Significance
Although many animal species have demonstrated an ability to differentiate between more and less when presented with different amounts of food, they have done so primarily using vision. In this study, by contrast, elephants showed that they can detect differences between various quantities of food using only their sense of smell. Thus, elephants may be unique in their use of olfaction in cognitive tasks. This research suggests that it is important for psychologists to incorporate into experimental designs the ways in which different animals interact with their environment using all of their senses. Such species-specific paradigms would ensure comparisons about cognition across taxa are fair and relevant.
Keywords: comparative cognition, relative quantity judgment, elephants, elephant cognition, numerosity
Abstract
Animals often face situations that require making decisions based on quantity. Many species, including humans, rely on an ability to differentiate between more and less to make judgments about social relationships, territories, and food. Habitat-related choices require animals to decide between areas with greater and lesser quantities of food while also weighing relative risk of danger based on group size and predation risk. Such decisions can have a significant impact on survival for an animal and its social group. Many species have demonstrated a capacity for differentiating between two quantities of food and choosing the greater of the two, but they have done so based on information provided primarily in the visual domain. Using an object-choice task, we demonstrate that elephants are able to discriminate between two distinct quantities using their olfactory sense alone. We presented the elephants with choices between two containers of sunflower seeds. The relationship between the amount of seeds within the two containers was represented by 11 different ratios. Overall, the elephants chose the larger quantity of food by smelling for it. The elephants’ performance was better when the relative difference between the quantities increased and worse when the ratio between the quantities of food increased, but was not affected by the overall quantity of food presented. These results are consistent with the performance of animals tested in the visual domain. This work has implications for the design of future, cross-phylogenetic cognitive comparisons that ought to account for differences in how animals sense their world.
At the grocery store, the “10 items or less” checkout line is always the shortest; as we go to pay for our goods, we quickly check our cart to see if we qualify. Whether we are shopping for groceries or splitting a bill at dinner, we make decisions daily based on quantities. Many studies have investigated our own capacity for understanding numbers and discriminating between relative and absolute quantities (1, 2). The ability to differentiate between two different quantities is evolutionarily significant, as animals rely on quantity judgment to make routine decisions that impact their physical and social lives. For example, they may select habitats with greater food resources (3), pursue larger groups of prey (4), seek locations with more potential mates (5), or assess group size when challenging conspecific competitors or defending against predators (6–9).
In tasks that ask an animal to differentiate between two different amounts of food (an individual may be presented with these different quantities based on predetermined ratios, for example, 1:2 or 3:4), species generally perform well, making relative quantity judgments in favor of the larger quantity [e.g., nonhuman primates (10–12), pinnipeds and cetaceans (13, 14), birds (15, 16), canines (17), bears (18), elephants (19–21)]. Remarkably, however, quantity discrimination testing has almost always focused on the visual domain [but see studies on beetles (22) and voles (23) that tested for this capacity in a limited social, olfactory context, on chimpanzees for tests in the auditory domain (24), and on dogs for tests in which they failed to differentiate quantities of food when they could smell but not see it (25)]. This tendency is largely due to the field of comparative cognition’s long history of drawing species-level comparisons from a primate-centric, and thus primarily visual, perspective (26). The result is that it becomes difficult to draw direct comparisons across taxa; failure on a given cognitive task may be due not to a lack of a capacity but to an experimental bias that assumes a particular animal relies on vision.
Most investigations into how complex cognition has evolved convergently in taxa that have no recent common ancestor have centered on animals that share comparable visual perspectives, such as nonhuman primates and corvids (27, 28). But, as large-brained, socially complex animals, elephants are also an interesting model species for psychologists interested in convergent cognitive evolution. In addition, studying the elephant’s capacity for quantity discrimination has ecological validity. Elephants often travel to find high-quality food and water, depending on seasonal availability, social status, human risk, and environmental change (29–31). It would thus be evolutionarily advantageous for them to be able to make calculated decisions about food availability that result in the conservation of time and energy.
However, while elephants do use vision [for example, in close, social contexts where they may react to each other’s body language (32)], they use it primarily as a complement to their more dominant senses of hearing, smell, and touch. This sensory difference makes it challenging to design experiments to investigate the elephant’s cognition (33–35). In previous quantity-discrimination tasks based on visual cues, elephant studies have produced conflicting results (19–21). In addition, while it is well-documented that elephants use a complex array of acoustic signaling in social communication (36–38), their sense of smell and how they use it to navigate their physical environment has received little attention to date (but see refs. 34 and 39–42). Thus, we investigated whether elephants have the capacity for quantity discrimination in a sensory domain of high ecological relevance to them: olfaction.
Results and Discussion
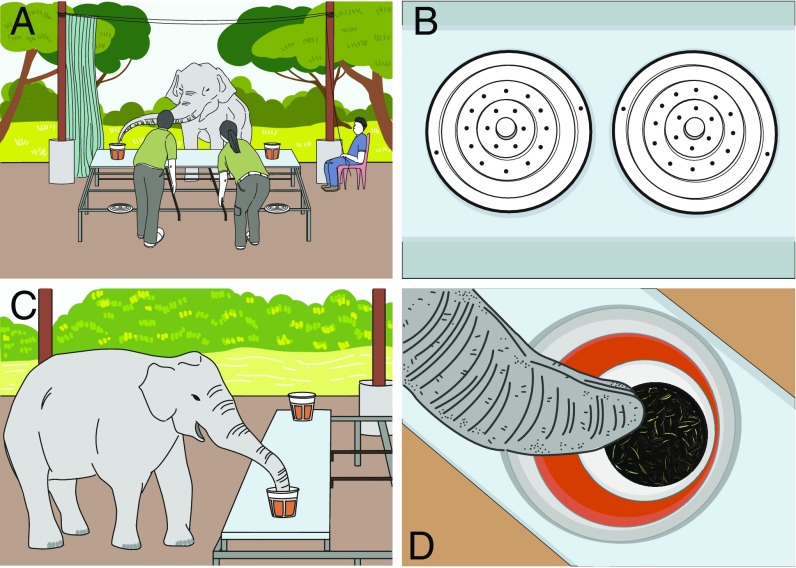
We tested six Asian elephants (Elephas maximus) for their capacity to make relative quantity judgments using olfactory information alone at a facility in northern Thailand (SI Appendix, Tables S1 and S2). The elephants in this study are captive animals living in a tourism-based facility with a high degree of animal welfare standards and a full-time veterinary staff. A sliding table was used to present each subject with two buckets containing a varying amount of sunflower seeds. First, the elephants had an opportunity to smell the two locked buckets on the table through perforated lids. Then, the buckets were withdrawn, unlocked, and presented again so the elephants could choose one of the two (Fig. 1). The elephants were first pretested to ensure that they could recognize 4 g of sunflower seeds—the smallest amount of food tested across the study—and would choose this quantity over none. The elephants all reached criterion (80% correct within a set of 12 trials) in three or fewer sets (mean = 1.67 sets). Next, the elephants were given a “solid-lid” control condition to rule out the potential effect of the elephants perceiving nonolfactory information about the quantities of food presented (one set of 12 trials with solid lids only, 24 g of seeds vs. none). They were then trained on a small ratio (1:8, i.e., 4 vs. 32 g of seeds) to ensure that they understood that both buckets could contain food [these elephants had extensive previous experience with object-choice tasks in which only one bucket had food (34, 43)]. The elephants all reached criterion (80% correct within a set of 12 trials) in eight or fewer sets (mean = 2.67 sets).
Fig. 1.
General setup during the experimental condition. (A) Two experimenters pushed the sliding table containing two buckets up to the elephant so that he/she could investigate each bucket using his/her trunk alone. (B) In the investigation phase of each trial, the bucket lids were locked and opaque, so that the only sensory information the elephants could gather about the food was olfactory and they could only gather this information by smelling through the small holes in the bucket lids. (C) After smelling the bucket lids, the table was withdrawn and then, after the lids were unlocked and replaced with upside-down solid lids, pushed back up to the elephant again so he/she could make a choice by removing the lid of a single bucket. (D) The elephant’s eye view of a bucket after a choice had been made shows a small container with sunflower seeds, an inner, orange pail to prevent visual information from being obtained from the outside, and an outer, opaque bucket onto which the lids were attached. Illustrations by Nuttayapond Doungcharoen (artist).
The elephants then proceeded to complete experimental test trials in which we investigated whether they could discriminate between pairs of quantities by smell. Six test quantities (multiples of 4 g up to 24 g of seeds) were used. We constructed the overall experiment to evaluate the elephants’ performance distinguishing between pairs of quantities with varying ratio values (e.g., 12 g vs. 16 g has a ratio value of 3:4 or 0.75) and varying ratio disparities (i.e., the absolute difference between the two numbers in the ratio; the ratio 1:3 has a disparity of 2 units, for instance). Subjects completed 10 sets, with each set consisting of one trial of each of the 11 ratios produced by pairing the six test quantities (1:2, 1:3, 1:4, 1:5, 1:6, 2:3, 2:5, 3:4, 3:5, 4:5, 5:6). For three of these ratios (1:2, 1:3, 2:3), multiple pairings of the six test quantities were possible; for example, 1:2 could be presented as 4 g vs. 8 g or 12 g vs. 24 g. Given this, each subject completed five sets with the lower-quantity pairings for these three ratios (4 g vs. 8 g for 1:2; 4 g vs. 12 g for 1:3; 8 g vs. 12 g for 2:3) and five sets with the higher-quantity pairings for these three ratios (12 g vs. 24 g for 1:2; 8 g vs. 24 g for 1:3; 16 g vs. 24 g for 2:3). The use of different quantities of the same ratio allowed us to determine whether the total quantity or magnitude of food present influenced the elephants’ performance. Although a third pairing (8 g vs. 16 g) was possible for the ratio 1:2, we chose not to test this intermediate pairing to standardize analysis by including two pairings for each of the three ratios. For 1:2, to increase the odds of identifying an existing magnitude effect, we opted to use the two pairings with the greatest discrepancy between them (4 g vs. 8 g compared with 12 g vs. 24 g).
After all 10 sets of the experimental condition, the subjects completed four different conditions in the following order: (i) a repeat of the solid-lid condition to determine if the elephants had learned to follow any inadvertent cues about the food’s location (one set of 12 “0 g vs. 24 g” trials), (ii) a “metal-bucket” condition that controlled for potential confounds of residual olfactory cues in the plastic testing buckets by testing in metal buckets instead (two sets of the 11 different ratio trials), (iii) a “double-blind” condition that controlled for the potential of an experimenter cuing inadvertently toward the greater quantity by ensuring the experimenters at the sliding table did not know which bucket contained which quantity (two sets of the 11 different ratio trials), and (iv) a “residual-odor” condition that further controlled for the potential effect of accumulated residual odor within the baited containers used in the test trials (two sets of 12 “14 g vs. 14 g” trials). These conditions were conducted after the experimental condition for two reasons: (i) to avoid confusion on the part of the elephants due to too many changes to the paradigm in the middle of the experiment, and (ii) in the case of the metal-bucket and double-blind conditions, to confirm that the results would be consistent regardless of bucket material and experimenter bias, respectively.
To analyze the elephants’ success in choosing the higher quantity by both ratio value and ratio disparity, we constructed two logistic regression mixed models to test for an effect of each (see Materials and Methods for details). The modeling approach we used included data from all trials and used a random term for individual ID to allow for variation between individuals in intercept. This retains the structure of the experimental design and, subsequently, none of the models failed to converge (see SI Appendix, Statistical Analyses for details on nonsignificant covariate terms).
The experimental, metal-bucket, and double-blind condition sets each consisted of one trial of each of the 11 ratios, and thus were included alongside all other trials in the models and analyzed as a factor with three levels. First, there was no significant difference between success in the experimental condition and either the metal-bucket or double-blind conditions across trials [success/ratio model: likelihood ratio test (LRT) χ2 = 0.29, df = 2, P = 0.87; success/disparity model: LRT χ2 = 0.33, df = 2, P = 0.85], indicating that success for the elephants was not affected by container type or experimenter cues. This allowed us to look at success by ratio, disparity, and magnitude across all three of these conditions combined.
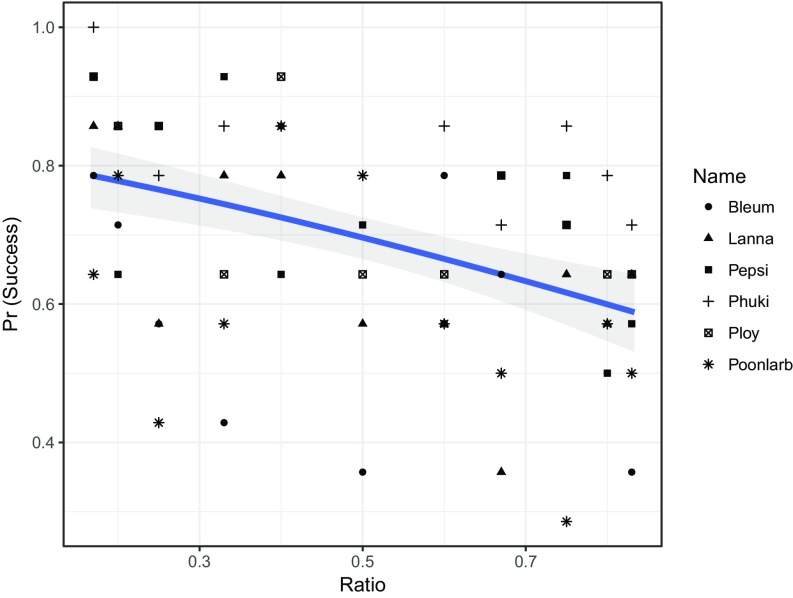
Overall, the elephants chose the greater quantity of food across the different ratio values using olfaction alone. The likelihood of success (selecting the bucket with more food) over the 154 trials varied significantly by ratio (Fig. 2 and SI Appendix, Table S3). Specifically, the elephants’ likelihood of success decreased as the quantities of food in the ratio became closer (i.e., the overall ratio value approached 1; likelihood ratio test for model with terms for linear and quadratic ratio and model without those terms: χ2 = 21.86, df = 2, P < 0.0001). When testing the model with both linear and quadratic terms for ratio against the model with a linear term only, there was no significant difference, indicating a linear relationship between food ratio and success (SI Appendix, Table S3; LRT χ2 = 0.14, df = 1, P = 0.70). The elephants as a group scored significantly better than chance on 5 out of 11 ratios (1:3, 1:5, 1:6, 2:5, 3:5; SI Appendix, Table S1). Although none of the covariates in the model significantly improved the model fit, sex (male vs. female) was significant within the ratio model (SI Appendix, Table S3).
Fig. 2.
Probability of success by ratio of food as a decimal value. Points indicate raw data, while the line represents predicted values based on the model of success by ratio. Both raw data and predicted probabilities are displayed, with the former shown by individual elephant. Shaded coloration indicates predicted variation in success around each ratio based on the model. Raw data are indicated by symbol, with each elephant represented only once at each ratio value.
To assess magnitude effects, we compared whether the elephants performed differently when presented with single vs. double or triple (e.g., 4 g vs. 12 g and 8 g vs. 24 g, or 4 g vs. 8 g and 12 g vs. 24 g) versions of the same food ratios in the experiments (e.g., 1:3 and 1:2, respectively). Magnitude was a nonsignificant term [LRT χ2 = 1.18, df = 1 (as the variable was treated as a two-level factor with levels single and double/triple), P = 0.28], indicating that at the same ratio, the combined magnitude of food presented did not influence the likelihood of success.
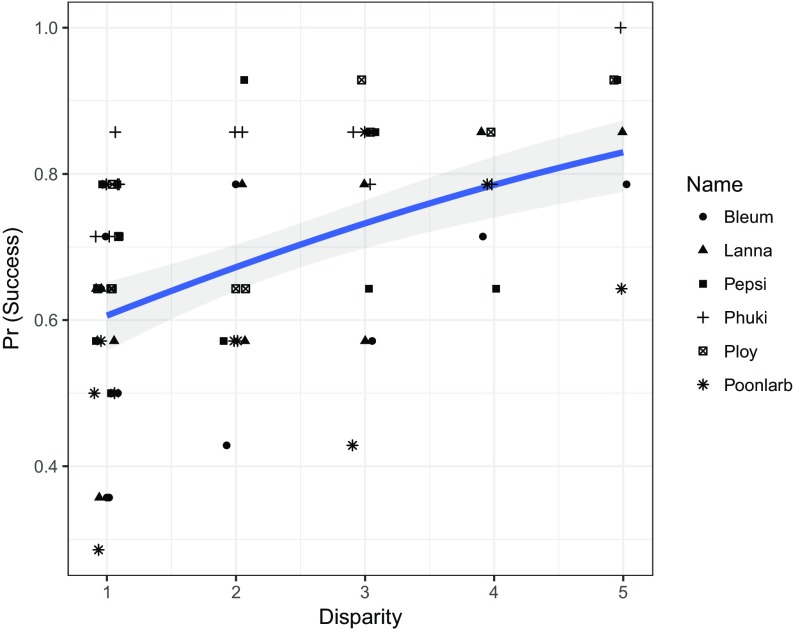
We also tested for an association between probability of success and disparity between the two food quantities. Results showed that overall, the elephants’ likelihood of success varied significantly across different pairwise disparities; there was a significant increase in success as disparity increased (LRT χ2 = 27.70, df = 1, P < 0.0001; Fig. 3 and SI Appendix, Table S4). As in the ratio model, none of the covariates in the disparity model significantly improved the model fit, but sex (male vs. female) was significant within the disparity model as well (SI Appendix, Table S4).
Fig. 3.
Probability of success by disparity of food quantities. Points indicate raw data, while the line represents predicted values based on the model of success by disparity. Both raw data and predicted probabilities are displayed, with the latter shown by individual elephant. Shaded coloration indicates predicted variation in success around each disparity based on the model. Raw data are indicated by symbol; each elephant may be represented more than once at each disparity, as several ratio values shared the same disparity. A jitter of 0.1 has been added to better visualize the data points, but all disparities are whole-number values.
The solid-lid (0 g vs. 24 g) and residual-odor (14 g vs. 14 g) control conditions tested the same ratios across all trials. Thus, these conditions were analyzed separately from the aforementioned models. With the solid-lid condition, we were interested in whether the elephants could find food when olfactory information was obstructed (i.e., odors from the food currently present in the bucket were trapped under a solid lid). With the residual-odor condition, we were interested in whether the elephants could find food when olfactory information was manipulated (i.e., different amounts of residual odor from previously stored food were introduced). The elephants performed significantly better with the ratio 1:6 (the next-largest disparity after 0:6) across the experimental + metal-bucket + double-blind conditions (mean = 12/14, SD = 1.79) than they did in the solid-lid control (Wilcoxon paired-samples test, one-tailed: W = 21, n = 6, P = 0.016). One-tailed tests were used for Wilcoxon tests because of strong a priori predictions that elephants would score significantly better on experimental than control trials, specifically due to the fact that the latter aimed to exclude or control for the olfactory cues that we predicted would promote the elephants’ ability to discriminate quantity. As a group, their mean performance on solid-lid controls of 12.17 out of 24 trials correct (SD = 1.94) approximated chance levels (12 out of 24 trials correct), suggesting the elephants could not find the greater quantity when olfactory information was occluded. When comparing the mean success for each elephant across all of the ratios in the experimental + metal-bucket + double-blind conditions with their success in the residual-odor control, the elephants as a group performed significantly better in the former (W = 19, n = 6, P = 0.031). As a group, their mean performance of 12.5 out of 24 trials correct (SD = 1.76) on residual-odor trials approximated chance levels (12 out of 24 trials correct), suggesting the elephants could not perceive an olfactory difference when the same food quantities were presented within two buckets that had accumulated disparate residual odors.
Our findings show that elephants are able to discriminate between quantities using olfactory information alone. As a group, the elephants’ performance was worse with an increase in ratio value, better with an increase in ratio disparity, and remained consistent when the magnitude of the quantities increased at the same ratio. Thus, using olfaction, the elephants performed similarly to other species tested with vision and appeared to both make approximations about food quantity and recognize the relative difference between two amounts (44–46). Prior research suggests that animals are able to cognitively represent quantity through approximation, and thus recognize the relative difference between two amounts rather than the exact quantity each represents (e.g., refs. 44 and 45). An alternative, yet not necessarily mutually exclusive, model, referred to as “object file,” suggests that individuals can represent small numbers (four or less) as separate memory files and thus differentiate smaller quantities as exact amounts rather than as approximations (47). Interestingly, previous results for elephants were mixed in this regard. Some research suggests that Asian elephants may have a larger object-file capacity (19) or some other unique counting mechanism (21) that may allow for greater precision within quantity judgments. On the other hand, two studies on African elephants (20, 48) suggest that the elephants perform similarly, through approximation, to other animals. Most importantly, Irie-Sugimoto et al. (19) and Irie et al. (21) argue that Asian elephants’ performance on relative quantity judgments do not seem to be affected by disparity, magnitude, or ratio value.
Our results, however, follow the approximation model and thus Weber’s law; the elephants’ performance increased as the disparity increased, but increases in magnitude were not associated with diminished performance. Thus, our research shows that within the olfactory domain, the Asian elephants’ performance is similar to most other species tested in the visual domain. Differences in the results across these studies on Asian and African elephants may be due to discrepancies in methodology or possible species-level differences (19–21, 48), but our research suggests that greater attention to olfaction is nonetheless needed in cognitive tasks across elephant species.
In terms of biological variables that could be associated with success in the experiments, none of the covariates in the models, including age and sex, significantly improved the models’ fit. Sex (male vs. female), however, was significant within both the ratio and disparity models (SI Appendix, Tables S3 and S4). Although it is difficult to interpret these results given the small sample size, this would be a very interesting area for future research with a larger sample size of both males and females. If this sex difference exists in the wild, it may be due to sex-specific nutritional and reproductive differences: Larger-bodied male elephants may have consistently greater energy needs (excluding the high-energy periods of peak lactation and gestation in females) and need to locate estrous females over long distances (7, 49, 50). If these are contributing factors, the potential sex differences in quantity discrimination may either disappear or reverse in social olfactory tasks due to the female-centric nature of elephant social dynamics.
Interestingly, an animal’s ability to count or approximate the quantity of objects in an array likely differs mechanistically between the visual and olfactory domains (51). While an exact count of discrete objects (e.g., sunflower seeds) is possible visually, it seems unlikely that an object’s odor could be “countable” as a discrete unit. Olfaction is also a difficult domain in which to test cognition because, unlike vision, olfactory information cannot easily be removed following its presentation. One additional concern we had in implementing this study was that residual olfactory information could have hindered each elephant’s ability to choose larger quantities across trials. The elephants’ poor performance on the residual-odor condition and nonsignificant difference in performance between the experimental and metal-bucket conditions, however, suggest this was not the case. The results across all four control conditions indicate the elephants (i) were only using olfactory information presented in the buckets to find food (the solid-lid condition), (ii) were not using inadvertent cues provided by the experimenters (the double-blind condition), and (iii) were not affected by olfactory cues other than those resulting from the actual presence of the two different quantities of sunflower seeds (the metal-bucket and the residual-odor conditions). These results, taken together, suggest the elephants were able to discriminate between the two discrete quantities using only the olfactory difference between them.
The primary purpose of this study was to investigate whether or not the elephants could discriminate quantity using smell; one remaining question is how they did it. One potential answer to this question is that the elephants simply smelled the quantity closer to their trunk, as the higher quantities would be slightly higher in the bucket. This is unlikely, given their success on relatively small differences in the presented quantities where the height difference was negligible. In addition, in a post hoc control done with two of the tested elephants in which we raised presented quantities to the same height within the buckets, the elephants still chose the larger quantity significantly above chance (both Bleum and Lanna chose the larger quantity in 19 of 24 trials: binomial test, P = 0.007). Future research would be crucial in understanding the precise mechanisms that elephants and other olfactory animals use to discriminate quantities, and the ecological significance of such an ability. For instance, elephants’ acute sense of smell could help them make important foraging and social decisions from far-enough distances so as to mitigate potential risk in human-dominated landscapes (52). It would also be important to study these effects in wild elephants, as the current study focused on captive animals living in unique environments with access to diverse food resources provided by human caretakers. Although we expect that the capacity for olfactory-based relative quantity judgment would be consistent across individuals within the Asian elephant species regardless of life experience, differences in how this capacity is expressed and how it affects the elephant’s decision-making process would be interesting to compare in wild and captive animals.
As the study of cognition in animals continues to grow as a field, it is becoming increasingly important that experimental designs become more species-specific. Research into the minds of animals should account for differences in sensory perspectives to ensure fair comparisons of cognitive capacity, rather than rely on approaches that are unfairly biased toward the primate-centric, visual perspective. In addition, understanding how elephants use their sense of smell in the cognitive decision-making process could be applied to human–elephant conflict-mitigation strategies in Asia and Africa that must balance the ecological and behavioral needs of both humans and elephants to be successful in the long term (52).
Materials and Methods
Subjects.
We tested six elephants (4 females: Bleum, Lanna, Ploy, and Poonlarb, and 2 males: Pepsi and Phuki), ranging in age from 12 to 45 y old, at the Golden Triangle Asian Elephant Foundation in Chiang Saen, Chiang Rai, Thailand. These elephants live on the property of the Anantara Golden Triangle Elephant Camp and Resort. Each elephant’s mahout (the daily caretaker), full-time staff veterinarians, and senior management provide daily care and ensure proper elephant welfare practice is in place. This research was approved by the National Research Council of Thailand and by the University of Cambridge Zoology Animal Users Committee (Z003/2011).
Apparatus and Materials.
A sliding table was used to extend and retract baited buckets toward and away from the subject. The table, measuring 2.97 × 0.90 m, was fitted with wheels that rolled within grooves on a support frame. The square frame measured 3 m along each side and stood 0.54 m off of the ground (0.67 m with the table). Two cylindrical arms (2.02 m), at the rear of the table, served as push/pull handles (Fig. 1).
A pair of opaque buckets (tapered from a lip diameter of 26 cm to a base diameter of 19 cm) sat within two metal baskets (21.5 cm in diameter) and were bolted together to the top of the table, one at either end (2.46 m apart). Slightly smaller, orange pails were inserted into the opaque buckets (to obscure any visual cues), while smaller containers were nested within the orange pails. For simplicity, we only refer to the buckets and the containers below, as the insertion of the orange pails was consistent throughout the experiment. In the one-quantity conditions, the 4-g pretest and the solid-lid control, two smaller, nested containers always had food and were used in alternating trials as the baited container, whereas a third one never had food. This procedure helped control for residual olfactory cuing: The two containers that were alternatingly used as the baited container would have accumulated equal residual odor from these one-quantity tests and so could later be used simultaneously in two-quantity conditions.
Two concentric rings of pencil-sized holes were burned into the opaque bucket lids. Ten holes made up the inner ring (8-cm diameter), while 16 holes made up the outer ring (13-cm diameter). A curtain (length 4.66 m, height 2.77 m) was rigged up on a pulley system, a distance of 1.24 m to the front of the table frame (positioned between the subject and the table; Movies S1 and S2). In every condition, two experimenters manipulated the table—one positioned at each bucket—while a third individual operated the curtain.
Training and Testing.
In an initial one-quantity 4-g pretest, we aimed to investigate whether subjects could locate the smallest quantity (4 g) of sunflower seeds by olfaction. With the curtain drawn closed, one experimenter deposited 4 g of seeds into the baited container (behind and directly central to the table) and inserted it into one of the buckets. The empty container was inserted into the opposite bucket. The perforated lid of each bucket was secured into place with two cable ties, the curtain was open, and the table was pushed toward the subject. In each trial, the subject was given 10 s to investigate the two buckets (the “investigation phase”). This time frame commenced as soon as the subject used his/her trunk to contact a bucket. The table was then pulled back, the cable ties were cut, and the perforated lids were replaced with solid lids positioned upside down on top of the buckets [subjects were previously trained in earlier experiments to remove the lids from the buckets when the lids were placed upside-down on top of the openings (34, 43)]. The table was again extended, allowing the subject to remove the lid from one bucket to potentially retrieve the reward (the “choice phase”). If the subject selected the baited container, the experimenter located behind the corresponding bucket rewarded the subject further by delivering a handful of sunflower seeds directly to the elephant in proximity to the selected bucket. The table remained extended during this reward period and was withdrawn once the supplementary seeds had been delivered. This measure was taken to ensure the subjects remained motivated throughout a session.
Subjects completed sets of 12 trials (up to four sets) until they reached the criterion of over 80% (i.e., successfully choosing the baited container on at least 10 trials in a set). Trials were pseudorandomized so that each bucket contained the baited container in 6 of the 12 trials, and the baited container was never inserted into the same bucket in more than three consecutive trials.
Solid-lid condition.
The procedure for this one-quantity condition followed that of the 4-g pretest, with three exceptions: (i) the smallest test quantity (4 g) was substituted with the largest test quantity (24 g), (ii) during the investigation phase, olfactory access to the odor of 24 g of seeds was obscured, and (iii) supplementary seeds were not delivered under any circumstances. Both the baited container and empty container were covered with fitted solid lids before being put inside the buckets. The subject’s access to olfactory information about the food reward was further obscured by cable-tying solid lids instead of perforated lids on the buckets. Upon completion of the investigation phase, lids were removed from both of the smaller containers and the solid lids were turned upside down as in the 4-g pretest.
Subjects completed two sets of 12 trials. Subjects (except for Lanna) completed set 1 after reaching the criterion on the 4-g pretest and set 2 soon after completing the experimental condition. Due to experimenter error, Lanna completed both sets after completing the latter condition. Regardless, performance on this control condition should not have been affected by completion of the two-quantity conditions, as it should not be sensitive to any potential order effects.
Two-quantity training.
In this two-quantity condition, subjects were habituated to trials in which both buckets were baited with two different quantities (4 and 32 g). The procedure followed that of the 4-g pretest, with three exceptions: (i) both of the buckets held a baited container, (ii) subjects were required to investigate both buckets, and (iii) supplementary seeds were not delivered under any circumstances. In each trial, 4 g of seeds was deposited inside one baited container and 32 g inside the other baited container. Trials were pseudorandomized so that each bucket contained the greater quantity in half of the total trials and the greater quantity was never inserted into the same bucket in more than three consecutive trials. This ensured the higher and lower quantities were on each side of the elephant in an equal number of trials, and the elephants did not have an opportunity to develop a side bias due to one side having the larger quantity in too many consecutive trials. The quantity 32 g, greater than the largest test quantity (24 g), was used as the larger quantity to avoid giving the subjects unequal exposure to one of the experimental condition ratios (see below), and in an attempt to exaggerate the difference between the quantities presented.
Unlike in one-quantity conditions (i.e., the 4-g pretest and solid-lid control), in two-quantity conditions subjects were required to compare the magnitudes of the odors presented. As such, it was not sufficient for subjects to only investigate one bucket. The same 10-s investigation phase was implemented, but if the subject failed to contact both buckets within the 10-s interval, the table was pulled back for a 15-s delay and subsequently extended for another 10-s interval. This pattern persisted until the subject had contacted both buckets within a given trial, thereby fulfilling the requirements of the investigation phase.
Subjects completed sets of 12 trials until they reached the criterion of over 80% (i.e., they successfully chose the larger, 32-g quantity on at least 10 trials in a set). For five of the six subjects, this criterion was reached by the second set. Poonlarb required eight sets, due to her difficulty adjusting to this initial two-quantity condition and the requirement that she investigate both buckets without direction from the experimenters.
Experimental condition.
Upon reaching the training criterion, subjects proceeded to the magnitude-discrimination tests to investigate whether they could effectively discriminate between pairs of quantities solely by using olfactory information. This two-quantity condition (i.e., both buckets on the table had food) followed the procedure of the two-quantity training, with two exceptions: (i) quantities 1 to 6 (4, 8, 12, 16, 20, and 24 g) were used in place of quantities 1 and 8 (4 and 32 g), and (ii) each set consisted of 11 trials instead of 12.
Each set of 11 trials consisted of one occurrence of each of the possible ratios produced by pairing two of the six quantities. The ratio pairs were as follows: 1:2, 1:3, 1:4, 1:5, 1:6, 2:3, 2:5, 3:4, 3:5, 4:5, and 5:6. The ratios 1:2, 1:3, and 2:3 were generated by multiple quantity pairings. To investigate any effects related to total magnitude (the sum of both quantities presented), half of the subjects were first exposed to smaller quantity pairings for the ratios 1:2, 1:3, and 2:3, while the other half of the subjects were first exposed to larger quantity pairings for these ratios. Therefore, one group (group A) encountered 4 g vs. 12 g, 4 g vs. 8 g, and 8 g vs. 12 g in sets 1 to 5, while the other (group B) encountered 8 g vs. 24 g, 12 g vs. 24 g, and 16 g vs. 24 g in sets 1 to 5. Groups switched for sets 6 to 10. A third combination yielding the 1:2 ratio (8 g vs. 16 g) was not tested. Subjects completed 10 sets of this condition. The 11 ratios were pseudorandomized in each set so that each bucket (i) contained the greater quantity in a particular ratio in half of the trials, and (ii) never contained the greater quantity in more than three consecutive trials.
Metal-bucket condition.
This two-quantity condition investigated the potential effect that the accumulated residual odor within the containers could have had on the elephants’ performance. The procedure followed that of the experimental condition, with the single exception that small metal buckets replaced the plastic containers as depositories for the sunflower seeds. This condition allowed for direct comparison with the data yielded by the experimental condition, to see if residual odor amplified the “true odor” of the quantity of food present to any discernable degree.
Subjects completed two sets of 11 trials after completion of the experimental condition. For group A subjects, the first set included 4 g vs. 12 g, 4 g vs. 8 g, and 8 g vs. 12 g. For group B subjects, the first set included the greater quantity pairings associated with the same three ratios (8 g vs. 24 g, 12 g vs. 24 g, and 16 g vs. 24 g). Groups switched for the second set.
Double-blind condition.
This two-quantity condition investigated the possibility of a “Clever Hans effect” of subjects locating the sunflower seeds by following signals unintentionally given by experimenters. Here again, to allow for a direct comparison of data across conditions, the procedure followed that of the experimental condition, with two exceptions: (i) none of the experimenters operating the table or the curtain were aware of which bucket contained which quantity, and (ii) the metal buckets were again used as seed depositories in place of the plastic containers. The presentation of ratios for groups A and B followed the procedure of the metal-bucket condition.
Residual-odor condition.
In this condition, we further investigated whether residual odors were confounding the true odors associated with the quantities of food present. In each trial, two equal quantities (14 g) were presented in two new plastic containers, identical to those used in previous tests. While the quantity presented in each container was the same, the two containers had previously held different quantities of seeds and thus may have accumulated contrasting residual odors. The residual-odor condition thus probed the effects of the unequal accumulation of residual odor between two containers.
The procedure followed that of the two-quantity conditions completed previously, in that a baited container was inserted within both buckets and the subject was required to investigate both options. Before the first trial in a set, two new containers were baited—one with 4 g of seeds and the other with 24 g. The containers sat for 2 min (the approximate duration of a trial), after which their contents were emptied and 14 g (the midpoint between 4 and 24 g—1:6) was deposited within each. These two containers were presented to the subject while a second pair of new containers was baited with 4 and 24 g, respectively. Between each trial, the two containers, which were set aside to accumulate the residual odor from each of the two quantities, were interchanged with the two containers on the table that each held 14 g of seeds. The same container within each pair consistently accumulated residual odor from 4 and 24 g, respectively, while set aside.
Subjects completed two sets of 12 trials. Four new containers were used for each subject, but not each set. Trials were pseudorandomized such that each bucket contained the baited container with 4-g residual odor in 6 of the 12 trials. Likewise, the baited container with 4-g residual odor was never inserted into the same bucket in more than three consecutive trials.
Supplementary Material
Acknowledgments
We thank the mahouts, staff, and elephants of the Golden Triangle Asian Elephant Foundation, the Anantara Golden Triangle Elephant Camp and Resort, and the Four Seasons Tented Camp in Chiang Rai, Thailand, for their participation and support. We also thank Elsa Loissel for her contribution to data collection and discussion, and the volunteers of the Earthwatch Institute for their assistance. We are grateful to the National Research Council of Thailand for their guidance and support. Finally, we thank three anonymous reviewers and the editor for constructive feedback that helped improve the quality of this manuscript. This study was funded in part by a Newton International Fellowship provided by the Royal Society and British Academy (to J.M.P.), Think Elephants International, the Earthwatch Institute, and the Golden Triangle Asian Elephant Foundation.
Footnotes
Conflict of interest statement: J.M.P. is the founder of Think Elephants International and was head of research for the Golden Triangle Asian Elephant Foundation, two charities that provided partial funding support for this project.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818284116/-/DCSupplemental.
References
- 1.Dehaene S. (1997) The Number Sense (Oxford Univ Press, New York: ). [Google Scholar]
- 2.Lipton JS, Spelke ES (2003) Origins of number sense. Large-number discrimination in human infants. Psychol Sci 14:396–401. [DOI] [PubMed] [Google Scholar]
- 3.van Beest FM, Mysterud A, Loe LE, Milner JM (2010) Forage quantity, quality and depletion as scale-dependent mechanisms driving habitat selection of a large browsing herbivore. J Anim Ecol 79:910–922. [DOI] [PubMed] [Google Scholar]
- 4.Botham MS, Kerfoot CJ, Louca V, Krause K (2005) Predator choice in the field; grouping guppies, Poecilia reticulata, receive more attacks. Behav Ecol Sociobiol 59:181–184. [Google Scholar]
- 5.Kitchen DM, Cheney DL, Seyfarth RM (2004) Factors mediating intergroup encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour 141:197–218. [Google Scholar]
- 6.McComb K, Packer C, Pusey A (1994) Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim Behav 47:379–387. [Google Scholar]
- 7.Moss CJ, Croze H, Lee PC, eds (2011) The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal (Univ Chicago Press, Chicago: ). [Google Scholar]
- 8.Wilson ML, Hauser MD, Wrangham RW (2001) Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzee? Anim Behav 61:1203–1216. [Google Scholar]
- 9.Bonanni R, Natoli E, Cafazzo S, Valsecchi P (2011) Free-ranging dogs assess the quantity of opponents in intergroup conflicts. Anim Cogn 14:103–115. [DOI] [PubMed] [Google Scholar]
- 10.Call J. (2000) Estimating and operating on discrete quantities in orangutans (Pongo pygmaeus). J Comp Psychol 114:136–147. [DOI] [PubMed] [Google Scholar]
- 11.Evans TA, Beran MJ, Harris EH, Rice DF (2009) Quantity judgments of sequentially presented food items by capuchin monkeys (Cebus apella). Anim Cogn 12:97–105. [DOI] [PubMed] [Google Scholar]
- 12.Garland A, Beran MJ, McIntyre J, Low J (2014) Relative quantity judgments between discrete spatial arrays by chimpanzees (Pan troglodytes) and New Zealand robins (Petroica longipes). J Comp Psychol 128:307–317. [DOI] [PubMed] [Google Scholar]
- 13.Abramson JZ, Hernández-Lloreda V, Call J, Colmenares F (2011) Relative quantity judgments in South American sea lions (Otaria flavescens). Anim Cogn 14:695–706. [DOI] [PubMed] [Google Scholar]
- 14.Abramson JZ, Hernández-Lloreda V, Call J, Colmenares F (2013) Relative quantity judgments in the beluga whale (Delphinapterus leucas) and the bottlenose dolphin (Tursiops truncatus). Behav Processes 96:11–19. [DOI] [PubMed] [Google Scholar]
- 15.Emmerton J. (1998) Numerosity differences and effects of stimulus density on pigeons’ discrimination performance. Anim Learn Behav 26:243–256. [Google Scholar]
- 16.Garland A, Low J, Burns KC (2012) Large quantity discrimination by North Island robins (Petroica longipes). Anim Cogn 15:1129–1140. [DOI] [PubMed] [Google Scholar]
- 17.Ward C, Smuts BB (2007) Quantity-based judgments in the domestic dog (Canis lupus familiaris). Anim Cogn 10:71–80. [DOI] [PubMed] [Google Scholar]
- 18.Vonk J, Beran MJ (2012) Bears ‘count’ too: Quantity estimation and comparison in black bears (Ursus americanus). Anim Behav 84:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irie-Sugimoto N, Kobayashi T, Sato T, Hasegawa T (2009) Relative quantity judgment by Asian elephants (Elephas maximus). Anim Cogn 12:193–199. [DOI] [PubMed] [Google Scholar]
- 20.Perdue BM, Talbot CF, Stone AM, Beran MJ (2012) Putting the elephant back in the herd: Elephant relative quantity judgments match those of other species. Anim Cogn 15:955–961. [DOI] [PubMed] [Google Scholar]
- 21.Irie N, Hiraiwa-Hasegawa M, Kutsukake N (2019) Unique numerical competence of Asian elephants on the relative numerosity judgment task. J Ethol 37:111–115. [Google Scholar]
- 22.Carazo P, Font E, Forteza-Behrendt E, Desfilis E (2009) Quantity discrimination in Tenebrio molitor: Evidence of numerosity discrimination in an invertebrate? Anim Cogn 12:463–470. [DOI] [PubMed] [Google Scholar]
- 23.Ferkin MH, Pierce AA, Sealand RO, Delbarco-Trillo J (2005) Meadow voles, Microtus pennsylvanicus, can distinguish more over-marks from fewer over-marks. Anim Cogn 8:182–189. [DOI] [PubMed] [Google Scholar]
- 24.Beran MJ. (2012) Quantity judgments of auditory and visual stimuli by chimpanzees (Pan troglodytes). J Exp Psychol Anim Behav Process 38:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz A, Hecht J, Dedrick A (2013) Smelling more or less: Investigating the olfactory experience of the domestic dog. Learn Motiv 44:207–217. [Google Scholar]
- 26.MacLean EL, et al. (2014) The evolution of self-control. Proc Natl Acad Sci USA 111:E2140–E2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery NJ, Clayton NS (2004) The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science 306:1903–1907. [DOI] [PubMed] [Google Scholar]
- 28.Plotnik JM, Clayton NS (2015) Convergent cognitive evolution across animal taxa: Comparisons of chimpanzees, corvids and elephants. Concepts: New Directions in the Study of Concepts, eds Margolis E, Laurence S (MIT Press, Cambridge, MA: ), pp 29–56. [Google Scholar]
- 29.Campos-Arceiz A, Blake S (2011) Megagardeners of the forest—The role of elephants in seed dispersal. Acta Oecol 37:542–553. [Google Scholar]
- 30.Moss CJ, Poole JH (1983) Relationships and social structure of African elephants. Primate Social Relationships: An Integrated Approach, ed Hinde R. (Blackwell Scientific, Oxford: ), pp 315–325. [Google Scholar]
- 31.Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I (2007) Social dominance, seasonal movements, and spatial segregation in African elephants: A contribution to conservation behavior. Behav Ecol Sociobiol 61:1919–1931. [Google Scholar]
- 32.Poole JH. (1989) Announcing intent: The aggressive state of musth in African elephants. Anim Behav 37:140–152. [Google Scholar]
- 33.Byrne RW, Bates LA, Moss CJ (2009) Elephant cognition in a primate perspective. Comp Cogn Behav Rev 4:1–15.26516391 [Google Scholar]
- 34.Plotnik JM, Shaw RC, Brubaker DL, Tiller LN, Clayton NS (2014) Thinking with their trunks: Elephants use smell but not sound to locate food and exclude nonrewarding alternatives. Anim Behav 88:91–98. [Google Scholar]
- 35.Irie N, Hasegawa T (2009) Elephant psychology: What we know and what we would like to know. Jpn Psychol Res 51:177–181. [Google Scholar]
- 36.McComb K, Reby D, Baker L, Moss C, Sayialel S (2003) Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav 65:317–329. [Google Scholar]
- 37.de Silva S. (2010) Acoustic communication in the Asian elephant, Elephas maximus maximus. Behaviour 147:825–852. [Google Scholar]
- 38.O’Connell-Rodwell CE. (2007) Keeping an “ear” to the ground: Seismic communication in elephants. Physiology (Bethesda) 22:287–294. [DOI] [PubMed] [Google Scholar]
- 39.Bates LA, et al. (2007) Elephants classify human ethnic groups by odor and garment color. Curr Biol 17:1938–1942. [DOI] [PubMed] [Google Scholar]
- 40.Miller AK, et al. (2015) African elephants (Loxodonta africana) can detect TNT using olfaction: Implications for biosensor application. Appl Anim Behav Sci 171:177–183. [Google Scholar]
- 41.Von Dürckheim KEM, et al. (2018) African elephants (Loxodonta africana) display remarkable olfactory acuity in human scent matching to sample performance. Appl Anim Behav Sci 200:123–129. [Google Scholar]
- 42.Schmitt MH, Shuttleworth A, Ward D, Shrader AM (2018) African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim Behav 141:17–27. [Google Scholar]
- 43.Plotnik JM, et al. (2013) Visual cues given by humans are not sufficient for Asian elephants (Elephas maximus) to find hidden food. PLoS One 8:e61174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beran MJ, McIntyre JM, Garland A, Evans TA (2013) What counts for ‘counting’? Chimpanzees, Pan troglodytes, respond appropriately to relevant and irrelevant information in a quantity judgment task. Anim Behav 85:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantlon JF, Platt ML, Brannon EM (2009) Beyond the number domain. Trends Cogn Sci 13:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feigenson L, Carey S, Hauser M (2002) The representations underlying infants’ choice of more: Object files versus analog magnitudes. Psychol Sci 13:150–156. [DOI] [PubMed] [Google Scholar]
- 47.Pylyshyn ZW, Storm RW (1988) Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spat Vis 3:179–197. [DOI] [PubMed] [Google Scholar]
- 48.Irie N. (2012) Numerical cognition of elephants: Relative quantity judgment by an African forest elephant (Loxodonta cyclotis) and an African savannah elephant (Loxodonta africana). Elephants: Ecology, Behavior and Conservation, eds Aranovich M, Dufresne O (Nova Science, New York: ), pp 145–151. [Google Scholar]
- 49.Poole JH. (1987) Rutting behavior in African elephants: The phenomenon of musth. Behaviour 102:283–316. [Google Scholar]
- 50.Poole JH, Moss CJ (1989) Elephant mate searching: Group dynamics and vocal and olfactory communication. Symp Zool Soc Lond 61:111–125. [Google Scholar]
- 51.Firestein S. (2001) How the olfactory system makes sense of scents. Nature 413:211–218. [DOI] [PubMed] [Google Scholar]
- 52.Mumby HS, Plotnik JM (2018) Taking the elephants’ perspective: Remembering elephant behavior, cognition and ecology in human-elephant conflict mitigation. Front Ecol Evol 6:122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.