ABSTRACT
The objective of this study was to determine the effects of protease origin and dosage on the prececal (pc) amino acid (AA) digestibility and the influence on composition of the microbial community in the small intestine. In addition, the effects of phytase supplementation were investigated. A total of 8 dietary treatments were included. The basal diet contained mainly corn and soybean meal. Three protease products were added to the basal diet, each at the level recommended by the supplier and at an 8-fold level. Phytase was supplemented in another dietary treatment. Each dietary treatment was allocated to 8 replicates of 15 birds each. The experimental diets were offered from day 15 to 21 for ad libitum consumption. The effect of protease supplementation on the pc AA digestibility depended on the protease product type and the amount supplemented. The pc AA digestibility was significantly increased by 1 protease product when supplemented at high level and when phytase was supplemented. In all the other treatments, protease supplementation had no significant influence or it decreased pc AA digestibility, when compared with the treatment with no enzymes added. In general, Firmicutes was the most abundant phylum among the ileal microbiota across all the treatments. Significant effects on microbiota composition were observed at the genus level for some but not all protease treatments and phytase supplementation. The genera Streptococcus, Lactobacillus, and uncultured Clostridiaceae were responsible for these differences. Furthermore, microbial networks established for each diet showed either high or low number of intergeneric interactions, but without a consistent enzyme effect. We conclude that enzyme supplementation effects were evident in the terminal small intestine microbiota composition, and to a lesser extent, in pc AA digestibility. However, the changes in microbiota composition and pc AA digestibility could not be correlated, indicating absence of a causal relationship.
Keywords: amino acids, broiler chickens, digestibility, enzymes, microbiota
INTRODUCTION
Increasing the nutrient utilization efficiency in broiler chickens is an effective approach to minimize nutrient intake for growth and meat production, and reduce N excretion. The utilization of CP is subject of many studies because of its economic impact on the industry and detrimental effects of N excretion on the environment.
Protease supplements have been suggested to potentially achieve increased prececal (pc) amino acid (AA) digestibility in broiler chickens, and thereby reduce the dietary CP level. The effects of protease supplementation on pc CP and AA digestibility have been found to be inconsistent. Studies on broiler chickens and turkeys showed that the pc digestibility was increased for all AA (Vieira et al., 2013; Stefanello et al., 2016; Cowieson et al., 2017) or some AA (Bertechini et al., 2009; Angel et al., 2011; Vieira et al., 2013). Whereas, in some other studies, no effects (Boguhn et al., 2011; Kaczmarek et al., 2014; Rada et al., 2016; Erdaw et al., 2017) or decreasing effects (Walk et al., 2018) of protease supplementations on pc digestibility were described. Divergent results may be caused by differences in the composition of the experimental diet (Selle et al., 2016; Toghyani et al., 2017), supplementation level (Angel et al., 2011), or concurrent supplementation of other enzymes (Lee et al., 2018).
Characteristics of the supplemented protease likewise contributed to the divergent results as the efficacy of protease is influenced by the environment of the surrounding medium, including pH and temperature (Ghazi et al., 2002; Mahmood et al., 2017). Hence, differences in the efficacy of proteases have been reported. Ghazi et al. (2002) reported no effect of a protease isolated from a Bacillus species on pc CP digestibility, whereas it increased with the addition of a protease isolated from an Aspergillus species. In another study, supplementation of proteases derived from Aspergillus niger and Bacillus subtilis had no effect on the total tract CP digestibility (Mahmood et al., 2017). In a screening of several proteases derived from various bacteria and fungi, the effect on pc AA digestibility differed widely (Walk et al., 2018). These authors reported that protease supplementation in most cases had no effect on the pc AA digestibility. They found that pc digestibility of some AA was increased, whereas it was decreased for others, depending on the supplemented protease and the experiment. However, in their study all diets contained a phytase supplement (Walk et al., 2018).
Phytase is primarily used to increase degradation of phytate and utilization of phosphorus, and additionally has potential to increase pc AA digestibility. Similar to protease, the effects of phytase supplementation on pc AA digestibility are variable, with some studies reporting an increasing effect (Amerah et al., 2014; Sommerfeld et al., 2018) and others without any effect (Rodehutscord et al., 2004; Manangi et al., 2009). Should phytase have a similar effect like protease and if such effects are not additive, then it is possible that the overall addition of phytase in the study by Walk et al. (2018) masked possible effects of protease supplementation. Furthermore, the study by Walk et al. (2018) did not include different dosages of protease, but doses necessary to achieve increased pc AA digestibility might vary between proteases.
The supplementation of enzymes can influence the microbiota composition in the intestine. Phytase supplementation increased the total number of microbial counts in the small intestine and increased relative abundance of bacteria such as Lactobacillus and Enterococcus (Ptak et al., 2015; Witzig et al., 2015). To the best of our knowledge, the effects of protease supplementation on microbial ecology have not been investigated using Next Generation Sequence (NGS) techniques. However, different scenarios of consequences of protease supplementation on the microbiota can be deduced from the literature. In a study that used qPCR methodology to target specific microbial groups, protease seemed to increase the presence of Lactobacillus spp. but decrease the presence of Clostridium perfringens in the ileum (Giannenas et al., 2017). In another study, protease supplemented in combination with α-amylase and glucoamylase increased the relative abundance of Bifidobacterium, Staphylococcus, Bacteroides, and Megamonas (Yin et al., 2018), which usually are considered to be beneficial bacteria. It also is possible that protease supplements alter the microbiota composition by modifying the substrates that the microorganisms access. For instance, higher availability of AA was shown to be either beneficial or harmful to the growth of certain microorganisms (Dahiya et al., 2007). Other metabolites like short chain fatty acids, amines, and AA derivatives were also shown to impact the microorganisms (Hemarajata and Versalovic, 2013). Therefore, effects of protease supplementation on pc digestibility might partly be explained by a shift in the microbial composition. To our knowledge, such a relationship has not been investigated to date.
Hence, our main objective was to investigate the effects of different proteases at 2 dosage levels on the pc AA digestibility and the composition of the microbiota in the terminal small intestine. We also aimed to examine whether effects on pc AA digestibility, if caused by proteases, could be found upon supplementation of phytase in a separate treatment. We hypothesized that enzymes affect the pc AA digestibility in a dose-dependent manner and that the microbiota composition in the terminal small intestine is altered due to enzyme supplementation.
MATERIALS AND METHODS
Experimental Diets
The study comprised of 8 dietary treatments. The basal diet (BD) did not contain any enzyme supplement and was mainly based on corn and solvent-extracted soybean meal (Table 1). For other 6 treatments, the BD was supplemented with 3 different proteases at 3 levels each: Aspergillus Acid Protease (Protease A) (Meiji Seika Pharma Co., Ltd., Japan) produced from A. niger with a declared protease activity of not less than 950,000 U/g at pH 2.6, supplemented at 25 or 200 mg/kg of diet; CIBENZA DP100 (Protease B) (Novus International Inc., MO, USA) produced from Bacillus licheniformis with a declared minimum protease activity of 600,000 U/g supplemented at 500 or 4000 mg/kg of diet; RONOZYME PROACT (Protease C) (DSM Nutritional Products AG, Kaiseraugst, Switzerland) produced from a genetically engineered B. licheniformis strain with a declared minimum protease activity of 75,000 U/g, supplemented at 200 or 1600 mg/kg of diet. The lower dosage of the proteases was chosen based on supplier recommendations and the other dosage was set at 8 times the recommended dosage. For the eighth treatment, Natuphos E (Phy) (BASF SE, Germany) was supplemented to provide 1500 FTU/kg of diet. The calculated phytase level was verified by analysis (1410 FTU/kg). Titanium dioxide (TiO2) was included as an indigestible dietary marker (5 g/kg). All diets were adequate in phosphorus concentration. The CP concentration of the diets was uniform and ranged from 245 to 248 g/kg DM (Table 2). Diets were manufactured at Research Diet Services (Hoge Maat 10, 3961 NC Wijk bij Duurstede, Netherlands). Application of all enzymes in this experiment was approved by the Regierungspräsidium Tübingen, approval number 34/8302.31.
Table 1.
Composition of the experimental diets and supplementation levels of the enzyme products (g/kg unless otherwise stated).
| Basal diet | Protease A | Protease B | Protease C | Phytase | ||||
|---|---|---|---|---|---|---|---|---|
| Supplementation level1 | No enzyme | 25 mg/kg | 200 mg/kg | 500 mg/kg | 4000 mg/kg | 200 mg/kg | 1600 mg/kg | 1500 FTU/kg |
| Corn | 560 | 560 | 560 | 560 | 560 | 560 | 560 | 560 |
| Soybean meal2 | 371 | 371 | 371 | 371 | 371 | 371 | 371 | 371 |
| Soybean oil | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Calcium carbonate | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 |
| Sodium chloride | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Monocalcium phosphate | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Choline chloride | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Vitamin- and mineral mix3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| TiO2 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
1The respective amount of enzyme was added on top of the mixtures; the lower supplementation of each protease product complies with the recommendations of the suppliers, the other dosage is 8-fold higher.
2Solvent-extracted soybean meal; trypsin inhibitor activity: 1.36 g/kg; urease activity: < 0.02 mg N/g (min, 30°C); nitrogen solubility index: 12%; Lys: 29.0 g/kg; reactive Lys: 24.4 g/kg (all on as-is basis).
3Provided per kg of mixed feed: vitamin A (retinyl acetate): 12,000 IE; vitamin D3 (cholecalciferol): 2,500 IE; vitamin E (dl-α-tocopherol): 50 mg; vitamin K3 (menadione): 1.5 mg; Vitamin B1 (thiamine): 2.0 mg; vitamin B2 (riboflavine): 7.5 mg; vitamin B6 (pyridoxine): 3.5 mg; vitamin B12 (cyanocobalamin): 20 μg; niacine: 35 mg; pantothenic acid: 12 mg; choline chloride: 460 mg; folic acid: 1.0 mg; biotine: 0.2 mg; iron: 80 mg; copper: 12 mg; manganese: 85 mg; zinc: 60 mg; iodine: 0.8 mg; selenium: 0.15 mg, anti-oxidant: 125 mg.
Table 2.
Analyzed chemical composition of the experimental diets (g/kg dry matter).
| Basal diet | Protease A | Protease B | Protease C | Phytase | ||||
|---|---|---|---|---|---|---|---|---|
| Supplementation level1 | No enzyme | 25 mg/kg | 200 mg/kg | 500 mg/kg | 4000 mg/kg | 200 mg/kg | 1600 mg/kg | 1500 FTU/kg |
| CP | 246 | 246 | 246 | 248 | 245 | 248 | 246 | 245 |
| Ether extract | 71 | –2 | – | – | – | – | – | 70 |
| Crude fiber | 27 | – | – | – | – | – | – | 27 |
| aNDFom | 118 | – | – | – | – | – | – | 117 |
| ADFom | 44 | – | – | – | – | – | – | 45 |
| Ala | 12.2 | 12.4 | 12.3 | 12.1 | 12.1 | 12.2 | 12.3 | 12.4 |
| Arg | 16.7 | 16.8 | 16.7 | 16.6 | 16.3 | 16.7 | 16.6 | 16.8 |
| Asp/Asn | 26.4 | 26.8 | 26.5 | 26.1 | 26.2 | 26.5 | 26.5 | 26.9 |
| Cys | 3.7 | 3.8 | 3.7 | 3.7 | 3.7 | 3.7 | 3.9 | 3.8 |
| Glu/Gln | 46.0 | 46.5 | 46.1 | 45.5 | 45.5 | 45.9 | 46.0 | 46.6 |
| Gly | 10.3 | 10.5 | 10.4 | 10.3 | 10.2 | 10.4 | 10.4 | 10.5 |
| His | 7.2 | 7.3 | 7.3 | 7.1 | 7.3 | 7.3 | 7.4 | 7.5 |
| Ile | 10.6 | 10.5 | 10.3 | 10.4 | 9.8 | 10.4 | 10.3 | 10.2 |
| Leu | 21.4 | 21.6 | 21.4 | 21.2 | 21.0 | 21.2 | 21.3 | 21.5 |
| Lys | 13.4 | 13.5 | 13.3 | 13.3 | 13.1 | 13.4 | 13.3 | 13.6 |
| Met | 3.6 | 3.6 | 3.6 | 3.5 | 3.5 | 3.6 | 3.6 | 3.6 |
| Phe | 12.5 | 12.6 | 12.5 | 12.4 | 12.2 | 12.5 | 12.5 | 12.6 |
| Pro | 14.9 | 15.1 | 15.4 | 15.0 | 15.3 | 15.0 | 15.2 | 14.8 |
| Ser | 12.9 | 13.3 | 13.2 | 12.9 | 13.1 | 13.1 | 13.2 | 13.4 |
| Thr | 9.8 | 10.0 | 9.9 | 9.7 | 9.7 | 9.8 | 9.9 | 10.0 |
| Tyr | 8.5 | 8.6 | 8.6 | 8.5 | 8.5 | 8.7 | 8.5 | 8.6 |
| Val | 11.5 | 11.2 | 11.1 | 11.2 | 10.6 | 11.2 | 11.1 | 10.9 |
| Calcium | 10.9 | – | – | – | – | – | – | 11.0 |
| Phosphorus | 5.7 | – | – | – | – | – | – | 5.8 |
1The lower supplementation of each protease product complies with the recommendations of the suppliers, the other dosage is 8-fold higher.
2– = Not analyzed.
Birds and Experimental Procedures
The experiment was conducted in the Agricultural Experiment Station of Hohenheim University, location Lindenhöfe, Eningen, Germany. All the animal procedures were in accordance with the German Animal Welfare Legislation and were approved by the Regierungspräsidium Tübingen (approval number HOH34-15TE).
A total of 960 unsexed broiler chicken hatchlings of the strain Ross 308 were allocated to 64 pens of 15 birds each on a wood shavings bedding. Lighting in the barn was permanent and the temperature was 34°C for the first 2 d of the experiment. Following this, the lighting schedule was adjusted to 18 h of light and 6 h of dark, and the temperature was decreased continuously to reach 19°C until day 21.
From day 1 to day 14 post-hatching, birds received a commercial starter diet that was calculated to be adequate in ME and all nutrients according to the recommendations of the Gesellschaft für Ernährungsphysiologie (1999) (Club Mastkükenstarter 4150020, Deutsche Tiernahrung Cremer GmbH & Co. KG, Germany; contained according to the manufacturer data sheet per kg 215 g CP, 10.5 g Ca, 5.5 g P, 12.5 MJ ME, 110 mg coccidiostat monensin sodium, 10 IU endo-1.4-β-xylanase (EC 3.2.1.8), and 750 FTU 6-phytase (EC 3.1.3.26)). On day 15, 8 pens were allocated to each of the dietary treatments in a randomized complete block design. The experimental diets were provided for 7 d in mash form for ad libitum consumption.
Bird weight and feed consumption were determined on day 14 and day 21 of the experiment. Dead birds were weighed and feed consumption up to the day of removal of the bird was recorded. Determination of ADFI for 1 pen with low level of Protease A supplementation and 2 pens with high level of Protease C supplementation did not deliver plausible results. The determined level of ADFI of these observations was untrustworthily low (18 g/d and 39 g/d) or high (92 g/d). These observations together with their related values of final BW, ADG, and G:F were excluded from the data evaluation to ensure the comparability of the results for all traits. On day 21, birds were euthanized by carbon dioxide asphyxiation following anesthesia in a gas mixture (Zeller et al., 2015b). The terminal two-thirds of a section of the small intestine between the Meckel's diverticulum and 2 cm anterior to the ileo-ceco-colonic junction were isolated. Approximately, 2 cm from this section (randomly taken) was dissected and longitudinally opened. The randomization was practiced in order to make sure that, on average of the pen, samples were from the same section as samples for AA analysis. Digesta from this 2-cm piece was aseptically collected with a sterile spoon, pooled on a pen-basis, and stored at −80°C for microbiota analysis. From the remaining part of the chosen intestine section, digesta was flushed out using deionized water, pooled on a pen basis, and immediately frozen at -20°C until freeze-drying.
Chemical Analyses
A vibrating disc mill (Fritsch Pulverisette 9, Fritsch GmbH, Germany) was used to grind the diet and digesta samples for AA and Ti analysis. Samples were ground using a centrifugal mill (Retsch ZM200, Retsch GmbH, Germany) and passed through a 0.5 mm sieve for all other analyses. All analyses were performed in duplicate.
The German official methods for nutrient analyses of the Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (2007) were followed for the analyses of DM (no. 3.1), CP (no. 4.1.1), crude ash (no. 8.1), ether extract (no. 5.1.1), crude fiber (no. 6.1.1), neutral detergent fiber after pre-treatment with α-amylase without residual ash (aNDFom; no. 6.5.1), and acid detergent fiber without residual ash (ADFom; no. 6.5.2). Vadopest and Fibretherm analysis systems (C. Gerhardt GmbH & Co. KG, Germany) were used for Kjeldahl digestion and crude fiber, ADFom, and aNDFom analysis, respectively. Concentrations of Ti, phosphorus and calcium were analyzed using an ICP-OES following wet digestion as described by Zeller et al. (2015a). Amino acids were analyzed as described previously (Rodehutscord et al., 2004) with minor laboratory modifications (Zuber et al., 2016). Briefly, samples were oxidized in an ice bath with a mixture of hydrogen peroxide, phenolic formic acid solution, and phenol prior to hydrolysis in acidic conditions at 113°C for 24 h in a mixture containing hydrochloric acid and phenol. Norleucine was used as the external standard. Separation and detection of AA was done using the L-8900 AA analyzing system (VWR/Hitachi Ltd, Japan) after post-column derivatization using ninhydrin. The oxidation procedure might slightly affect the calculated concentration of His, Phe, and Tyr (Mason et al., 1980). Asn and Gln were determined together with Asp and Glu, respectively, due to the loss of amide residue from the side group of Asn and Gln during acid hydrolysis and convertion to Asp and Glu, respectively (Fontaine, 2003).
DNA Extraction, Illumina Amplicon Sequencing and Data Analysis
DNA was extracted using the FastDNA SPIN Kit for soil (MP Biomedicals LLC, OH, USA), following the manufacturer's instructions. DNA was quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, MA, USA) and stored at −20°C. Illumina library was prepared according to Kaewtapee et al. (2017). In brief, the V1–2 region of the 16S rRNA gene was amplified in a 20 μl reaction. About 1 μl of the first PCR product was used as a template in the second PCR with multiplexing and indexing primers as described previously (Camarinha-Silva et al., 2014). Amplicons were verified by agarose gel electrophoresis, purified, and normalized using the SequalPrep Normalization Kit (Invitrogen Inc., CA, USA). Samples were pooled and sequenced using the 250 bp paired-end sequencing chemistry on an Illumina MiSeq platform.
Raw reads were checked for quality, assembled, and aligned using Mothur pipeline tool (Kozich et al., 2013). A total of 28,151 ± 2,736 reads were obtained per sample. The UCHIME program included in Mothur pipeline was used to identify possible chimeras (Edgar et al., 2011). Reads were clustered at 97% identity into 1,021 operational taxonomic units (OTU). Only OTU with an average abundance higher than 0.0001% and a sequence length >250 bp were considered for further analysis. The closest representative was manually identified using seqmatch from the Ribosomal Database Project (RDP) (Wang et al., 2007). Sequences were submitted to the European Nucleotide Archive (accession number PRJEB26340).
Calculations and Statistical Analysis
The pc digestibility of CP and AA was calculated on a pen basis using the following equation:
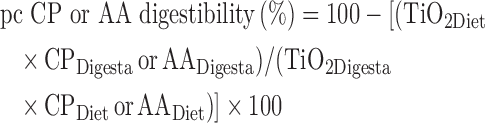 |
(1) |
where CPDigesta or AADigesta and CPDiet or AADiet are the concentrations of CP or AA in the digesta and diets, respectively, and TiO2Diet and TiO2Digesta are the concentrations of TiO2 in the diets and digesta, respectively.
Statistical evaluation of growth performance, pc CP, and pc AA digestibility was done using the MIXED procedure of SAS for Windows (Version 9.3, SAS Institute, Cary, NC). Data were analyzed considering the fact that the observations recorded for some traits related to growth performance were unbalanced by one-way analysis of variance (ANOVA) using the following statistical model:
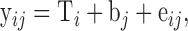 |
(2) |
with yij as the dependent traits, T as the fixed effect of treatment i, b as the random effect of block j, and eij as the residual error. Treatment effects were considered significant if P < 0.050.
Illumina amplicon sequencing data were analyzed using PRIMER (PRIMER-E version 7.0.9, Plymouth Marine Laboratory, UK) as described by Clarke and Warwick (2001). Data were standardized, square-root transformed, and a sample similarity matrix was created using Bray–Curtis coefficient (Bray and Curtis, 1957). Alpha-diversity was calculated based on Shannon diversity index at 97% of identity (Paul et al., 2015). Beta-diversity was studied based on community similarity structure and depicted through non-metric multi-dimensional scaling plots (nMDS) (Clarke and Warwick, 2001). Similarity percentage analysis (SIMPER) was used to identify the genera responsible for the differences observed between the treatments (Clarke and Warwick, 2001). PERMANOVA routine was used to study the significant differences observed when the dietary treatments were investigated using a permutation method under a reduced model. Pearson correlation was calculated using GraphPad Prism 6 (GraphPad Software Inc., CA, USA). Correlations were considered significantly different at P < 0.050.
Co-occurrence network analysis was done considering OTU with more than 0.1% abundance and clustered at the genus level as proposed (Manasson et al., 2018). Correlations were estimated based on the sparse correlation for compositional data approach (Friedman and Alm, 2012; Ramayo-Caldas et al., 2016), which determines the co-abundance and co-exclusion of bacteria present in the absolute abundance (Zhang et al., 2018). Two-sided pseudo P-values were obtained considering 10 iterations and 100 bootstraps. Non-significant correlations (P > 0.050) were ignored. Cytoscape software version 3.6.0 (Shannon et al., 2003) was used to build the network, with each node representing a genus and the edges denoting the strongest positive and negative association of all possible pairs (Ramayo-Caldas et al., 2016).
RESULTS
Growth Performance and Prececal Amino Acid Digestibility
The average initial BW on day 14 was 405 (SD 14.5) g/bird and was not significantly different between the treatments. Compared with the BD, protease supplementation did not significantly influence ADG, except for a significant reduction in ADG when fed the high level of Protease B supplementation (Table 3). When Phy was supplemented, ADG was significantly higher than that in all the other treatments. Supplementation of Phy and the higher level of Protease C significantly increased the G:F values. There was no significant difference in G:F of the other protease-supplemented treatments when compared with that of the BD.
Table 3.
Growth performance of broiler chickens in the 7-d-experimental period (8 replicates per treatment unless otherwise stated).
| Basal diet | Protease A | Protease B | Protease C | Phytase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supplementation | No | 25 | 200 | 500 | 4000 | 200 | 1600 | 1500 | Pooled | P-value |
| level | enzyme | mg/kg1 | mg/kg | mg/kg | mg/kg2 | mg/kg | mg/kg | FTU/kg | SEM | ANOVA |
| Final BW (g/bird) | 692b,c | 674c | 690b,c | 689b,c | 670c | 708b | 702b | 741a | 9.0 | <0.001 |
| ADG (g/d) | 40.9c,d | 39.6d,e | 40.8c–e | 41.3b–d | 38.5e | 41.9b,c | 43.0b | 46.3a | 0.78 | <0.001 |
| ADFI (g/d) | 72.9b,c | 72.8b,c | 71.6c,d | 72.5b,c | 69.3d | 74.9a,b | 70.8c,d | 77.8a | 1.19 | <0.001 |
| G:F (g/g) | 0.560b,c | 0.545c | 0.570b | 0.570b | 0.557b,c | 0.559b,c | 0.609a | 0.595a | 0.010 | <0.001 |
a–eValues without a common superscript within 1 row are significantly different (P < 0.050).
17 replicates.
26 replicates.
Higher level of supplementation of Protease C and Phy significantly increased the pc digestibility of not only CP but also of all measured AA compared with that of the BD (Table 4). No significant differences in the pc CP and AA digestibility were found between supplementation of Protease C and Phy. The supplementation of Protease A and B had no significant influence on the pc CP and AA digestibility when compared with that of the BD in most cases. Exceptions include among others a significantly higher pc Ser digestibility observed for the lower supplementation level of Protease A and a significantly lower pc Ile and Val digestibility for Protease B at both supplementation levels. Compared with BD, the lower supplementation level of Protease C significantly decreased the pc digestibility of AA except for His, Met, Pro, and Tyr.
Table 4.
Prececal crude protein and amino acid digestibility (%) of the experimental diets (8 replicates per treatment).
| Basal diet | Protease A | Protease B | Protease C | Phytase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supplementation | No | 25 | 200 | 500 | 4000 | 200 | 1600 | 1500 | Pooled | P-value |
| level | enzyme | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | FTU/kg | SEM | ANOVA |
| CP | 79.9b | 80.4b | 79.9b | 78.6c | 79.7b,c | 78.5d | 81.9a | 81.9a | 0.61 | <0.001 |
| Ala | 80.8b | 81.6b | 80.7b | 79.2c | 80.4b,c | 79.4c | 83.2a | 83.7a | 0.71 | <0.001 |
| Arg | 88.0b,c | 88.3b | 88.0b,c | 86.9d | 87.4c,d | 86.9d | 89.5a | 89.5a | 0.34 | <0.001 |
| Asp/Asn | 78.8b,c | 79.5b | 79.1b,c | 77.1d | 78.1c,d | 77.1d | 80.9a | 81.2a | 0.50 | <0.001 |
| Cys | 66.9b | 67.5b | 67.6b | 64.6c | 67.0b | 64.8c | 71.9a | 70.8a | 0.91 | <0.001 |
| Glu/Gln | 86.1b,c | 86.6b | 86.2b,c | 84.8d | 85.6c,d | 85.0d | 87.7a | 88.0a | 0.41 | <0.001 |
| Gly | 75.4b,c | 76.1b | 75.5b,c | 73.7d,e | 74.9c,d | 73.5e | 77.9a | 78.3a | 0.69 | <0.001 |
| His | 79.8b,c | 80.6b | 80.1b | 78.1d | 80.1b | 78.7c,d | 82.7a | 83.0a | 0.67 | <0.001 |
| Ile | 82.5b | 82.5b | 81.8b,c | 80.6d | 80.5d | 80.8c,d | 84.3a | 84.4a | 0.58 | <0.001 |
| Leu | 82.7b,c | 83.3b | 82.6b,c | 81.1d | 82.1c,d | 81.3d | 85.1a | 85.4a | 0.62 | <0.001 |
| Lys | 84.6b,c | 84.9b | 84.2b–d | 83.3d | 83.8c,d | 83.2d | 85.9a | 86.8a | 0.53 | <0.001 |
| Met | 84.1b | 84.6b | 83.6b,c | 82.4c | 83.7b,c | 83.3b,c | 86.8a | 87.4a | 0.73 | <0.001 |
| Phe | 83.0b,c | 83.7b | 83.1b,c | 81.5d | 82.2b,c | 81.9d | 85.6a | 85.6a | 0.55 | <0.001 |
| Pro | 79.6b,c | 80.6b | 80.6b | 78.2d | 80.2b | 78.5c,d | 83.0a | 81.9a | 0.66 | <0.001 |
| Ser | 77.8c | 79.0b | 78.4b,c | 76.4d | 77.9b,c | 76.4d | 81.2a | 81.6a | 0.63 | <0.001 |
| Thr | 73.3b | 74.2b | 73.5b | 71.3c | 72.9b | 71.0c | 76.1a | 76.6a | 0.78 | <0.001 |
| Tyr | 80.6b,c | 81.5b | 80.9b,c | 79.1d | 80.7b,c | 80.0c,d | 84.1a | 83.5a | 0.64 | <0.001 |
| Val | 80.6b | 80.2b | 79.7b | 78.4c | 78.5c | 78.4c | 82.2a | 82.3a | 0.68 | <0.001 |
a–eValues without a common superscript within 1 row are significantly different (P < 0.050).
Microbial Communities in the Terminal Small Intestine
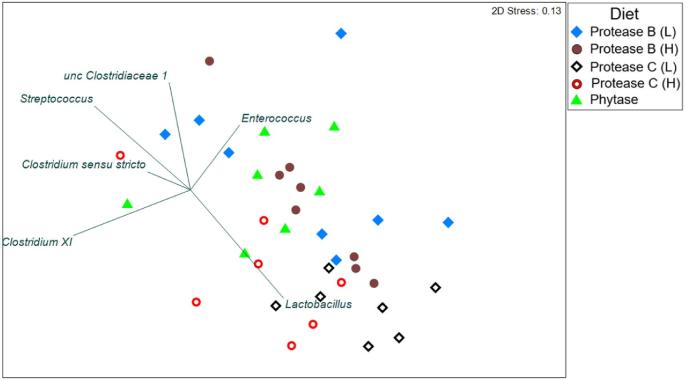
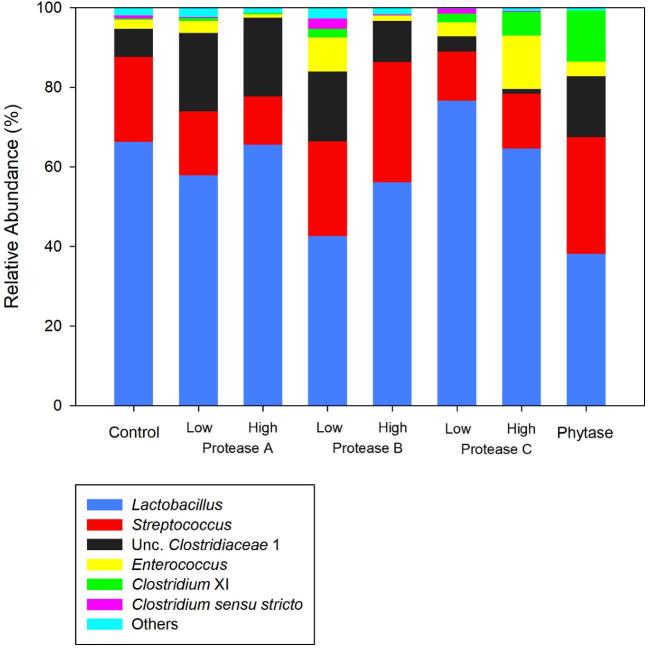
A total of 1,021 OTU were identified from the entire dataset. Firmicutes were the most abundant phylum, commonly observed across all diet treatments (>98%). A significant difference between the bacterial profiles at the genus level was observed between treatments (P = 0.024) (Table S1). Regardless of dosage, the microbial communities in the terminal small intestine were significantly different between the treatments with Protease B and Protease C supplementation and between Protease C at low level and Phy (P < 0.050). The clustering of OTU in cases where Protease C was fed at both supplementation levels was influenced by a higher presence of the genus Lactobacillus, whereas supplementation of Phy and both dosages of Proteases B grouped further apart and may be caused by the abundance of Enterococcus and uncultured Clostridiaceae 1 (Figure 1). Additionally, diets supplemented with Phy and the lower level of Protease B resulted in the numerically highest diversity index among all diets. Significant differences in the diversity index were found between the supplemented Phy and Protease C, and between Protease B and Protease C both at low level (Figure 2, Table S1). Lactobacillus genus was the most abundant in all treatments (Figure 3). With Protease C supplementation at both levels, Lactobacillus accounted for 77% and 64% of the total community, whereas it was only 38% in the Phy diet, 43% in Protease B at low level, and 56% at the high level. The most relevant OTU identified were Lactobacillus salivarius (OTU 53, 77, and 40) and Lactobacillus gallinarum (OTU 86).
Figure 1.
Non-metric multi-dimensional scaling plot illustrating the global bacterial community structure of dietary treatments that showed a statistical difference among each other (low (L) and high (H) supplementation levels of protease). The symbols represent one pooled sample from each pen comprising all Operational Taxonomic Units clustered at genus level.
Figure 2.
Shannon diversity obtained for the experimental diets at genus level. The plot indicates the second (box) and third quartiles (whiskers), and the median value is represented by the vertical line. Values without common letters are significantly different (P < 0.050).
Figure 3.
Relative abundance of microbes at the genus level detected in the terminal small intestine of broiler chickens for the experimental diets (8 replicates per treatment).
Streptococcus counts increased with both dosages of Protease B (24% and 30% for the low and high supplementation levels) when compared with 13% abundance in diets containing Protease C (Figure 3). The OTU 79, related to Streptococcus alactolyticus (Table S2), contributed to 20% of the total community in the low supplementation levels of Protease B and Protease C. In the Phy-supplemented diet, OTU 79 caused a significant difference with Protease C supplementation at low level (P < 0.050), where it accounted only for 30% abundance.
The genus Clostridium XI was more abundant when Phy or the higher level of Protease C was supplemented (13% and 6%, respectively) than in the other treatments (ranging from 0.4% to 3%). In the Phy treatment, OTU 13 (uncultured Clostridium XI) was negatively correlated with OTU related to the genus Lactobacillus (4, 57, 72, 90, and 98). Also, Clostridium sensu stricto was not highly abundant in this study. The Enterococcus genus was highly abundant when the high level of Protease C was supplemented (14%), being mainly represented by OTU 8 (Enterococcus azikeevi).
With special consideration to the 2 treatments, Protease C at the high level and Phy that had significantly higher AA digestibility than the other treatments, SIMPER analysis revealed that Phy supplementation increased the fold change (FC) of S. alactolyticus (OTU 79, FC = 1.9), uncultured Clostridium XI (OTU 13, FC = 2.2), and uncultured Clostridiaceae 1 (OTU 52, FC = 16) in comparison to the high supplementation level of Protease C. Upon supplementation of Protease C at the higher, level the principal OTU observed were the uncultured Lactobacillus (OTU 53, FC = 2.4), L. salivarius (OTU 40, FC = 2.7), Lactobacillus taiwanensis (OTU 77, FC = 1.4), and E. azikeevi (OTU 8, FC = 7).
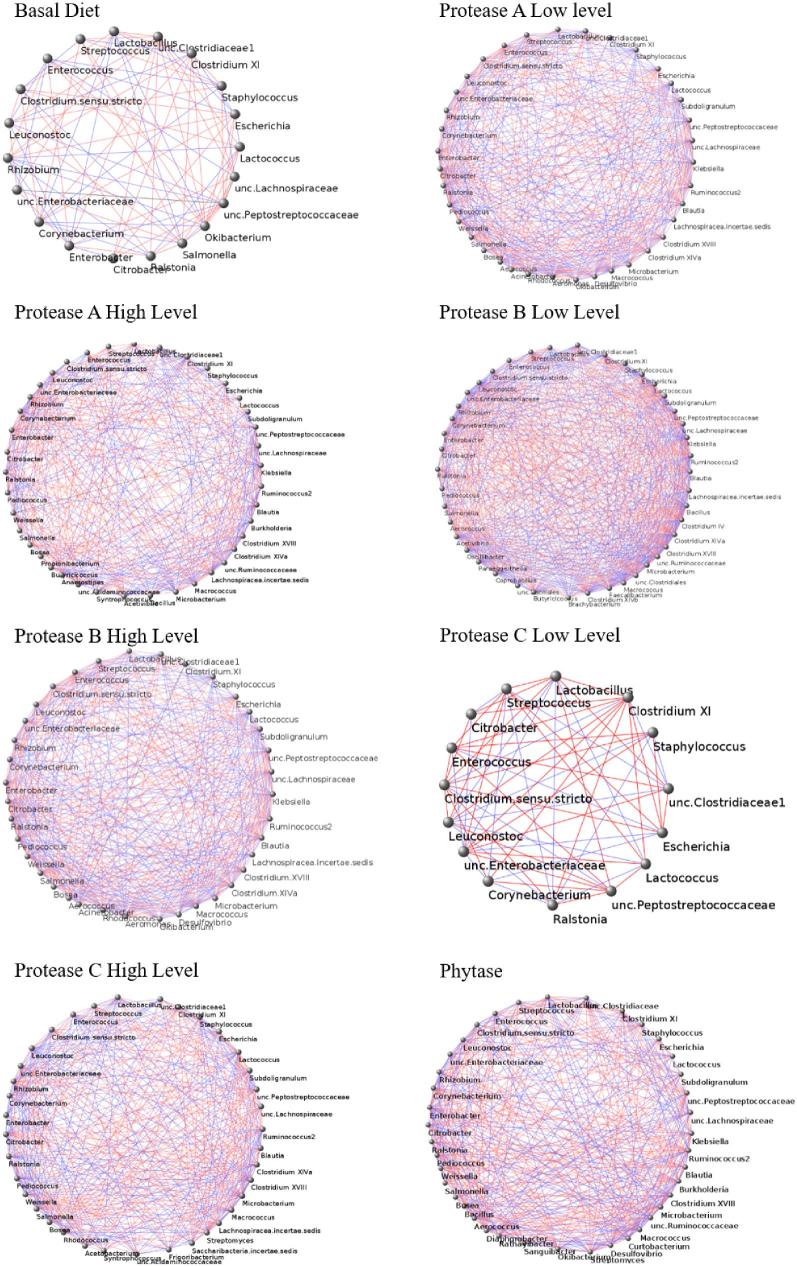
Microbial networks revealed different levels of connectivity between the microbes as reflected by the significant interactions observed among them (Figure 4). The total number of negative correlations was found to be higher than the positive ones. The BD and Protease C at low level had fewest correlations (185 and 128, respectively) and a smaller number of genus (nodes) (15 and 20, respectively) (Figure 4). When Protease B was supplemented at the high level, we observed multiple correlations (1,187 edges) in the 43 genera. The other diets (both dosages of Protease A, Protease B at the low level, Protease C at the high level, and Phy addition) all yielded similar quantity of edges and nodes (on average 724 and 37, respectively). This co-occurrence analysis also showed that Lactobacillus was negatively correlated to other abundant genera in the small intestine.
Figure 4.
Microbial network at genus level for the experimental treatments (8 replicates per treatment). Significant interactions are indicated by the connective lines (edges) between genus (nodes). Negative and positive interactions are shown in red and blue, respectively.
DISCUSSION
Prececal Amino Acid Digestibility
The results of this study show that the effect of protease supplementation on pc AA digestibility depended on the protease product and supplementation level. Protease A and B at both supplementation levels did not increase the pc AA digestibility. For some AA, supplementing these proteases even reduced pc digestibility. Protease C had no influence or decreased pc AA digestibility when supplemented at the recommended level. Supplementation of Protease C at an 8 times higher level, however, increased pc digestibility of all measured AA by an average of 2.6 percentage points. These results overall are in accordance with previous results, which showed that the supplementation of different proteases at a certain level can either decrease or increase the pc AA digestibility (Walk et al., 2018). However, classifying distinct protease products with respect to their effectiveness in increasing pc CP and AA digestibility is difficult. The protease products and concentrations used in this study did not increase pc AA digestibility, but had increasing effects on pc CP digestibility in other studies (Liu et al., 2013; Selle et al., 2013).
The present study also showed that the effect of protease supplementation was dose-dependent. Whereas, there was no effect of Protease B at the higher supplementation level, the lower supplementation level tended to decrease pc AA digestibility. Supplementation of 1,600 mg/kg of Protease C increased pc CP and AA digestibility, but no effect or a decreasing effect was observed at the dosage of 200 mg/kg. A dose-dependent effect of Protease C was also determined by Angel et al. (2011), who found the effect of protease supplementation to be fully expressed at the 200 mg/kg supplementation level. This shows that protease product and supplementation levels can explain divergent effects on pc CP and AA digestibility reported in the literature. Other possible influences on the efficacy of protease supplementation should be investigated to obtain more predictable results. Likewise, responses to protease supplementation can be affected by the choice of raw materials used, especially protein sources. For instance, effects due to diet composition were reported by Cowieson et al. (2016). For the present study, the diets used were based on corn and soybean meal, whereas sorghum, wheat bran, and canola meal have been used by Liu et al. (2013) and Selle et al. (2013). The diets used by Angel et al. (2011) were based on corn and soybean meal similar to the diets used in the present study, but the proportions of feedstuffs varied. Dietary composition may also contribute to the differences in the efficacy of protease supplementation between experiments observed by Walk et al. (2018). These authors used diets based on corn and soybean meal in one experiment, whereas a wheat-soybean-meal-based diet was used in another experiment. Such differences alter the substrate and might also modify gastro-intestinal conditions relevant for enzyme activity, such as the pH in the digestive tract. In the present study, supplementation of Phy increased the pc AA digestibility by about the same extent achieved with Protease C supplementation at the higher level (2.7 percentage points on average).
Increase in pc AA digestibility due to protease supplementation has been attributed in part to a reduction of basal endogenous AA loss (Cowieson and Roos, 2016). Among the basal endogenous AA lost, the proportions of Asp/Asn, Cys, Glu/Gln, Pro, Ser, and Thr are relatively high (Kluth and Rodehutscord, 2009; Adedokun et al., 2011; Adeola et al., 2016). For some of these AA, namely Asp/Asn, Cys, and Thr the median of increase in pc AA digestibility by Protease C supplementation was above the median of the increase of all AA, whereas it was below the median value for others (Glu/Gln, Pro, and Ser). Therefore, based on the present results, the observed increase in pc AA digestibility cannot be simply explained by a reduction of basal endogenous AA loss. In regard to phytase supplementation, increased pc AA digestibility through reduced basal endogenous AA loss has also been described (Selle et al., 2016). Upon phytase supplementation in the present study, the increase in pc digestibility of those AA with a high concentration in basal endogenous losses was above the median increase of all AA for Asp/Asn, Cys, Pro, Ser, and Thr, but markedly below for Glu/Gln. Basal endogenous AA losses are affected by ADFI (Adedokun et al., 2011; Adeola et al., 2016), which was influenced by phytase supplementation in the present study. This means that basal endogenous AA losses may be affected by Phy, either directly by the enzyme or by feed intake, or both. Hence, our results do not clarify if phytase supplementation led to an increase in the pc AA digestibility through reduction of basal endogenous AA losses.
Microbial Communities in the Terminal Small Intestine
Protease supplements altered the overall microbial composition. This change was mostly observed with the higher diversity obtained for Phy and the low supplementation level of Protease B (Figure 2). A similar finding was reported in a study testing fecal protease activity in humans, in which higher protease activity was reported to result in a lower number of bacterial species and a decreasing diversity index (Carrol et al., 2013).
Streptococcus commonly found in the small intestine during the growth of broiler chickens (Han et al., 2016; Ranjitkar et al., 2016) was higher in abundance upon supplementation of Protease B when compared with that of Protease A and C. The presence of Streptococcus has been related to an increase in the density of CD8+ T cells influencing the immune functions in the intestine (Huang et al., 2013), and can be involved in the reduction of pathogens (Dahiya et al., 2007). Enterococcus also increased with high level of Protease C supplementation, and low level of Protease B supplementation. This genus is usually found in low abundance in the small intestine of broiler chickens (Lu et al., 2003). Also, a probiotic mixture of Enterococcus and Lactobacillus increased the number of mucosal adherent bacteria in the terminal small intestine apart from increasing the goblet cells and mucous layer (Chichlowski et al., 2007). Furthermore, strains from this genus are able to synthesize bacteriocins that are active against pathogens like Eimeria spp., making these bacteria a potential probiotic candidate (Ivanova et al., 2004; Pan and Yu, 2014).
The uncultured Clostridiaceae 1 was the lowest in abundance for Protease C at both supplementation levels in comparison with that of the other treatments. The high percentage of sequences (around 11% of the total abundance) of this uncultured bacterium demonstrated that there is still a need for culturing and better characterizing the microbiota of the digestive tract of chickens. A better characterization offers a clearer view of the microbial abundance and the effects of supplementing diets with enzymes (Borda-Molina et al., 2018).
The microbial composition after providing the high level of Protease C and Phy that caused increased pc AA digestibility was different when compared with that of the other treatments. Most of quantified OTU in Protease C at low and high dosages belonged to Lactobacillus species. The high presence of Lactobacillus increased the production of extracellular proteins with adhesive properties in the study of Spivey et al. (2014). This adhesion influences gut health and the population dynamics in the gut through the synthesis of compounds such as bacteriocins that are active against Gram-positive bacteria (Fasina et al., 2016). Based on the high dominance, it was estimated that this genus assimilates 3% to 6% of the protein ingested by the chicken (Apajalahti and Vienola, 2016).
The Clostridium genus in the small intestine was reported to have less than 20% in abundance (Mohd Shaufi et al., 2015) similar to observations in the present study. Species belonging to clusters IV, XI, and XIVa are able to increase the growth of chickens due to butyrate production, which is an indispensable source of energy for the gut wall and mediator of immune responses (Pourabedin and Zhao, 2015; Sun et al., 2018). These clusters showed different significant interactions in the co-occurrence network and in the case of Phy, Clostridium XI was found to increase in abundance with potential benefits to the host.
Network co-occurrence analysis was performed to deduce significant interactions between the microorganisms. A higher diversity index observed in diets supplemented with Protease B at low level and with Phy may have influenced the higher presence of significant interactions visualized in the microbial network. Except for Protease C at low level, different supplementation of Protease enzymes and Phy increased the connectivity within microbiota in the terminal small intestine of broiler chickens. It is important to highlight that with the approach applied it was not possible to identify a “hub” or dominant genus (Mandal et al., 2015). Perhaps the reason is a higher rate of absorption of substrates from the broiler chickens with Protease supplementation (except Protease C at low level) and Phy. This rate of absorption could be influenced by the action of the enzymes because they start to increase the AA digestibility even as early as in the proximal jejunum (Selle et al., 2016) and the distal jejunum (Liu et al., 2013). Also, this fact would imply possible consequences in the modification of the substrate before they arrive at the terminal small intestine.
Modified microbiota composition could be attributed to the different modes of action of the enzymes. Protease A is obtained from the fungi A. niger, whereas Protease B and C are derived from the bacteria B. licheniformis. Enzymes synthesized from different microorganisms catalyze precise reactions that are influenced by the case-specific evolution of the protein (López-Otín and Bond, 2008). An influence on pc AA digestibility probably is specific for certain sources of proteases. A study testing 2 proteases in degrading whey protein found that a more significant extent of protein hydrolysis occurred at higher concentrations of the enzymes (Pintado et al., 1999). Furthermore, a single type of protease action resulted in a hydrolysate richer in peptides, whereas in others it was richer in AA (Pintado et al., 1999). Another potential influencing factor on enzyme activity is the substrate concentration. A protease isolated from B. licheniformis had a reduced rate of hydrolysis and enzyme selectivity with increased substrate concentration (Butré et al., 2014). On the contrary, protease from A. niger revealed that at least 30% of the activity could be increased if optimal conditions are provided (Mandal et al., 2005). These facts can lead to the different availability of products that do not affect the measurements of digestibility but may impact the microbial composition.
In line with the effects on the microbiota from the protease supplementation, an antimicrobial effect could be speculated. A protease derived from B. licheniformis is capable of removing the biofilm produced from Bacillus cereus and Pseudomonas aeruginosa (Morvay et al., 2011). The mechanisms behind are related to the breakdown of extracellular polymeric substances that can be produced in the digestive tract by members of the genus Lactobacillus. Until now, there is no literature discussing antimicrobial activity of proteases from A. niger. Whether or not these effects were relevant to the present study and what they mean for pc AA digestibility cannot be answered at this time.
Connections between pc AA digestibility and microbiota composition in the terminal small intestine could not be clearly established in our study. It cannot be ruled out that closer connections exist in the other sections of the digestive tract or on the basis of functionality rather than abundance. Our study showed that protease effects in principle exist. Hence, it should be considered as a pilot study that needs to be verified through other experiments and exploring deeper in phylogeny and functionality of the microbiota.
In conclusion, the effect of protease supplementation on pc AA digestibility in broiler chickens depended on protease product and supplementation level. Supplementation of Phy resulted in an increased pc AA digestibility. The microbiota composition and interactions between microbial groups were different between treatments. However, no clear relationship between pc AA digestibility and microbiota composition was detected.
SUPPLEMENTARY DATA
Table S1. One-way PERMANOVA analysis of the effects of diets based on enzyme supplementation, and one-way ANOVA for the difference across treatments considering the Shannon diversity index (8 replicates per treatment).
Table S2. Taxonomic assignment of the most relevant Operational Taxonomic Units present in the terminal small intestine of broiler chickens. The assignment was performed in the Seqmatch function of the Ribosomal Database Project (https://rdp.cme.msu.edu/) database for type and non-type strain.
ACKNOWLEDGMENTS
The authors acknowledge the support by the State of Baden-Württemberg through bw-HPC. We would also like to thank Yuliaxis Ramayo-Caldas for assistance in constructing the microbial networks.
Notes
Presented in part at the annual meeting of the Society of Nutrition Physiology, Göttingen, Germany, March 13–15, 2018. Borda-Molina, D., Zuber, T., Camarinha-Silva, A., Feuerstein, D., Rodehutscord, M. 2018. Protease and phytase supplementation effects on microbiota composition in the ileum and amino acid digestibility in broiler chickens. Proc. Soc. Nutr. Physiol. 27:46.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Adedokun S. A., Adeola O., Parsons C. M., Lilburn M. S., Applegate T. J.. 2011. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult. Sci. 90:1737–1748. [DOI] [PubMed] [Google Scholar]
- Adeola O., Xue P. C., Cowieson A. J., Ajuwon K. M.. 2016. Basal endogenous losses of amino acids in protein nutrition research for swine and poultry. Anim. Feed Sci. Technol. 221:274–283. [Google Scholar]
- Amerah A. M., Plumstead P. W., Barnard L. P., Kumar A.. 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 93:906–915. [DOI] [PubMed] [Google Scholar]
- Angel C. R., Saylor W., Vieira S. L., Ward N.. 2011. Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poult. Sci. 90:2281–2286. [DOI] [PubMed] [Google Scholar]
- Apajalahti J., Vienola K.. 2016. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 221:323–330. [Google Scholar]
- Bertechini A. G., Carvalho J. C. C., Mesquita F. R., Castro S. F., Meneghetti C., Sorbara J. O. B.. 2009. Use of a protease to enhance the utilization of soybean meal amino acids by broilers. Poult. Sci. 88:69. [Google Scholar]
- Boguhn J., Broz J., Rodehutscord M.. 2011. Amino acid digestibility of soybean meal and DDGS without and with supplementation of a protease in turkeys. Proc. 18th Eur. Symp. Poult. Nutr., Çeşme/Izmir, Turkey, 542–544. [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A.. 2018. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 16:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. R., Curtis J. T.. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27:325–349. [Google Scholar]
- Butré C. I., Sforza S., Gruppen H., Wierenga P. A.. 2014. Determination of the influence of substrate concentration on enzyme selectivity using whey protein isolate and Bacillus licheniformis protease. J. Agric. Food Chem. 62:10230–10239. [DOI] [PubMed] [Google Scholar]
- Camarinha-Silva A., Jáuregui R., Chaves-Moreno D., Oxley A. P., Schaumburg F., Becker K., Wos-Oxley M. L., Pieper D. H.. 2014. Comparing the anterior nare bacterial community of two discrete human populations using Illumina amplicon sequencing. Environ. Microbiol. 16:2939–2952. [DOI] [PubMed] [Google Scholar]
- Carroll I. M., Ringel-Kulka T., Ferrier L., Wu M. C., Siddle J. P., Bueno L., Ringel Y.. 2013. Fecal protease activity is associated with compositional alterations in the intestinal microbiota. PLoS One 8:e78017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M., Croom W. J., Edens F. W., McBride B. W., Qiu R., Chiang C. C., Daniel L. R., Havenstein G. B., Koci M. D.. 2007. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult. Sci. 86:1121–1132. [DOI] [PubMed] [Google Scholar]
- Clarke K. R., Warwick R. M.. 2001. Change in marine communities: an approach to statistical analyses and interpretation. 2nd ed PRIMER-E, Plymouth. [Google Scholar]
- Cowieson A. J., Roos F. F.. 2016. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed Sci. Technol. 221:331–340. [Google Scholar]
- Cowieson A. J., Lu H., Ajuwon K. M., Knap I., Adeola O.. 2016. Interactive effects of dietary protein source and exogenous protease on growth performance, immune competence and jejunal health of broiler chickens. Anim. Prod. Sci. 57:251–261. [Google Scholar]
- Cowieson A. J., Zaefarian F., Knap I., Ravindran V.. 2017. Interactive effects of dietary protein concentration, a mono-component exogenous protease and ascorbic acid on broiler performance, nutritional status and gut health. Anim. Prod. Sci. 57:1058–1068. [Google Scholar]
- Dahiya J. P., Hoehler D., Van Kessel A., Drew M. D.. 2007. Effect of different dietary methionine sources on intestinal microbial populations in broiler chickens. Poult. Sci. 86:2358–2366. [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R.. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdaw M. M., Wu S., Iji P. A.. 2017. Growth and physiological responses of broiler chickens to diets containing raw, full-fat soybean and supplemented with a high-impact microbial protease. Asian-Australas J. Anim. Sci. 30:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y. O., Newman M. M., Stough J. M., Liles M. R.. 2016. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 95:247–260. [DOI] [PubMed] [Google Scholar]
- Fontaine J. 2003. Amino acid analysis in feeds. Pages 41–70 in Amino Acids in Animal Nutrition. D’Mello J. P. F. ed., 2nd edn CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- Friedman J., Alm E. J.. 2012. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 8:e1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesellschaft für Ernährungsphysiologie. 1999. Energie- und Nährstoffbedarf Landwirtschaftlicher Nutztiere. 7. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler). DLG-Verlag, Frankfurt a. M., Germany. [Google Scholar]
- Ghazi S., Rooke J. A., Galbraith H., Bedford M. R.. 2002. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br. Poult. Sci. 43:70–77. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Bonos E., Anestis V., Filioussis G., Papanastasiou D. K., Bartzanas T., Papaioannou N., Tzora A., Skoufos I.. 2017. Effects of protease addition and replacement of soybean meal by corn gluten meal on the growth of broilers and on the environmental performances of a broiler production system in greece. PLoS One 12:e0169511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. G., Kim E. B., Lee J., Lee J. Y., Jin G., Park J., Huh C. S., Kwon I. K., Kil D. Y., Choi Y. J., Kong C.. 2016. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus 5:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemarajata P., Versalovic J.. 2013. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 6:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.-N., Okawara T., Watanabe M., Kawai Y., Kitazawa H., Ohnuma S., Shibata C., Horii A., Kimura K., Taketomo N., Xiao J.-Z., Iwatsuki K., Saito T.. 2013. New screening methods for probiotics with adhesion properties to sialic acid and sulphate residues in human colonic mucin using the Biacore assay. J. Appl. Microbiol. 114:854–860. [DOI] [PubMed] [Google Scholar]
- Ivanova E. P., Flavier S., Christen R.. 2004. Phylogenetic relationships among marine Alteromonas-like proteobacteria: emended description of the family Alteromonadaceae and proposal of Pseudoalteromonadaceae fam. nov., Colwelliaceae fam. nov., Shewanellaceae fam. nov., Moritellaceae fam. nov., Ferrimonadaceae fam. nov., Idiomarinaceae fam. nov. and Psychromonadaceae fam. nov. Int. J. Syst. Evol. Microbiol. 54:1773–1788. [DOI] [PubMed] [Google Scholar]
- Kaczmarek S. A., Rogiewicz A., Mogielnicka M., Rutkowski A., Jones R. O., Slominski B. A.. 2014. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult. Sci. 93:1745–1753. [DOI] [PubMed] [Google Scholar]
- Kaewtapee C., Burbach K., Tomforde G., Hartinger T., Camarinha-Silva A., Heinritz S., Seifert J., Wiltafski M., Mosenthin R., Rodenfelder-Kuon P.. 2017. Effect of Bacillus subtilis and Bacillus licheniformis supplementation in diets with low- and high-protein content on ileal crude protein and amino acid digestibility and intestinal microbiota composition of growing pigs. J. Anim. Sci. Biotechnol. 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth H., Rodehutscord M.. 2009. Effect of inclusion of cellulose in the diet on the inevitable endogenous amino acid losses in the ileum of broiler chicken. Poult. Sci. 88:1199–1205. [DOI] [PubMed] [Google Scholar]
- Kozich J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D.. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. A., Bedford M. R., Walk C. L.. 2018. Meta-analysis: explicit value of mono-component proteases in monogastric diets. Poult. Sci. 97:2078–2085. [DOI] [PubMed] [Google Scholar]
- Liu S. Y., Selle P. H., Court S. G., Cowieson A. J.. 2013. Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion. Anim. Feed Sci. Technol. 183:175–183. [Google Scholar]
- López-Otín C., Bond J. S.. 2008. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283:30433–30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J. J., Lee M. D.. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 69:6816–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood T., Mirza M. A., Nawaz H., Shahid M.. 2017. Effect of different exogenous proteases on growth performance, nutrient digestibility, and carcass response in broiler chickens fed poultry by-product meal-based diets. Livest. Sci. 200:71–75. [Google Scholar]
- Manangi M. K., Sands J. S., Coon C. N.. 2009. Effect of phytase on ileal amino acid digestibility, nitrogen retention and AMEn for broilers fed diets containing low and high phytate phosphorus. Int. J. Poult. Sci. 8:929–938. [Google Scholar]
- Manasson J., Shen N., Garcia Ferrer H. R., Ubeda C., Iraheta I., Heguy A., Von Feldt J. M., Espinoza L. R., Garcia Kutzbach A., Segal L. N., Ogdie A., Clemente J. C., Scher J. U.. 2018. Gut microbiota perturbations in reactive arthritis and postinfectious spondyloarthritis. Arthritis Rheumatol. 70:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal C., Gudi R. D., Suraishku G. K.. 2005. Multi-objective optimization in Aspergillus niger fermentation for selective product enhancement. Bioprocess Biosyst. Eng. 28:149–164. [DOI] [PubMed] [Google Scholar]
- Mandal R. S., Saha S., Das S.. 2015. Metagenomic surveys of gut microbiota. Genomics Proteomics Bioinform. 13:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason V. C., Rudemo M., Bech-Andersen S.. 1980. Hydrolysate preparation for amino acid determinations in feed constituents. Z. Tierphysiol. Tierer. 43:35–48. [PubMed] [Google Scholar]
- Mohd Shaufi M. A., Sieo C. C., Chong C. W., Gan H. M., Ho Y. W.. 2015. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvay A. A., Decun M., Sala C., Morar A., Galbenu-Morvay P. L.. 2011. The ability of Bacillus licheniformis protease to remove Bacillus cereus and Pseudomonas aeruginosa biofilm. Bulletin UASVM Animal Science and Biotechnologies68:245–250. [Google Scholar]
- Pan D., Yu Z.. 2014. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Kumbhare S. V., Mhatre S. S., Chowdhury S. P., Shetty S. A., Marathe N. P., Bhute S., Shouche Y. S.. 2015. Exploration of microbial diversity and community structure of Lonar Lake. The only hypersaline meteorite crater lake within basalt rock. Front. Microbiol. 6:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintado M. E., Pintado A., Malcata F. X.. 1999. Controlled whey protein hydrolysis using two alternative proteases. J. Food Eng. 42:1–13. [Google Scholar]
- Pourabedin M., Zhao X.. 2015. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 362, doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Ptak A., Bedford M. R., Świątkiewicz S., Żyła K., Józefiak D.. 2015. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS One 10:e0119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada V., Lichovníková M., Foltyn M., Šafařík I.. 2016. The effect of exogenous protease in broiler diets on the apparent ileal digestibility of amino acids and on protease activity in jejunum. Acta Univ. Agric. Silvic. Mendelianae Brun. 64:1645–1652. [Google Scholar]
- Ramayo-Caldas Y., Mach N., Lepage P., Levenez F., Denis C., Lemonnier G., Leplat J. J., Billon Y., Berri M., Doré J., Rogel-Gaillard C., Estellé J.. 2016. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 10:2973–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R. M.. 2016. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 82:2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodehutscord M., Kapocius M., Timmler R., Dieckmann A.. 2004. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 45:85–92. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Truong H. H., McQuade L. R., Moss A. F., Liu S. Y.. 2016. Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient utilisation and digestive dynamics in broiler chickens. Anim. Nutr. 2:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P. H., Liu S. Y., Cai J., Cowieson A. J.. 2013. Steam-pelleting temperatures, grain variety, feed form and protease supplementation of mediumly ground, sorghum-based broiler diets: influences on growth performance, relative gizzard weights, nutrient utilisation, starch and nitrogen digestibility. Anim. Prod. Sci. 53:378–387. [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T.. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M.. 2018. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 97:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey M. A., Dunn-Horrocks S. L., Duong T.. 2014. Epithelial cell adhesion and gastrointestinal colonization of Lactobacillus in poultry. Poult. Sci. 93:2910–2919. [DOI] [PubMed] [Google Scholar]
- Stefanello C., Vieira S. L., Rios H. V., Simões C. T., Sorbara J. O. B.. 2016. Energy and nutrient utilisation of broilers fed soybean meal from two different Brazilian production areas with an exogenous protease. Anim. Feed Sci. Tech. 221:267–273. [Google Scholar]
- Sun Y.-L., Li W.-Q., Ding P.-X., Wang Z.-W., Wei C.-H., Ma X.-X., Zhang R.-F., Wu Y., Zhou L., Liang R.-P., Zhang Y.-P., Zhao Y.-P., Zhu R.-T., Li J.. 2018. Specific alterations in gut microbiota are associated with prognosis of Budd–Chiari syndrome. Oncotarget 9:3303–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghyani M., Wu S. B., Pérez-Maldonado R. A., Iji P. A., Swick R. A.. 2017. Performance, nutrient utilization, and energy partitioning in broiler chickens offered high canola meal diets supplemented with multicomponent carbohydrase and mono-component protease. Poult. Sci. 96:3960–3972. [DOI] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten. 2007. Handbuch der landwirtschaftlichen versuchs- und untersuchungsmethodik (VDLUFA-methodenbuch). In Die Chemische Untersuchung von Futtermitteln. Vol. III VDLUFA-Verlag, Darmstadt, Germany. [Google Scholar]
- Vieira S. L., Angel C. R., Miranda D. J. A., Favero A., Cruz R. F. A., Sorbara J. O. B.. 2013. Effects of a monocomponent protease on performance and protein utilization in 1- to 26-day-of-age turkey poults. J. Appl. Poult. Res. 22:680–688. [Google Scholar]
- Walk C. L., Pirgozliev V., Juntunen K., Paloheimo M., Ledoux D. R.. 2018. Evaluation of novel protease enzymes on growth performance and apparent ileal digestibility of amino acids in poultry: enzyme screening. Poult. Sci. doi: 10.3382/ps/pey080. [DOI] [PubMed] [Google Scholar]
- Wang Q. G. M. Garrity, Tiedje J. M., Cole J. R.. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig M., Camarinha-Silva A., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L. E., Rodehutscord M.. 2015. Spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One 10:e0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Yin X., Wang X., Lei Z., Wang M., Guo Y., Aggrey S. E., Nie W., Yuan J.. 2018. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M.. 2015a. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L. E., Rodehutscord M.. 2015b. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018–1029. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu W., Lee Y. K., Xie J., Zhang H.. 2018. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front. Microbiol. 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber T., Maurer H. P., Möhring J., Nautscher N., Siegert W., Rosenfelder P., Rodehutscord M.. 2016. Variability in amino acid digestibility of triticale grain from diverse genotypes as studied in cecectomized laying hens. Poult. Sci. 95:2861–2870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.