Abstract
Background
Colorectal cancer (CRC) is the third most common cancer in the world. The cornerstone of CRC treatment is surgical resection. However, patients in the same TNM stage show different recurrence rates and survival. Of patients with a local disease without lymph node or a distant metastasis, 20–25% still develop recurrence. There is evidence that inflammatory reaction is one of the key elements in tumour development.
Materials and methods
We reviewed literature on colorectal cancer and its relationships with the immune system, with special focus on local and systemic inflammatory reaction. The Pubmed and ClinicalKey databases were searched using the key words colorectal cancer, local inflammation, systemic inflammation, markers of inflammation. The relevant literature was reviewed and included in the article.
Results
The immune system has two-sided relationships with cancer, so it not only performs anti-tumour activities, but can also promote tumour growth and spread. Research has shown that signs of local inflammation are associated with a better prognosis in CRC. Systemic inflammation has been associated with more aggressive behaviour and a worse prognosis for patients with several cancers, including CRC.
Conclusions
Recent findings in tumour biology have improved our understanding of colorectal cancer and of the natural course of this disease. Several markers of local and systemic inflammatory reaction have been identified. The next step is to find the most accurate and applicable marker, so that this promising tool can be used in clinical practice and aid in decision making.
Keywords: colorectal cancer, local inflammation, systemic inflammation, markers of inflammation
Abstract
SERGANČIŲJŲ KOLOREKTALINIU VĖŽIU SISTEMINIS IR VIETINIS UŽDEGIMAS
Santrauka
Įvadas. Kolorektalinis vėžys (CRC) – trečias iš labiausiai paplitusių vėžio lokalizacijų pasaulyje. Kolorektalinio vėžio gydymo pagrindas – chirurginė rezekcija. Tačiau tos pačios TNM stadijos pacientams būdinga skirtingas ligos atsinaujinimas ir išgyvenamumas. 20–25 % pacientų, sergančių neišplitusia liga, nustatomas ligos recidyvas. Įrodyta, kad uždegiminė reakcija yra vienas pagrindinių navikų vystymosi elementų.
Medžiaga ir metodai. Analizuota literatūra apie kolorektalinį vėžį ir jo ryšį su imunine sistema, išskirtinį dėmesį skiriant vietiniam ir sisteminiam uždegimui. „Pubmed“ ir „ClinicalKey“ duomenų bazėse buvo vykdyta paieška naudojant raktinius žodžius: kolorektalinis vėžys, vietinis uždegimas, sisteminis uždegimas, uždegimo žymenys.
Rezultatai. Imuninės sistemos paskirtis vėžio atveju yra dvejopa: viena vertus, ji kovoja su vėžiu, kita vertus, gali skatinti naviko augimą bei plitimą. Tyrimų rezultatai parodė, kad vietinio uždegimo požymiai yra susiję su geresne kolorektalinio vėžio (KRV) prognoze. Sisteminis uždegimas buvo susijęs su agresyvesniu elgesiu ir blogesne prognoze pacientams, sergantiems įvairiomis vėžio formomis, įskaitant KRV.
Išvados. Naujausi auglio biologijos tyrimai suteikė papildomų žinių apie kolorektalinį vėžį ir natūralią šios ligos eigą. Nustatyti keli vietinės ir sisteminės uždegiminės reakcijos žymenys. Kitas žingsnis – surasti tiksliausią, tinkamiausią ir perspektyviausią žymenį, kuris galėtų būti panaudotas klinikinėse praktikose, padėtų priimant sprendimus.
Raktažodžiai: kolorektalinis vėžys, vietinis uždegimas, sisteminis uždegimas, uždegimo žymenys
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the world, with approximately one million new cases and 50,000 deaths every year (1). Although mortality in Europe has decreased by 13% in men and 27% in women during the past two decades (2), this trend will surely plateau in the near future without serious advances in treatment. The cornerstone of CRC treatment is surgical resection, but despite the efforts of modern surgery, recurrence is possible and metastatic cancers need other modalities to improve survival.
Most CRCs develop from adenomatous polyps in the so-called adenoma-carcinoma cycle, which takes 10–15 years in the case of sporadic cancer (3). The process starts in the stem cells at the base of intestinal crypts and develops as these cells accumulate genetic mutations (4). Just a small fraction (5%) of CRCs develop as a hereditary cancer syndrome.
It is proposed that up to 20% of all cancers in humans are a result of chronic inflammation and persistent infections (5). In this regard, CRCs can be classified as either sporadic, with inflammation following cancer onset, or colitis-associated CRCs, which are induced by chronic inflammation. Two inflammatory bowel diseases, ulcerative colitis and Crohn’s disease, have a clear correlation with a significantly increased CRC risk, which indicates the role of chronic inflammation in cancerogenesis (6). During the whole process of cancerogenesis, the immune system, depending on the type, intensity, and timing of reactions, can either suppress or promote tumour growth (7).
Up until recently little attention was paid to the local (LIR) and systemic inflammatory reaction (SIR) in the case of cancer. Nowadays, however, there is growing evidence that suggests the inflammatory reaction as one of key elements in tumour development. In recent studies, hallmarks of LIR and SIR have shown superiority in predicting overall survival and recurrence (1).
MATERIALS AND METHODS
We reviewed literature on colorectal cancer and its relationships with the immune system, with special focus on the local and systemic inflammatory reaction. The Pubmed and ClinicalKey databases were searched using keywords colorectal cancer, local inflammation, systemic inflammation, markers of inflammation. The relevant literature was reviewed and included in the article.
Review of literature
Innate and adaptive immunity
Two fundamental components of immune response are innate and adaptive immunity. While these are usually discussed separately, both innate and adaptive immune mechanisms work synergistically to eliminate pathogens and foreign molecules. Several factors of innate immunity have a crucial role in successful function of adaptive immunity, and vice versa (8).
Innate immunity is the first line of defence and is non-specific, meaning that it will eliminate or limit the threat immediately after contact but will do it in a predefined manner not considering specific antigens of the pathogen, which might not be sufficient. Main cellular elements of the innate immune system are macrophages, dendritic cells (DCs), natural killer (NK) cells, and granulocytes. It also includes bioactive molecules present in biological fluids (either constitutively or following release after cell activation) like the complement system, defensins, cytokines, chemokines, and reactive oxygen species. Finally, part of the innate immune system consists of membrane-bound receptors and cytoplasmic proteins that recognize the so-called pathogen-associated molecular patterns that help to identify and destroy infectious agents (8–9). However, the immune system is somehow able to distinguish between beneficial commensal and pathogenic organisms. Therefore, the response that follows recognition can differ depending on other factors (10). One of the drawbacks of innate immunity is the lack of memory. Reactions are the same after each exposure (11).
Adaptive immunity, on the contrary, works in a highly specific manner against target antigens and is carried out by T and B lymphocytes and their antigen-specific receptors expressed on the cell surface (8). However, to provide such a targeted response, clonal expansion of antigen-specific T and B lymphocytes is necessary. Therefore, it might take up to five days to produce enough memory T and B cells to provide a sufficient adaptive response. As opposed to the innate response, adaptive immunity has a memory, so the response is enhanced after repeated exposure (11). It is worth noting that adaptive immunity is a luxury that only vertebrates possess, so most organisms on our planet have only innate immunity (11).
Immunosurveillance
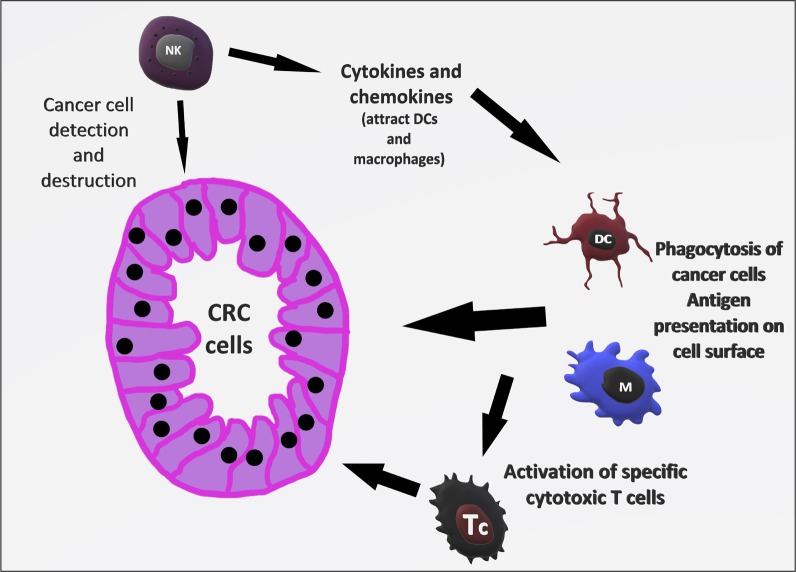
The immune response to cancer cells is somewhat similar to the reaction against infectious agents (12). Innate immune cells such as NK can detect cancer cells by their lack of MHC-I antigen on the cell surface. Following recognition, NK can destroy these cells and produce cytokines and chemokines that attract other innate immune cells, namely DCs and macrophages, which then phagocytose cancer cells and present their antigens on the cell surface to activate specific cytotoxic T cells (TC) (13) (Fig. 1).
As it is with most diseases, inflammation in CRC can be both beneficial and harmful. The idea that immunity has protective properties against cancer was first proposed by Ehrlich in 1909. Half a century later, the term immunosurveillance (IS) was used to describe the ability of the immune system to detect cancer cell-specific antigens and eliminate these cells before growth and clinical expression (1, 12).
Although the existence of IS was initially questioned, clinical evidence that supports this entity comes from immune-deficient patients, for example, patients after organ transplantation (14). There is a significantly higher incidence of various tumours, including CRC, in this population (1, 12). The existence and the important role of IS in cancer development has been proven in several animal models with knockout mice (1).
Besides the NK cells, others are also involved in IS. For example, CD8+ T lymphocytes can directly lyse cancer cells and produce cytotoxic cytokines; CD4+ Th1 lymphocytes can facilitate the reactions of TC lymphocytes. However, besides NK cells, most of the IS agents can also stimulate cancer growth and spread, depending on other cofactors and tumour microenvironment (12).
These reactions, collectively known as anti-tumour immune response (ATIR), have been extensively studied, and their hallmarks could be used to improve prognostication before and after surgery, or even aid in early diagnosis of colorectal cancer. For example, the extent of tumour infiltrating lymphocytes (TIL) has been shown to correlate with overall survival (OS), with better outcomes noted in high TIL group patients (15–16). Patients with CRC have a higher proportion of regulatory T lymphocytes (suppress anti-tumour immunity) in peripheral blood than healthy controls (17). In addition to prognostic determination, ATIR is a promising target for immunotherapy, as many scientists are trying to find ways to facilitate this natural defence (12).
Fig. 1.
Illustration of immunosurveillance
NK – natural killer; DC – dendritic cell; M – macrophage; Tc – T cytotoxic lymphocyte; CRC – colorectal cancer
Immunoediting and escape
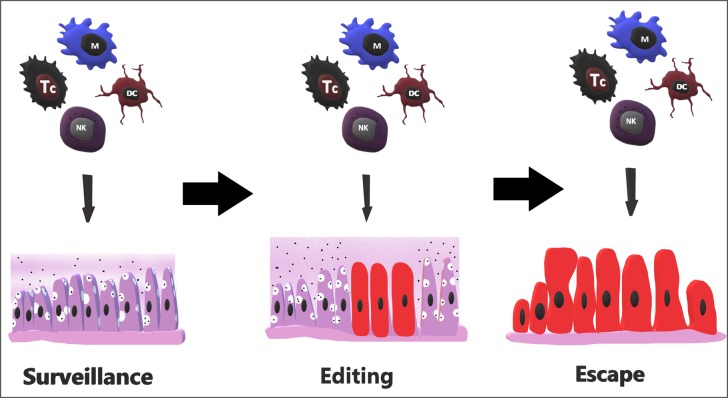
After the concept of IS was developed, additional studies revealed how cancer evades these ATIRs in a process called immune editing. It has three phases: elimination, equilibrium, and escape. In the first phase, IS with innate and adaptive responses eliminates tumour cells and prevents clinical expression (18). This is the preferred state in which the immune system has control over defective cells and their growth. As this interaction between cancer cells and the immune system moves to the equilibrium phase, defence mechanisms are no longer capable of destroying all the dividing cells but can do enough to prevent expansion and metastasis (12, 18). The process of tumour development can be halted at the equilibrium phase for a lifetime of the host. However, if the process progresses, tumour cells that are more immunogenic are recognized and eliminated, but those that are able to escape immune detection and elimination are selected and remain resistant against immunity and the disease therefore enters the escape phase (Fig. 2). In this phase, tumour cells divide in an unrestrained manner in an immunosupressive microenvironment (18).
Immune escape formation performed by cancer cells are following: (1) cancer cells express macrophage scavenger receptors thus gaining functional and phenotypic characteristics of immune cells; (2) aberrant activation of oncogenic pathways aids in interaction with stromal cells; (3) local production of anti-inflammatory cytokines and metabolites reduces immunogenicity and creates a favourable TMI (19). Most of CRCs, (around 70%) reduce the expression of MHC-I antigens on the cell surface.
The theory of immune editing has been applied in practice by mice tumour transplant studies. After transplanting tumour from immunodeficient mice into wild type mice, these tumours are more immunogenic (compared to the tumours from wild type mice), because of the lack of prior immune editing and selection for less immunogenic tumour cells (12). Furthermore, a considerable part of cancers coming from immunodeficient mice can be eliminated if transplanted into immunocompetent mice (12).
Fig. 2.
Sequential steps of immune editing and escape
NK – natural killer; DC – dendritic cell; M – macrophage; Tc – T cytotoxic lymphocyte
Immune escape is nowadays considered a hallmark of cancer. It must be kept in consideration that nearly all of the knowledge about tumour cell antigens comes from studies of immunoedited cancers in humans and mice, so very little is actually known about their antigen properties prior to this selection process (20).
Gut microbiota and CRC
A recent theory by Yamauchi et al. proposes that CRCs differ molecularly in various parts of the large intestine and that intestinal microorganisms, along with factors like biochemical substances, epithelial cells, and innate immunity, could have a direct or indirect (via influence on TMI) effect on tumour development (19, 21).
There is evidence to support the assumption that certain microorganisms can facilitate tumour development or growth by causing chronic inflammatory reaction in the large intestine (6). One example is Streptoccucus gallolyticus gallolyticus infection, which, if present, corresponds to a 65% probability of colorectal neoplasia (6, 22). The possible pathogenesis is that CRC precursors, adenomas, create favourable metabolic and nutritional environment for this opportunist, which in return causes chronic inflammatory reaction with high cyclooxygenase-2 (COX-2) expression. COX-2 stimulates epithelial proliferation, neoangiogenesis, and inhibits apoptosis, and is highly expressed in about 85% of CRCs. That is the reason why non-steroidal anti-inflammatory medication has a protective effect in CRC patients. However, tests to determine Streptococcus colonization and facilitate early CRC diagnosis have proven to be of limited value due to low sensitivity of available multi-antigen tests (6).
Pro-tumour immune response
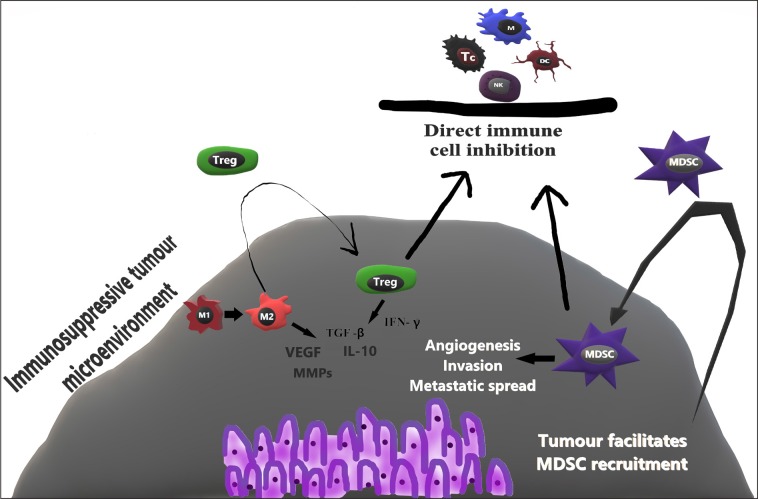
Unfortunately, the immune system has two-sided relationships with cancer, so it not only performs anti-tumour activities, but can also promote tumour growth and spread (12, 23). The mechanisms by which tumour shifts the activity of the immune system to its favour are numerous and we are only beginning to elucidate them. Some of the ways the immune system stimulates tumour growth and spread are noted in this section. Notably, TAMs, T-regulatory cells (Tregs), myeloid-derived suppressor cells (MDSC), and tumour microenvironment (TMI) have been shown to influence tumour aggressiveness, metastatic spread, and prognosis (1, 12, 23).
TAMs can be divided in two types – M1 (killer) and M2 (healer) (23). M1s are classically activated by Th1 cytokines and are involved in the innate immune response, secrete proinflammatory molecules (IL-6, IL-12, IL-23 and TNFα) and facilitate TC lymphocyte functions. M2 TAMs, however, have immune suppressive properties (produce IL-10 and TGFβ) and provide maintenance and repair after damage in healthy tissue. M2 TAMs also attract Tregs that help to create an immunosuppressive environment. M2 TAMs facilitate angiogenesis (produce VEGF and other factors), increase tumour invasiveness by secreting matrix metalloproteases, and sustain chronic inflammation (via COX-2 secretion). It has been shown that TAMs are flexible and can change from M1 to M2 phenotype, and that TMI could shift TAMs to a more favourable M2 phenotype (12, 23).
There is strong evidence that supports the importance of TMI in directing innate and adaptive immune cells from anti-tumour to pro-tumour functions and activities. This process is called oncotraining. After immune escape, cancer cells that resisted immune elimination create a microenvironment that causes the arriving immune cells to lose their phagocytotic and cytotoxic functions while maintaining other tumour promoting activities, such as tissue repair, stimulation of proliferation, angiogenesis, and epithelial-mesenchymal transition (acquiring invasive, migratory mesenchymal cell phenotype) (23, 24).
One of the more recently discovered T cell populations, Tregs, play a major role in self-tolerance and prevent hyperactive immune reactions. These cells are characterised by expression of CD4, CD25, and Foxp3, which is a “master regulator” gene vitally important in the development and function of this cell population (25). Tregs modulate tumour and pathogen-induced immune reactions as well as protect host from developing auto-immune diseases and allergies (1, 25). With these functions, Tregs can supress activation, proliferation, and functioning of other immune cells, such as CD4+ and CD8+ T cells, B lymphocytes, NKs and antigen presenting cells. Thus, Tregs are important in recovery from inflammation and in sustaining immune homeostasis but can also be used by CRC and other tumours to avoid immune clearance and facilitate progression (1, 6, 25). The proportion of the infiltrating effector CD3+ T cells to Tregs has been shown to influence disease-free survival, with a high effector T cell ratio having a more favourable outcome (6). Several studies reported increased Treg counts in circulating blood, draining lymph nodes, and CRC itself. This has been shown in other malignancies as well (25). There is evidence that Foxp3 expression, which is a marker of Tregs suppressive capacity, and not just the amount of Tregs is increased in CRC patients. Furthermore, one year after surgery, Foxp3 expression in peripheral Tregs decreases substantially and becomes comparable with healthy controls, thus indicating that CRC could increase suppressive capacity of Tregs (6). Both mice and human studies have documented that several cancers, including CRC, can stimulate Treg proliferation and accumulation in tumour and peripheral blood (25). Proposed mechanisms by which Tregs suppress anti-tumour immunity are secretion of IFN-γ, TGF-β, IL-10 and direct immune cell inhibition. Based on these findings, immunotherapy directed against tumour-induced Tregs has been studied as a potential way to improve anti-tumour immunity and prognosis in CRC patients (26). The role of Tregs could be stage-dependent. In the early stages, Tregs aid in suppressing tumour and bacteria-induced pro-inflammatory reactions, whereas in later stages CRC causes a phenotype change in Tregs so that these cells prevent anti-tumour immunity and facilitate cancer growth and spread (25, 27).
MDSC is a heterogynous group of cells comprised of myeloid progenitors, immature macrophages, immature granulocytes, and DCs. The differentiation of these myeloid cells is interrupted at different stages therefore MDSCs have phenotypical features of granulocytes and monocytes. The amount of MDSCs increases in peripheral blood and TMI in CRC patients, suggesting that cancer preferentially recruits these cells (28). Research shows that MDSCs have immunosuppressive properties targeted against T-cell immune reactions and innate responses. In addition to immunosuppressive properties, MDSCs promote tumour angiogenesis, invasion, and metastasis (23). Following activation by IFN-γ, IL-4, IL-10, TGF-β and toll-like receptor ligands, MDSCs inhibit T-cell activation and proliferation, migration to lymph nodes, CD8+ effector T cell migration to the tumour, and NK cell activity. In addition, MDSCs expand Tregs population and stimulate their differentiation in inducible Tregs, which favour tumour growth (29) (Fig. 3).
Local inflammatory markers in CRC
Numerous publications emphasize the importance of host immune response in CRC. Research has led to conclusion that local inflammation, measured by densities of TILs, is associated with better prognosis in CRC (7). More specifically, T cell populations like TH1, TC and memory T cells have been shown to associate with better disease-free and OS (30).
Thus far, tumour staging and prognosis establishment has been based on the American Joint Committee on Cancer (AJCC/UICC) TNM staging system, which also determines which patients receive adjuvant chemotherapy after surgical resection. However, patients in the same TNM stage show different recurrence rates and survival. For example, 20–25% of patients with local disease without lymph node or distant metastasis still develop recurrences (30). This clearly indicates that TNM system alone is insufficient and additional information is necessary to improve prediction and results (31).
A tool to measure local inflammation after surgical resection of CRC called immunoscore was developed. To determine the immunoscore, densities of CD3+ T cells, CD8+ T cells, and/or CD45RO+ memory T cells are measured. Two of these populations are examined in the cancer core and invasive margin to determine the immunoscore. The score ranges from 0 to 4. In one cohort study (n = 602), patients with immunoscore 0 had a 5-year recurrence of 72% and only 27.5% survived past five years, whereas patients with a score of 4 had 4.8% recurrence rate and 5-year survival of 86.2% (30). In a study of 599 patients, immune score proved to be a superior predictor of cancer recurrence than a TNM stage (32). Based on these and other findings that consolidate this belief, there is a proposal to routinely assess this immune score and incorporate it in everyday practice to improve staging and prognostication. However, there are concerns about standardization and automatization of this method before it can be used in clinical practice (33).
Fig. 3.
Illustration of protumour immune response
NK – natural killer; DC – dendritic cell; M – macrophage; Tc – T cytotoxic lymphocyte; M1–M1 (killer) macrophage; M2–M2 (healer) macrophage; Treg – regulatory T cell; MDSC – myeloid-derived suppressor cell; VEGF – Vascular endothelial growth factor; MMPs – Matrix metalloproteinases; TGF-β – Transforming growth factor beta; IFN-γ – Interferon gamma; IL-10 – Interleukin 10
Systemic inflammation markers in CRC
While intensive local inflammation with high density of TILs is associated with anti-tumour immune response and a better prognosis, systemic inflammation has been associated with pro-tumour immune response with more aggressive behaviour and worse prognosis for patients with several cancers, including CRC (7, 34). Some of the mechanisms by which systemic inflammation favours cancer growth and spread include increased vascular permeability, increased cancer cell infiltration through blood and lymphatic vessels, increased adhesion to endothelium at metastatic sites (35). It is, however, unclear if systemic inflammation is the cause of oncogenesis or a desired effect of cancer development (7).
There are several surrogate markers of systemic inflammation in cancer patients – acute-phase proteins (most notably, C-reactive protein (CRP)), circulating immune cells and their ratios, and circulating cytokines (36).
CRP is secreted by hepatocytes following pro-inflammatory stimulus, especially IL 6, which also decreases albumin production in the liver (35, 36). CRP is non-specific, therefore increased levels can be noted during such states as infection, tissue trauma and ischemia, chronic inflammation and also cancer (37). Studies show that elevated pre-diagnostic CRP levels have a dose-dependent relationship with increased risk of subsequent CRC development in men (37, 38). After disease has already developed, preoperative CRP levels show prognostic properties. Several studies have shown association between elevated preoperative CRP levels and worse cancer-specific survival (CSS) of CRC patients after surgical resection. This association is present in all stages (39). Higher CRP values are noted in patients who are found to have high-risk adenomas during screening colonoscopies, indicating the role of CRP throughout the polyp-adenoma-carcinoma cycle of CRC (40).
Because of its role as a marker of systemic inflammatory response, CRP has been used along with albumin to create a simple prognostic score – Glasgow Prognostic Score (GPS). Originally, a score of 2 was given if there was both CRP>10 mg/dL and albumin <35 g/L. A score of 1 and 0 was given if only one or none of the criteria was met, respectively. The GPS was modified (mGPS) after recognition that low albumin does not per se imply reduced survival. Therefore, mGPS is 0 in the case of low albumin and low CRP (35). There are several studies, including recent meta-analysis, which indicate a clear association between high GPS and mGPS and poor OS and CSS irrespective of TNM stage and tumour differentiation (41–44, 7).
Circulating innate and adaptive immune cells have been extensively studied as a potential tool to assess a systemic inflammatory reaction (SIR). Namely, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) have shown association with OS and CSS in patients with CRC. Important factors in favour of these markers are that they are easily available, cheap, and simple.
There is significant variation among different studies as to the most accurate biomarker and the optimal cut-off value. The majority of publications, including a recent meta-analysis (45) conclude that the most useful and accurate biomarker of SIR is NLR. Regarding 5-year survival rates, a systematic review comprised of more than 10,000 patients showed a 76.6% survival for patients with NLR<5 and only 49.9% for those with NLR>5 (46). The cut-off value varies significantly in different studies, with majority of them choosing a NLR of 2–5 as a cut-off. In addition to OS, recurrence rates also differ significantly between patients with higher and lower NLR. Importantly, this association was shown also in patients with early (stage I–II) disease, indicating that NLR could be used to predict recurrence and guide therapy in early CRC (47). Similarly, advanced CRC, among other solid cancers, shows a statistically significant association between higher NLR and worse OS, CSS, and progression-free survival (48–49).
Another extensively studied marker of systemic inflammation is PLR. Several publications state an association between elevated PLR and worse prognosis in CRC patients, namely, OS, CSS, disease-free and recurrence-free survival (50–52) in both localized and metastatic disease. There are several known mechanisms for increased PLR in cancer patients. Hypercoagulation is a known feature of malignancy. As noted earlier, elevation of several cytokines accompanies cancerogenesis, including IL-6, which stimulates platelet production in the bone marrow. Platelets, in return, produce several cytokines and growth factors that favour angiogenesis and tumour growth. Which of the two, thrombocytosis or cancer growth, comes first and which follows is not clear (50, 53). The other component, lymphocytes, play a fundamental role in the anti-tumour immune response, as discussed in previous sections. Therefore, a low lymphocyte count is a negative prognostic factor for patients with CRC (54). Based on these mechanisms, it is not surprising that elevated PLR is associated with a more advanced tumour stage, grade, and worse prognostic features. In a study comparing PLR among CRC patients, healthy people, and patients with neoplastic polyps, PLR was significantly elevated in the CRC group compared to the other two (55). This indicates a potential role of PLR as a tool for early diagnosis of CRC.
Finally, LMR has also been studied as a marker of SIR. Monocytes function as a source of macrophages, which have several pro-tumour properties, like increased angiogenesis, invasion, and metastatic spread of CRC (12, 56). As noted earlier, reduced lymphocyte count is a negative factor in CRC patients, therefore, low LMR should also imply worse prognosis. This has been shown in two recent meta-analysis (n = 9045 and n = 8626), in which the authors present low LMR to be associated with reduced OS and disease-free survival in localized and metastatic CRC (57, 58).
CONCLUSIONS
Despite the improvements in CRC treatment, results remain inadequate for large numbers of patients. Inflammation and immune reactions, both anti-tumour and pro-tumour responses, are a fundamental part of CRC and have a significant influence on tumour development, growth, and spread. In addition, gut microbiota might have a role in CRC development due to induction of chronic inflammation.
The anti-tumour immune response, carried out mainly by NKs and CD8+ T lymphocytes, has a beneficial effect and its hallmarks can be used to improve prognostication. Furthermore, efforts are made to find ways to facilitate these reactions and used them as an immune therapy tool.
On the contrary, pro-tumour immune responses mediated by TAMs, regulatory T cells, MDSCs and TMI have been shown to facilitate cancer progression. It is therefore a promising area of research that could result in understanding the way we can shift the balance of anti- and pro-tumour responses in our favour and improve CRC treatment.
Local inflammatory reaction has proved to be beneficial in the case of CRC. Its intensity can be assessed with tools like the immunoscore to improve prognostication in addition to the TNM staging system. In contrast, the systemic inflammatory reaction is associated with a pro-tumour response and a worse prognosis for CRC patients. In addition to such already established SIR scores as mGPS, more recently studied cell ratios like NLR, PLR and LMR have shown good prognostic value. The next step is to elucidate the most accurate marker and determine its cut-off value, so that this promising tool can be used in clinical practice and aid decision making.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Roberts Rumba, Sandra Cipkina, Fanija Cukure, Andrejs Vanags
References
- Pernot S Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, et al. . Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014; 20(14): 3738–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouakrim AD, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri E, et al. . Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015; 351: h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Ponce C, Serrano R, Sánchez-Heras AB, Teulé A, Chirivella I, Martín T, et al. . Clinical guideline SEOM: hereditary colorectal cancer. Clin Transl Oncol. 2015; 17: 962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. . Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009; 457: 608–11. [DOI] [PubMed] [Google Scholar]
- Wang K, Karin M.. Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res. 2015; 128: 173–96. [DOI] [PubMed] [Google Scholar]
- Formica V, Cereda V, Nardecchia A, Tesauro M, Roselli M.. Immune reaction and colorectal cancer: Friends or foes? World J of Gastroenterol. 2014; 20(35): 12407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Tie J, Lee B, Desai J, Gibbs P, Tran B.. Systemic inflammation – impact on tumor biology and outcomes in colorectal cancer. J Clin Cell Immunol. 2015; 6: 377. [Google Scholar]
- Chaplin DD. Overview of the Immune Response. J Allergy Clin Immunol. 2010; 125 Suppl 2: S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang C.. Innate recognition of microbial derived signals in immunity and inflammation. Sci China Life Sci. 2016; 59: 1210–7. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R.. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015; 16(4): 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey SE, Broide DH. Innate Immunity. J Allergy Clin Immunol. 2010; 125 Suppl 2: S24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol. 2015; 6(2): 208–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EM, Roberti MP, Mordoh J.. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011; 2011: 676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF Jr, Engel EA.. Risk of colorectal cancer after solid organ transplantation in the United States. Am J Transplant. 2016; 16: 960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. . Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014; 110(110): 1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Lee JH, Kim HR.. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012; 147(4): 366–72. [DOI] [PubMed] [Google Scholar]
- Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, et al. . Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007; 7: 7. [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Schreiber RD. . Cancer immunoediting: antigens, mechanisms and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013; 1284(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancione M, Giordano G, Remo A, Febbraro A, Sabatino L, Manfrin E, et al. . Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res. 2014; 2014: 686879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. . Colorectal cancer: a tale of two sides or a continuum? Gut. 2012; 61(6): 794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij A, van Gelder MM, Swinkels DW, Tjalsma H.. Clinical importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and metaanalysis. Clin Infect Dis. 2011; 53: 870–8. [DOI] [PubMed] [Google Scholar]
- Chimal-Ramírez GK, Espinoza-Sánchez NA, Fuentes-Pananá EM. Protumor activities of the immune response: insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J Oncol. 2013; 2013: 835956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119(119): 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kelaria S, Kerstetter J, Wang J.. The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol. 2015; 6(3): 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh D, Larmonier N.. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014; 74: 2663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012; 22: 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor SM, Syed Khaja AS, El Salhat H, Bekdache O, Kanbar E, Jaloudi M, et al. . Increased levels of circulating and tumor-infiltrating granulocytic myeloid cells in colorectal cancer patients. Front Immunol. 2016; 7: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umansky V, Blattner C, Gebhardt C, Utikal J.. The role of myeloid-derived suppressor cells in cancer progression. Whiteside TL, editor. Vaccines. 2016; 4(4): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. . Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012; 10: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CH, Roxburgh CSD, Powell AG, Foulis AK, Horgan PG, McMillan DC. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer. 2014; 50(2): 309–19. [DOI] [PubMed] [Google Scholar]
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. . Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011; 29: 610–8. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol. 2013; 3: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. . Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017; 23(34): 6261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Basso M, Strippoli A, Schinzari G, D’Argento E, Larocca M, et al. . Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017; 16(4): 264–74. [DOI] [PubMed] [Google Scholar]
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014; 15(11): e493–503. [DOI] [PubMed] [Google Scholar]
- Wu J, Cai Q, Li H, Cai H, Gao J, Yang G, et al. . Circulating C-reactive protein and colorectal cancer risk: a report from the Shanghai men’s health study. Carcinogenesis. 2013; 34(12): 2799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Shu B, Yang J, Liu J, Xi T, Xing Y.. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control. 2014; 25(10): 1397–405. [DOI] [PubMed] [Google Scholar]
- Kersten C, Louhimo J, Ålgars A, Lahdesmaki A, Cvancerova M, Stenstedt K, et al. . Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013; 52(8): 1691–8. [DOI] [PubMed] [Google Scholar]
- Lee HM, Cha JM, Lee JL, Jeon JW, Shin HP, Joo KR, et al. . High C-reactive protein level is associated with high-risk adenoma. Intest Res. 2017; 15(4): 511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He X, Pan J, Chen S, Wang L.. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017; 7(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Hong SW, Chang YG, Lee WY, Lee B, Paik IW, et al. . Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treat Res. 2014; 86(6): 309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozoe T, Matono R, Ijichi H, Ohga T, Ezaki T.. Glasgow prognostic score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg. 2014; 99(5): 512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DS, Foulis AK, et al. . An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011; 104(4): 726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, et al. . The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer. 2017; 17: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017; 115(4): 470–9. [DOI] [PubMed] [Google Scholar]
- Galizia G, Lieto E, Zamboli A, De Vita F, Castellano P, Romano C, et al. . Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surg. 2015; 158(1): 112–20. [DOI] [PubMed] [Google Scholar]
- Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. . Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2018; 58: 1–13. [DOI] [PubMed] [Google Scholar]
- Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. . Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106(6). [DOI] [PubMed] [Google Scholar]
- Lu C, Gao P, Yang Y, Chen X, Wang L, Yu D, et al. . Prognostic evaluation of platelet to lymphocyte ratio in patients with colorectal cancer. Oncotarget. 2017; 8(49): 86287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Li W, Huang K, Yang W, Huang L, Cong T, et al. . Increased platelet-lymphocyte ratio closely relates to inferior clinical features and worse long-term survival in both resected and metastatic colorectal cancer: an updated systematic review and meta-analysis of 24 studies. Oncotarget. 2017; 8(19): 32356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-X, Lin K, He BS, Pan YQ, Hu XX, Xu T, et al. . Platelet-to-lymphocyte ratio could be a promising prognostic biomarker for survival of colorectal cancer: a systematic review and meta-analysis. FEBS Open Bio. 2016; 6(7): 742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Shibutani M, Otani H, Nagahara H, Ikeya T, Iseki Y, et al. . Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015; 7(8): 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zhu J, Jia H, Huang L, Li D, Li Q, Li X.. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget. 2016; 7: 1014–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir S, Aydin M, Can G, Bali I, Yildirim O, Öznur M, et al. . Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015; 19(19): 3613–8. [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006; 124: 263–6. [DOI] [PubMed] [Google Scholar]
- Wu Q, Hu T, Zheng E, Deng X, Wang Z.. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Zhang. D, ed. Medicine. 2017; 96(22): e7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Wang K, Zhang R, Zou S.. Prognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: A meta-analysis. Amornyotin S, ed. Medicine. 2016; 95(49): e5540. [DOI] [PMC free article] [PubMed] [Google Scholar]