Abstract
Background
Gastric antral vascular ectasia (GAVE) is currently recognized as an important cause of upper gastrointestinal (GI) haemorrhage, being responsible for about 4% of non-variceal upper GI haemorrhages and typically presents in middle-aged females. GAVE, also called “watermelon stomach”, is diagnosed through esophagogastroduodenoscopy and is characterized by the presence of visible columns of red tortuous enlarged vessels along the longitudinal folds of the antrum. The pathogenesis is still obscure and many hypotheses have been proposed such as mechanical stress, humoral and autoimmune factors. In the last two decades, numerous therapeutic strategies have been proposed, including surgical, endoscopic, and medical choices, yet successful treatment of GAVE continues to be a challenge. Currently, given the rapid response, safety, and efficacy, endoscopic ablative modalities have largely usurped medical treatments as first-line therapy, particularly using argon plasma coagulation. The actual GAVE prevalence in patients with end-stage renal disease (ESRD) is not clear, yet in difficult cases it should be considered as a cause of erythropoietin resistance.
Case presentation
We report four clinical cases of GAVE syndrome patients diagnosed with stage 4 to 5 chronic kidney disease. All patients presented with anaemia and GI haemorrhage, the origin of which turned out to be GAVE syndrome.
Conclusions
GAVE syndrome is a serious condition in ESRD patients, especially in those presenting with treatment-refractory anaemia. Realization of its aetiology and characteristics is essential to suspect, diagnose, and treat gastric ectasia. Only proper diagnosis and well-timed disease treatment can significantly improve a patient’s medical condition and future prognosis.
Keywords: gastric antral vascular ectasia, GAVE, watermelon stomach, end-stage renal disease, ESRD, hemodialysis
Abstract
SKRANDŽIO URVO KRAUJAGYSLIŲ EKTAZIJA – ATSPARIOS GYDYMUI ERITROPOETINU ANEMIJOS PRIEŽASTIS
Santrauka
Įvadas. Skrandžio urvo kraujagyslių ektazija yra svarbi viršutinės virškinimo trakto hemoragijos priežastis, dėl kurios išsivysto maždaug 4 % viršutinio virškinimo trakto kraujavimų be varikozės. Paprastai tai pasireiškia vidutinio amžiaus moterims. Skrandžio urvo kraujagyslių ektazija, taip pat vadinama „Arbūziniu pilvu“, diagnozuojama ezofagogastroduodenoskopijos metu: endoskopiškai matoma išilginės kraujagyslių juostos prievarčio urve. Patogenezė vis dar yra neaiški, tačiau yra siūlomos hipotezės, tokios kaip mechaninis stresas, humoraliniai ar autoimuniniai veiksniai. Per paskutinius du dešimtmečius buvo pasiūlyta daugybė gydymo strategijų, tačiau sėkmingas skrandžio urvo kraujagyslių ektazijos gydymas vis dar yra iššūkis. Šiuo metu, atsižvelgiant į greitą reagavimą, saugumą ir veiksmingumą, endoskopinės abliacijos metodas yra pirmo pasirinkimo gydymas, ypač naudojant argono plazmos koaguliaciją. Skrandžio urvo kraujagyslių ektazijos paplitimas tarp pacientų, sergančių lėtine inkstų liga, ypač galutinės stadijos, nėra aiškus, tačiau sudėtingose situacijose tai gali lemti atsparios gydymui eritropoetinu anemiją.
Klinikinių atvejų pristatymas. Šiame darbe aprašomi klinikiniai atvejai 4 pacientų, sergančių 4–5 stadijos lėtine inkstų liga bei skrandžio urvo kraujagyslių ektazija. Visiems pacientams pasireiškė anemija ir virškinimo trakto hemoragija, kurios priežastis buvo skrandžio urvo kraujagyslių ektazijos sindromas.
Išvada. Skrandžio urvo kraujagyslių ektazijos sindromas yra gyvybiškai pavojinga būklė, pacientams sergantiems toli pažengusia lėtine inkstų liga, ypač tiems, kuriem pasireiškia gydymui atspari anemija. Etiologijos ir ypatybių supratimas yra būtinas, norint įtarti, diagnozuoti ir gydyti skrandžio ektaziją. Tik tinkama diagnozė ir savalaikis gydymas gali žymiai pagerinti paciento sveikatos būklę ir ateities perspektyvas.
Raktažodžiai: skrandžio urvo kraujagyslių ektazija, arbūzinis pilvas, galutinės stadijos lėtinė inkstų liga, hemodializės
INTRODUCTION
Gastric antral vascular ectasia (GAVE) syndrome, also known as “watermelon stomach”, is recognized as a rare cause of upper gastrointestinal bleeding (1) and can be a cause of severe blood effusion. Gastric antral vascular ectasia is often found with other disorders, such as liver cirrhosis, autoimmune connective tissue disorder, bone marrow transplantation, ischemic heart disease, valvular heart disease, hypertension, hypothyroidism, diabetes, or acute myeloid leukaemia (2–6). Additionally, recent studies show that patients with end stage renal disease (ESRD) are more likely to have GAVE syndrome (7). We report four clinical cases of GAVE syndrome in patients diagnosed with stage 2 to 5 chronic kidney disease undergoing continuous ambulatory haemodialysis. All patients presented with anaemia and GI haemorrhage, the origin of which turned out to be GAVE syndrome.
CASE REPORTS
Case 1
A 66-year-old female patient presented with complaints of general weakness and melena for five days in a row. She had been on haemodialysis for three years after diagnosis of ESRD due to hypertension and diabetes. On examination, patient’s condition was stable, blood pressure (BP) 160/80 mmHg, heart rate 78 bpm. Rectal examination confirmed melena. As a result of continuous loss of blood in stool, laboratory investigations revealed iron-deficiency anaemia with haemoglobin value 60 g/l, despite continuous administration of intravenous iron and erythropoietin previously during each dialysis session. Upper gastrointestinal endoscopy showed multiple linear gastric vascular malformations with signs of bleeding. APC was performed to which the patient responded with positive dynamics of increased RBC (3.9 × 1012/l), haemoglobin (126 g/l), and platelets counts (268 × 109/l). As the patient’s condition became stable, she was discharged on a conservative line of management.
Fig. 1.
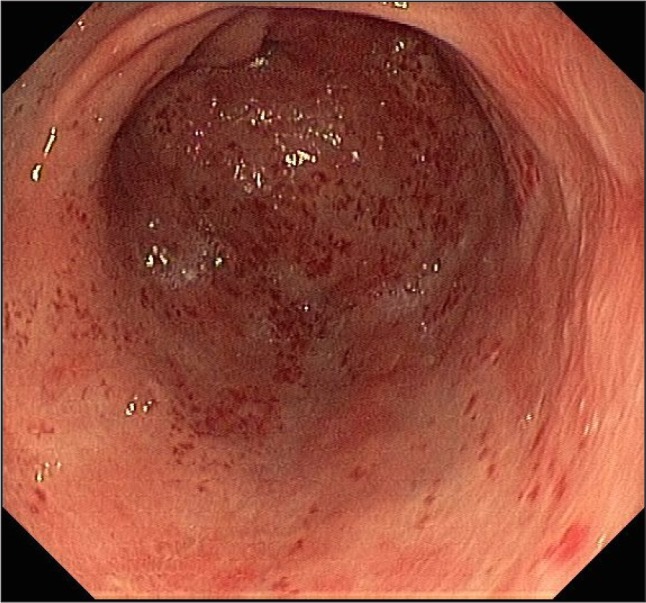
“Watermelon stomach” – longitudinal flat rows and reddish stripes diverging from the pylorus into the antrum
Fig. 2.
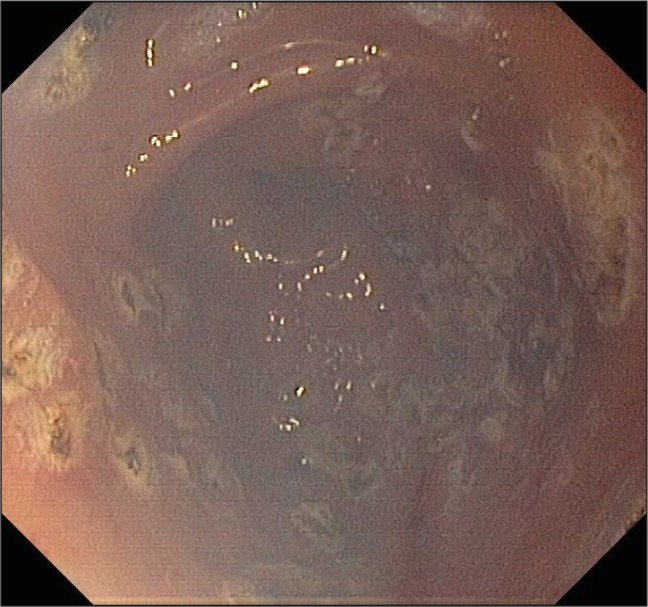
Argon plasma coagulation treatment of GAVE
Case 2
A 75-year-old male patient with hypertensive nephropathy on chronic haemodialysis for five years was referred to the hospital with complaints of general weakness and melena present for four days. His BP was 148/80 mmHg, heart rate 80 bpm. Laboratory investigations revealed slight iron-deficiency anaemia and haemoglobin value of 118 g/l. The patient also had a history of myocardial infarction, heart insufficiency and coronary artery bypass. Upper gastrointestinal endoscopy found multiple linear gastric vascular malformations in the antrum, with 3 mm lesions and no signs of bleeding. During the procedure, bleeding occurred and adrenaline injections were given. APC was performed for GAVE syndrome and the patient responded well, with positive dynamics showing increased red blood cell (5.1 × 1012/l), haemoglobin (136 g/l) and platelets (268 × 109/l) counts.
Fig. 3.
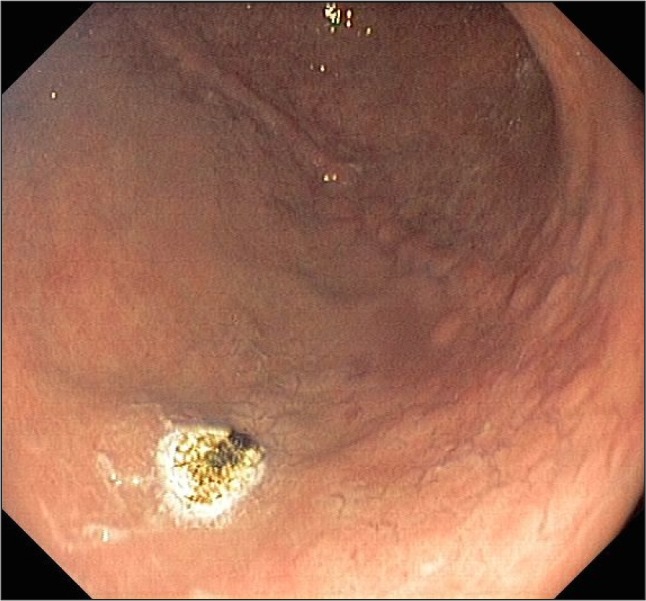
Argon plasma coagulation treatment of GAVE
Case 3
A 64-year-old male patient with ESRD due to chronic pyelonephritis, on maintenance haemodialysis for five years presented with complaints of general weakness and shortness of breath for four days in a row. On examination, the patient’s condition was unstable with weakened vesicular breathing sounds, peripheral leg oedema, severe pallor and indolence, BP 105/60 mmHg, heart rate 96 bpm. The patient also had a history of two myocardial infarctions, severe heart insufficiency, coronary artery bypass, and cardiac stimulator implantation surgery. Rectal examination was positive for melena and, as a result of continuous loss of blood in stool, laboratory investigations revealed iron-deficiency anaemia with haemoglobin of 98 g/l. As chest radiograph showed pleural effusion on both sides of the lungs, right lung puncture was performed with 1.5 l of transudate retrieved. After upper gastrointestinal endoscopy, the patient was found to have multiple linear gastric vascular malformations in the antrum without any signs of bleeding. Due to pleural effusion and peripheral leg oedema remaining during the hospital stay, the patient was given additional haemodialysis course. Despite maximal doses of erythropoietin therapy along with blood transfusion and intravenous iron in view of the severe iron deficiency, this patient remained anaemic. APC was performed, and one month after endoscopic therapy there was no need for transfusion of RBC, haemoglobin concentration stabilized. The patient responded well with increased blood counts (red blood cell 3.6 × 1012/l, haemoglobin 126 g/l) and was discharged on a conservative line of management.
Fig. 4.
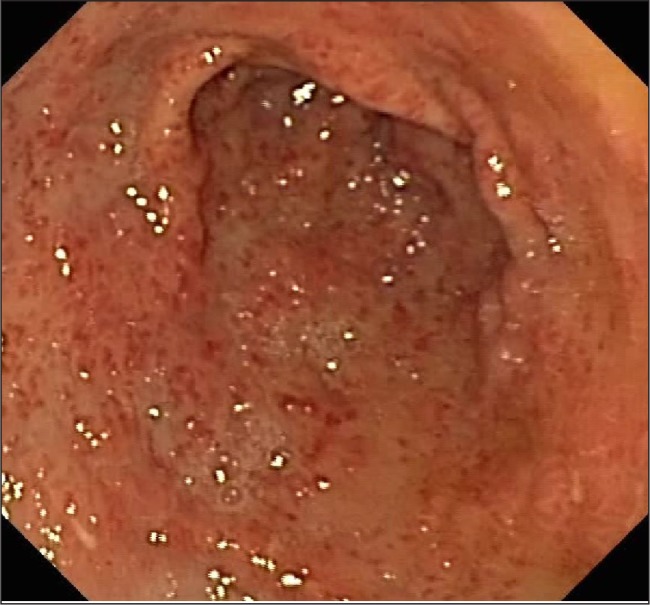
“Watermelon stomach” – linear, intensely erythematous lesions at the apices of longitudinal antral folds radiating to the pylorus
Fig. 5.
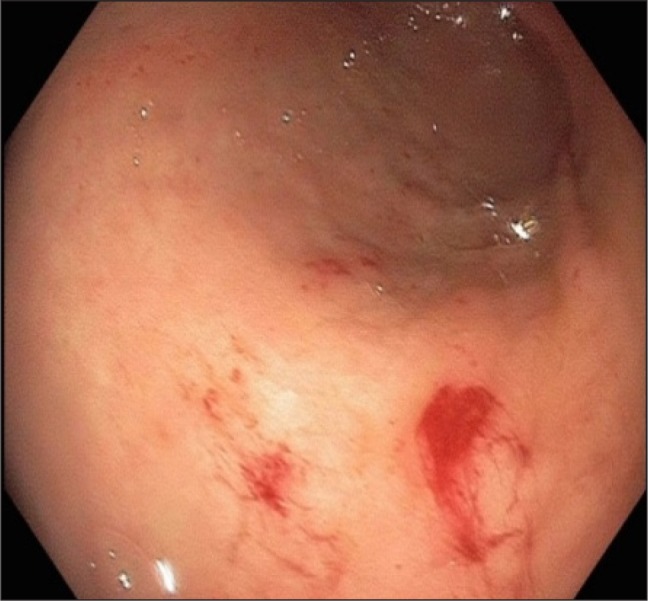
Small multiple linear gastric vascular malformations in the antrum with moderate bleeding
Case 4
An 80-year-old male patient with CKD stage G4 of unknown origin was referred with complaints of general weakness, shortness of breath, and blood in stool for five days in a row, with severe worsening of the symptoms. He denied previous history of liver or autoimmune disease, and this was the first episode of melena. On physical examination, the patient’s condition was stable with notable mucosal pallor, shortness of breath, rhythmic heart activity, vesicular breathing sounds, and lack of peripheral oedema, BP 85/58 mmHg, heart rate 82 bpm. In the past, myocardial infarction and atrial fibrillation were diagnosed; heart ablation surgery and cardiac stimulator implantation were performed. Rectal examination was positive for melena and laboratory investigations revealed iron-deficiency anaemia with haemoglobin of 77 g/l. Urea blood level was 8.2 mmol/l, creatinine 190 µmol/l and glomerular filtration rate 28 ml/min/1.73 m2. During hospital stay, the patient was treated with erythropoietin therapy, blood transfusion, and intravenous iron therapy. Endoscopy found multiple linear gastric vascular malformations in the antrum with small signs of bleeding. Argon plasma coagulation was performed for GAVE syndrome, to which the patient responded well with increased red blood cell (4.8 × 1012/l) and haemoglobin (123 g/l) counts. The altered blood in stool decreased and the patient was discharged on a conservative line of management.
Table.
Overview of presented case reports
| CASE 1 | CASE 2 | CASE 3 | CASE 4 | |
|---|---|---|---|---|
| CKD grade | 5 | 5 | 5 | 4 |
| Haemodialysis | For the last 3 years, 3 times per week | For the last 5 years, 3 times per week | For the last 5 years, 3 times per week | No dialysis required |
| Complaints | General weakness, blood in stool | General weakness, blood in stool | General weakness, shortness of breath, peripheral leg oedema, severe pallor, indolence | Pallor, shortness of breath, blood in stool |
| Erythropoetin dose | 20,000 IU/week | 20,000 IU/week | 100 mkg/week | 100 mkg/week |
| Intravenous iron administration | + | + | + | + |
| Haemoglobin (before/after treatment) | 60 g/l/126 g/l | 118 g/l/136 g/l | 98 g/l/126 g/l | 77 g/l/123 g/l |
| Underlying conditions | High BP, diabetes | Hypertension, myocardial infarction, heart failure, coronary artery bypass | Chronic pyelonephritis myocardial infarctions, heart failure, coronary artery bypass, cardiac pacemaker implantation | Myocardial infarction, atrial fibrillation, heart ablation surgery, cardiac pacemaker implantation |
| Treatment | APC, erythropoietin therapy, blood transfusion, intravenous iron | APC | APC, erythropoietin therapy, blood transfusion, intravenous iron | APC, erythropoietin therapy, blood transfusion, intravenous iron therapy |
DISCUSSION
Although GAVE syndrome is involved in only 4% of non-variceal upper gastrointestinal bleeding developments (1), more and more case reports describe ESRD coexistence with GAVE syndrome (11). The way to suspect and anticipate the occurrence of this syndrome is very important, especially for patients with ESRD. If a patient has iron or erythropoietin resistance anaemia, undetected bleeding loss can quickly reduce the patient’s iron reserves (15). There are theories trying to explain the origin of the syndrome.
It has been suggested that in patients with chronic kidney disease, increased mechanical stress of the antrum in uraemia-induced weakening of gastric emptiness is thought to contribute to the development of GAVE. Enhanced gastrin levels also increase gastric vasodilatation (9). Uraemia-induced platelet dysfunction might also be incriminated as a risk factor for antral bleeding (10). Prostaglandin E2 is another hormone with vasoactive functions; in a single study, it was found to be significantly higher in cirrhosis patients with GAVE than in those without. In another study, histological examination showed that neuroendocrine cells containing 5-hydroxytryptamine and vasoactive intestinal peptide were found close to the ectatic gastric mucosal vessels (9). An impaired renal excretory function and decreased removal of these vasoactive substances from the circulatory system in patients with CKD may be associated with the emergence of gastric vascular ectasia (11–17). These pathological mechanisms were also noticed in the cases reported in the present paper. Three of the four patients suffered from stage 5D CKD and had been treated with haemodialysis three times per week for the past three to five years. The average time from the beginning of dialysis to the first bleeding episode was 52 months, although a larger sample size would be needed to be able to generalize this finding to the general population. However, every case should be approached individually as the time GAVE takes to manifest varies.
Typical initial presentations of the syndrome range from occult bleeding, causing transfusion-dependent chronic iron-deficiency anaemia, to severe acute upper gastrointestinal bleeding (8). All four described patients presented with anaemia levels between 60 and 118 g/l, as well as symptoms such as weakness, pallor, and shortness of breath. Three patients developed evident GI haemorrhage confirmed by rectal examination.
Literature suggests that lesions are typically located in the gastric antrum, and the four described cases were in line with this. Gastric lesions slightly raised above the mucosal surface, cherry red in colour and 2–10 mm in size were seen. GAVE syndrome appears as endoscopic forms of “watermelon stomach”, characterized by longitudinal flat rows and reddish stripes diverging from the pylorus into the antrum. Endoscopy findings were sufficient to diagnose GAVE syndrome and histological investigation was not necessary.
The initial form of treatment was APC (17, 18), which is considered the preferred and the most frequently used method of endoscopic therapy. In our four patients endoscopic haemostatic therapy using APC was performed for the ectatic lesions; the procedure was well tolerated without any further complications associated with the therapy. After the endoscopic procedure, GAVE lesions were significantly diminished. All patients required additional erythropoietin therapy, blood transfusions, and intravenous iron. After the therapy, patients’ condition became stable and they were discharged on a conservative line of management.
According to the documented cases of patients with GAVE syndrome undergoing APC therapy, appropriate monitoring is required as there is a risk of eventual recurrence. Thus, in recurrent bleeding, repeat endoscopy is useful and surgery is necessary.
CONCLUSIONS
In conclusion, GAVE syndrome is an important cause of upper gastrointestinal bleeding and has a higher prevalence in patients with chronic kidney disease. Verification of the aetiology and characteristics are essential for suspecting, diagnosis, and treatment of gastric ectasia in patients, especially in those with more than one risk factor and showing signs of treatment refractory anaemia. Only proper diagnosis and well-timed treatment can significantly improve patient’s medical condition and future prognosis.
Laurynas Rimševičius, Domantas Galkauskas, Julius Lavinskas, Evelina Šestelinska, Ernesta Mačionienė, Agnė Laučytė-Cibulskienė, Skirmantė Rėkutė, Marius Miglinas
References
- Dulai GS, Jensen DM, Kovacs TO, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004; 36(1): 68–72. [DOI] [PubMed] [Google Scholar]
- Ward EM, Raimondo M, Rosser BG, Wallace MB, Dickson RD. Prevalence and natural history of gastric antral vascular ectasia (GAVE) in patients undergoing orthoptic liver transplantation. J Clin Gastroenterol. 2004; 38: 898–900. [DOI] [PubMed] [Google Scholar]
- Tobin RW, Hackman RC, Kimmey MB, Durtschi MB, Hayashi A, Malik R, McDonald MF, McDonald GB. Bleeding from gastric antral vascular ectasia in marrow transplant patients. Gastrointest Endosc. 1996; 44: 223–9. [DOI] [PubMed] [Google Scholar]
- Sebastian S, O’Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003; 18: 157–65. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Miya T, Oki M, Sugawara N, Yoshimoto M, Tsujisaki M. Severe hemorrhage from gastric antral vascular ectasia developed in a patient with AML. Int J Hematol. 2006; 83: 467–8. [DOI] [PubMed] [Google Scholar]
- Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992; 15: 256–63. [DOI] [PubMed] [Google Scholar]
- Junichiro AI, Kazama J, Komatsu M, Kaneko Y, Iino Noriaki, Narita Ichiei. Three cases of gastric antral vascular ectasia in chronic renal failure. Case Rep Nephrol Urol. 2011; 1: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008; 77: 131–7. [DOI] [PubMed] [Google Scholar]
- Lowes JR, Rode J. Neuroendocrine cell proliferations in gastric antral vascular ectasia. Gastroenterology. 1989; 97: 207–12. [DOI] [PubMed] [Google Scholar]
- Stefanidis I, Liakopoulos V, Kapsoritakis AN, Ioannidis I, Eleftheriadis T, Mertens PR, Winograd R, Vamvaka E, Psychos AK. Potamianos SP. Gastric antral vascular ectasia (watermelon stomach) in patients with ESRD. Am J Kidney Dis. 2006; 47: e77–e82. [DOI] [PubMed] [Google Scholar]
- Liberski SM, McGarrity TJ, Hartle RJ, Varano V, Reynolds D. The watermelon stomach: long–term outcome in patients treated with Nd:YAG laser therapy. Gastrointest Endosc. 1994; 40: 584–7. [DOI] [PubMed] [Google Scholar]
- Kaaroud H, Fatma LB, Beji S, Boubaker K, Hedri H, Ben Hamida F, El Younsi F, Ben Abdallah T, Ben Maiz H, Kheder A. Gastrointestinal angiodysplasia in chronic renal failure. Saudi J Kidney Dis Transplant. 2008; 19(5): 809–12. [PubMed] [Google Scholar]
- Jinga M, Checherita IA, Becheanu G, Jinga V, Peride I, Niculae A. A rare case of watermelon stomach in woman with continuous ambulatory peritoneal dialysis and systemic lupus erythematosus. Rom J Morphol Embryol. 2013; 54(3 Suppl): 863–5. [PubMed] [Google Scholar]
- Iguchi A, Kazama JJ, Komatsu M, Kaneko Y, Iino N, Goto S, Narita I. Three cases of gastric antral vascular ectasia in chronic renal failure. Case Rep Nephrol Urol. 2011; 1: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Fragata J, Pestana JO, Draibe S, Canziani ME, Cendoroglo M, Goes MA., Jr Erythropoietin resistance in end–stage renal disease patient with gastric antral vascular ectasia. J Bras Nefrol. 2015; 37(3): 410–3. [DOI] [PubMed] [Google Scholar]
- Lata S, Gupta V, Nandwani A, Sharma P. Watermelon stomach in end-stage renal disease patient. Indian J Nephrol. 2012; 22: 477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001; 53(7): 764–770. [DOI] [PubMed] [Google Scholar]
- Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol. 2006; 43: 191–5. [DOI] [PubMed] [Google Scholar]


