. Realization in vitro of quail-chicken chimera mesonephros]] C R Acad Sci Hebd Seances Acad Sci D 272:737–739. [Article in French]. [PubMed] [Google Scholar]
- 52.Hamburger V, Hamilton HL. 1951. A series of normal stages in the development of the chick embryo. J Morphol 88:49–92. 10.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- 53.Hamidu JA, Uddin Z, Li M, Fasenko GM, Guan LL, Barreda DR. 2011. Broiler egg storage induces cell death and influences embryo quality. Poult Sci 90:1749–1757. 10.3382/ps.2011-01361. [DOI] [PubMed] [Google Scholar]
- 54.Hatada Y, Stern CD. 1994. A fate map of the epiblast of the early chick embryo. Development 120:2879–2889. [DOI] [PubMed] [Google Scholar]
- 55.Henning AL, Jiang MX, Yalcin HC, Butcher JT. 2011. Quantitative 3D imaging of live avian embryonic morphogenesis via micro–computed tomography. Dev Dyn 240:1949–1957. 10.1002/dvdy.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho E, Shimada Y. 1978. Formation of the epicardium studied with the scanning electron microscope. Dev Biol 66:579–585. 10.1016/0012-1606(78)90263-4. [DOI] [PubMed] [Google Scholar]
- 57.Hutson JM, Donahoe PK. 1984. Improved histology for the chick-quail chimera. Stain Technol 59:105–111. 10.3109/10520298409113839. [DOI] [PubMed] [Google Scholar]
- 58.Icardo JM, Fernandez-Terán A. 1987. Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat (Basel) 130:264–274. 10.1159/000146455. [DOI] [PubMed] [Google Scholar]
- 59.Itasaki N, Bel-Vialar S, Krumlauf R. 1999. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nat Cell Biol 1:E203–E207. 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- 60.Iyer D, Gambardella L, Bernard WG, Serrano F, Mascetti VL, Pedersen RA, Talasila A, Sinha S. 2015. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development 142:1528–1541. 10.1242/dev.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeter JR, Jr, Cameron IL. 1971. Cell proliferation patterns during cytodifferentiation in embryonic chick tissues: liver, heart and erythrocytes. J Embryol Exp Morphol 25:405–422. [PubMed] [Google Scholar]
- 62.Jones SP, Kennedy SW. 2009. Chicken embryo cardiomyocyte cultures–a new approach for studying effects of halogenated aromatic hydrocarbons in the avian heart. Toxicol Sci 109:66–74. 10.1093/toxsci/kfp039 [DOI] [PubMed] [Google Scholar]
- 63.Kain KH, Miller JW, Jones-Paris CR, Thomason RT, Lewis JD, Bader DM, Barnett JV, Zijlstra A. 2014. The chick embryo as an expanding experimental model for cancer and cardiovascular research. Dev Dyn 243:216–228. 10.1002/dvdy.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaiser A, Willer T, Steinberg P, Rautenschlein S. 2017. Establishment of an in vitro intestinal epithelial cell culture model of avian origin. Avian Dis 61:229–236. 10.1637/11524-110216-Reg.1. [DOI] [PubMed] [Google Scholar]
- 65.Kalluri R, Weinberg RA. 2009. The basics of epithelial–mesenchymal transition. J Clin Invest 119:1420–1428. 10.1172/JCI39104. Erratum in: J Clin Inves 2010 120:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamimura T, Yamagishi T, Nakajima Y. 2018. Avian coronary endothelium is a mosaic of sinus venosus—and ventricle–derived endothelial cells in a region-specific manner. Dev Growth Differ 60:97–111. 10.1111/dgd.12422. [DOI] [PubMed] [Google Scholar]
- 67.Kawasumi-Kita A, Ohtsuka D, Morishita Y. 2017. Morphometric staging of organ development based on cross sectional images. J Theor Biol 440:80–87. 10.1016/j.jtbi.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Kelder TP, Duim SN, Vicente-Steijn R, Vegh AM, Kruithof BP, Smits AM, van Bavel TC, Bax NA, Schalij MJ, Gittenberger-de Groot AC, DeRuiter MC, Goumans MJ, Jongbloed MR. 2015. The epicardium as modulator of the cardiac autonomic response during early development. J Mol Cell Cardiol 89:251–259. 10.1016/j.yjmcc.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 69.Kelder TP, Vicente-Steijn R, Harryvan TJ, Kosmidis G, Gittenberger-de Groot AC, Poelmann RE, Schalij MJ, DeRuiter MC, Jongbloed MR. 2015. The sinus venosus myocardium contributes to the atrioventricular canal: potential role during atrioventricular node development? J Cell Mol Med 19:1375–1389. 10.1111/jcmm.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirby ML, Aronstam RS, Buccafusco JJ. 1985. Changes in cholinergic parameters associated with failure of conotruncal septation in embryonic chick hearts after neural crest ablation. Circ Res 56:392–401. 10.1161/01.RES.56.3.392. [DOI] [PubMed] [Google Scholar]
- 71.Kirby ML, Gale TF, Stewart DE. 1983. Neural crest cells contribute to normal aorticopulmonary septation. Science 220:1059–1061. 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 72.Krainock M, Toubat O, Danopoulos S, Beckham A, Warburton D, Kim R. 2016. Epicardial epithelial–to–mesenchymal transition in heart development and disease. J Clin Med 5:1–14. 10.3390/jcm5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kue CS, Tan KY, Lam ML, Lee HB. 2015. Chick embryo chorioallantoic membrane (CAM): an alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp Anim 64:129–138. 10.1538/expanim.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulesa PM, Bailey CM, Cooper C, Fraser SE. 2010. In ovo live imaging of avian embryos. Cold Spring Harb Protoc 2010:1–8. doi: 10.1101/pdb.prot5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuo BR, Erickson CA. 2010. Regional differences in neural crest morphogenesis. Cell Adh Migr 4:567–585. 10.4161/cam.4.4.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuo BR, Erickson CA. 2011. Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest. Dev Dyn 240:2084–2100. 10.1002/dvdy.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurantowicz N, Sawosz E, Halik G, Strojny B, Hotowy A, Grodzik M, Piast R, Pasanphan W, Chwalibog A. 2017. Toxicity studies of 6 types of carbon nanoparticles in a chicken-embryo model. Int J Nanomedicine 12:2887–2898. 10.2147/IJN.S131960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawson TB, Scott-Drechsel DE, Chivukula VK, Rugonyi S, Thornburg KL, Hinds MT. 2018. Hyperglycemia alters the structure and hemodynamics of the developing embryonic heart. J Cardiovasc Dev Dis 5:1–12. 10.3390/jcdd5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Douarin NM. 1980. The ontogeny of the neural crest in avian embryo chimaeras. Nature 286:663–669. 10.1038/286663a0. [DOI] [PubMed] [Google Scholar]
- 80.Lee HJ, Lee HC, Kim YM, Hwang YS, Park YH, Park TS, Han JY. 2016. Site-specific recombination in the chicken genome using Flipase recombinase-mediated cassette exchange. FASEB J 30:555–563. 10.1096/fj.15-274712. [DOI] [PubMed] [Google Scholar]
- 81.Leong HS, Chambers AF, Lewis JD. 2012. Assessing cancer cell migration and metastatic growth in vivo in the chick embryo using fluorescence intravital imaging. Methods Mol Biol 872:1–14. 10.1007/978-1-61779-797-2_1. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Li W. 2015. Epithelial–mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther 150:33–46. 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Lim J, Thiery JP. 2012. Epithelial–mesenchymal transitions: insights from development. Development 139:3471–3486. 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 84.Linask KK, Knudsen KA, Gui YH. 1997. N–cadherin–catenin interaction: necessary component of cardiac cell compartmentalization during early vertebrate heart development. Dev Biol 185:148–164. 10.1006/dbio.1997.8570. [DOI] [PubMed] [Google Scholar]
- 85.Lincoln J, Alfieri CM, Yutzey KE. 2004. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230:239–250. 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 86.Liu M, Xie S, Zhou J. 2018. Use of animal models for the imaging and quantification of angiogenesis. Exp Anim 67:1–6. 10.1538/expanim.17-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee. 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 121:586–613. 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 88.Männer J. 1992. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 186:379–385. 10.1007/BF00185988. [DOI] [PubMed] [Google Scholar]
- 89.Männer J. 1999. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 255:212–226. . [DOI] [PubMed] [Google Scholar]
- 90.Männer J. 2000. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259:248–262. . [DOI] [PubMed] [Google Scholar]
- 91.Männer J, Merkel N. 2007. Early morphogenesis of the sinuatrial region of the chick heart: a contribution to the understanding of the pathogenesis of direct pulmonary venous connections to the right atrium and atrial septal defects in hearts with right isomerism of the atrial appendages. Anat Rec (Hoboken) 290:168–180. 10.1002/ar.20418. [DOI] [PubMed] [Google Scholar]
- 92.Markwald RR, Fitzharris TP, Manasek FJ. 1977. Structural development of endocardial cushions. Am J Anat 148:85–119. 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- 93.Martinsen BJ. 2005. Reference guide to the stages of chick heart embryology. Dev Dyn 233:1217–1237. 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- 94.Masters M, Riley PR. 2014. The epicardium signals the way towards heart regeneration. Stem Cell Res 13:683–692. 10.1016/j.scr.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matrone G, Tucker CS, Denvir MA. 2017. Cardiomyocyte proliferation in zebrafish and mammals: lessons for human disease. Cell Mol Life Sci 74:1367–1378. 10.1007/s00018-016-2404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McLennan R, Dyson L, Prather KW, Morrison JA, Baker RE, Maini PK, Kulesa PM. 2012. Multiscale mechanisms of cell migration during development: theory and experiment. Development 139:2935–2944. 10.1242/dev.081471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikawa T, Gourdie RG. 1996. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 174:221–232. 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 98.Mohammed RH, Anderton H, Brameld JM, Sweetman D. 2017. Effects of insulin like growth factors on early embryonic chick limb myogenesis. PLoS One 12:1–13. https://doi.org/10.1371/journal.pone.0185775. Erratum: PLoS One 2017. https://doi.org/10.1371/journal.pone.0189395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morse DE, Rogers CS, McCann PS. 1984. Atrial septation in the chick and rat: a review. J Submicrosc Cytol 16:259–272. [PubMed] [Google Scholar]
- 100.Nakamura H, Funahashi J. 2012. Electroporation: past, present and future. Dev Growth Differ 55:15–19. 10.1111/dgd.12012. [DOI] [PubMed] [Google Scholar]
- 101.Nakaya Y, Sheng G. 2008. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ 50:755–766. 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- 102.Nakaya Y, Sheng G. 2013. EMT in developmental morphogenesis. Cancer Lett 341:9–15. 10.1016/j.canlet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 103.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. 1982. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1:841–845. 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.New DA. 1959. The adhesive properties and expansion of the chick blastoderm. J Embryol Exp Morphol 7:146–164. [PubMed] [Google Scholar]
- 105.Norris A, Streit A. 2014. Morpholinos: studying gene function in the chick. Methods 66:454–465. 10.1016/j.ymeth.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Odani N, Ito K, Nakamura H. 2008. Electroporation as an efficient method of gene transfer. Dev Growth Differ 50:443–448. 10.1111/j.1440-169X.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 107.Ogura T. 2002. In vivo electroporation: a new frontier for gene delivery and embryology. Differentiation 70:163–171. 10.1046/j.1432-0436.2002.700406.x. [DOI] [PubMed] [Google Scholar]
- 108.Ohta S, Schoenwolf GC, Yamada G. 2010. The cessation of gastrulation: BMP signaling and EMT during and at the end of gastrulation. Cell Adh Migr 4:440–446. 10.4161/cam.4.3.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. 2016. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep 6:1–10. 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ombrato L, Malanchi I. 2014. The EMT universe: space between cancer cell dissemination and metastasis initiation. Crit Rev Oncog 19:349–361. 10.1615/CritRevOncog.2014011802. [DOI] [PubMed] [Google Scholar]
- 111.Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djousse L, Clark CR. 2016. Ideal cardiovascular health and incident cardiovascular events. Am J Prev Med 51:502–506. 10.1016/j.amepre.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pardanaud L, Altmann C, Kitis P, Dieterlen-Lievre F, Buck CA. 1987. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349. [DOI] [PubMed] [Google Scholar]
- 113.Pennisi DJ, Ballard VL, Mikawa T. 2003. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn 228:161–172. 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 114.Pennisi DJ, Rentschler S, Gourdie RG, Fishman GI, Mikawa T. 2002. Induction and patterning of the cardiac conduction system. Int J Dev Biol 46:765–775. [PubMed] [Google Scholar]
- 115.Pérez-Pomares JM, Carmona R, González-Iriarte M, Atencia G, Wessels A, Muñoz-Chápuli R. 2002. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol 46:1005–1013. [PubMed] [Google Scholar]
- 116.Pérez-Pomares JM, Macias D, González-Iriarte L, Muñoz-Chápuli R. 1998. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol 200:57–68. 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 117.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. 2011. Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080. 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qayyum SR, Webb S, Anderson RH, Verbeek FJ, Brown NA, Richardson MK. 2001. Septation and valvar formation in the outflow tract of the embryonic chick heart. Anat Rec 264:273–283. 10.1002/ar.1162. [DOI] [PubMed] [Google Scholar]
- 119.Quiring DP. 1933. The development of the sino-atrial region of the chick heart. J Morphol 55:81–118. 10.1002/jmor.1050550106. [DOI] [Google Scholar]
- 120.Reijrink IA, Meijerhof R, Kemp B, Graat EA, van den Brand H. 2009. Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poult Sci 88:2649–2660. 10.3382/ps.2008-00523. [DOI] [PubMed] [Google Scholar]
- 121.Ridenour DA, McLennan R, Teddy JM, Semerad CL, Haug JS, Kulesa PM. 2014. The neural crest cell cycle is related to phases of migration in the head. Development 141:1095–1103. 10.1242/dev.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rine J. 2014. A future of the model organism model. Mol Biol Cell 25:549–553. 10.1091/mbc.e12-10-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruiz-Villalba A, Simón AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sánchez-Domínguez R, Segovia JC, Prosper F, Pérez-Pomares JM. 2015. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol 65:2057–2066. 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 124.Rulands S, Simons BD. 2016. Tracing cellular dynamics in tissue development, maintenance and disease. Curr Opin Cell Biol 43:38–45. 10.1016/j.ceb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 125.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. 2011. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 108:51–59. 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sauka-Spengler T, Barembaum M. 2008. Gain—and loss-of-function approaches in the chick embryo. Methods Cell Biol 87:237–256. 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- 127.Scherson T, Serbedzija G, Fraser S, Bronner-Fraser M. 1993. Regulative capacity of the cranial neural tube to form neural crest. Development 118:1049–1062. [DOI] [PubMed] [Google Scholar]
- 128.Schlueter J, Brand T. 2013. Subpopulation of proepicardial cells is derived from the somatic mesoderm in the chick embryo. Circ Res 113:1128–1137. 10.1161/CIRCRESAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- 129.Schmitz M, Nelemans BK, Smit TH. 2016. A Submerged filter paper sandwich for long-term ex ovo time-lapse imaging of early chick embryos. J Vis Exp 28:1–12.doi:10.3791/54636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schultheiss TM, Lassar AB. 1999Vertebrate heart induction, p 51–62. Chapter 4. In: Harvey RP, Rosenthal N. Heart development. New York (NY): Academic Press; doi: 10.1016/B978-012329860-7/50006-4 [Google Scholar]
- 131.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. 2000. Developmental patterning of the myocardium. Anat Rec 258:319–337. . [DOI] [PubMed] [Google Scholar]
- 132.Sejima H, Isokawa K, Shimizu O, Morikawa T, Ootsu H, Numata K, Fukai M, Kubota S, Toda Y. 2001. Possible participation of isolated epicardial cell clusters in the formation of chick embryonic epicardium. J Oral Sci 43:109–116. 10.2334/josnusd.43.109. [DOI] [PubMed] [Google Scholar]
- 133.Selleck MA, Bronner-Fraser M. 1995. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development 121:525–538. [DOI] [PubMed] [Google Scholar]
- 134.Serbedzija GN, Bronner-Fraser M, Fraser SE. 1989. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development 106:809–816. [DOI] [PubMed] [Google Scholar]
- 135.Serbedzija GN, Burgan S, Fraser SE, Bronner-Fraser M. 1991. Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development 111:857–866. [DOI] [PubMed] [Google Scholar]
- 136.Serbedzija GN, Fraser SE, Bronner-Fraser M. 1990. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development 108:605–612. [DOI] [PubMed] [Google Scholar]
- 137.Shaw PM, Chandra V, Escobar GA, Robbins N, Rowe V, Macsata R. 2018. Controversies and evidence for cardiovascular disease in the diverse Hispanic population. J Vasc Surg 67:960–969. 10.1016/j.jvs.2017.06.111. [DOI] [PubMed] [Google Scholar]
- 138.Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM. 2017. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 357:811–815. 10.1126/science.aai7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith SM, Flentke GR. 2017. The avian embryo as a model for fetal alcohol spectrum disorders. Biochem Cell Biol 96:98–106. https://doi.org/10.1139/bcb-2017-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song J, Yue Q, Münsterberg A. 2011. Time-lapse imaging of chick cardiac precursor cells. Methods Mol Biol 769:359–372. 10.1007/978-1-61779-207-6_24. [DOI] [PubMed] [Google Scholar]
- 141.Spear PC, Erickson CA. 2012. Apical movement during interkinetic nuclear migration is a two-step process. Dev Biol 370:33–41. 10.1016/j.ydbio.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stalsberg H, DeHaan RL. 1969. The precardiac areas and formation of the tubular heart in the chick embryo. Dev Biol 19:128–159. 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- 143.Streit A, Stern CD. 2014. Transplantation of neural tissue: quail-chick chimeras. Methods Mol Biol 1082:235–251. 10.1007/978-1-62703-655-9_16. [DOI] [PubMed] [Google Scholar]
- 144.Suarez ME, Wilson HR, McPherson BN, Mather FB, Wilcox CJ. 1996. Low temperature effects on embryonic development and hatch time. Poult Sci 75:924–932. 10.3382/ps.0750924. [DOI] [PubMed] [Google Scholar]
- 145.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. 2003. Fibroblast growth factor FGF4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol 258:252–263. 10.1016/S0012-1606(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 146.Suzuki T. 2013. How is digit identity determined during limb development? Dev Growth Differ 55:130–138. 10.1111/dgd.12022. [DOI] [PubMed] [Google Scholar]
- 147.Tam PPL, Schoenwolf GC. 1999. Cardiac fate maps: lineage allocation, morphogenetic movement, and cell commitment, p 3–8. Chapter 1. In: Harvey RP Rosenthal N, Heart development. New York (NY): Academic Press; 10.1016/B978-012329860-7/50003-9. [DOI] [Google Scholar]
- 148.Tanor MA, Leeson S, Summers JD. 1984. Effect of heat stress and diet composition on performance of White Leghorn hens. Poult Sci 63:304–310. 10.3382/ps.0630304. [DOI] [PubMed] [Google Scholar]
- 149.Teillet MA, Ziller C, Le Douarin NM. 2008. Quail-chick chimeras. Methods Mol Biol 461:337–350. 10.1007/978-1-60327-483-8_24. [DOI] [PubMed] [Google Scholar]
- 150.Theveneau E, Mayor R. 2012. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol 366:34–54. 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 151.Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial–mesenchymal transitions in development and disease. Cell 139:871–890. 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 152.Torlopp A, Schlueter J, Brand T. 2010. Role of fibroblast growth factor signaling during proepicardium formation in the chick embryo. Dev Dyn 239:2393–2403. 10.1002/dvdy.22384. [DOI] [PubMed] [Google Scholar]
- 153.VAN DER Saag MR, Ward MP, Kirkland PD. 2017. Application of an embryonated chicken egg model to assess the vector competence of Australian Culicoides midges for bluetongue viruses. Med Vet Entomol 31:263–271. 10.1111/mve.12231. [DOI] [PubMed] [Google Scholar]
- 154.van Wijk B, Gunst QD, Moorman AF, van den Hoff MJ. 2012. Cardiac regeneration from activated epicardium. PLoS One 7:1–14. 10.1371/journal.pone.0044692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AF, van den Hoff MJ. 2009. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res 105:431–441. 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verberne ME, Gittenberger-de Groot AC, Poelmann RE. 1998. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat Embryol (Berl) 198:171–184. 10.1007/s004290050175. [DOI] [PubMed] [Google Scholar]
- 157.Véron N, Qu Z, Kipen PA, Hirst CE, Marcelle C. 2015. CRISPR mediated somatic cell genome engineering in the chicken. Dev Biol 407:68–74. 10.1016/j.ydbio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 158.Vicente Steijn R, Sedmera D, Blom NA, Jongbloed M, Kvasilova A, Nanka O. 2018. Apoptosis and epicardial contributions act as complementary factors in remodeling of the atrioventricular canal myocardium and atrioventricular conduction patterns in the embryonic chick heart. Dev Dyn 247:1033–1042. 10.1002/dvdy.24642. [DOI] [PubMed] [Google Scholar]
- 159.Vieira JM, Riley PR. 2011. Epicardium-derived cells: a new source of regenerative capacity. Heart 97:15–19. 10.1136/hrt.2010.193292. [DOI] [PubMed] [Google Scholar]
- 160.Virágh S, Szabó E, Challice CE. 1989. Formation of the primitive myo- and endocardial tubes in the chicken embryo. J Mol Cell Cardiol 21:123–137. 10.1016/0022-2828(89)90856-0. [DOI] [PubMed] [Google Scholar]
- 161.Voiculescu O, Stern CD. 2017. Manipulating gene expression in the chick embryo. Methods Mol Biol 1565:105–114. 10.1007/978-1-4939-6817-6_9. [DOI] [PubMed] [Google Scholar]
- 162.von Gise A, Pu WT. 2012. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 110:1628–1645. 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. 1999. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 199:367–378. 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 164.Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. 1998. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 196:129–144. 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 165.Waldo KL, Willner W, Kirby ML. 1990. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat 188:109–120. 10.1002/aja.1001880202. [DOI] [PubMed] [Google Scholar]
- 166.Walewska M, Dolka I, Malek A, Wojtalewicz A, Wojtkowska A, Zbikowski A, Lechowski R, Zabielska-Koczywas K. 2017. Experimental tumor growth of canine osteosarcoma cell line on chick embryo chorioallantoic membrane (in vivo studies). Acta Vet Scand 59:1–5. 10.1186/s13028-017-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wallis JW, Aerts J, Groenen MA, Crooijmans RP, Layman D, Graves TA, Scheer DE, Kremitzki C, Fedele MJ, Mudd NK, Cardenas M, Higginbotham J, Carter J, McGrane R, Gaige T, Mead K, Walker J, Albracht D, Davito J, Yang SP, Leong S, Chinwalla A, Sekhon M, Wylie K, Dodgson J, Romanov MN, Cheng H, de Jong PJ, Osoegawa K, Nefedov M, Zhang H, McPherson JD, Krzywinski M, Schein J, Hillier L, Mardis ER, Wilson RK, Warren WC. 2004. A physical map of the chicken genome. Nature 432:761–764. 10.1038/nature03030. [DOI] [PubMed] [Google Scholar]
- 168.Watanabe Y, Yaginuma H. 2015. Tangential cell migration during layer formation of chick optic tectum. Dev Growth Differ 57:539–543. 10.1111/dgd.12238. [DOI] [PubMed] [Google Scholar]
- 169.Weeke-Klimp A, Bax NA, Bellu AR, Winter EM, Vrolijk J, Plantinga J, Maas S, Brinker M, Mahtab EA, Gittenberger-de Groot AC, van Luyn MJ, Harmsen MC, Lie-Venema H. 2010. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J Mol Cell Cardiol 49:606–616. 10.1016/j.yjmcc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 170.Wei Y, Mikawa T. 2000. Fate diversity of primitive streak cells during heart field formation in ovo. Dev Dyn 219:505–513. . [DOI] [PubMed] [Google Scholar]
- 171.Willemsen H, Li Y, Willems E, Franssens L, Wang Y, Decuypere E, Everaert N. 2011. Intermittent thermal manipulations of broiler embryos during late incubation and their immediate effect on the embryonic development and hatching process. Poult Sci 90:1302–1312. 10.3382/ps.2011-01390. [DOI] [PubMed] [Google Scholar]
- 172.Willetts L, Bond D, Stoletov K, Lewis JD. 2016. Quantitative analysis of human cancer cell extravasation using intravital imaging. Methods Mol Biol 1458:27–37. 10.1007/978-1-4939-3801-8_3. [DOI] [PubMed] [Google Scholar]
- 173.Wunsch AM, Little CD, Markwald RR. 1994. Cardiac endothelial heterogeneity defines valvular development as demonstrated by the diverse expression of JB3, an antigen of the endocardial cushion tissue. Dev Biol 165:585–601. 10.1006/dbio.1994.1278. [DOI] [PubMed] [Google Scholar]
- 174.Wynn ML, Rupp P, Trainor PA, Schnell S, Kulesa PM. 2013. Follow-the-leader cell migration requires biased cell-cell contact and local microenvironmental signals. Phys Biol 10:1–17 10.1088/1478-3975/10/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yalcin HC, Shekhar A, Rane AA, Butcher JT. 2010. An ex–ovo chicken embryo culture system suitable for imaging and microsurgery applications. J Vis Exp 44:1–4.doi: 10.3791/2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, Virani SS, Maziak W, Agatston AS, Veledar E, Nasir K. 2016. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and Non–US Populations. Mayo Clin Proc 91:649–670. 10.1016/j.mayocp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 177.Zabielska-Koczywąs K, Wojtkowska A, Dolka I, Malek A, Walewska M, Wojtalewicz A, Żbikowski A, Lechowski R. 2017. 3D chick embryo chorioallantoic membrane model as an in vivo model to study morphological and histopathological features of feline fibrosarcomas. BMC Vet Res 13:1–12. 10.1186/s12917-017-1114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. 2007. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282:23337–23347. 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 179.Zhang D, Ighaniyan S, Stathopoulos L, Rollo B, Landman K, Hutson J, Newgreen D. 2014. The neural crest: a versatile organ system. Birth Defects Res C Embryo Today 102:275–298. 10.1002/bdrc.21081. [DOI] [PubMed] [Google Scholar]
- 180.Zuo Q, Wang Y, Cheng S, Lian C, Tang B, Wang F, Lu Z, Ji Y, Zhao R, Zhang W, Jin K, Song J, Zhang Y, Li B. 2016. Site-directed genome knockout in chicken cell line and embryos can use CRISPR/Cas gene editing technology. G3 (Bethesda) 6:1787–1792. 10.1534/g3.116.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]