ABSTRACT
The objective of this study was to evaluate the effects of 2 Mexican oregano essential oils (MOO), from Poliomintha longiflora Gray (PLG) and Lippia berlandieri Schauer (LBS), in drinking water (DWt) on the performance, slaughter variables, and meat quality of broilers over a 40 D period of growth. A total of 180 non-sexed Ross-308 broilers (1-day-old) were randomly assigned to 3 treatments with 6 replications each (10 birds per replicate): CON = DWt control (without MOO); PLG = DWt + 400 mg/L of PLG; and LBS = DWt + 400 mg/L of LBS. The CON, PLG, and LBS broilers body weights were similar (P > 0.05) at all times. Feed intake (FI) was different by treatment (P < 0.05) at 7 and 28 D, and water intake (WI) was different (P < 0.05) at day 28. The CON treatment was highest (P < 0.05) at 28 D for FI and WI, whereas LBS was lowest (P < 0.05). Weight gain (WG) for CON, PLG, and LBS broilers was similar (P > 0.05), although WG for CON was slightly higher. In CON broilers, slaughter weight was highest (P < 0.05), but thigh yield was lowest (P < 0.05). The CON and PLG treatments were lower (P < 0.05) in thigh and leg cooking losses. The PLG treatment presented the highest values (P < 0.05) for breast-meat redness, saturation index, shear force, odor, taste, and overall sensory acceptability. The LBS was higher (P < 0.05) for breast-meat shear force, cohesiveness and resilience, but lower (P < 0.05) for sensory attributes. Mexican oregano essential oils at 400 mg/L can serve as natural alternative additives in DWt to improve broiler production and meat quality.
Keywords: Mexican oregano oil, growth, meat color, texture, sensory
INTRODUCTION
The use of antibiotics in the intensive production of animal husbandry products continues as a significant and integral part of food animal industrial technology (Amiranashvili et al., 2017). The addition of antibiotics into broiler production improves growth performance, disease control, and bird health. However, targeted and non-targeted bacteria risk development of resistance to these additives, presenting a potential hazard to consumer health as a result of eating enteropathogen-contaminated broiler-meat products. As a consequence, natural options, such as the use of plant essential oils, are being examined in broiler production.
Oregano essential oil (OEO) has emerged as one of the most popular natural strategies used in broiler production and has been examined in several studies (Ghazi et al., 2015; Silva-Vázquez et al., 2015, 2018; Peng et al., 2016; Skoufos et al., 2016; Méndez-Zamora et al., 2017; Reyer et al., 2017. These authors have indicated and recommended OEO as a growth promoter, natural antibiotic, improver of beneficial bacteria in the digestive tract, and that OEO can be used to improve meat quality. Globally, 2 types of oregano are known and studied in broiler production, the European or Mediterranean oregano varieties Origanum vulgare L. and Origanum onites sp. A. sativum L., and the Mexican oregano varieties Lippia graveolens, Lippia berlandieri, and Poliomintha longiflora (Mata-González and Meléndez-González, 2005; Karimi et al., 2010). These OEO contain carvacrol and thymol as the principal compounds, and lutein and β-carotene as the main carotenoids (Young et al., 2003). Mexican oregano essential oil (MOO) from L. graveolens, L. berlandieri, and P. longiflora also contains these compounds, which are characterized as exhibiting antimicrobial and antioxidant properties (Calvo-Irabien, 2018). Furthermore, these Mexican oregano plants are used as common ingredients in local cuisine, rendering seasoning flavors and textures similar to the European Origanum spp.
The OEO extracts have been incorporated into broiler diets to evaluate effects on growth performance, carcass traits, and meat quality. An alternative to dietary presentation is the addition of OEO to the drinking water (DWt) for the examination of effects on broiler production. Drinking water as a carrier of additives has been studied for probiotic administration (Jin et al., 1996; Timmerman et al., 2006; Karimi Torshizi et al., 2010; Liu et al., 2012; Amiranashvili et al., 2017). These studies indicated that supplementation in DWt may contribute to broiler performance and can be a more practical method than the conventional in-feed supplementation method. Pelicano et al. (2003) concluded that the combination of presenting probiotics in DWt and feed increased meat quality (e.g., color, pH, and tenderness). In addition, OEO has antioxidant and antimicrobial properties that when added could improve water quality. Amaral (2004) indicated that the use of water with adequate physical, chemical, and microbiological quality could aid disease prevention in chicks, influencing cost and yield at grow-out.
Oregano oil was applied to DWt by Gámez-Piñón et al. (2015), who demonstrated improved broiler performance and meat quality. Additionally, Adaszyńska-Skwirzyńska and Szczerbińska (2018) evaluated lavender essential oil extract (0.4 mL/L) in DWt and found that the addition of this oil extract had positive effects on broiler production performance and ileal microbiota. In contrast, Silva-Vázquez et al. (2018) used P. longiflora Gray and L. berlandieri Schauer MOO in broiler diets and concluded that these 2 MOO exhibit positive effects on broiler performance, blood profiles, carcass traits, and meat composition. These findings could indicate that MOO can be used in DWt as an enhancer of the broiler performance and meat quality.
The objective of the current study was to evaluate, during growth to market age, the effects of the MOO from Poliomintha longiflora Gray (PLG) and Lippia berlandieri Schauer (LBS) given in the DWt on broiler performance, carcass variables, physicochemical traits, texture, and sensory evaluation.
MATERIALS AND METHODS
The experiment was carried out at the Marin Experimental Farm, Universidad Autonoma de Nuevo Leon, Marin, Nuevo Leon, Mexico (INEGI, 2018). The study was conducted following the national standard on animal use (NOM-062-ZOO, 1999).
Broilers and Experimental Design
A total of one hundred and eighty 1-day-old Ross-308 mixed-sex broilers (46.92 ± 0.96 g) were purchased from a commercial hatchery and distributed 10 birds each in 18 floor pens (1.20 × 1.20 × 0.80 m) and on fresh wood shaving (6 pens per treatment). Broilers were randomly assigned to 3 treatments (diets), in which 2 MOO derived from PLG and LBS were added to the DWt: CON = control DWt (without MOO), PLG = DWt + 400 mg/L of PLG, and LBS = DWt + 400 mg/L of LBS. Mexican oregano oil was prepared by steam distillation (Natural Solutions Company SMI, Jimenez, Chihuahua, Mexico), and was emulsified (w/w %) in Tween 20 (Millikan, S.A. de C.V., Tlalnepantla, Estado de Mexico, Mexico) for incorporation into the DWt: 40:60 (MOO: emulsifier) for PLG and 50:50 for LBS. Mixing was done manually for 8 min and treatment DWt was prepared weekly according to the MOO; emulsifier ratios were then adjusted for the addition of 400 mg/L of PLG and LBS treatments. The treatments were supplied in a broiler DWt container per pen and were monitored daily. Preliminary tests were carried out to evaluate the blend stability and to establish the blend ratios. Emulsion blend storage stability was evaluated over several months at 26°C for the development of a creamy layer or droplets over time. The mixture was considered unstable at the moment layers or droplets appeared in the emulsion. Components of MOO were determined by gas chromatography (Clarus 600 and MS SQ8 PerkinElmer Inc., Waltham, MA) according to Silva-Vázquez et al. (2017), with carvacrol and thymol being the principal active components. The PLG presented 13.80% carvacrol and 28.40% thymol, and LBS contained 60.00% carvacrol and 3.91% thymol. Results were similar to those obtained by Silva-Vázquez et al. (2018).
Starter (1 to 21 D) and finisher (22 to 40 D) diets were the same for all treatments and were formulated according to NRC (1994) and as used by Silva-Vázquez et al. (2015, 2018) and Méndez-Zamora et al. (2017). Likewise, husbandry practices were applied according to these authors. Feed and water were provided ad libitum throughout the experiment.
Growth Performance
The initial broiler weights (IW; g) were determined at the beginning of the experiment. Broiler body weight (BW), feed intake (FI; g intake per week/number of broilers per pen), and water intake (WI; g intake per week/number of broilers per pen) were evaluated at 7, 14, 21, 28, 35, and 40 D. These variables were used to estimate the weekly body weight gain (WBWG; g (BWcurrent-BWprevious)/days per period) and feed efficiency (FE; FI/WBWG) and were determined at the same periods. Weights of offered and rejected feed were recorded to estimate these variables.
Slaughter Variables
The slaughter process was carried out according to the Official Mexican Standard (NOM-033-SAG/ZOO, 2014) and the method of Méndez-Zamora et al. (2015a). A total of thirty 40-day-old chicks at the end of the growth period on day 40 from each treatment (5 birds per pen) were randomly selected for slaughter by cervical dislocation. Slaughter weight (SW), and hot (HCW; after removal of the head, feathers, and internal organs) and cold (CCW; 24 h post-mortem) carcass weights were recorded to calculate hot (HCY; (HCW/SW) × 100) and cold (CCY; (CCW/SW) × 100) carcass yields. Breast meat (BY), thigh (TY), and leg (LY) yields were estimated using piece weight and SW (piece yield = (piece weight/SW) × 100) (5 birds per pen).
Physicochemical Variables
Breast meat pH, color, and water holding capacity (WHC) were measured, and cooking loss (CL) was evaluated in breast (CLB), thigh (CLT), and leg (CLL) meat, 24 h post-mortem. Meat pH was determined with a puncture electrode (HI 99,163, Hanna Instruments WoonSocket, RI). Breast meat surface color values for lightness (L*), redness (a*), yellowness (b*), chroma (saturation index), and hue angle were measured with a colorimeter (CR-400 Konica Minolta, Tokyo, Japan; Illuminant/Observer: D65/10), set on the CIE Lab System (CIE, 1976). The equipment was calibrated with a standard white plate. Meat total color change (ΔE) and browning index (BI) were calculated according to equations used by Bozkurt and Bayram (2006) and Ledesma et al. (2016), and the colorimeter calibration values 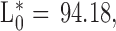
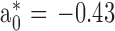 y
y 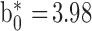 . The breast-meat WHC was determined using the compression method according to Tsai and Ockerman (1981) and Méndez-Zamora et al. (2015b). Approximately 300 ± 0.1 mg of each meat sample was placed between 2 pieces of filter paper, between 2 acrylic-plastic plates, applying a force of 4 kg for 20 min, and the final weight was obtained as: WHC = 100–(((initial weight—final weight)/initial weight) x 100). To determine CL, the pieces were vacuum-packed (Koch 800, Kansas City, MO) in vacuum bags (Zubex Industrial SA de CV, Monterrey, Nuevo Leon, Mexico) and cooked by immersion in water at 75.0 ± 0.1°C for 1 h. Then the samples were cooled by immersion in water at 4°C for 20 min. The pieces were removed from the bags, carefully drained, and weighed. Raw and cooked weights of each piece were recorded to evaluate the percentage CL (% CL = ((raw weight piece-cooked weight piece)/raw weight piece) × 100). All these variables were measured in duplicate for 12 pieces from each treatment, randomly selected (2 pieces/pen/treatment).
. The breast-meat WHC was determined using the compression method according to Tsai and Ockerman (1981) and Méndez-Zamora et al. (2015b). Approximately 300 ± 0.1 mg of each meat sample was placed between 2 pieces of filter paper, between 2 acrylic-plastic plates, applying a force of 4 kg for 20 min, and the final weight was obtained as: WHC = 100–(((initial weight—final weight)/initial weight) x 100). To determine CL, the pieces were vacuum-packed (Koch 800, Kansas City, MO) in vacuum bags (Zubex Industrial SA de CV, Monterrey, Nuevo Leon, Mexico) and cooked by immersion in water at 75.0 ± 0.1°C for 1 h. Then the samples were cooled by immersion in water at 4°C for 20 min. The pieces were removed from the bags, carefully drained, and weighed. Raw and cooked weights of each piece were recorded to evaluate the percentage CL (% CL = ((raw weight piece-cooked weight piece)/raw weight piece) × 100). All these variables were measured in duplicate for 12 pieces from each treatment, randomly selected (2 pieces/pen/treatment).
Meat Texture Analysis
Breast meat shear force (SF; g) and texture profile analysis (TPA) were carried out with a TA.XT.Plus texturometer (Stable Micro Systems, Serrey, England) in 2 sections on each side of the breast per replicate (n = 24/treatment; 2 breast/pen/treatment) at 4°C. A Warner-Bratzler shear blade with a triangular slot cutting edge and rectangular slices (3.5 cm long x 1.0 cm wide x 1.0 cm high for each breast) were used to evaluate SF; sample cuts were made parallel to the direction of the muscle fibers. Test conditions used in the instrument were a velocity of 2 mm/s pre-test, 2 mm/s during the test, 10 mm/s post-test, and a distance of 15 mm. The SF value was calculated from the maximum point of the curve generated. The TPA was determined using standardized cylinders (1.5 cm high and 2.5 cm in diameter), oriented perpendicular to the direction of the muscle fibers. A cylindrical piston (75 mm in diameter) was used to compress the sample during 2 test cycles, compressing the sample up to 60% from the original height within a time span of 5 s between the cycles. Force–time curves of deformation were obtained from the conditions established in the texturometer. The velocities used were 2.0 mm/s pre-test, 5.0 mm/s during the test, and 5.0 mm/s post-test. The following parameters were recorded according to Bourne (1978): hardness (Hard; g), adhesiveness (Adhes; g/s), springiness (Spring; mm), cohesiveness (Cohes; dimensionless), gumminess (Gum; g), chewiness (Chew; g mm), and resilience (Resil; dimensionless).
Sensory Evaluation
An affective sensory test of breast meat attributes was conducted to measure the satisfaction level of 30 panelists. The breasts (1 breast/pen/treatment) were vacuum-packed and cooked by immersion in water at 75.0 ± 0.1°C for 1 h. Each panelist evaluated four 1.5-cm cut cubes chosen at random per treatment. Samples for evaluation were maintained at 30°C and were presented in small plastic cups codified with 3 random numbers. The attributes evaluated were odor, taste, juiciness, softness, and overall acceptability. A 7-point hedonic scale was used, where 7 = liked very much and 1 = disliked very much (Anzaldúa-Morales, 1994; Meilgaard et al., 2006).
Statistical Analysis
The growth performance data were analyzed by day (at 7, 14, 21, 28, 35, and 40 D) using the GLM procedure of SAS (2006) and the following statistical model (Wang and Goonewardene, 2004): yijk = μ + Ti + λ + ϵijk, where yijk = production variables measured during the experiment, μ = general mean, Ti = effect of the ith treatment (CON, PLG, and LBS), λ = effect of the covariate IW, and ϵijk = random error normally distributed with mean zero and variance σ2 (ϵijk ∼ N (0, σ2)). Slaughter and meat quality variables were analyzed with the GLM procedure (SAS, 2006), and the same statistical model was used to analyze growth performance. The sensorial data were analyzed with a complete random block design and the statistical model yij = μ + Ti + βj+ ϵj. The treatments (Ti) represented the fixed effects and each consumer was the block (βj). A significance level of P < 0.05 was used to detect the significant statistical difference, and when the P-value was less than 0.05 in fixed effect, the means were compared using the instruction Tukey test (SAS, 2006).
RESULTS AND DISCUSSION
Growth Performance
Some studies with MOO in feed at 400 and 600 mg/kg (Silva-Vázquez et al., 2015), 300 and 600 mg/kg (Peng et al., 2016), and 400 mg/kg (Méndez-Zamora et al., 2017; Silva-Vázquez et al., 2018) showed effects on broiler growth performance. Those authors indicated that MOO could serve as a substitute for growth promoters and antibiotics due to similar effects on improved broiler production. Comparisons of growth performance variables of broilers, given MOO supplemented in DWt, are given in Table 1. Broiler BW was not different (P > 0.05) between treatments on each day and at the end of the growth period (40 D). The CON group BW was statistically similar to PLG and LBS, with differences of 21.6 and 102.8 g, respectively, at 40 D (Table 1). Similar BW results were obtained by Mohiti-Asli and Ghanaatparast-Rashti (2015) at 29 to 35 D for 300 and 500 mg/kg of OEO in feed. Peng et al. (2016) found effects for OEO at 300 and 600 mg/kg of feed in the grower and finisher phases. Basmacıoğlu et al. (2004) obtained differences over time and in the grower and finisher phases with OEO at 150 and 300 mg/kg of feed. Results from those studies presented high BW yields for high levels of OEO, and in contrast with BW gains with LBS and PLG MOO levels used in the current study at 400 mg/L. Differences in effects obtained with different levels of essential oils may be due to OEO in most studies being incorporated in diets, whereas in the current study MOO was made available in DWt.
Table 1.
Growth performance parameters over the 40 D growth period in broilers supplemented with Mexican oregano oils in the drinking water.
| Days | |||||||
|---|---|---|---|---|---|---|---|
| Treatment1 | 1 (IW)2 | 7 | 14 | 21 | 28 | 35 | 40 |
| BW (g) | |||||||
| CON | 46.83 | 125.25 | 263.29 | 475.87 | 764.71 | 1,241.13 | 1,659.63 |
| PLG | 46.92 | 115.28 | 277.61 | 462.42 | 773.96 | 1,188.38 | 1,638.03 |
| LBS | 47.00 | 115.90 | 241.19 | 431.13 | 689.02 | 1,069.47 | 1,556.79 |
| SEM | 0.41 | 5.97 | 13.65 | 24.29 | 31.33 | 47.84 | 54.59 |
| P-value | 0.9607 | 0.4383 | 0.2003 | 0.4326 | 0.1469 | 0.0640 | 0.3984 |
| FI (g) | 7 | 14 | 21 | 28 | 35 | 40 | 1 to 40 |
| CON | 88.22a,b | 291.11 | 429.45 | 649.32a | 966.32 | 785.61 | 3,210.03 |
| PLG | 86.70b | 323.10 | 456.83 | 627.69a,b | 930.74 | 819.60 | 3,244.65 |
| LBS | 115.57a | 298.90 | 437.84 | 574.46b | 889.00 | 880.42 | 3,196.18 |
| SEM | 7.95 | 21.53 | 18.11 | 17.85 | 50.00 | 44.33 | 100.18 |
| P-value | 0.0379 | 0.5619 | 0.5623 | 0.0284 | 0.5639 | 0.3389 | 0.9400 |
| WI (g) | 7 | 14 | 21 | 28 | 35 | 40 | 1 to 40 |
| CON | 224.06 | 521.40 | 825.53 | 1,302.87a | 1,973.75 | 1,640.37 | 6,487.98 |
| PLG | 218.62 | 540.02 | 818.43 | 1,244.74a,b | 1,727.23 | 1,663.44 | 6,212.48 |
| LBS | 190.97 | 448.30 | 740.76 | 1,036.15b | 1,550.89 | 1,548.23 | 5,515.31 |
| SEM | 16.39 | 29.14 | 46.70 | 59.70 | 121.13 | 137.56 | 275.29 |
| P-value | 0.3398 | 0.0973 | 0.3888 | 0.0173 | 0.0788 | 0.8241 | 0.0667 |
1Treatments CON = control drinking water (DWt; without Mexican oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS).
2IW = initial weight; BW = body weight; FI = feed intake; WI = water intake.
a,bMeans (n = 6 replicate pens with 10 chicks per treatment) in columns and with different superscripts are significantly different (P < 0.05).
Feed intake was different (P < 0.05) at 7 and 28 D, with highest FI at 7 D for LBS and lowest for PLG, but, on day 28, CON was highest (P < 0.05) and LBS lowest (P < 0.05). In contrast to results in the current study, Silva-Vázquez et al. (2015) obtained FI differences at 7, 14, 21, 35 and 39 D with 400 to 1600 mg/kg of MOO in feed, and Peng et al. (2016) found effect at 22 to 42 D and 1 to 42 D, with FI being highest with 600 mg/kg. These contrasts could be due to intake type, diet vs. DWt. Particularly, Silva-Vázquez et al. (2018) indicated that the difference in broiler performance may be correlated with the levels of feed additives used in the diets in relation to their chemical composition. In the current study, results obtained with 400 mg/L in DWt indicated a slight decrease in FI with the LBS treatment, although FI with LBS was not different from the CON group. Accordingly, Reyer et al. (2017) suggested that increases in apparent ileal digestibility could be due to feed additives and their effects on intestine epithelial function, and increases in membrane recruitment of sodium-glucose transport protein 1 and peptide transporter 1.
Regarding WI, treatments were different (P < 0.05) on day 28 (Table 1). The CON group gave the highest (P < 0.05) WI values and LBS presented the lowest (P < 0.05) values. In contrast, over the full growth period (1 to 40 D), WI was not different (P > 0.05) between treatments; however, PLG and LBS did present slightly lower (P > 0.05) WI values over the full growth period. Few studies have reported on WI with MOO; however, Silva-Vázquez et al. (2015) found slight increases in WI on days 14, 21, 35, and 39 with LBS in diets, and these authors suggested that these results could be due to the thymol and carvacrol levels of MOO used in diets, which could affect the sensory properties. Additionally, results from the current study with MOO in DWt suggest changes in the sensory traits of the water as indicated by decreases in WI for PLG and LBS, and those sensory changes may influence FI. In contrast, Adaszyńska-Skwirzyńska and Szczerbińska (2018) detected no differences in WI in broiler chickens when evaluating the addition of lavender essential oil (LEO; 0.4 mL/L). These contrasts could be due to the chemical composition differences of MOO and LEO, where MOO has carvacrol (60.00%) and thymol (28.40%) as major components, whereas LEO presents linalool (35.17%) and linalool acetate (46.25%) (Adaszyńska-Skwirzyńska and Szczerbińska, 2018).
Production efficiency is shown in Table 2, illustrating that WBWG was different (P < 0.05) on day 14, with the PLG group being highest (P < 0.05) and LBS lowest (P < 0.05). Additionally, WBWG was not different (P > 0.05) between groups over the growth period of 1 to 40 D. Feed efficiency was not different (P > 0.05) at each time between treatment groups, as well as between groups over the full growth period (1 to 40 D). In contrast to these findings, Ghazanfari et al. (2015) found effects for weight gain and feed conversion ratio at 1 to 10, 25 to 42, and 1 to 42 D when evaluating feed containing 100, 200, and 300 mg/kg of coriander essential oil. Those authors indicated that the essential oil improved digestive enzyme activities and nutrient digestibility, enhancing the feed conversion ratio and greater growth rate. In addition, Basmacıoğlu et al. (2004) obtained differences for body weight gain but not for feed conversion ratio when testing 150 and 300 mg/kg of oregano oil in feed. The results in the current study were similar to those of Silva-Vázquez et al. (2015), who indicated that MOO as a diet supplement improved broiler production and depended on the MOO levels tested. In the current study, MOO added to DWt did not affect the FE, and this observation contrasts with Silva-Vázquez et al. (2015), who used MOO in diets, which suggests a different mechanism of action when the oregano oil intake is in feed or water.
Table 2.
Growth efficiency over the 40 D growth period of broilers supplemented with Mexican oregano oils in the drinking water.
| Days | |||||||
|---|---|---|---|---|---|---|---|
| Treatment1 | 7 | 14 | 21 | 28 | 35 | 40 | 1 to 40 |
| WBWG (g)2 | |||||||
| CON | 78.33 | 138.04a,b | 212.58 | 288.83 | 476.43 | 418.50 | 268.79 |
| PLG | 68.36 | 162.34a | 184.80 | 311.55 | 414.42 | 449.64 | 265.19 |
| LBS | 68.99 | 125.28b | 189.94 | 257.90 | 380.44 | 487.32 | 251.64 |
| SEM | 5.96 | 9.70 | 16.27 | 19.16 | 25.22 | 58.59 | 9.10 |
| P-value | 0.4383 | 0.0490 | 0.4591 | 0.1756 | 0.0509 | 0.7142 | 0.3984 |
| FE | |||||||
| CON | 1.15 | 2.12 | 2.04 | 2.35 | 2.03 | 1.98 | 1.95 |
| PLG | 1.33 | 2.04 | 2.71 | 2.02 | 2.31 | 2.08 | 2.08 |
| LBS | 1.73 | 2.44 | 2.34 | 2.23 | 2.35 | 2.13 | 2.20 |
| SEM | 0.17 | 0.20 | 0.25 | 0.14 | 0.12 | 0.36 | 0.10 |
| P-value | 0.0868 | 0.3775 | 0.2043 | 0.2901 | 0.1528 | 0.9558 | 0.2386 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS).
2WBWG = weekly body weight gain; FE = feed efficiency.
a,bMeans (n = 6 replicate pens with 10 chicks per treatment) in columns and with different superscripts are significantly different (P < 0.05).
Slaughter Variables
As shown in Table 3, SW and TY presented differences (P < 0.05) between treatments, but other slaughter variables were not different (P > 0.05). Slaughter weight was highest (P < 0.05) for the CON group and lowest (P < 0.05) for LBS, but TY was highest (P < 0.05) for the LBS and PLG groups. Slaughter weight and yield values in the current study were lower than those obtained by Méndez-Zamora et al. (2017) and Silva-Vázquez et al. (2018) evaluating 0.40 g/kg of MOO in diets, respectively. Additionally, Gámez-Piñón et al. (2015) obtained higher values for SW and yields when examining 0.10, 0.20, and 0.40 g/L of OEO in DWt and for broilers at the end of a 42-D growth period in respect to 40 D in the current study. These differences could be due to the total growth period difference of 2 D, where the OEO action could have a major effect on broiler performance, as well as on slaughter variables SW and TY. Comparing results from other studies where essential oils were supplemented in diets (Peng et al., 2016; Chowdhury et al., 2018; Silva-Vázquez et al., 2018), slaughter values were higher than those from the current study, which could be due to 3 factors, supplementation type (feed or water), type and level of essential oil, and growth period.
Table 3.
Slaughter variables at the end of the 40 D growth period for broilers supplemented with Mexican oregano oils in the drinking water.
| Yields (%)2 | ||||||
|---|---|---|---|---|---|---|
| Treatment1 | SW (kg) | HCY | CCY | BY | TY | LY |
| CON | 1.75a | 68.91 | 67.95 | 24.24 | 10.70b | 12.99 |
| PLG | 1.70a,b | 70.90 | 69.80 | 25.23 | 11.49a | 10.43 |
| LBS | 1.57b | 67.20 | 69.40 | 24.07 | 11.68a | 10.31 |
| SEM | 0.04 | 1.53 | 1.39 | 0.44 | 0.16 | 1.65 |
| P-value | 0.0144 | 0.2376 | 0.6154 | 0.1434 | < 0.0001 | 0.4364 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS). SEM = standard error of the mean.
2SW = slaughter weight; HCY = hot carcass yield; CCY = cold carcass yield; BY = breast yield; TY = thigh yield; LY = leg yield.
a,bMeans (n = 30 per treatment) in columns and with different superscripts are significantly different (P < 0.05).
Physicochemical Variables
Breast meat pH and WHC, and cooking loss of breast, thigh, and leg are shown in Table 4. Breast meat values for pH, WHC, and CLB were not different (P > 0.05) between treatments, but those for CLT and CLL were different (P < 0.05), showing improvements for CON and PLG treatments (low values). Méndez-Zamora et al. (2015b) and Gámez-Piñon et al. (2015) evaluated 0.40 g/kg of OEO in the diet and DWt (0.4 g/L), respectively, finding similar values for pH and WHC to those from the current study. Additionally, Kirpinar et al. (2014) obtained similar pH values using 0.15 and 0.30 g/kg of OEO in diets, as well as Gámez-Piñón et al. (2015) found similar results with 0.10, 0.20, and 0.40 g/L of OEO in DWt. Few studies with oil extracts have reported WHC and CL for breast, thigh, and leg meat; however, Park et al. (2014) did not find effects on CLB when testing 0.2% (w/v) of 3 plant extracts (Saposhnikovia divaricata, Lonicera japonica, and Chelidonium majus). The CLT and CLL were improved by PLG treatment, but not by LBS, which indicated that the essential oil type in DWt can influence muscle metabolism, increasing pH at the cellular level and the post-mortem metabolism in muscles.
Table 4.
Broiler breast, thigh and leg meat pH, water holding capacity, and cooking loss at the end of the 40 D growth period for broilers supplemented with Mexican oregano oils in the drinking water.
| Breast meat2 | Cooking loss (%) | ||||
|---|---|---|---|---|---|
| Treatment1 | pH | WHC (%) | CLB | CLT | CLL |
| CON | 5.88 | 61.60 | 12.32 | 21.10b | 12.85b |
| PLG | 5.94 | 63.25 | 12.48 | 20.61b | 13.05b |
| LBS | 5.95 | 59.55 | 12.61 | 23.54a | 16.50a |
| SEM | 0.02 | 1.06 | 1.43 | 0.65 | 0.96 |
| P-value | 0.0605 | 0.0555 | 0.9896 | 0.0056 | 0.0159 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS). SEM = standard error of the mean.
2WHC = water holding capacity, CLB = cooking loss breast, CLT = cooking loss thigh, CLL = cooking loss leg.
a,bMeans (n = 12 per treatment; 2 replicates by pen) in columns and with different superscripts are significantly different (P < 0.05).
The color parameter assessments of breast meat are shown in Table 5. Redness (a*), chroma, and BI were different (P < 0.05) between treatments with the CON group presenting the lowest (P < 0.05) values and PLG and LBS the highest (P < 0.05). The other variables were not different (P > 0.05), however, PLG or LBS generally presented high values and CON presented low values. Hong et al. (2012) and Gámez-Piñon et al. (2015) did not find an effect on breast meat color parameters. Contrasting effects on breast meat color variables were presented by Méndez-Zamora et al. (2015b) for L* and b*; however, Kirkpinar et al. (2014) found statistical effects on b* similar to those from the current study but not on a*. Those authors noted the changes in color attributes as minor and that the high carotenoid content of oregano could affect the color parameters. The differences between treatments found in the current study on BI (calculated using L*, a*, and b* values) suggest that pigments present in MOO could be deposited in the breast meat, principally for the LBS group. For example, Young et al. (2003) reported that the main carotenoids in pure oregano were lutein (21.3 ± 0.2 μg/g) and β-carotene (3.9 ± 0.4 μg/g). Some studies indicated that the more yellow color (b*) in the pectoralis major could be attributed to the high carotenoid content of oregano (Young et al., 2003; Symeon et al., 2009; Kirkpinar et al., 2014).
Table 5.
Color parameters of breast meat at the end of the 40 D growth period for broilers supplemented with Mexican oregano oils in the drinking water.
| Variables2 | |||||||
|---|---|---|---|---|---|---|---|
| Treatment1 | L* | a* | b* | Hue | Chroma | ΔE | BI |
| CON | 57.50 | 10.91b | 13.36 | 39.03 | 17.39b | 39.64 | 40.17b |
| PLG | 58.55 | 12.69a | 13.58 | 42.76 | 18.72a | 39.25 | 41.81a,b |
| LBS | 57.29 | 12.38a | 14.15 | 39.73 | 18.64a | 40.46 | 43.89a |
| EEM | 0.53 | 0.53 | 0.31 | 1.40 | 0.29 | 0.54 | 1.04 |
| P-value | 0.2019 | 0.0444 | 0.1920 | 0.1447 | 0.0032 | 0.2737 | 0.0457 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS). SEM = standard error of the mean.
2L* = lightness; a* = redness; b* = yellowness; Hue = Hue angle; Chroma = saturation index; ΔE = total color change; BI = browning index.
a,bMeans (n = 12 per treatment; 2 breast by pen) in columns and with different superscripts are significantly different (P < 0.05).
Meat Texture Analysis
The texture analysis evaluates meat softness and structure performance. Values of SF, Cohes, and Resil were different (P < 0.05) between experimental groups (Table 6). Shear force was higher (P < 0.05) for broilers receiving PLG than those receiving LBS oil; however, PLG was lower (P < 0.05) and LBS higher (P < 0.05) for Cohes and Resil. Although differences were not obtained for other breast meat texture variables, the CON treatment generally presented high values for Hard, Adhes, Gum, and Chew. These results indicated that MOO supplemented in DWt could have effects on the breast meat texture, principally on SF as a function of the muscle fiber and water content. Studies have reported effects of OEO on carcass characteristics, meat physicochemical variables (pH, WHC, color), breast meat chemical, and fatty acid composition, but SF and texture profiles had minimal to no effects when OEO was added to diets. Particularly, Park et al. (2014) found effects on SF and Adhes and indicated the reasons for these results as unknown. Being that there is a lack of information regarding texture effects of plant extracts, those authors indicated that more study is needed to determine the effects of natural extracts on broiler breast meat texture traits. The current study presents more results on broiler breast meat texture profile and when MOO was supplemented in DWt, finding effects on SF, Cohes, and Resil, which could indicate that MOO has effects on the meat texture. Similarly, Cázares-Gallegos et al. (2019) obtained differences for Hard, Cohes, and Resil using 200, 400, 600, 800, and 1000 mg/kg of LBS incorporated in the diet. Those authors indicated that those results, and results obtained by Park et al. (2014), demonstrated that essential oil extracts in diets could influence texture properties of broiler meat. Additionally, Sandercock et al. (2001) indicated that ante-mortem physiological phenomena would appear to be associated with changes in post-mortem breast muscle and meat characteristics, and a decline in pH may be the consequence of a greater degree of post-mortem glycolytic metabolism in more mature muscles. Furthermore, those authors indicated that heat stress-induced ante-mortem alterations in muscle membrane permeability and concomitant changes in muscle metabolism in broilers may influence post-mortem meat quality. Additionally, changes in muscle membrane integrity may be involved in mechanisms of post-mortem intracellular water loss, and elevations in plasma creatine kinase, reflecting membrane muscle damage, and an association with higher rates of pH decline. In the current study, results obtained for breast meat pH when MOO was added to DWt may indicate that MOO could alter ante-mortem physiological processes due to stress or natural physiology reactions. As a consequence, these alterations may slow down the rapid pH decline when the muscle is still warm. The rapid decrease in pH causes denaturation (loss of functionality and water-binding ability) of many proteins (Huff-Lonergan and Lonergan, 2005). These possible restrictive effects of MOO on ante-mortem metabolism may have prevented major damage in myofibrillar proteins and breast-meat structure, and resulted in improvements in thermal analysis results, SF, Cohes, and Resil for the PLG group.
Table 6.
Texture analysis at the end of the 40 D growth period of breast meat from broilers supplemented with Mexican oregano oils in the drinking water.
| Variables2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment1 | SF (gf) | Hard (g) | Adhes (g s−1) | Spring (mm) | Cohes | Gum (g) | Chew (g mm) | Resil |
| CON | 1381.57a,b | 5133.41 | −23.73 | 0.50 | 0.36a,b | 1989.67 | 1006.72 | 0.16b |
| PLG | 1615.27a | 4736.80 | −26.95 | 0.51 | 0.34b | 1611.79 | 845.65 | 0.16b |
| LBS | 1286.74b | 4622.35 | −30.88 | 0.51 | 0.39a | 1828.09 | 966.44 | 0.19a |
| SEM | 78.93 | 257.97 | 5.24 | 0.01 | 0.01 | 128.03 | 70.41 | 0.01 |
| P-value | 0.0135 | 0.3549 | 0.6301 | 0.7807 | 0.0165 | 0.1193 | 0.2494 | 0.0093 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS). SEM = standard error of the mean.
2SF = shear force; Hard = hardness; Adhes = adhesiveness; Spring = springiness; Cohes = cohesiveness (dimensionless); Gum = gumminess; Chew = chewiness; Resil = resilience (dimensionless).
a,bMeans (n = 24 sections per treatment; 2 sections on each side of the breast by pen) in columns and with different superscripts are significantly different (P < 0.05).
Sensory Evaluation
Meat sensory evaluation is a method to examine preference attributes perceived by consumers. Sensory evaluations of breast meat from broilers supplemented with oregano oils in DWt are presented in Table 7. Odor, taste, and overall acceptability presented significant effects (P < 0.05) by treatment. These attributes were more preferred for PLG breast meat than meat from the LBS-treated group. Few studies have evaluated sensorial traits with OEO in broilers at grow-out, and results have generated some controversies. Symeon et al. (2009) did not find significant differences in the acceptance test regarding tenderness, juiciness, taste, and overall acceptance when evaluating 100 and 250 mg/kg of Origanum vulgare L. essential oils; however, those authors indicated that any effect found must depend on the herb selected or the dose administered. In the current study, MOO from the 2 plant sources used in DWt elicited differences in breast meat odor, taste, and overall acceptability. Similarly, significant statistical effects were obtained by Hong et al. (2012) and Kirkpinar et al. (2014) for appearance, juiciness, flavor, and overall acceptability. Hong et al. (2012) indicated that improvement in overall consumer acceptance was related to the antioxidant properties of the polyphenols and flavonoids that would limit the level of protein and lipid oxidation. Therefore, consumer acceptability may be influenced by the high levels of the characteristics of the potent antioxidants carvacrol and thymol of oregano oils (Silva-Vázquez et al., 2017), which improved the oxidative stability of chicken meat and hence the sensory values (Kirkpinar et al., 2014).
Table 7.
Sensory evaluation at the end of the 40 D growth period of breast meat from broilers supplemented with Mexican oregano oils in the drinking water.
| Sensory attributes2 | |||||
|---|---|---|---|---|---|
| Treatment1 | Odor | Taste | Juiciness | Softness | Overall acceptability |
| CON | 5.37a,b | 5.37a | 5.37 | 5.63 | 5.40a,b |
| PLG | 5.43a | 5.57a | 5.80 | 5.97 | 5.80a |
| LBS | 4.73b | 4.63b | 5.03 | 6.03 | 4.97b |
| SEM | 0.19 | 0.21 | 0.24 | 0.19 | 0.17 |
| P-value | 0.0255 | 0.0069 | 0.1010 | 0.3190 | 0.0054 |
1Treatments CON = control drinking water (DWt; without oregano oils), PLG = DWt + 400 mg/L of Poliomintha longiflora Gray (PLG) and LBS = DWt + 400 mg/L of Lippia berlandieri Schauer (LBS). SEM = standard error of the mean.
2A 7-point hedonic scale where 7 = liked very much and 1 = disliked very much.
a,bMeans (n = 30 consumers) in columns and with different superscripts are significantly different (P < 0.05).
CONCLUSIONS
Essential oils derived from the Mexican oregano PLG and LBS at 400 mg/L in the DWt presented broiler BW that were not different from the CON group. The LBS treatment presented broiler low feed and WI, and, along with the PLG treatment, resulted in the best thigh yield, whereas slaughter weight was higher for the CON group. Thigh and leg cooking loss for the CON and PLG treatments gave better results over LBS. For broiler breast meat quality traits, redness, saturation and BI, SF, Cohes, and Resil benefited from PLG and LBS supplementation. The sensory results indicated that breast meat from the PLG group was most acceptable by the panel. Mexican oregano essential oil can serve as an alternative and natural additive in DWt to improve broiler production; however, other studies are needed to evaluate the mechanism of action of MOO in the DWt on growth performance and the effects on broiler-meat quality.
ACKNOWLEDGMENTS
The current study was supported by the Programa de Apoyo a la Investigacion Cientifica y Tecnologica (PAICYT-2015; code CT253-15), and Facultad de Agronomia of the Universidad Autonoma de Nuevo Leon.
REFERENCES
- Adaszyńska-Skwirzyńska M., Szczerbińska D.. 2018. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult. Sci. Accessed August 2018. 10.3382/ps/pey385. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Amaral L. A. do. 2004. Drinking water as a risk factor to poultry health. Rev. Bras. Cienc. Avic. 6:191–199. [Google Scholar]
- Amiranashvili L. L., Gagelidze N. A., Makaradze L. A., Varsimashvili K. I., Tolordava L. L., Tinikashvili L. M., Amashukeli N. V., Sachaneli-Qadagishvili T. Z., Kirtadze E. G.. 2017. The effect of homoprobiotic preparation “Probiogeo” supplemented with drinking water and feed on survivability and growth performance of broiler-chickens. Anna. Agrar. Sci. 15:476–479. [Google Scholar]
- Anzaldúa-Morales A. 1994. La evaluación sensorial de los alimentos en la teoría y la práctica. Editorial Acribia S.A. Zaragoza, Spain. [Google Scholar]
- Basmacıoğlu H., Tokuşoğlu O., Ergül M.. 2004. The effect of oregano and rosemary essential oils or alpha-tocopheryl acetate on performance and lipid oxidation of meat enriched with n-3 PUFA’s in broilers. S. Afr. J. Anim. Sci. 34:197–210. [Google Scholar]
- Bourne M. C. 1978. Texture profile analysis. Food Technol. 32:62–66. [Google Scholar]
- Bozkurt H., Bayram M.. 2006. Colour and textural attributes of sucuk during ripening. Meat Sci. 73:344–350. [DOI] [PubMed] [Google Scholar]
- Calvo-Irabien L. M. 2018. Native Mexican aromatic flora and essential oils: Current research status, gaps in knowledge and agro-industrial potential. Ind. Crops Prod. 111:807–822. [Google Scholar]
- Cázares-Gallegos R., Silva-Vázquez R., Hernández-Martínez C. A., Gutiérrez Soto J. G., Kawas-Garza J. R., Hume M. E., Méndez-Zamora G.. 2019. Performance, carcass variables, and meat quality in broilers supplemented with dietary Mexican oregano oil. Braz. J. Poult. Sci. 21:001–010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Prasad Mandal G., Kumar Patra A.. 2018. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 236:86–97. [Google Scholar]
- CIE 1976. International Commission on Illumination. Colorimetry. Bureau Central de la CIE, Vienna, Austria: Accessed August 2018. http://www.cie.co.at/. [Google Scholar]
- Gámez-Piñón J. R., Rentería-Monterrubio A., Durán-Meléndez L. A., Chávez-Martínez A., Alarcón-Rojo A. D., Aguilar-Palma N. G., Silva-Vázquez R.. 2015. Efecto del aceite esencial de orégano en el rendimiento y las propiedades fisicoquímicas y microbiológicas de la carne de pollo. Invest. Cien. 23:5–11. [Google Scholar]
- Ghazanfari S., Mohammadi Z., Moradi A. M.. 2015. Effects of coriander essential oil on the performance, blood characteristics, intestinal microbiota and histological of broilers. Rev. Bras. Cienc. Avic. 17:419–426. [Google Scholar]
- Ghazi S., Amjadian T., Norouzi S.. 2015. Single and combined effects of vitamin C and oregano essential oil in diet, on growth performance, and blood parameters of broiler chicks reared under heat stress condition. Int. J. Biometeorol. 59:1019–1024. [DOI] [PubMed] [Google Scholar]
- Hong J. C., Steiner T., Aufy A., Lien T. F.. 2012. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 144:253–262. [Google Scholar]
- Huff-Lonergan E., Lonergan S. M.. 2005. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 71:194–204. [DOI] [PubMed] [Google Scholar]
- INEGI (Instituto Nacional de Estadística y Geografía). 2018. Compendio de Información Geográfica Municipal. Accessed August 2018. http://www.inegi.org.mx/geo/contenidos/topografia/compendio.aspx.
- Jin L. Z., Ho Y. W., Abdullah N., Jalaludin S.. 1996. Influence of Bacillus subtilis and Lactobacilli cultures on intestinal microflora and performance in broilers. Asian-Australas. J. Anim. Sci. 9:397–403. [Google Scholar]
- Karimi A., Yan F., Coto C., Park J. H., Min Y., Lu C., Gidden J. A., Lay J. O. Jr., Waldroup P. W.. 2010. Effects of level and source of oregano leaf in starter diets for broiler chicks. J. Appl. Poult. Res. 19:137–145. [Google Scholar]
- Karimi Torshizi M. A., Moghaddam A. R., Rahimi S., Mojgani N.. 2010. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 51:178–184. [DOI] [PubMed] [Google Scholar]
- Kırkpınar F., Ünlü B. H., Serdaroğlu M., Turp Y. G.. 2014. Effects of dietary oregano and garlic essential oils on carcass characteristics, meat composition, colour, pH and sensory quality of broiler meat. Br. Poult. Sci. 55:157–166. [DOI] [PubMed] [Google Scholar]
- Ledesma E., Laca A., Rendueles M., Díaz M.. 2016. Texture, colour and optical characteristics of a meat product depending on smoking time and casing type. LWT - Food Sci. Technol. 65:164–172. [Google Scholar]
- Liu X., Yan H., Lv L., Xu Q., Yin C., Zhang K., Wang P., Hu J.. 2012. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 25:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-González R., Meléndez-González R.. 2005. Growth characteristics of Mexican oregano (Lippia berlandieri Schauer) under salt stress. Southwest. Nat. 50:1–6. [Google Scholar]
- Meilgaard M. C., Civille G. V., Carr B. T.. 2006. Sensory Evaluation Techniques. 4th ed.CRC Press, Boca Raton, FL. [Google Scholar]
- Méndez-Zamora G., García-Macías J. A., Durán-Meléndez L. A., Herman-Lara E., Santellano-Estrada E., Silva-Vázquez R.. 2015a. Aceite esencial de orégano (Lippia berlandieri Schauer) en variables de calidad de la canal de pollo. Ecosistemas Recur. Agropecuarios. 2:41–51. [Google Scholar]
- Méndez-Zamora G., García-Macías J. A., Santellano-Estrada E., Durán-Meléndez L. A., Silva-Vázquez R.. 2015b. Aceite de orégano sobre la calidad de pechuga de pollos de engorda. Invest. Cien. 65:5–12. [Google Scholar]
- Méndez-Zamora G., Durán-Meléndez L. A., Hume M. E., Silva-Vázquez R.. 2017. Performance, blood parameters, and carcass yield of broiler chickens supplemented with Mexican oregano oil. R. Bras. Zootec. 46:515–520. [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M.. 2015. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 120:195–202. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 1994. Nutritional Requirements of Poultry. 9th ed.Natl. Acad. Sci.: Washington, DC, USA. [Google Scholar]
- NOM-033- SAG/ZOO-2014. 2014. Norma Oficial Mexicana. Métodos para dar muerte a los animales domésticos y silvestres. Accessed August 2018. http://www.economia-noms.gob.mx/noms/consultasAction.do.
- NOM-062-ZOO-1999. 1999. Norma oficial Mexicana, especificaciones técnicas para la producción, cuidado y uso de animales de laboratorio. Accessed August 2018. http://www.economia-noms.gob.mx/noms/consultasAction.do.
- Park J. H., Kang N. S., Chu M. G., Jin K. S.. 2014. Growth performance, blood cell profiles, and meat quality properties of broilers fed with Saposhnikovia divaricata, Lonicera japonica, and Chelidonium majus extracts. Livest. Sci. 165:87–94. [Google Scholar]
- Pelicano E. R. L., de Souza P. A., de Souza H. B. A., Oba A., Norkus E. A., Kodawara L. M., de Lima T. M. A.. 2003. Effect of different probiotics on broiler carcass and meat quality. Rev. Bras. Cienc. Avic. 5:207–214. [Google Scholar]
- Peng Q. Y., Li J. D., Li Z., Duan Z. Y., Wu Y. P.. 2016. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 214:148–153. [Google Scholar]
- Reyer H., Zentek J., Männer K., Youssef I. M. I., Aumüller T., Weghuber J., Wimmers K., Müller A.. 2017. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J. Agric. Food Chem. 65:6821–6830. [DOI] [PubMed] [Google Scholar]
- Sandercock D. A., Hunter R. R., Nute G. R., Mitchel M. A., Hocking P. M.. 2001. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult. Sci. 80:418–425. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2006. SAS/STAT user's Guide. SAS Institute Inc. Cary, NC. [Google Scholar]
- Silva-Vázquez R., Durán-Meléndez L. A., Santellano-Estrada E., Rodríguez-Muela C., Villalobos-Villalobos G., Méndez-Zamora G., Hume M. E.. 2015. Performance of broiler chickens supplemented with Mexican oregano oil (Lippia berlandieri Schauer). R. Bras. Zootec. 44:283–289. [Google Scholar]
- Silva-Vázquez R., Duran-Melendez L. A., Mendez-Zamora G., Santellano-Estrada E., Xie M., Dunford N. T., Goad C.. 2017. Antioxidant activity of essential oils from various Mexican oregano ecotypes and oil fractions obtained by distillation. JSM Chem. 5:1046. [Google Scholar]
- Silva-Vázquez R., Duran-Meléndez L. A., Hernández-Martínez C. A., Gutiérrez-Soto J. G., Hume M. E., Méndez-Zamora G.. 2018. Effects of two sources of Mexican oregano oil on performance, blood profile, carcass variables, and meat of broilers. Rev. Bras. Zootecn. 47:e20170198, Accessed Aug. 2018, doi:10.1590/rbz4720170198. [Google Scholar]
- Skoufos I., Giannenas I., Tontis D., Bartzanas T., Kittas C., Panagakis P., Tzora A.. 2016. Effects of oregano essential oil and attapulgite on growth performance, intestinal microbiota and morphometry in broilers. S. Afr. J. Anim. Sci. 46:77–88. [Google Scholar]
- Symeon G. K., Zintilas C., Ayoutanti A., Bizelis J. A., Deligeorgis S. G.. 2009. Effect of dietary oregano essential oil supplementation for an extensive fattening period on growth performance and breast meat quality of female medium-growing broilers. Can. J. Anim. Sci. 89:331–334. [Google Scholar]
- Timmerman H. M., Veldman A., van den Elsen E., Rombouts M. F., Beynen C. A.. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85:1383–1388. [DOI] [PubMed] [Google Scholar]
- Tsai T. C., Ockerman H. W.. 1981. Water binding measurement of meat. J. Food Sci. 46:697–701. [Google Scholar]
- Wang Z., Goonewardene L. A.. 2004. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 84:1–11. [Google Scholar]
- Young J. F., Stagsted J., Jensen S. K., Karlsson A. H., Henckel P.. 2003. Ascorbic acid, alpha-tocopherol, and oregano supplements reduce stress-induced deterioration of chicken meat quality. Poult. Sci. 82:1343–1351. [DOI] [PubMed] [Google Scholar]


