Abstract
Purpose
Immune checkpoint inhibitors designed to revert tumor-induced immune suppression have emerged as potent anti-cancer therapies. Tryptophan metabolism represents an immune checkpoint and targeting this pathway’s rate limiting enzyme IDO1 is actively being investigated clinically. Here, we studied the intermediary metabolism of tryptophan metabolism in glioblastoma and evaluated the activity of the IDO1 inhibitor GDC-0919, both alone and in combination with radiation (RT).
Experimental Design
LC/GC-MS and expression profiling was performed for metabolomic and genomic analyses of patient-derived glioma. Immune competent mice were injected orthotopically with genetically engineered murine glioma cells and treated with GDC-0919 alone or combined with RT. Flow-cytometry was performed on isolated tumors to determine immune consequences of individual treatments.
Results
Integrated cross-platform analyses coupling global metabolomic and gene-expression profiling identified aberrant tryptophan metabolism as a metabolic node specific to the mesenchymal and classical subtypes of glioblastoma. GDC-0919 demonstrated potent inhibition of this node and effectively crossed the blood brain barrier. Although GDC-0919 as a single agent did not demonstrate anti-tumor activity, it had a strong potential for enhancing RT response in glioblastoma, which was further augmented with a hypofractionated regimen. RT response in glioblastoma involves immune stimulation, reflected by increases in activated and cytotoxic T-cells, which was balanced by immune checkpoint reactivation, reflected by an increase in IDO1 expression and Tregs. GDC-0919 mitigated RT-induced Tregs and enhanced T-cell activation.
Conclusion
Tryptophan metabolism represents a metabolic node in glioblastoma and combining RT with IDO1 inhibition enhances therapeutic response by mitigating RT-induced immune suppression.
Introduction
Glioblastoma is the most common adult primary brain tumor (1). Despite continued advances in surgery and combined chemoradiotherapy, clinical outcomes remain poor. An overwhelming majority of tumors recur within a year of definitive therapy and a very small percentage of patients survive beyond three years of diagnosis. Unfortunately, concerted efforts to improve clinical outcomes, including integration of molecularly targeted agents, angiogenesis inhibitors, and vaccines to standard therapy, have all failed. Therefore, developing innovative treatment strategies to improve outcome in glioblastoma is of clear importance (2).
Recent clinical advancements using immune checkpoint inhibitors designed to target tumor-mediated immune tolerance have revolutionized our approach to cancer therapy and offers strong promise in glioblastoma. Immune tolerance is important in normal physiology to prevent over-reactivity of stimulated immune responses to external pathogens and other stimuli. Dysfunction of these immune response “brakes” may lead to a variety of disorders, including autoimmune diseases, type 1 diabetes, inflammatory bowel disease, asthma and allergies (3). Tumors have co-opted multiple mechanisms to activate these “brakes” by inducing immune suppressive signaling pathways, creating a tolerogenic microenvironment allowing evasion of the host immune response despite the presence of recognizable antigens. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1), which negatively regulate T-cell activation, represent two specific immune checkpoints that have received recent attention, with inhibitors targeting these immune pathways demonstrating unprecedented clinical activity in a variety of solid tumors (4,5).
The central nervous system (CNS) has been previously considered an immune privileged site, however, growing data support a dynamic interaction between the CNS and the systemic immune system. Although the CNS lacks a traditional lymphatic system, recent findings identified a rich lymphatic network in the dura mater that is able to absorb and transport CSF into deep cervical lymph nodes where CNS antigens have been reported (6,7). Further, glioblastomas produce an array of chemokines such as IL-8, CCL2, CXCL12 that are able to recruit immunosuppressive tumor associated macrophages (TAMs) and myeloid derived suppressive cells (MDSCs), furthering the tolerogenic tumor microenvironment (8,9). This paradigm shift has contributed towards a growing interest in the evaluation of immunotherapeutic approaches in glioblastoma, although early studies have demonstrated limited clinical activity when used alone or in combination with bevacizumab (10).
Emerging studies have identified multifaceted strategies by which alterations in tumor metabolism may also contribute to a potent tolerogenic immune environment, thereby representing a line of next-generation immune checkpoints (11,12). One of the most clinically advanced with particular relevance to glioblastoma is tryptophan metabolism, whose most notable physiologic role has been attributed to peripheral immune tolerance and fetal protection from maternal immune rejection in the placenta (13). Tryptophan is metabolized to kynurenine by the rate limiting enzymes indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO) (14,15) contributing towards an immune tolerant environment at many levels. Increased metabolism of tryptophan results in depletion of this critical metabolite in the microenvironment, resulting in cell cycle arrest and/or anergy in effector T cells, while simultaneously fostering maturation and activation of regulatory T cells (Tregs) (16). In addition to this passive consequence of tryptophan metabolism, the resulting kynurenine is exported from the cell into the microenvironment, where it has the capacity to balance regulatory and effector responses of the immune system. This includes binding and activation of aryl hydrocarbon receptors (AHRs), a cytoplasmic transcription factor, resulting in reduced proliferation and infiltration of effector T cells (14). Kynurenine-activated AHRs are also responsible for providing a tolerogenic phenotype in dendritic cells (DCs), resulting in increased production of Tregs and reduced type 1 T helper (Th1) cells (17,18). Activation of AHRs also results in the induction of various cytokines such as TGF-β, IL-6, and IL-1β, that are responsible for converting CD4+ T cells to inducible T regs (iTregs) and maintaining the suppressive ability of myeloid-derived suppressor cells (MDSCs) (14,19,20).
Interestingly, a variety of tumors, including glioblastoma, have evolved mechanisms to co-opt this potent mode of immune tolerance to evade the host immune system (21). This relationship between cancer and elevated tryptophan catabolism was first recognized in the 1950s with the identification of its metabolic intermediaries in the urine of cancer patients (22,23). The IDO1 pathway was later proposed to play a more direct role in tumor immune evasion, demonstrating a more robust T-cell response and delayed growth in vivo following pathway inhibition (24). Several recent studies have demonstrated that tryptophan catabolism, through IDO1 and/or TDO upregulation, is particularly relevant in glioblastoma. TDO-mediated pathway activation in a panel of glioma cell lines resulted in inhibition of T-cell proliferation and modulating this pathway influenced tumor growth (14). Wainwright et al. demonstrated significant IDO1 expression in glioblastoma that promoted an immunosuppressive environment through recruitment of Tregs (15) and went on to show that the therapeutic efficacy of IDO1 inhibition can be significantly enhanced when combined with other immune checkpoint inhibitors (25).
The majority of studies evaluating the immune consequences of aberrant tryptophan metabolism have largely focused on expression of its rate limiting enzymes rather than the individual metabolites involved in immune suppression. In this study, using global metabolomic profiling as a framework, we explore this immune modulatory pathway from a metabolic perspective, further validating its relevance in glioblastoma, and evaluate the anti-tumor potential of the potent IDO1 inhibitor GDC-0919 in a novel, immune competent preclinical model of adult glioblastoma.
Methods
Study approval
All mice were housed in the AAALAC-accredited Animal facility located at William Beaumont Research Institute. All animal studies were carried out under protocols approved by the Institutional Animal Care and Use Committee at William Beaumont Research Institute. Patient-derived tumors were obtained from the H. Lee Moffitt Cancer Center, following Institutional Review Board and Human Subjects approval received from the H Lee Moffitt Cancer Center Tissue Core Facility as previously described (26).
Human tumor samples and metabolomic profiling
All tumors used in this analysis were newly diagnosed. Tumors were fresh-frozen and their integrity and histology confirmed by a staff pathologist before aliquoting samples (26). Metabolomic studies were conducted at Metabolon Inc. (Morrisville, NC) using methods previously described (26). For box plots, raw area count was normalized and rescaled to set the median equal to one, followed by inputting minimum to missing values. Rescaled data was used to calculate fold change by comparing GBM to LGG.
Microarray and Database Analysis
For details, see Supplementary Methods
Cells culture and reagents
Human glioblastoma cell lines U87, T98G, (obtained from ATCC, Manassas, VA), and U251 (obtained from CLS Cell Lines Service GmbH, Eppelheim, Germany) were cultured in Minimum Essential Media (MEM). Human glioblastoma neural stem cells (GNS) MES326 and PN19 were generated and cultured in DMEM-F12 media with Glutamax, heparin and B27 supplement as previously described (27). The genetically engineered murine GBM cell line TRP was cultured in MEM (28–30). Primary cell culture of mouse splenocyte (T cells) and murine GBM tumor lines GL261 (obtained from NCI DTP, DCTD tumor repository, Frederick, MD) was performed in RPMI-1640. All cell culture reagents were purchased from GIBCO/Thermo (Grand Island, NY) and Corning Life Sciences (New York, NY). Tryptophan, kynurenine, p-dimethylaminobenzaldehyde, glacial acetic acid, TCA and IDO1 inhibitors 1-L-Methyl Tryptophan (1-L-MT) were purchased from Sigma Aldrich (St. Louis, MO). GDC-0919 was provided by Genentech (San Francisco, CA).
Western Blot, Flow cytometry, Magnetic sorting and ELISA
For details, see Supplementary Methods
Metabolite Extraction and Targeted Liquid Chromatography Mass Spectrometry
For details, see Supplementary Methods
Immune competent tumor models
C57BL/6 (H-2b, CD45.2) mice were exposed to 4 Gy total body irradiation (TBI) one-day prior to implant to help tumor engraftment. Mice were injected with 2×105 TRP (murine GBM) tumor cells in the brain for intracranial tumor model or 2 × 106 TRP tumor cells in right flank for subcutaneous tumor model. MRI was performed on 6–8 days post tumor implant. After verification of tumors, mice were randomized into described treatment arms. Detailed procedure is provided in supplementary materials and methods.
T cell suppression assay
For details, see Supplementary Methods
Kynurenine estimation
For details, see Supplementary Methods
Statistical analysis
Comparisons across two different groups were performed on original data using 2 tailed student’s t test. A log rank test was used for survival analyses. Comparisons between tumor volumes in the subcutaneous tumor model was performed using 2 way ANOVA in combination with post-hoc operations to generate p values between RT and RT+GDC-0919. ROC curve were constructed using metabolomics data for kynurenine and kynurenine/kynurenine acid ratios. Highest concentrations of kynurenine and kynurenine/kynurenine acid ratios in LGG were used as thresholds for predicting glioblastoma from LGG. All statistical analyses were performed using Origin Pro 2016 software (Origin Lab Corporation; Northampton, MA).
Results
Metabolomic profiling identifies aberrant tryptophan metabolism in glioblastoma
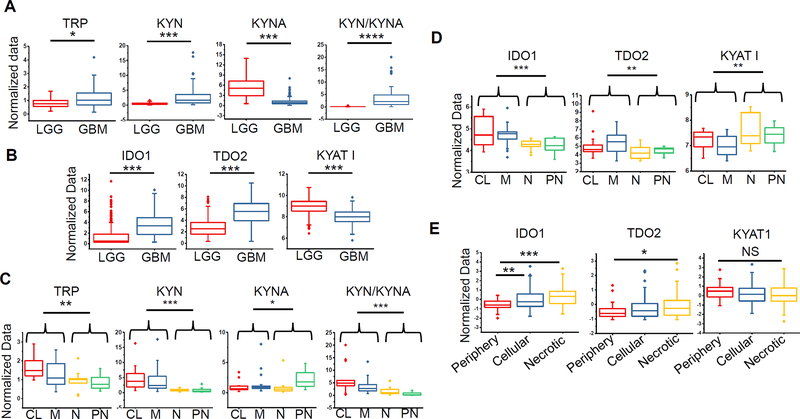
Metabolomic profiling was performed in glioma using a robust library of standards enriched with intermediates of tryptophan metabolism, comparing patient-derived glioblastoma (n=80) to low-grade glioma (LGG; n=28). Tryptophan levels were modestly elevated in glioblastoma (1.44-fold, p≤0.011; Fig. 1A). Tryptophan has several metabolic fates independent of the IDO1/TDO2 pathway, including serving as a mediator for the formation of serotonin and indoles (Supplementary Fig. 1A). Global metabolomic profiling identified several indoles, which may serve as both catabolic byproducts and anabolic precursors of tryptophan, elevated in glioblastoma (Supplementary Fig. 1B). Of the identified metabolites associated with tryptophan metabolism, kynurenine demonstrated the highest accumulation in glioblastoma when compared to LGG (6.07-fold, p≤0.0002; Fig. 1A). Interestingly, kynurenate, a metabolite immediately downstream of kynurenine, was significantly lower in glioblastoma when compared to LGG (0.23-fold, p≤0.0004) and the resulting kynurenine/kynurenate ratio demonstrated the highest degree of sensitivity (91.1%) and specificity (96.5%) in discriminating between glioblastoma and LGG in comparison to using kynurenine alone (sensitivity 60.8%; specificity 91.6%) (p<.00001; Fig. 1A and Supplementary Fig. 1C). Using the TCGA database, we then sought to determine if aberrant expression of enzymes involved with tryptophan metabolism recapitulated these metabolic findings in glioma. Both IDO1 and TDO2 were elevated in glioblastoma when compared to LGG and consistent with the observed accumulation of kynurenate in LGG, we identified increased expression of its biosynthetic enzyme kynurenine aminotransferase I (KYAT1) in these tumors when compared to glioblastoma (Fig. 1B).
Figure 1. Aberrant tryptophan metabolism represents a metabolic node in glioblastoma.
(A) Metabolomic profiling performed on patient-derived low grade glioma (LGG; n=28) and glioblastoma (n=80) demonstrates differential accumulation of tryptophan (TRP), kynurenine (KYN), kynurenate (KYNA) and KYN/KYNA ratio. (B) Expression analysis of enzymes involved in tryptophan metabolism in LGG and glioblastoma was performed using The Cancer Genome Atlas (TCGA). (C/D) Cross platform analysis was performed coupling metabolomic profiles with gene expression arrays in glioblastoma (n=56). The relative accumulation of intermediaries of tryptophan metabolism (C) and enzymes driving tryptophan metabolism (D) were evaluated in the context of the tumors’ molecular subtype, classified as classical (CL), mesenchymal (M), neural (N) or proneural (PN). (E) Expression of enzymes involved in tryptophan metabolism in the context of their anatomic structure derived from the Ivy GAP database. Regions were classified as periphery (leading edge and infiltrating tumor), cellular tumor, and necrotic core (perinecrotic and pseudopalisading necrosis). ****=p<0.00005, ***= p<0.0005; **= p<0.005; *= p<0.05.
Tryptophan metabolism is linked to molecular subtypes of glioblastoma
We next sought to determine if aberrant tryptophan metabolism was unique to a specific glioblastoma molecular subtype. Transcriptional profiling has revealed considerable intertumoral heterogeneity in glioblastoma (31). These include the proneural and mesenchymal subtypes, which have been identified most consistently in glioblastoma. Their transcriptional profiles are mutually exclusive and can be applied to approximately one half of all tumors. The proneural subtype is characterized by mutations in isocitrate dehydrogenase 1 (IDH1), frequent alterations in expression of p53 and platelet derived growth factor receptor, alpha polypeptide (PDGFR-α), and a transcriptional signature typically present in LGG. In contrast mesenchymal glioblastoma is characterized by mesenchymal gene expression, including CD44 and chitinase 3-like 1 (YKL40) and attributed to more aggressive disease. Other subtypes include classical and neural, which are characterized by epidermal growth factor receptor and the expression of neuronal markers, respectively. Of the 80 glioblastoma specimens metabolomically profiled, 56 had paired tissue available for transcriptional profiling, allowing for cross-platform analysis. Following molecular subtyping using methods previously described (31) we demonstrated that tryptophan and kynurenine accumulation was specific to classical and mesenchymal subtypes, while kynurenate accumulation, the metabolite elevated in LGG, was only evident in the proneural subtype. The kynurenine/kynurenate ratio was able to discriminate between individual molecular subtypes, with highest ratios present in the classical and mesenchymal subtypes when compared to neural and proneural tumors (Fig. 1C). The expression levels of enzymes involved in tryptophan metabolism corroborated these metabolic findings, with higher levels of IDO1 and TDO2 in classical and mesenchymal subtypes, while neural and proneural subtypes demonstrated elevated expression of KYAT1 (Fig. 1D). Using a unique data repository generated by the Ivy GAP, which used laser-capture microdissection to isolate RNA from different structural regions of individual tumors that was subsequently molecularly profiled using RNA-seq, we recently uncovered a relationship between subtype heterogeneity in glioblastoma and its unique tumor microenvironment. In this study, we demonstrated that cells obtained from the infiltrating, leading edge of a tumor nearly exclusively harbored a proneural signature, while the mesenchymal subtype was observed in perinecrotic regions within an individual tumor (32). Based on these findings, we sought to determine if aberrant tryptophan metabolism was unique in specific regions within an individual tumor. Consistent with our previous findings, increased IDO1/TDO2 expression was present in the necrotic and central core of tumor when compared to the periphery, which corresponds to the mesenchymal/classical and proneural molecular subtypes, respectively (Fig. 1E).
The IDO1 pathway is activated in glioblastoma cell lines
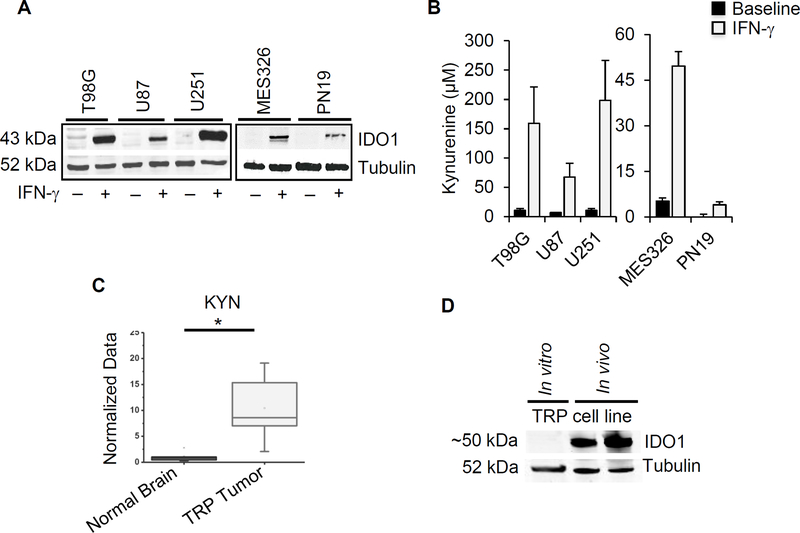
As cross-platform analysis coupling metabolomics with transcriptional profiling identified aberrant tryptophan metabolism as a metabolic phenotype in glioblastoma, we extended investigations to preclinical models. IDO1, which is typically undetectable in cells at baseline, is rapidly induced by pro-inflammatory stimuli. Due to the multiple interferon-stimulated response elements in the IDO1 promoter region, in vitro treatment of cultured cells with the T effector cells and pro-inflammatory cytokine interferon gamma (IFN-γ) leads to robust expression of this enzyme (33). Accordingly, baseline expression of IDO1 was low/undetectable in established human glioblastoma cell lines and robustly expressed following treatment with IFN-γ (Fig. 2A). Similar findings were observed when extended to novel, molecular subtype specific glioblastoma neural stem cells (GNS), with more robust expression in the mesenchymal GNS when compared to proneural, which is consistent with our findings from patient-derived tumors. To determine if IFN-γ induced expression of IDO1 corresponded to increased tryptophan metabolism, kynurenine levels were evaluated. Consistent with increased IDO1 expression, IFN-γ induced accumulation of kynurenine in the media of glioblastoma cell lines grown in culture, confirming pathway activation (Fig. 2B). TDO2, on the other hand, was inconsistently expressed in cell lines and interestingly, appeared to be down-regulated by IFN-γ in several of the lines tested (Supplementary Fig. 2A).
Figure 2. The IDO1 pathway is activated in glioblastoma cell lines.
(A) Human glioblastoma cell lines (T98G, U87, U251, MES326 and PN19) were cultured in the presence or absence of 50 ng/ml of human IFN-γ for three days and evaluated by western blot. (B) Human cell lines were cultured in 250 μM tryptophan and presence or absence of 50 ng/ml of IFN-γ. Media supernatant was collected after three days for kynurenine estimation using Ehrlich’s method. Data represent mean ± SD. (C) Orthotopic TRP tumors grown in C57BL/6 mice were analyzed for kynurenine using LC/MS and compared to normal murine brain. (D) IDO1 expression was analyzed in TRP tumors isolated from C57BL/6 mice using western blot. Results are representative of at least 3 independent experiments. *= p<0.05
To begin to explore the immune consequence of aberrant tryptophan metabolism in glioblastoma, we extended investigations to an adult astrocytic, genetically-engineered mouse (GEM) cell line to allow for in vivo studies using an immune competent model. Specifically, primary astrocyte lines were generated from a series of conditional GEM in which core glioblastoma pathways were genetically targeted (30). Briefly, after Cre-mediated recombination in vitro, lines express (1) a truncation mutant of SV40 large T antigen (T) from the human Gfap promoter that inactivates all 3 Rb family proteins, (2) a constitutively active KrasG12D mutant (R), and/or (3) a homozygous Pten deletion (P). As an initial investigation, we performed metabolomic profiling of TRP cells grown orthotopically, which harbor all 3 of these mutations and displays the most aggressive phenotype (30), which confirmed pathway activation with high levels of kynurenine when compared to normal brain (Fig 2C). Although we were unable to induce IDO1 expression and kynurenine production in the TRP line and the mouse glioblastoma cell line GL261 in vitro using a variety of methods, including both human and mouse IFNγ (Supplementary Fig. 2B), expression was clearly evident in tumors grown in vivo (Fig. 2D).
GDC-0919 demonstrates potent inhibition of tryptophan metabolism in glioblastoma and achieves biologically relevant concentrations in the brain
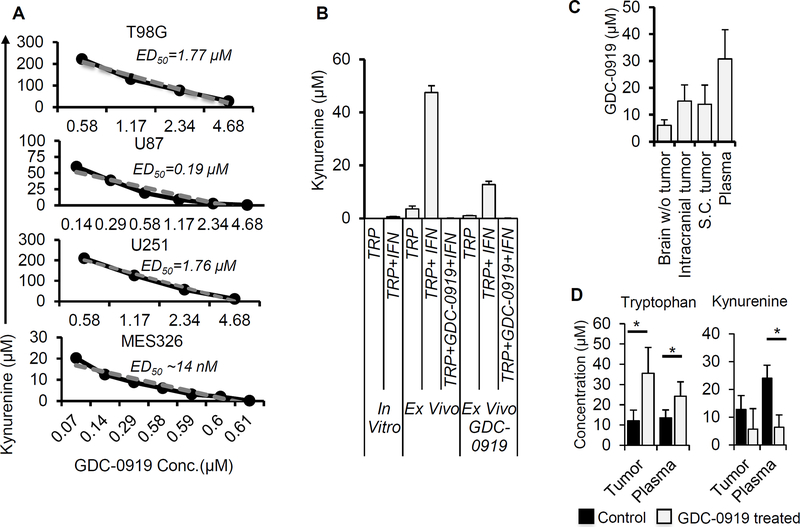
As we established aberrant tryptophan metabolism in patient-derived glioblastoma, which was recapitulated in preclinical models, we next sought to determine the potential for molecularly targeting this immune modulatory pathway using the potent and direct small molecule IDO1 inhibitor GDC-0919 (navoximod), which is actively being investigated in clinical trials (34). As an initial investigation, we evaluated levels of kynurenine following graded concentrations of GDC-0919 in our human glioblastoma cell lines in vitro. GDC-0919 demonstrated potent inhibition of kynurenine production, with median effective dose (ED50) concentrations ranging from 7 nM - 1.77 μM and near complete inhibition at 5 μM (Fig. 3A). As we were unable to induce IDO1 expression in vitro in our mouse glioblastoma models, we implemented an ex vivo approach to evaluate the potential of GDC-0919 to inhibit kynurenine production in the TRP line. In these studies, TRP tumors grown subcutaneously in C57BL/6 mice were excised and disaggregated in cell culture media using DNase I and collagenase IV. Dissociated tumors were then passed through a 70μM cell strainer to obtain a uniform cell suspension. The cell suspension was treated with IFN-γ and supernatant was collected after 24 hours for kynurenine estimation. Using this approach, we demonstrated that TRP cells endogenously produced kynurenine ex vivo, which was significantly increased with IFN-γ. Kynurenine production was completely inhibited in TRP ex vivo by GDC-0919 (5 μM). Interestingly, in a parallel study where mice were treated with GDC-0919 (200 mg/kg, BID for 3 days) in vivo prior to tumor excision, TRP cells demonstrated diminished production of both baseline and IFN-γ-induced kynurenine (Fig. 3B).
Figure 3. GDC-0919 demonstrates potent inhibition of tryptophan metabolism in glioblastoma and achieves biologically relevant concentrations in the brain.
(A) Human GBM cell lines (T98G, U87, U251 and MES326) were cultured with human IFN-γ (50 ng/ml) and of tryptophan (250 μM) and graded concentrations of the IDO1 inhibitor GDC-0919. The supernatant was collected after three days and evaluated for kynurenine. Data was plotted and used to calculate ED50 values for individual cell lines. (B) Murine TRP tumors (in vitro and ex vivo tumors isolated from C57BL/6 mice and C57BL/6 mice treated with GDC-0919 (200 mg/kg twice a day for 3 days given orally) were cultured in the presence and absence of mouse IFN-γ (100 ng/ml) and tryptophan (250 μM). The supernatant was collected after three days and analyzed for kynurenine. (C/D) C57BL/6 mice with subcutaneous or intracranial TRP tumors were treated with GDC-0919 (200 mg/kg twice a day for 3 days given orally). Tissue and plasma were isolated after three days of treatment and analyzed for (C) GDC-0919 and (D) tryptophan and kynurenine (in intracranial tumors) using LC/MS. Results obtained in mg/kg were converted to μM by assuming mg/ml ratio to be 1. Data represent mean ± SD. *= p<0.05. A minimum of 4 tissue samples were used for each experiment.
Next, we performed pharmacokinetic/pharmacodynamic (PK/PD) studies to determine the potential of GDC-0919 to cross the blood brain barrier (BBB) and achieve biologically relevant concentrations in the brain. Using LC-MS, we quantified GDC-0919 levels in normal brain (with no tumor implanted), intracranial tumors, subcutaneous tumors and plasma of mice following treatment with GDC-0919 (200 mg/kg BID) for 3 days, with tissue being extracted 2 hours after the last dose. Importantly, GDC-0919 achieved biologically relevant concentrations in intracranial tumors (~15 μM), which was comparable to levels achieved in subcutaneous tumors. Further, GDC-0919 achieved biologically relevant concentrations in the normal brain (~5 μM), which is particularly important in glioblastoma, based on the infiltrative nature of this malignancy (Fig. 3C). In addition, tryptophan and kynurenine levels were quantified in both intracranial tumors and plasma from mice treated with the above-described schedule, confirming drug activity with increased levels of tryptophan and decreased levels of kynurenine following GDC-0919 treatment (Fig. 3D).
GDC-0919 enhances radiation response in glioblastoma
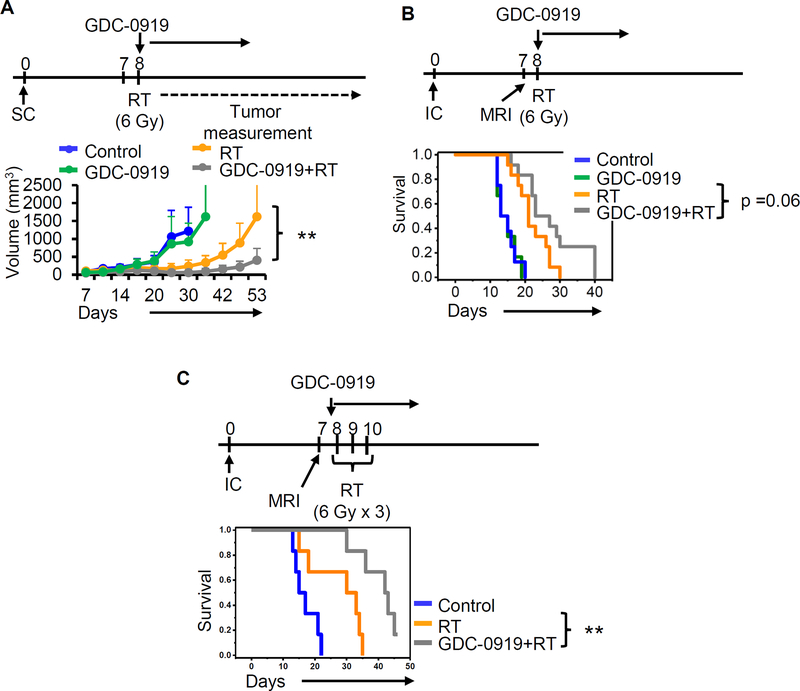
Next, we determined the anti-tumor potential of IDO1 inhibition using GDC-0919 in glioblastoma. As an initial investigation, we evaluated for growth delay in TRP cells grown in a subcutaneous xenograft model. As a single agent, GDC-0919 did not demonstrate anti-tumor activity (Supplementary Fig. 3A). These findings were recapitulated using a first generation IDO1 inhibitor 1-L-MT (Supplementary Fig. 3B). As radiation therapy (RT) is a standard treatment in glioblastoma and emerging data has identified a potential for synergy when combining RT with immune checkpoint blockade, we went on to evaluate the potential of GDC-0919 to enhance RT response. Continuing with the subcutaneous TRP model, GDC-0919 alone again demonstrated no anti-tumor activity, however when combined with RT (6 Gy x 1), demonstrated a significant growth delay when compared to RT alone (p<0.005; Fig. 4A). Next, to allow for translating these findings clinically, we extended studies to an orthotopic, intracranial TRP tumor model. MRI was performed using our small animal-imaging platform prior to initiating therapy to confirm both presence of intracranial tumors and equal volumes between treatment arms (Supplementary Fig. 4A/B). Similar to results in the subcutaneous model, IDO1 inhibition using GDC-0919 alone did not demonstrate anti-tumor activity, however, a trend towards improved survival when combined with RT (6 Gy x 1; p=0.06; Fig. 4B) was evident. As fractionation has been described to further potentiate the synergy between RT and immune checkpoint agents, we extended studies using a hypofractionated regimen (6 Gy x 3). As demonstrated in Fig. 4C, IDO1 inhibition demonstrated potent anti-tumor activity when combined with fractionated RT, with approximately one third of mice demonstrating complete regression of tumors confirmed by MRI (Supplementary Fig. 4C) and increased survival when compared to fractionated RT alone (p<0.005).
Figure 4. GDC-0919 enhances radiation response in glioblastoma.
TRP tumors were grown (A) subcutaneously (SC) or (B) & (C) intracranially (IC) in immune competent C57BL/6 mice. MRI was obtained to confirm the establishment of IC tumors prior to the initiation of therapy. Following establishment of tumors, mice were randomized to treatment as described in schema. GDC-0919 was given orally at a concentration of 200 mg/kg twice a day (six days a week) for 2–3 weeks. Each treatment arm consisted of a minimum of 6 mice. ** = p<0.005.
Tryptophan metabolism contributes to radiation-induced immune checkpoint reactivation in glioblastoma
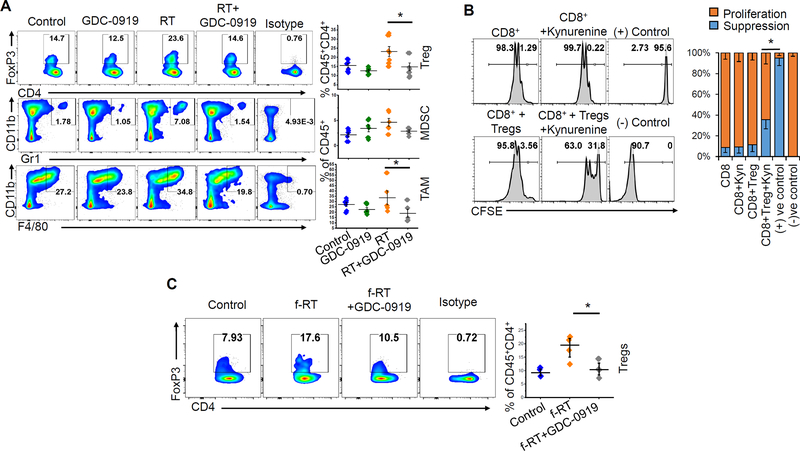
We went on to evaluate immunologic factors underlying the interface between tryptophan metabolism and radiation response. As an initial investigation, correlative studies were performed using the experimental design detailed in Fig. 4B, consisting of mice with intracranial tumors treated with vehicle control, GDC-0919, single fraction RT, or the combination. Mice were sacrificed 5 d following initiation of therapy and flow cytometry was performed on macro-dissected tumors to evaluate for a panel of immunologic markers. Similar to above efficacy studies, GDC-0919 alone did not appear to modulate immune response in orthotopic TRP tumors, demonstrating no changes in percentage of Tregs (CD4+FoxP3+CD25+), MDSCs (CD11b+Gr1+), or TAMs (CD11b+F4/80+) (Fig. 5A). As GDC-0919 alone did not appear to influence the number of Tregs quantitatively in our model, we extended our studies to determine if IDO1/kynurenine upregulation could contribute towards Treg function using a CD8+ T cell proliferation/suppression assay. Briefly, naïve CD8+ T cells and Tregs (CD4+CD25+ T cells) were isolated from splenocytes and CD8+ T cells were then stained with cell proliferation dye (CFSE). These cells were then activated (using anti-CD3 and anti-CD28 antibodies) and co-cultured in the presence or absence of Tregs, kynurenine, or the combination, for 3 days. Interestingly, neither kynurenine nor Tregs alone appeared to suppress the proliferation of CD8+ T cells. However, kynurenine appeared to impart suppressiveness to Tregs, as the combination led to diminished proliferation of CD8+ T cells (Fig. 5B).
Figure 5. Tryptophan metabolism contributes to radiation-induced immune suppression in glioblastoma.
(A) Intracranial tumors were macrodissected from mice 5 days following the GDC-0919 and/or RT treatment schedule described in Fig. 4B and analyzed for immune correlates by flow cytometry, including presence of regulatory T cells (Tregs; CD4+FoxP3+CD25+), myeloid-derived suppressor cells (CD45+CD11b+Gr1+) and tumor-associated macrophages (CD45+CD11b+F4/80+). Scatter plot represents data from 5–6 tumors from each group. (B) To analyze the suppressive ability of Tregs, splenocytes from C57BL/6 mice were used for isolating CD8+ T cells and Tregs (CD4+CD25+) using magnetic sorting. CFSE labeled CD8+ T cells were activated using plate-bound anti-CD3/CD28 antibody for three days in presence or absence of Tregs (CD8:Tregs=1:3) and kynurenine (50μM) and represented as flow histogram plots as CFSE dilution (proliferation) or no proliferation (suppression). The bar graph represents cumulative data from three experiments. (C) Intracranial tumors were macrodissected from mice 7 days following the GDC-0919 and/or fractionated RT treatment schedule described in Fig. 4C and analyzed for immune correlates by flow cytometry, including presence of Tregs (CD4+FoxP3+CD25+). Scatter plot represents data as mean ± SEM from 4 tumors in each group. *= p<0.05
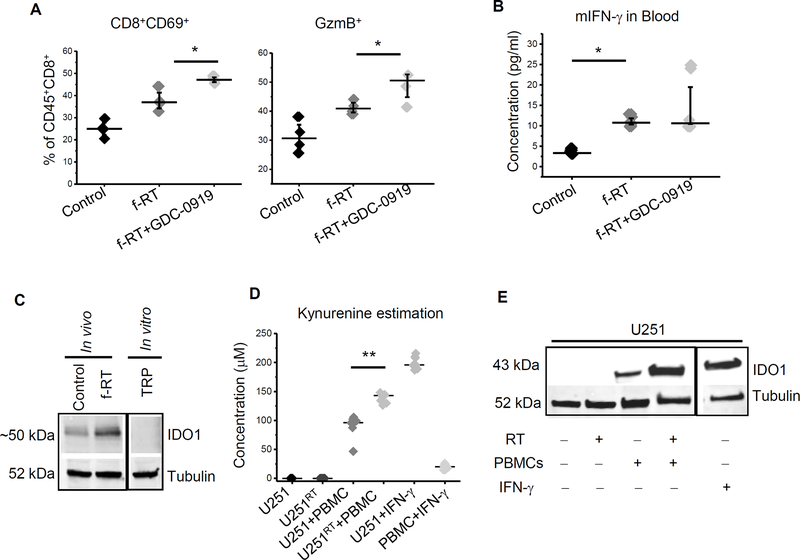
Interestingly, we found that RT alone appeared to contribute towards an immune suppressive environment in glioblastoma, including over a 50% increase in Tregs (from 14.7% to 23.6% of CD45+CD4+ cells). A trend in increase of MDSCs and TAMs was also observed. When combined with RT, GDC-0919 appeared to mitigate this immune suppressive response, normalizing Tregs, MDSCs, and TAMs back to baseline levels (Fig. 5A). Next, we extended this line of investigation to studies using a fractionated RT regimen, a combination that led to a significant survival advantage in our model (Fig. 4C). Similar to a single fraction schedule, fractionated RT also led to an increase in Tregs, which was attenuated by GDC-0919 (Fig. 5C). In addition to immune suppression, we sought to determine if GDC-0919, RT, or the combination influenced immune activation. No changes in the percentage of T effector cells (CD8+ T cells) or helper T cells (CD4+ T cells) were observed in either treatment arm (Supplementary Fig 5A and 5B). As radiation induces formation of various neoantigens, thereby enhancing activation of cytotoxic T cells (35), we extended our studies to determine if changes in activated or cytotoxic T cells (CTLs) could be observed with the RT and GDC-0919 combination. Interestingly, fractionated RT alone led to robust increases in both activated T cells (CD8+CD69+) and cytotoxic T cells (CD8+GzmB+) in the tumor (Fig. 6A). This RT induced immune activation was also reflected by increased IFN-γ levels in the plasma of mice receiving fractionated RT in comparison to controls (Fig 6B). When combined with RT, GDC-0919 led to further increases in both activated and cytotoxic T cells, which supports the observed enhanced anti-tumor response by this combination (Fig. 6A). We went on to demonstrate increased expression of PD-1 in resected tumors following RT, suggesting an increase in tumor-specific tumor infiltrating lymphocytes (TILs) (Supplementary Fig. 5C) (36).
Figure 6. Radiation induces antitumor immune reactivation.
(A) Intracranial TRP tumors described in Fig. 4C were macrodissected from mice 7 days following the GDC-0919 and/or fractionated RT treatment schedule and analyzed for activated (CD8+CD69+) and cytotoxic (GzmB+CD8+) T cells. Scatter plot represents data as mean ± SEM from 4 tumors in each group. (B) Plasma from mice harboring intracranial TRP tumors described in Fig. 4C were analyzed for mouse IFN-γ using ELISA. Scatter plot represents data from 4 mice from each group (C) IDO1 expression was analyzed in orthotopic TRP tumors isolated from C57BL/6 control mice and mice treated with f-RT and used to perform western blot for IDO1. Tubulin was used as loading control. (D) Human PBMCs (HLA-A2+) were co-cultured with U251 (HLA-A2+) and 250μM of tryptophan for 3 days. Cell culture supernatant was analyzed for kynurenine and (E) Cells were used for western blotting analysis for IDO1. Results are representative of at least 3 independent. **= p<0.005; *= p<0.05.
We went on to explore potential mechanism(s) underlying the potentiation of RT response with IDO1 inhibition. As both the anti-tumor and immune response following IDO1 inhibition appeared RT-specific in our glioblastoma model, as an initial investigation, we sought to determine if RT could reactivate or further activate this immune checkpoint. As demonstrated in Fig. 6C, western blot performed on intracranial TRP tumors following fractionated RT demonstrated increased IDO1 expression when compared to controls, confirming RT-induced pathway activation. As we have shown that RT stimulates both T cell activation and cytotoxicity, we next sought to link this observed T cell activation with increased IDO1 expression by determining if activated T cells could in turn induce tryptophan metabolism, thereby contributing towards RT-induced immune suppression. To test this possibility, we co-cultured irradiated and control U251 cells with HLA-A2+ naïve human peripheral blood mononuclear cells (PBMCs). As expected, neither control nor irradiated U251 cells demonstrated IDO1 pathway activation (Fig 6D). Interestingly, co-culturing U251 cells with naïve PBMCs appeared to stimulate T cell activation, leading to IDO1 pathway activation (Fig 6E) determined by increased kynurenine production. Pathway activation was significantly potentiated when PBMCs were co-cultured with irradiated U251 cells, providing further support linking RT response with tryptophan metabolism in glioblastoma.
Discussion
There have been several recent reports implicating aberrant tryptophan metabolism with glioblastoma immune tolerance (14,23,37). A majority of these studies have indirectly evaluated this pathway through expression of its rate limiting enzymes IDO1 and TDO. Results from this line of investigation were somewhat inconsistent, reporting IDO1 expression in 8–61.5% of glioblastoma using immunohistochemical (IHC) staining in 60 and 39 tumors, respectively (38,39). Further, the determination of increased IDO1 expression in glioblastoma is also mixed, ranging from medium to strong enzyme expression observed in 61.5% of these tumors when using IHC to 29% when measured by mRNA levels. Our study is amongst the first to delineate this pathway at the level of the individual metabolites contributing to immune modulation. In addition to further validating the relevance of this pathway in gliomagenesis, evaluating levels of individual metabolites provides a far more striking distinction between glioblastoma and LGG than when determined through enzyme expression. For example, when quantifying relative values of kynurenine and using the highest level of kynurenine detected in LGG as a threshold to define pathway activation, ~60% of glioblastoma demonstrated aberrant tryptophan metabolism. Further, through global metabolomic profiling, we uncovered a previously undescribed, inverse relationship between kynurenine levels and its downstream metabolic intermediate kynurenate in glioma. A ratio generated by coupling these two metabolites led to a highly significant distinction between LGG and glioblastoma, with over 90% of glioblastoma having a kynurenine/kynurenate ratio above the defined threshold (Supplementary Fig. 1C). These findings have unique clinical applications. For example, the differential accumulation of tryptophan-related metabolites in LGG and glioblastoma can potentially be imaged through MR spectroscopy, and therefore has diagnostic implications. In addition, such imaging correlates may be extended into clinical trials, allowing for the enrichment in patients with tumors likely to respond to IDO1 pathway inhibition or by supporting Phase 0 investigations designed to confirm target engagement of a specific compound designed to inhibit this pathway.
In addition to evaluating the IDO1 pathway from a metabolic perspective, integrated analyses coupling these global metabolomic signatures with gene expression profiles performed on matched tumors provided further insight into the biologic implications of this pathway. These cross-platform studies demonstrated that tryptophan metabolism is specific to the mesenchymal and classical molecular subtypes of glioblastoma, which is consistent with recent work identifying immune heterogeneity between these individual subtypes (40). Our group has recently reported that rather than representing intertumoral heterogeneity, molecular subtypes in glioblastoma were reflective of regional intratumoral heterogeneity, with the proneural subtype comprising the infiltrative edge of an individual tumor, while the mesenchymal subtype was specific to the perinecrotic core (31). Consistent with these findings, evaluating regional expression of IDO1 using the Ivy GAP atlas demonstrated highest expression of this enzyme in the perinecrotic core and lowest in the infiltrative edge of individual tumors. As increased expression of IDO1 has been recently described as a prognostic factor in glioblastoma (23), the observed differential expression of this enzyme may contribute towards the therapeutic resistance typically attributed to this region. In addition, these findings may imply that aberrant tryptophan metabolism is a late event in gliomagenesis and rational combinatorial strategies designed to also utilize agents specifically targeting the unique immune and/or signaling associated with tumor cell populations expanding in the infiltrative edge may lead to more durable control in these otherwise resistant tumors.
Aberrant tryptophan metabolism identified in patient-derived glioblastoma was recapitulated in our preclinical models, with robust pathway activation observed in all glioblastoma cell lines tested. The IDO1 inhibitor GDC-0919 demonstrated potent inhibition of tryptophan metabolism in these models, which was measured by inhibition of kynurenine production, and importantly, PK/PD studies demonstrated the strong potential for this agent to cross the BBB and achieve biologically relevant concentrations in the brain, supporting further preclinical investigations with this compound in glioblastoma. To determine the potential of GDC-0919 to serve as a novel immune checkpoint inhibitor in glioblastoma, we extended studies in vivo using an adult non-germline GEM astrocyte cell line that recapitulated both the genotype and aggressive phenotype of glioblastoma (29,30) and displayed aberrant tryptophan metabolism. Using this model, IDO1 inhibition with GDC-0919 did not demonstrate anti-tumor activity as a single agent in subcutaneous and orthotopic tumors or modulate the tumors’ immune profile at baseline (41,42). Although molecular knockdown of IDO1 expression has been shown to influence glioblastoma growth (43), our results are consistent with other recent publications demonstrating minimal activity in glioblastoma when inhibiting IDO1 as a single targeted agent (44). Although we did not observe any changes in the absolute number of Tregs following IDO1 inhibition, we did uncover an important role by which kynurenine modulates their activity, serving as a critical metabolite required for Treg-mediated suppression of CD8+ T cell proliferation. Further work is needed to precisely define the role kynurenine and its downstream metabolic intermediates play in modulating Treg function and determine its mechanistic underpinnings.
Although, GDC-0919 did not demonstrate anti-tumor activity as a single agent in our glioblastoma model, we identified a unique synergy when combined with radiation, which was most notable when using a hypofractionated regimen. As recent strategies designed to incorporate this agent in cancer treatment have been largely negative (45), these findings, which are consistent with recent studies that have generated considerable interest in the potential for immune checkpoint agents to be used in concert with radiation (46,47), may provide a novel direction for continued clinical trial development. Our results provide a novel insight into the complex mechanisms that may be underlying these interactions, suggesting radiation may perturb cancer immunoediting at several levels. For example, in our working model, RT-induced tumor cell death results in the release of tumor-associated antigen/peptides, ensued by antigen processing and presentation to T cells (35,48). This, in turn, helps stimulate the immune system and clonal expansion as demonstrated by a robust increase in activated and cytotoxic T cells, contributing towards an immunologic shift from a state of ‘equilibrium’ or ‘escape’ back to an immunologic state of ‘elimination’ of tumor cells (49). However, this potential for an immunologic shift that would result in enhanced anti-tumor activity appears to be dampened by parallel increases in RT-induced immune suppression, most notably, a consistent increase in Tregs. Our data, along with another recent publication (50), suggests that the observed increase in activated and cytotoxic T cells following RT may in turn stimulate tryptophan metabolism, contributing to the observed increase in Tregs. Inhibiting tryptophan metabolism mitigates the observed accumulation of RT-induced Tregs and attenuates their suppressiveness. This results in a further increase in T cell activation and cytotoxicity, thereby, enhancing anti-tumor activity by tipping the immunologic balance towards a state of ‘elimination’ (37), thereby, providing a mechanistic link between IDO1 inhibition and enhanced radiation response. Focused studies involving depletion of Tregs, and other potential targets of IDO1 inhibition, including MDSCs, are still required to more definitively establish mechanisms governing potentiation of RT response following IDO1 inhibition.
In summary, aberrant tryptophan metabolism represents an important metabolic node in glioblastoma. Although its specific role in gliomagenesis remains unclear, this pathway appears to play a significant role in RT-induced immune checkpoint reactivation. GDC-0919 is a potent IDO1 inhibitor with the capacity of crossing the BBB and enhancing RT response by mitigating RT-induced immune suppression, thereby shifting the immunologic balance towards a state of ‘elimination’, which was particularly striking when using a hypofractionated approach. These encouraging findings support clinical efforts designed to combine IDO1 inhibition with hypofractionated RT in glioblastoma, offering the promise of effectively harnessing a patient’s own immune system to attack these otherwise recalcitrant tumors.
Supplementary Material
Translational Relevance.
Through integrative, cross-platform analyses coupling global metabolomic profiling with gene expression arrays in over 100 patient-derived tumors, we identified aberrant tryptophan metabolism as an important metabolic node and immune checkpoint in glioblastoma. We discovered that intermediaries associated with tryptophan metabolism demonstrated >90% accuracy in discriminating between low and high-grade glioma, offering novel diagnostic implications. We then extended these findings preclinically using a novel, immune competent, genetically engineered mouse model of adult astrocytoma with a potent, clinically relevant inhibitor of tryptophan metabolism/IDO1. Through these studies, we uncovered a potent synergy when combining an IDO1 inhibitor with hypofractionated radiation and a novel mechanism linking tryptophan metabolism with radiation-induced immune suppression involving immune checkpoint reactivation. These encouraging findings support future clinical efforts designed to combine IDO1 inhibition with hypofractionated radiation in glioblastoma, offering the promise of harnessing a patient’s immune system to attack these otherwise recalcitrant tumors.
Acknowledgements
This work was supported by the NIH/NINDS (R21NS090087), ACS (RSG-11-029-01), Bankhead-Coley Cancer Research Program and Cancer Research Seed Grant Awards from Beaumont Heath (P.C). CRM is supported by NIH/NCI (R01CA204136).
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interest.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-oncology 2017;19(suppl_5):v1–v88 doi 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine 2008;359(5):492–507 doi 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Kamradt T, Mitchison NA. Tolerance and autoimmunity. The New England journal of medicine 2001;344(9):655–64 doi 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine 2015;373(1):23–34 doi 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer 2012;12(4):252–64 doi 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends in immunology 2015;36(10):569–77 doi 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine 2015;212(7):991–9 doi 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig A, Schulte A, Schnack C, Hundhausen C, Reiss K, Brodway N, et al. Enhanced expression and shedding of the transmembrane chemokine CXCL16 by reactive astrocytes and glioma cells. Journal of neurochemistry 2005;93(5):1293–303 doi 10.1111/j.1471-4159.2005.03123.x. [DOI] [PubMed] [Google Scholar]
- 9.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2000;6(1):102–11. [PubMed] [Google Scholar]
- 10.Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro-oncology 2017;19(suppl_3):iii21–iii doi 10.1093/neuonc/nox036.071. [DOI] [Google Scholar]
- 11.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England journal of medicine 2016;375(18):1767–78 doi 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesarwani P, Kant S, Prabhu A, Chinnaiyan P. The interplay between metabolic remodeling and immune regulation in glioblastoma. Neuro-oncology 2017;19(10):1308–15 doi 10.1093/neuonc/nox079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998;281(5380):1191–3. [DOI] [PubMed] [Google Scholar]
- 14.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478(7368):197–203 doi 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 15.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(22):6110–21 doi 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of clinical investigation 2004;114(2):280–90 doi 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of immunology 2010;185(6):3190–8 doi 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America 2010;107(46):19961–6 doi 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer research 2008;68(10):3609–17 doi 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453(7191):65–71 doi 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 21.van Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Frontiers in immunology 2015;6:34 doi 10.3389/fimmu.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyland E, Williams DC. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. The Biochemical journal 1956;64(3):578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai L, Lauing KL, Chang AL, Dey M, Qian J, Cheng Y, et al. The role of IDO in brain tumor immunotherapy. Journal of neuro-oncology 2015;123(3):395–403 doi 10.1007/s11060-014-1687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. International journal of cancer Journal international du cancer 2002;101(2):151–5 doi 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 25.Zhai L, Ladomersky E, Dostal CR, Lauing KL, Swoap K, Billingham LK, et al. Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTLA-4/PD-L1 inhibition in mouse glioblastoma. Brain, behavior, and immunity 2017;62:24–9 doi 10.1016/j.bbi.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinnaiyan P, Kensicki E, Bloom G, Prabhu A, Sarcar B, Kahali S, et al. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer research 2012;72(22):5878–88 doi 10.1158/0008-5472.CAN-12-1572-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proceedings of the National Academy of Sciences of the United States of America 2013;110(21):8644–9 doi 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prabhu A, Sarcar B, Miller CR, Kim SH, Nakano I, Forsyth P, et al. Ras-mediated modulation of pyruvate dehydrogenase activity regulates mitochondrial reserve capacity and contributes to glioblastoma tumorigenesis. Neuro-oncology 2015;17(9):1220–30 doi 10.1093/neuonc/nou369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Zhang Q, Kutlu B, Difilippantonio S, Bash R, Gilbert D, et al. Evolutionary etiology of high-grade astrocytomas. Proceedings of the National Academy of Sciences of the United States of America 2013;110(44):17933–8 doi 10.1073/pnas.1317026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitucci M, Karpinich NO, Bash RE, Werneke AM, Schmid RS, White KK, et al. Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis. Neuro-oncology 2013;15(10):1317–29 doi 10.1093/neuonc/not084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell 2010;17(1):98–110 doi 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhu A, Kesarwani P, Kant S, Graham SF, Chinnaiyan P. Histologically defined intratumoral sequencing uncovers evolutionary cues into conserved molecular events driving gliomagenesis. Neuro-oncology 2017. doi 10.1093/neuonc/nox100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1991;5(11):2516–22. [PubMed] [Google Scholar]
- 34.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21(24):5427–33 doi 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer research 2004;64(21):7985–94 doi 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 36.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. The Journal of clinical investigation 2014;124(5):2246–59 doi 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. The Journal of clinical investigation 2013;123(7):2756–63 doi 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 2013;72(6):1031–8; discussion 8–9 doi 10.1227/NEU.0b013e31828cf945. [DOI] [PubMed] [Google Scholar]
- 39.Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer immunology research 2015;3(2):161–72 doi 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 40.Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer immunology research 2013;1(2):112–22 doi 10.1158/2326-6066.CIR-13-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11(2 Pt 1):728–34. [PubMed] [Google Scholar]
- 42.Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PloS one 2013;8(12):e84927 doi 10.1371/journal.pone.0084927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20(20):5290–301 doi 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. Journal for immunotherapy of cancer 2014;2:21 doi 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burris HA, Gordon MS, Hellmann MD, LoRusso P, Emens LA, Hodi FS, et al. A phase Ib dose escalation study of combined inhibition of IDO1 (GDC-0919) and PD-L1 (atezolizumab) in patients (pts) with locally advanced or metastatic solid tumors. Journal of Clinical Oncology 2017;35(15_suppl):105- doi 10.1200/JCO.2017.35.15_suppl.105. [DOI] [Google Scholar]
- 46.Formenti SC. Silvia Formenti on the promise of combining radiotherapy and immunotherapy to treat cancer. Oncology 2016;30(4):289–92. [PubMed] [Google Scholar]
- 47.Seyedin SN, Schoenhals JE, Lee DA, Cortez MA, Wang X, Niknam S, et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 2015;7(9):967–80 doi 10.2217/imt.15.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine 2006;203(5):1259–71 doi 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annual review of immunology 2004;22:329–60 doi 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 50.Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G, et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(21):6650–60 doi 10.1158/1078-0432.CCR-17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.