Abstract
CROMWELL, R. L., J. M. SCOTT, M. DOWNS, P. O. YARBOUGH, S. B. ZANELLO, and L. PLOUTZ-SNYDER. Overview of the NASA 70-day Bed Rest Study. Med. Sci. Sports Exerc., Vol. 50, No. 9, pp. 1909–1919, 2018.
Purpose:
The purpose of this article was to provide an overview of the National Aeronautics and Space Administration (NASA) 70-day Bed Rest Study. The integrated complement of investigations and the standardized bed rest environment that served as the platform for this study complement are described. Outcomes of the studies will not be presented here but will be reported in separate publications.
Methods:
A set of studies running in an integrated fashion along the entire period (pre–, in–, and post–bed rest) and using the same subjects is referred in this article as ‘‘the campaign’’ or ‘‘complement.’’ NASA selected eight individual studies to participate in the 70-d bed rest campaign. These studies were integrated to increase efficiency in the utilization of resources and to share common measures among the investigations. In addition to the individual studies addressing specific aims, a battery of standardized measures was included. Standard measures target a wide range of physiologic systems and represent some of the testing routinely done on astronauts. Bed rest subjects underwent rigorous medical and psychological screening. Standardized conditions included 70 d of bed rest in a 6° head-down tilt position. Subjects_ vital signs, body weight, and fluid intake and output were measured daily. A standardized diet was provided to ensure consistent nutritional intake across subjects. Exercising subjects were prescribed individualized aerobic and resistance training 6 dIwk−1 performed in a horizontal body position. Subjects in the testosterone supplementation countermeasure group received testosterone enanthate injections at 2-wk intervals during bed rest.
Conclusion:
Long-duration head-down tilt bed rest provided a suitable platform for examining physiologic effects of spaceflight and testing countermeasures in a ground-based model. Integrating studies into a complement is an effective way to support multiple investigations while minimizing the number of subjects to answer many research questions.
Keywords: BED REST, DECONDITIONING, SPACEFLIGHT, ADAPTABILITY, EXERCISE, TESTOSTERONE
The effects of weightlessness due to spaceflight are many. Multiple physiologic adaptations occur, which include bone loss, muscle atrophy, cardiovascular changes, and sensorimotor alterations (1–5). These changes could severely affect the ability of astronauts to perform mission-related tasks and increase the risk of injury. Therefore, one of the major goals of the National Aeronautics and Space Administration (NASA) is to protect the health of astronauts by developing countermeasures to mitigate the negative consequences of spaceflight. There are, however, significant challenges to implementing research studies in the spaceflight environment. These challenges include limitations on access to astronaut’s time, experimental control of the research environment, and subject sample size. To compensate for these limited resources, many studies are first completed in ground-based spaceflight analogs. The use of analogs permits more detailed study of physiologic mechanisms and greater development and refinement of countermeasures before testing them in spaceflight.
Bed rest has long been used as a spaceflight analog to simulate physiological changes that occur in response to weightlessness (6–9). In particular, the 6° head-down tilt platform has wide acceptance and was recently standardized for international use (10,11). In 2011, NASA used the 6° head-down tilt bed rest platform to implement the NASA 70-day Bed Rest Study. Studying the response to 70 d of bed rest provides information on what may be occurring physiologically for long-duration astronauts. Investigations included in this bed rest study examined multiple physiological systems over the 70-d bed rest period. The depth and breadth of knowledge resulting from these studies will significantly add to the body of literature on long-duration bed rest as related to spaceflight. Uniquely, three of these investigations also had companion studies on the International Space Station. Future comparisons of bed rest to spaceflight for these studies will provide insights into the validity of the bed rest model for the study spaceflight.
The purpose of this article was to provide an overview of the integrated research complement for the NASA 70-day Bed Rest Study, and to describe the standardized bed rest environment that served as the platform for these studies.Outcomes of the studies will not be presented here but will be reported in separate publications.
METHODS
Study Integration
NASA selected eight investigations addressing different specific aims to include in the 70-day Bed Rest Study. These investigations are outlined in Table 1. The short titles listed in Table 1 will be used to refer to each individual investigation throughout this manuscript. Because of the timing of selections by NASA, Functional Task Test (FTT), iRAT, and Vision investigations started in the spring of 2011. All other investigations except Flywheel followed shortly afterward. These investigations were integrated into a single complement and implemented as one large complement of studies. The Flywheel investigation was added in 2014 as the integrated complement was being completed. The Fly-wheel investigation, however, used control subjects from the integrated complement and is therefore included here. A timeline depicting studies as they entered the integrated complement is provided in Figure 1.
TABLE 1.
Investigator studies participating in the NASA 70-day Bed Rest Study and subjects used in each study.
| Primary Investigator | Institution | Study Title | Short Title | Subject Groups | Key Dependent Measures |
|---|---|---|---|---|---|
| J. Bloomberg | NASA Johnson Space Center, Houston, TX | Physiological Factors Contributing to Post Flight Changes in Functional Performance | Functional Task Test (FTT) | Control Exercise (iRAT) Exercise + Testosterone | Dynamic visual acuity Isometric strength and force control Isotonic power endurance Neuromuscular drive assessments |
| L. Ploutz-Snyder | University of Michigan, Ann Arbor, MI | Integrated Resistance and Aerobic Training Study | iRATS | Control Exercise (iRAT) Exercise + Testosterone | Muscle cross-sectional area Muscle biopsy Isometric strength and force control Isotonic power endurance Neuromuscular drive assessments |
| R. Urban | UTMB, Galveston, TX | Testosterone Supplementation as a Countermeasure against Musculoskeletal Losses during Space Exploration | Testosterone Supplementation | Control Exercise (iRAT) Exercise + Testosterone | Body composition Muscle cross-sectional area and volume Isokinetic muscle performance |
| J. Hunter | Cornell University, Ithaca, NY | Effects of Retronasal Smelling, Variety and Choice on Appetite & Satiety | Retronasal Smelling | Control Exercise (iRAT) | Anterior nasal rhinomanometry Acoustic rhinometry Smell acuity Food acceptability surveys |
| C. Miller | Smart Information Flow Technologies, LLC, Minneapolis, MN | AD ASTRA: Automated Detection of Attitudes and States through Transaction Recordings Analysis | AD ASTRA | Control Exercise(iRAT) Exercise + Testosterone | Psychological dimensions survey Automated analysis of journal entries |
| R. Seidler | University of Michigan, Ann Arbor, MI | Bed Rest as a Spaceflight Analog to Study Neuro-cognitive Changes: Extent, Longevity, and Neural Bases | Neuro-mapping | Control Exercise (iRAT) | Functional connectivity MRI Diffusion tensor imaging |
| G. Vizzeri | UTMB, Galveston, TX | Surveillance of Ocular Parameters and Visual Function in Bed Rest Subjects | Vision | Control Exercise (iRAT) Exercise + Testosterone | Visual acuity Cycloplegic refraction Intraocular pressure Optical coherence tomography |
| L. Ploutz-Snyder | University of Michigan, Ann Arbor, MI | Integrated Resistance and Aerobic Exercise Training with Small Compact Exercise Equipment | Flywheel | Control Exercise (Flywheel) | Muscle cross-sectional area Muscle biopsy Isometric strength and force control Isotonic power endurance Neuromuscular drive assessments |
FIGURE 1.
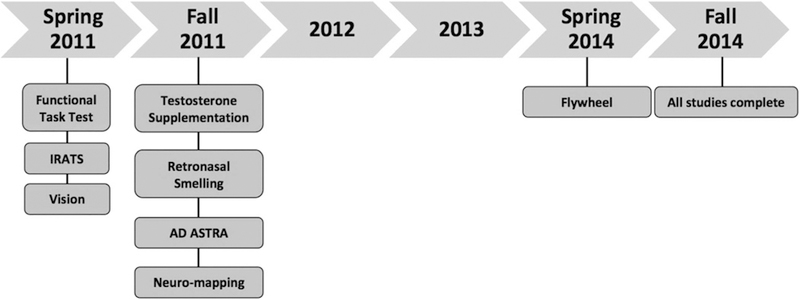
Timeline for studies entering the bed rest integrated complement.
In addition to the individual investigations, a battery of standardized measures was also included in the complement. Standard measures represent some of the testing routinely done on astronauts (7). These measures targeted a wide range of physiologic systems and helped to characterize the bed rest experience. They provide insights into physiological systems not intentionally targeted by countermeasures being studied in the bed rest complement. Data from standard measures were shared by the individual investigations to enhance interpretation of data. Although detailed descriptions of these tests were published previously (10,12–16), a brief description of each test is included in this overview paper. Individual investigations using the standard measures provided detailed methods in their manuscripts.
All of the individual investigations along with the standard measures were integrated as a single complement. Study integration took place over several investigator working group meetings. At these meetings, the required measures, controlled conditions, and testing constraints were determined for each individual study. After consideration of these individual study requirements, a comprehensive testing schedule was developed. This schedule was optimized to reflect the most efficient testing schedule without compromise to any individual study. A summary of the testing schedule is provided in Table 2.
TABLE 2.
Testing schedule summary.
| Testing Daysa |
|||
|---|---|---|---|
| Testing | Pre–Bed Rest | Bed Rest | Post–Bed Rest |
| Standard measures | |||
| Bone densitometry | −11 | 15, 32, 48, 65 | +2, +180, +365 |
| QCT | −3 | +4, +180, +365 | |
| Echocardiography | −6, −2 | 7, 21, 31, 70 | +0, +3, +13 |
| Cardiovascular tilt test | −4 | 70 | +3 |
| Plasma volume | −6, −2 | 7 | +0, +1, +3, +6, +13 |
| Aerobic capacity | −7 | 4, 25, 46, 68 | +0, +11 |
| Vertical jump | −6, −2 | +0, +13 | |
| Isokinetic testing | −11, −5 | +2, +12 | |
| Posture and balance | −12, −6, −2 | +0, +1, +6, +13 | |
| Reflex testing | −10, −4, −1 | 5, 20, 70 | +0, +3, +6 |
| Nutritional assessment | −10, −3 | 7, 14, 21, 28 | +0, +5 |
| Immunologic assessment | −10 | 28 | +0, +5 |
| Investigator measures | |||
| Treadmill locomotion/dynamic visual acuity test | −12, −6, −2 | +0, +1, +6, +13 | |
| Fine motor control test | −12, −6, −2 | +0, +1, +6, +13 | |
| FTT | −12, −6, −2 | +0, +1, +6, +13 | |
| Muscle performance measures | −20, −12, −6, −2 | +0, +1, +6, +13 | |
| Muscle size—MRI | −5 | 3, 7, 11, 15, 22, 29, 36, 53, 69 | +3, +6, +10 |
| Muscle size—ultrasound scanning | −5 | 3, 7, 11, 15, 22, 29, 36, 53, 69, | +3, +6, +10 |
| Muscle biopsy | 3, 57 | ||
| Mood and fatigue questionnaires | −20, −1 | Weekly | +0, +13 |
| Glucose tolerance test | −20, −1 | 37, 66 | +12 |
| Nasal patency | −10, −8, −6, −4, −2, −1 | 21 tests | +0, +1, +2, +4, +7, +10, +12, +180 |
| Odorant test | −10, −8, −6, −4, −2, −1 | 21 tests | +0, +1, +2, +4, +7, +10, +12, +180 |
| Smell acuity tests | −1 | 1, 3, 37, 65 | +2, +12 |
| Food questionnaires | Daily | Daily | Daily |
| Vision exam | −13, −5 | Weekly | +2, +9 |
| Optical coherence tomography and fundus photography | −13, −5 | 38 | +2, +9 |
| Behavioral assessment | −11, −7 | 8,50, 65 | +5, +11 |
| Brain MRI and fMRI | −12, −8 | 8, 50, 67 | +0, +6, +13 |
| Personality test | −21 | ||
| Journal entries | Daily | Daily | Daily |
| Psychological dimensions survey | Daily | Daily | Daily |
| Outgoing debrief | +12 | ||
fMRI, functional MRI.
A negative sign indicates the number of days before bed rest; a positive sign indicates the number of days after bed rest; no sign indicates the number of days in bed rest. Day +0 is the day subjects stood up from bed rest.
A protocol reflecting the integrated study complement was approved by the institutional review boards at the NASA Johnson Space Center and the University of Texas Medical Branch (UTMB). The integrated complement was implemented at the UTMB Institute for Translational Sciences, Clinical Research Center, in a dedicated hospital unit called the NASA Flight Analogs Research Unit.
Participants
Potential subjects were prescreened using an online application, or via telephone interviews by the nursing staff at the NASA Test Subject Screening Facility. Those that met minimum qualifying criteria such as age (24–55 yr), body mass index (18.5–30.0 kg∙m−2), nonsmoker, no prescription medications (including contraceptives), and absence of a medical condition that would preclude participation were invited to NASA Johnson Space Center to complete the screening process. Screening included a modified Air Force Class III physical, psychological examination, drug screening test, criminal background check, and fitness testing. NASA physicians and psychologists conducting examinations had significant experience in selection of bed rest subjects. Those subjects who failed to pass either the drug screening test or criminal background check were excluded.
During the physical examination, subjects reporting a history of thrombosis, thyroid dysfunction, renal stones, gastric ulcers, or metal implants that would interfere with magnetic resonance imaging (MRI) were excluded. Physical examination findings that would preclude study participation such as presence of cardiovascular disease, musculo-skeletal or neurological dysfunction, gastroesophageal reflux, osteopenia, or osteoporosis were also exclusionary.
Exclusion criteria for the psychological examination included a history of mental illness, presence of psychological disorders, or incompatibility with participation in a bed rest study. Factors that could cause a subject to be identified as incompatible include the inability to tolerate 70 d of confinement to bed rest, lack of tolerance for close spaces that would preclude MRI testing, or unwillingness to share a hospital room with another subject.
To ensure that subjects’ fitness levels were similar to the astronaut corps, qualified candidates then performed an upright peak cycle ergometry test and an isokinetic dynamometer test for knee extension strength. For the cycle ergometer test, subjects demonstrated a minimum level of cardiorespiratory fitness where their peak aerobic capacity was greater than 30 mL∙kg−1∙min−1 (17). Assessment of knee extension strength was made with the speed of the isokinetic dynamometer set at 60°∙s−1. The minimum criterion for knee extension strength relative to body mass was >2.0 N∙m∙kg−1 (18).
All subjects provided written informed consent for participation in the screening process. Those subjects who successfully passed screening were then consented for participation in the bed rest integrated complement. Table 3 provides subject demographics for the study participants.
TABLE 3.
Subject demographics and group assignment.
| Subject Group | Number (n) | Age (yr) | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| Controla | 11 | 36.5 ± 6.9 | 173.3 ± 6.6 | 78.5 ± 9.3 |
| Exercise (iRAT) | 10 | 33.9 ± 5.1 | 177.9 ± 4.1 | 76.5 ± 6.1 |
| Exercise + Testosterone | 8 | 32.9 ± 8.8 | 182.4 ± 7.4 | 77.5 ± 12.7 |
| Exercise (Flywheel) | 8 | 28.4 ± 3.7 | 176.7 ± 8.4 | 76.4 ± 13.2 |
| All subjects | 37 | 33.2 ± 7.0 | 177.2 ± 7.4 | 77.3 ± 10.5 |
A single female subject is included in the control group. All other subjects are males. Once the testosterone supplementation study entered the integrated complement, all subjects had to be males.
Because FTT, iRAT, Vision, and standard measures started before the other investigations in the complement, five subjects (one female and four males) completed those investigations before implementing the randomized double-blinded procedure necessary for the testosterone supplementation investigation. The next 24 subjects were randomly assigned to the nonexercising control group, the iRAT exercise group, or the exercise plus testosterone supplementation group. Both research subjects and investigators were blinded regarding the administration of testosterone. The key identifying those subjects who received testosterone and those who did not was held by the Flight Analogs Project scientist. Testosterone status of the subjects was known only to the Flight Analogs Project scientist and the attending physician who had oversight of the complement and subject health care, respectively. Subjects who were not assigned to the testosterone supplementation group received a placebo injection. Once the testosterone supplementation investigation joined the complement, all remaining subjects who entered the complement were males to facilitate comparison between groups for that investigation. Because the Flywheel investigation joined the complement at a later date, subjects were not randomized for this investigation. Instead, volunteers were accepted as they presented and were qualified.
Integration of investigations into a well-structured complement provided efficiencies and research benefits. Efficiencies included utilization of the same control group by all investigations and opportunities for consolidation of outcome measures. Research benefits included the ability for investigators to share outcome measures across investigations to broaden interpretation for each individual investigation. In addition, subject groups that were not originally planned for individual investigations could now be used to answer additional research questions. For example, the FTT investigation was originally planned to use only nonexercising subjects. By adding the iRAT and testosterone supplementation investigations to the complement, the FTT study could also determine the effects of these countermeasures on functional performance. Table 1 provides the subject groups that were used for each individual investigation.
Standardized Conditions
Standardized schedule.
The study consisted of three phases: pre–bed rest phase, bed rest phase, and post–bed rest phase. During the pre–bed rest phase, subjects lived on the unit and were ambulatory. Baseline data collection was completed, and subjects consumed a standardized diet. The pre–bed rest phase was 13 d for control subjects. For exercising subjects, an additional 7 d of pre–bed rest was added so that subjects could become familiarized with the exercise protocol and equipment. During the bed rest phase, subjects were confined to bed for 70 d in a 6° head-down tilt position. Subjects remained in this position for all testing that was completed in this phase. For exercise sessions during bed rest, specialized equipment was used to ensure subjects were maintained in a horizontal, supine, or prone position depending on the type of exercise. During the post–bed rest phase, subjects were ambulatory, and data collections were completed. Subjects were provided individualized reconditioning exercises. These exercises were designed to improve (for control subjects) or maintain (for exercising subjects) muscle strength and endurance and cardiorespiratory fitness and to assist with reorientation to vertical.
Head-down tilt bed rest.
Subjects followed a standard day–night cycle where they were awakened at 0600 h with lights out in the evening at 2200 h. Each morning subjects’ blood pressure, heart rate, respiration rate, body temperature, and body weight were assessed. Body weight during bed rest was measured using a patient-lift bed scale. Fluid intake and urine output were also measured and recorded for each 24-h period. Room temperature and humidity were maintained at 72°F ± 2°F and 70% ± 5%, respectively. All subject symptoms or complaints and medications administered were recorded.
No napping was permitting during waking hours. During bed rest, subjects were allowed to move within the plane of the bed (roll from side to side) but could not raise their heads from the pillow. The only exceptions to the head-down tilt position were during meals and exercise sessions. During meals, subjects were allowed to prop up on their elbow and support their heads for a 30-min period at each meal. This position was permitted as a safety measure to prevent the possibility of choking while eating. During exercise, subjects were maintained in a horizontal position. Toileting and showering were performed in the 6° head-down tilt position. Subjects were transferred to a head-down tilt shower gurney, and showering was completed in a dedicated shower room that accommodated the gurney.
Stretching exercises were performed twice daily during bed rest to maintain joint flexibility. A specialized stretching regimen was performed in the 6° head-down tilt position. Therapeutic massage was also provided every other day while subjects were in bed. Once subjects got up from bed rest, massage was provided daily during the first week.
Subjects’ health and study compliance were monitored 24 h∙d−1. The attending physician rounded daily with each subject and was available for any medical needs. The attending physician managed any medications needed by the subjects. Careful attention was paid to medications that could potentially confound testing. When this was the case, alternate medications were used or the testing schedule was adjusted to minimize any effects of the medications.
The Flight Analogs Research Unit was staffed with research nurses and patient care technicians who provided 24-h monitoring and health care for each subject. Research nurses also completed blood draws and urine collections used for medical monitoring and research purposes. Psychological support was provided once weekly during the study and was available on call when needed.
Subject monitors were stationed in the hallway just outside of subject rooms to monitor subject compliance during waking hours. Overnight, closed circuit, inroom cameras were viewed by a single subject monitor on a video monitor located in the nurses’ station.
Diet.
The standardized diet consisted of a 55% carbohydrate, 30% fat, and 15% protein macronutrient content. Coffee, cocoa, chocolate, tea, or herbal beverages were not permitted. All food was prepared in the metabolic kitchen located on the NASA Flight Analogs Research Unit. A detailed description of the nutrient content of the diet was published previously (19). Nutrient data for all foods were obtained using Nutritionist IV nutrient analysis software (First DataBank Inc., San Bruno, CA). A 10-d menu cycle was used to provide variety in the diet. Meals were served three times per day at approximately 0700, 1200, and 1700 h. The standardized diet was consumed throughout all phases of the study.
Subjects were required to consume all food served to them and ate only foods prepared by the dietary staff. Should any food be leftover, it was weighed and recorded so that actual nutritional intake could be determined. Subjects were required to drink a minimum of 28.5 mL∙kg−1 of body weight of water each day. Once subjects reached their required limit, they could drink water ad libitum for the rest of the day. This water requirement maintained hydration and helped to prevent the possibility of renal stones.
To supplement the 400 IU∙d−1 of vitamin D3 provided by the diet, 800 IU∙d−1 of vitamin D3 was provided throughout the bed rest phase to stabilize vitamin D levels for all subjects. The female subject and males whose ferritin levels fell below 35 ng∙ImL−1 received iron supplementation equivalent to 17.6 mg elemental iron orally each day.
Nutritional energy requirements were calculated using the Harris–Benedict equation, as described previously (19). For these calculations, an activity factor of 1.6 was used to determine energy requirements for the pre– and post–bed rest phases when subjects were ambulatory. During bed rest, an activity factor of 1.3 was used for control subjects to account for the reduced activity associated with bed rest. Exercising subjects maintained the ambulatory diet during bed rest to ensure adequate caloric intake to support the energy requirements of exercise.
Countermeasures
Exercise training.
Subjects assigned to either the iRAT exercise group or the exercise plus testosterone supplementation group underwent the same exercise regimen. The exercise countermeasure consisted of high-intensity interval and continuous aerobic training combined with resistive strength training (16). Subjects exercised 6 d∙wk−1. High-intensity interval aerobic exercise was completed every other day. On alternate days, continuous aerobic exercise was performed along with resistance exercise separated by 4–6 h. The interval aerobic exercise was completed on a vertical treadmill. This specialized treadmill was custom built to allow subjects to remain in the supine position during exercise (Fig. 2). Subjects were loaded at 75% of their body weight via the shoulder harness to maintain contact with the treadmill and perform an accurate running pattern. Continuous aerobic exercise was performed on a supine electronic cycle ergometer (Lode B.V., Groningen, the Netherlands). Resistance exercise was performed using a horizontal leg press (Quantum Fitness, Stafford, TX), prone leg curl (Cybex International, Medway, MA), and a custom-built horizontal squat device (Fig. 3). The horizontal squat device enabled performance of the squat exercise movement pattern in a supine position. For subjects who participated in the Flywheel investigation, the custom-built flywheel device (Fig. 4) was used for both aerobic and resistive exercise.
FIGURE 2.

Specialized vertical treadmill used for interval aerobic training for exercising participants. Subjects were supported by a harness and maintained in a supine, horizontal position during exercise.
FIGURE 3.

Horizontal squat device used for resistance exercise training. This NASA custom-built device enabled performance of a squat weightlifting exercise while maintaining a horizontal position.
FIGURE 4.
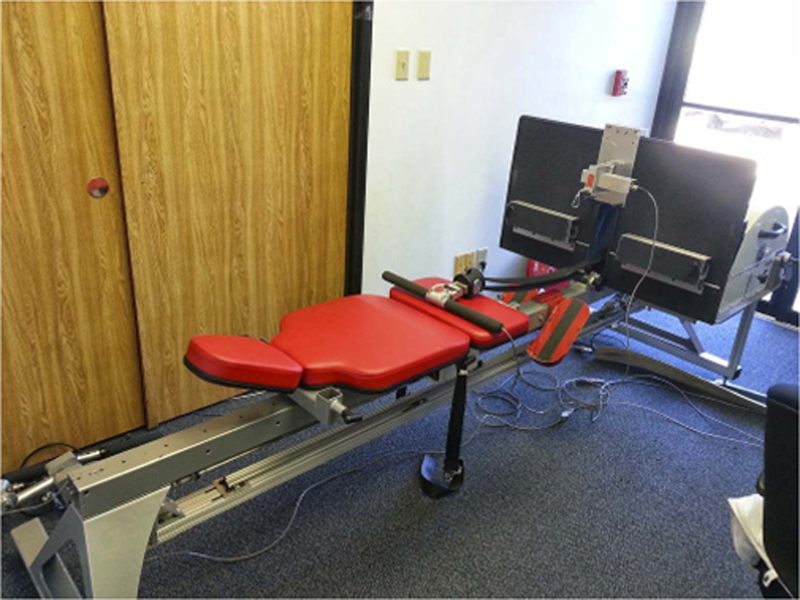
Flywheel exercise device was used for both aerobic and resistance exercise for subjects in the Flywheel study. Subjects were in a seated position for the duration of the flywheel exercise.
The pre–bed rest obtained from the aerobic capacity test was used to determine exercise intensities for the aerobic exercise completed during the bed rest phase. Exercise intensity for continuous aerobic exercise was targeted at 80% of . For the treadmill interval training, one of three interval protocols were performed: 1) 6 × 2-min stages at target intensities of 70%, 80%, 90%, 100%, 90%, and 80% of with a 2-min rest; 2) 8 × 30 s at maximal effort with a 15-s active rest; and 3) 4 × 4 min at a target intensity of 85% with a 3-min active rest. Each interval protocol was performed once per week. Heart rate was continuously monitored during training sessions. Resistance exercise sessions were prescribed using an undulating periodization schedule and consisted of three sets of 6, 8, or 12 repetitions for each of four lifts (supine squat, supine leg press, supine heel raise, and prone leg curl).
Flywheel subjects followed the same exercise prescription; however, modifications were made to accommodate the exercise equipment. All exercise completed on the fly-wheel was done in a seated position. Subjects were returned to the 6° head-down tilt position immediately after exercise. All aerobic exercise was performed using seated rowing. Flywheel resistance training was designed to achieve similar training stimuli as that used for the iRAT study. In particular, performing the squat exercise on the flywheel was not possible. Therefore, the number of sets for each of the other exercises was increased from three to four sets to maintain a similar resistance exercise volume. Flywheel subjects completed four sets of three exercises: heel raise, hamstring curl, and leg press. Subjects were instructed to perform using maximal effort for the number of repetitions as prescribed by the periodization schedule.
Testosterone supplementation.
The exercise plus testosterone supplementation group received a low-dose, intermittent testosterone regimen during the bed rest phase. Testosterone enanthate injections (100 mg∙wk−1, intramuscular) were administered in 2-wk intervals during bed rest. Injections occurred on the day before bed rest and then again on bed rest days 7, 28, 35, 56, and 63. A placebo, saline injection was provided to subjects who were not in the exercise plus testosterone group.
Other study considerations.
One consideration during the planning phase of this complement was the feasibility and tolerability of high-intensity aerobic and resistance training in subjects undergoing long-duration bed rest. To answer this question, a pilot study was conducted implementing the iRAT exercise protocol for subjects undergoing 14 d of bed rest in a horizontal position. Results of that pilot study determined that high-intensity aerobic exercise and resistance training were safe and feasible in healthy, bed rested subjects (16).
A second consideration was linked to an unanticipated medical condition that developed secondary to sweating during exercise. Early in the implementation of the complement, five subjects developed otitis externa. The unique condition of exercising in a horizontal position caused sweat droplets to collect in the ear canal trapping moisture in the ears. This was exacerbated by subjects who wore earbuds to listen to music during exercise. Further, showering after exercise and maintaining the 6- head-down tilt position did not give subjects’ ears adequate opportunity to drain and dry out, creating conditions similar to swimmer’s ear. Subjects who developed otitis externa were medically treated with Cortisporin Otic Suspension and continued in the study. Once the infection cleared, some subjects used swimmer’s ear drops as a drying aid. Precautionary measures to prevent otitis externa were implemented for all subsequent subjects. During intense exercise, subjects wore cotton balls in the ears to absorb excessive sweat. While showering, silicone ear plugs covered by a waterproof dressing were worn. No further cases of otitis externa developed.
Standard Measures
Bone densitometry (dual-energy X-ray absorptiometry).
Dual-energy X-ray absorptiometry (DXA) scans were performed using a GE Lunar iDXA (Lunar Product Division, Madison, WI) whole body densitometer. DXA scans provided a measure of areal bone mineral density (BMD) (g∙cm−1) for the whole body and specific important regions (lumbar spine, left hip and right hip [dual femur], calcaneus [heel], and forearm), as well as information on lean versus soft tissue of the whole body, trunk, and limbs. Testing took place once in pre–bed rest and once in post–bed rest, and scans were performed in triplicate for each region (three sets of each region).
Quantitative computed tomography.
Quantitative computed tomography (QCT) was used to evaluate bone mass and geometry. Scans of the lumbar spine (L1 and L2) and hip regions were performed to measure bone density, to analyze regional changes in bone, and to provide information for femoral volumetric BMD, geometry, and strength. To relate the hip QCT image units to equivalent concentration of calcium hydroxyapatite (and to provide simultaneous calibration), a bone mineral reference standard (four-sample calibration phantom; Image Analysis, Columbia KY) was placed under the participants’ hips and scanned simultaneously. QCT scans were analyzed to determine volumetric BMD, bone mineral content, and bone size. In addition to the bone mineral parameters, strength indices of the hip were estimated with analysis of the minimum femoral neck crosssection and also with finite element analysis.
Echocardiography.
An ultrasound probe was placed on the subject’s chest while lying on their back or left side to determine cardiac chamber size, systolic and diastolic function, blood flow parameters, and wall thickness (13). Hemodynamic assessment was performed with continuous wave, pulsed wave, and color flow Doppler. All four cardiac valves were evaluated for regurgitation and flow characteristics. Three-dimensional data sets were acquired for evaluation of left ventricular volume and mass. From these data sets, calculations of systolic function, including ejection fraction, stroke volume, and left ventricular mass, were performed. To address diastolic function, traditional echocardiographic measurements and indices were used, which included mitral E and A wave velocities and isovolemic relaxation time.
Tilt test.
The tilt test was used to assess orthostatic tolerance before and after bed rest (13). Subjects were instrumented while supine, and this position was maintained while baseline data were collected for 5 min. The table was then tilted to 80° placing subjects in a head-up tilt position at a rate of approximately 7°∙s−1. Subjects remain in this position for 15 min or until they exhibit symptoms of presyncope. With the subject lying on the tilt table, continuous measures of ECG, arterial pressure, and aortic blood flow (systolic velocity integral obtained by Doppler ultrasound [2 MHz probe] of the proximal ascending aorta) were recorded for 5 min. The subject was then tilted upright to 80° for 15 min. Beat-to-beat stroke volume (systolic velocity integral × cross-sectional area) (determined by two-dimensional ultrasound [2–4 MHz phased array probe] at cusp insertion), cardiac output (stroke volume × heart rate), and total peripheral resistance (mean arterial pressure/cardiac output) were calculated offline.
Plasma volume.
Blood (plasma) volume was measured using the carbon monoxide rebreathing technique (13). The subject breathed 100% oxygen on a closed system for 2 min while lying supine. Twenty-eight milliliters of carbon monoxide was then injected into the system and rebreathed for 10 min, after which a blood sample was drawn for analysis of hematocrit, total hemoglobin, and carboxyhemoglobin. After the first blood draw, 32 mL of carbon monoxide were injected into the system and rebreathed for 10 min, after which a second blood sample was drawn. Plasma volume was corrected by body surface area to derive plasma volume index (13).
Aerobic capacity.
Cycle ergometry (Lode Excalibur Sport, Lode B.V.) was used to assess aerobic fitness (16). Subjects warmed up for 3 min at 50 W, followed by 25-W increases each minute. Tests were terminated at volitional fatigue, and peak effort was verified by a plateau in despite a work rate increase, or a heart rate greater than 90% of age-predicted maximum accompanied by a respiratory exchange ratio greater than 1.10. Heart rate and rhythm were continuously monitored during testing (Q-Stress ECG monitor; Quinton Instruments, Seattle, WA). Expired gases were collected continuously and analyzed using a TrueOne metabolic cart (Parvo Medics, Sandy, UT).
Vertical jump test.
Muscle power was measured by vertical jump tests (10). In this testing, the subject performed vertical jumps, without shoes and with hands on the hips, on a ground reaction platform (Kistler Instrument Corp., Amherst, NY), recording height, weight, and force via computer interface. Data were collected with a custom LabVIEW software program (National Instruments Corp., Austin, TX) and sampled at 1000 Hz using a National Instruments data acquisition system (National Instruments Corp.). Attempts were made to obtain three maximal jumps, but subjects were free to perform less, especially after extended bed rest. Body mass, reaction force, and jump height were recorded, and jump power, peak acceleration, and velocity were calculated. Percentage changes from baseline were calculated in relation to the mean of the values obtained in the two pre–bed rest tests.
Isokinetic testing.
Isokinetic testing of the knee and ankle provided an assessment of muscle strength and endurance (16). These assessments were completed using a Biodex System 4 dynamometer (Biodex Medical Systems, Inc., Shirley, NY). The right limb was used for testing. As a warm-up before testing, subjects completed 5 min of light exercise (50 W) on a cycle ergometer. Knee flexion/extension strength was tested at 0°∙s−1, 60°∙s−1, 120°∙s−1, 180°∙s−1, 300°∙s−1, and 400°∙s−1. Endurance testing for knee flexion/ extension was assessed as the total work completed over 20 maximal repetitions at 180°∙s−1. Ankle plantar and dorsiflexion concentric strength were tested at 0°∙s−1, 60°∙s−1, 120°∙s−1, 180°∙s−1, 300°∙s−1, and 400°∙s−1. Eccentric strength was additionally tested at 30°∙s−1. Peak torque obtained at each speed was used for data analyses.
Posture and balance.
Sensorimotor balance control function was tested before and after bed rest using a NeuroCom Equitest Computerized Dynamic Posturography System (Clackamas, OR). During these sessions, the subject stood on a movable force-sensing support surface and within the movable visual enclosure of the EquiTest system. Movements of the support surface and/or visual enclosure under precise computer control were used to modify the sensory conditions and/or to impose unexpected perturbations to standing posture. Tests were performed in sets with eyes open, eyes closed, and head movements to challenge sensory systems while maintaining upright stance. The position of the head and other body segments were monitored using an Optotrak motion analysis system (NDI, Ontario, Canada) and together with the EquiTest system was used to determine the type of strategy the subject used to maintain balance.
Functional neurological assessment: T-reflex.
This test compares the effects of bed rest on the amplitude and latency of the stretch reflex (T-reflex) (14). Subjects lied in the prone position on a device developed by NASA specifically for rapid dorsiflexion of the foot about the ankle joint. The device consisted of a NeuroKinetics Inc. (Pittsburgh, PA) 80 ft. lb. DC servomotor controlled via position feedback. With the left ankle firmly attached to a footplate in a position of −5° of dorsiflexion (selected to preload the tendon stretch), the motor provided a 10- step input at a velocity of 250°∙s−1 in the dorsiflexion direction. The footplate returned to the starting position for a total of at least 20 trials. The subjects were instructed to provide no resistance to the torque, enabling collection of T-reflex data. EMG electrodes (Bagnoli-8 EMG amplifier; Delsys Inc., Boston, MA) with a high impedance probe were placed on the triceps surae (across both the lateral and the medial muscles, and at the interface between gastroc-nemius and soleus muscles) and tibialis anterior of the left leg. EMG data were then analyzed for both latencies and amplitudes.
Clinical nutritional assessment.
Blood samples were collected and processed to yield whole blood, plasma, or serum, depending on the specific analysis. All urine was collected from subjects and aliquoted thereafter. In addition, cellular mineral (magnesium and potassium) content was determined in sublingual epithelial cells using a commercially available kit (EXATEST, IntraCellular Diagnostics, Inc., Medford, OR). A detailed listing of measures used for the nutritional assessment is listed in Table 4.
TABLE 4.
Nutritional assessment battery.
| Sample Type |
||
|---|---|---|
| Nutritional Analysis | Blood | Urine |
| Body mass and composition | ||
| Body weight | ||
| Lean body mass | ||
| Protein status | ||
| Retinol binding protein | X | |
| Transthyretin | X | |
| Serum protein electrophoresis | X | |
| Total protein | X | |
| Album | X | |
| Alpha 1 | X | |
| Alpha 2 | X | |
| Beta | X | |
| Gamma | X | |
| 3-Methylhistidine | X | |
| Calcium/bone status | ||
| 25-Hydroxyvitamin D | X | |
| 1,25-Dihydroxyvitamin D | X | |
| Vitamin D binding protein | X | |
| Intact parathyroid hormone | X | |
| Osteocalcin | X | |
| Calcium | X | X |
| Alkaline phosphatase | X | |
| Ionized calcium | X | |
| Osteoprotegerin/OPG ligand | X | X |
| Urinary N-telopeptide | X | |
| Urinary pyridinoline | X | |
| Urinary deoxypyridinoline | X | |
| Helical peptide | X | |
| C-telopeptide | X | |
| Bone-specific alkaline phosphatase | X | |
| Undercarboxylated osteocalcin | X | |
| Antioxidant status | ||
| Total antioxidant capacity | X | |
| Superoxide dismutase | X | |
| Glutathione peroxidase | X | |
| 8-OH deoxyguanosine | X | |
| Protein carbonyls | X | |
| Glutathione (reduced and oxidized) | X | |
| Heme | X | |
| PGF2-a | X | |
| Malondialdehyde | X | |
| Total lipid peroxides | X | |
| Fat-soluble vitamin status | ||
| Retinol | X | |
| Retinyl palmitate | X | |
| β-Carotene | X | |
| α-Carotene | X | |
| Serum phylloquinone | X | |
| Urinary γ-carboxyglutamic acid | X | |
| α -Tocopherol | X | |
| γ -Tocopherol | X | |
| Tocopherol–lipid rate | X | |
| Water-soluble vitamin status | ||
| Erythrocyte transketolase stimulation | X | |
| Erythrocyte glutathione reductase activity | X | |
| Erythrocyte NAD/NADP | X | |
| Urinary N-methyl nicotinamide | X | |
| Urinary 2-pyridone | X | |
| Erythrocyte transaminase activity | X | |
| Urinary 4-pyridoxic acid | X | |
| Red cell folate | X | |
| Serum folate | X | |
| Vitamin C | X | |
| B6 metabolites (PLP, PL, and PA) | ||
| Homocysteine and related metabolites | ||
| Iron status | ||
| Hemoglobin | X | |
| Hematocrit | X | |
| Mean corpuscular volume | X | |
| Transferrin receptors | X | |
| Transferrin | X | |
| Ferritin | X | |
| Ferritin iron | X | |
| X | ||
| Mineral status | X | |
| Mineral profile | ||
| Iron | X | |
| Zinc | X | X |
| Selenium | X | X |
| Iodine | X | X |
| Ceruloplasmin | X | |
| Phosphorus | X | X |
| Magnesium | X | X |
| General | ||
| AST | X | |
| ALT | X | |
| Sodium | X | |
| Potassium | X | |
| Chloride | X | |
| Cholesterol | X | |
| HDL and LDL | X | |
| Triglyceride | X | |
| Creatinine | X | X |
| Urinary creatinine | ||
| IGF-1 | X | |
| Testosterone (free/total) | X | |
| Cortisol | X | |
| Leptin | X | |
| Cytokines (TNF- α, IL-1β, etc.) | X | |
| Fibrinogen | X | |
| C-reactive protein | X | |
| DHEA/DHEA-s | X | |
| Estradiol | X | |
| Total lipids | X | |
| pH | X | X |
Immune function assessment: general immune status.
A general immune assessment was performed, consisting of white blood cell count and differential analysis, immunophenotype distribution, T-cell function, and intra-cellular cytokine profiles. Regarding immunophenotype, the following peripheral leukocyte populations were assessed: leukocyte differential, lymphocyte subsets, T-cell subsets, T-cell subset memory/naϊve ratio, and levels of peripheral activated T cells. For T-cell function, whole blood cultures were pulsed with mitogenic stimuli, followed by detection of T-cell activation markers. Intracellular cytokine profiles consisting of IFNg and IL-2 expression in T-cell subsets after PMA + ionomycin stimulation in the presence of monensin was also performed.
For characterization of virus-specific T cells, flow cytometric assays (tetramer staining and intracellular cytokine staining) for virus Epstein–Barr virus (EBV)-specific T cells were performed. For latent viral reactivation, standard techniques (immunofluorescence assay) were used on serum/plasma specimens for determining immunoglobulin G/immunoglobulin M antibodies to EBV, viral capsid antigen, early antigen, EBV nuclear antigen, and cytomegalovirus (simultaneous detection of immediately early, early, and late antigens). Measurement of an irrelevant antibody for an acute virus infection (i.e., antimeasle virus) was performed to confirm the specificity of EBV–antibody titer changes. Viral load in blood, saliva, and urine samples was measured using PCR methodology.
Determination of the physiological stress response included assessment of plasma, urinary, and salivary cortisol.
DISCUSSION
The NASA 70-day Bed Rest Study was the most complex and highly integrated bed rest complement conducted by NASA to date. The 6° head-down tilt bed rest platform was used to induce physiological changes similar to spaceflight in an effort to study the effects of these changes on functional performance and test countermeasures to mitigate these changes. The use of the bed rest analog provided for a better controlled study, with a larger sample size than what is typically obtained in spaceflight research. The standardized controlled conditions using standard measures allowed for comparisons across investigations within the complement, and potentially with other NASA bed rest studies that collected the standard measures. Standard measures were also shared by individual investigators to enhance interpretation of their results. Because the standard measures represent testing routinely done on astronauts, these data can also be compared with astronaut data from long-duration spaceflights.
In contrast to the clinical models that use multiple centers to conduct a clinical trial, NASA uses a single center to conduct multiple investigations in an integrated fashion. By integrating investigations, efficiencies of subject use and optimization of measures are found, and the fewest number of subjects is placed at risk to obtain valuable spaceflight-related research data. Further, by integrating investigations, additional research questions can be addressed that were not originally planned for the individual investigations.
Although investigations were integrated in a manner that avoided confounds, data collection could not always be planned for the most preferred time for each test. For example, the day that subjects got out of bed (+0) was a highly preferred testing day. However, not all testing can be accomplished on that day due to time limitations and subject fatigue considerations. Therefore, tests such as bone densitometry, MRI, and vision testing were planned for the second and third day that subjects were out of bed. Optimizing the testing schedule is a difficult process. It is recommended to begin optimization with testing that carries the greatest number of constraints and build the schedule around these tests. Constraints may be factors like dietary or activity restrictions before or after testing, time of day needed for testing, or additional time requirements for transport to other hospital departments for testing.
CONCLUSION
Long-duration head-down tilt bed rest provided an excellent platform for examining physiological effects of spaceflight and testing countermeasures in a ground-based model. Integrating investigations into a complement is an effective way to support multiple investigations and use a minimum number of subjects to answer many research questions.
Acknowledgments
Study infrastructure and operations were supported by the NASA Human Research Program Flight Analogs Project and the UTMB, Institute for Translational Sciences–Clinical Research Center (ITS-CRC) through an NIH/NCATS Center for Translational Science Award (UL1TR000071). Individual research studies were supported by the NASA Human Research Program and the National Space Biomedical Research Institute. The authors wish to thank the research subjects for their commitment to this study. The unwavering support from the physicians, nurses, and staff at the UTMB ITS-CRC was gratefully appreciated. A study of this magnitude could not be completed without the dedicated support for planning and implementation from the NASA Flight Analogs Project.
Footnotes
The authors have no conflict of interest to declare. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Bloomberg JJ, Peters BT, Cohen HS, Mulavara AP. Enhancing astronaut performance using sensorimotor adaptability training. Front Sys Neurosci 2015;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang TF, LeBlanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res 2006;21(8):1224–30. [DOI] [PubMed] [Google Scholar]
- 3.Platts SH, Merz CN, Barr Y, et al. Effects of sex and gender on adaptation to space: cardiovascular alterations. J Womens Health 2014;23(11):950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 2005;93:463–8. [DOI] [PubMed] [Google Scholar]
- 5.Trappe S, Costill D, Gallagher P, et al. Exercise in space: human skeletal muscle after 6 months aboard the international space station. J Appl Physiol 2009;106:1159–68. [DOI] [PubMed] [Google Scholar]
- 6.Belav] DL, Bock O, Börst H, et al. The 2nd Berlin BedRest Study: protocol and implementation. J Musculoskelet Neuronal Interact 2010;10(3):207–19. [PubMed] [Google Scholar]
- 7.Meck JV, Dreyer SA, Warren LE. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med 2009;80(5 Suppl):A1–8. [DOI] [PubMed] [Google Scholar]
- 8.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 2007;101:143–94. [DOI] [PubMed] [Google Scholar]
- 9.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 1968;38:VII1–78. [PubMed] [Google Scholar]
- 10.Sundblad P, Orlov O (editors). Guidelines for Standardization of Bed Rest Studies in the Spaceflight Context International Academy of Astronautics; ISBN/EAN IAA: 9782917761342, 2015. Available from: http://www.nasa.gov/hrp/important_documents. [DOI] [PubMed] [Google Scholar]
- 11.Sundblad P, Orlov O, Angerer O, Larina I, Cromwell R. Standardization of bed rest studies in the spaceflight context. J Appl Physiol 2016;121:348–9. [DOI] [PubMed] [Google Scholar]
- 12.Crucian BE, Stowe RP, Mehta SK, et al. Immune status, latent viral reactivation, and stress during long-duration head-down bed rest. Aviat Space Environ Med 2009;80(5 Suppl):A37–44. [DOI] [PubMed] [Google Scholar]
- 13.Platts SH, Martin DS, Stenger MB, et al. Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med 2009;80(5 Suppl):A29–36. [DOI] [PubMed] [Google Scholar]
- 14.Reschke MF, Bloomberg JJ, Paloski WH, Mulavara AP, Feiveson AH, Harm DL. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviat Space Environ Med 2009;80(5 Suppl):A45–54. [DOI] [PubMed] [Google Scholar]
- 15.Zwart SR, Mathews Oliver SA, Fesperman JV, et al. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 2009;80(5 Suppl): A15–22. [DOI] [PubMed] [Google Scholar]
- 16.Ploutz-Snyder LL, Downs M, Ryder J, et al. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci Sports Exerc 2014;46:358–68. [DOI] [PubMed] [Google Scholar]
- 17.Moore AD, Downs ME, Lee SM, Feiveson AH, Knudsen P, Ploutz-Snyder L. Peak exercise oxygen uptake during and following long-duration spaceflight. J Appl Physiol 2014;117: 231–8. [DOI] [PubMed] [Google Scholar]
- 18.English KL, Lee SM, Loehr JA, Ploutz-Snyder RJ, Ploutz-Snyder LL. Isokinetic strength changes following long-duration spaceflight on the ISS. Aerospace Med Hum Perf; 86(12 Suppl): A68–77. [DOI] [PubMed] [Google Scholar]
- 19.Innis AM, Rice BL, Smith SM. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med 2009;80(5 Suppl): A9–14. [DOI] [PubMed] [Google Scholar]


