Abstract
In order to optimize outcomes in the face of uncertainty, one must recall past experiences and extrapolate to the future by assigning values to different choice outcomes. This behavior requires an interplay between memory and reward valuation, necessitating communication across many brain regions. At the anatomical nexus of this interplay is the perirhinal cortex (PRC). The PRC is densely connected to the amygdala and orbital frontal cortex, regions that have been implicated in reward-based decision making, as well as the hippocampus. Thus, the PRC could serve as a hub for integrating memory, reward, and prediction. The PRC’s role in value-based decision making, however, has not been empirically examined. Therefore, we tested the role of the PRC in a spatial delay discounting task, which allows rats to choose between a 1-second delay for a small food reward and a variable delay for a large food reward, with the delay to the large reward increasing after choice of each large reward and decreasing after each small reward. The rat can therefore adjust the delay by consecutively choosing the same reward or stabilize the delay by alternating between sides. The latter has been shown to occur once the ‘temporal cost’ of the large reward is established and is a decision-making process termed ‘exploitation’. When the PRC was bilaterally inactivated with the GABA(A) agonist muscimol, rats spent fewer trials successfully exploiting to maintain a fixed delay compared to the vehicle control condition. Moreover, PRC inactivation resulted in an increased number of vicarious trial and error (VTE) events at the choice point, where rats had to decide between the two rewards. These behavioral patterns suggest that the PRC is critical for maintaining stability in linking a choice to a reward outcome in the face of a variable cost.
Keywords: cognition, decision making, deliberation, medial temporal lobe, rat
INTRODUCTION
Decisions are often made in the face of dynamic contingencies. Optimization of decisions requires constant updating of cost versus reward representations in relation to outcomes (Pennartz, Berke, Graybiel, Ito, Lansink, van der Meer, Redish, Smith, and Voorn, 2009; Rangel, Camerer, and Montague, 2008; van der Meer, Kurth-Nelson, and Redish, 2012). The quintessential paradigm placing time and reward in a direct relationship is the “Stanford marshmallow test”, in which temporal delay directly interacts with reward magnitude (Mischel, Ebbesen, and Zeiss, 1972). Deliberation and decision-making theories suggest that the higher cognitive processes required for such intertemporal decisions are a distributed phenomenon, requiring orchestrated communication across brain regions including the medial temporal lobe and prefrontal cortices (for review, see Frost and McNaughton, 2017; Preston and Eichenbaum, 2013).
The intertemporal choice task requires optimizing outcomes based upon the magnitude of the reward and the cost associated with each option (Ainslie, 1975; Green, Myerson, Lichtman, Rosen, and Fry, 1996; Rachlin and Green, 1972). In rodent variations of this task, two options are made available: a small reward that arrives after a short delay or a larger reward following a variable delay (Evenden and Ryan, 1996; Mendez, Simon, Hart, Mitchell, Nation, Wellman, and Setlow, 2010; Rudebeck, Walton, Smyth, Bannerman, and Rushworth, 2006; Simon, LaSarge, Montgomery, Williams, Mendez, Setlow, and Bizon, 2010). In addition to quantifying the length of time a rat is willing to wait for a reward, this task (particularly when conducted in a maze apparatus , i.e., the “spatial delay discounting task”; Papale, Stott, Powell, Regier, and Redish, 2012) allows assessment of deliberation behavior including vicarious trial and error, or head turning that is believed to be indicative of an animal’s assessment of possible future outcomes (Tolman, 1939). Interestingly, this vicarious trial and error behavior is associated with hippocampal activation predicting a spatial course of action (Johnson and Redish, 2007; (Redish, 2016).
With the operant delay discounting experimental paradigm, the orbitofrontal cortex (OFC) has been demonstrated to be important for representing reward outcomes (Mobini, Body, Ho, Bradshaw, Szabadi, Deakin, and Anderson, 2002). Moreover, it has been found that rodents with OFC lesions prefer a small, immediate reward over a larger reward that comes after a delay (Mar, Walker, Theobald, Eagle, and Robbins, 2011; Rudebeck, Behrens, Kennerley, Baxter, Buckley, Walton, and Rushworth, 2008; Rudebeck et al., 2006). While the OFC is critical for normal performance on intertemporal choice tasks, this behavior has been shown to rely on a network of structures including the basolateral amygdala, ventral striatum, subthalamic nucleus and hippocampus (Abela and Chudasama, 2013; 2014; Bailey, Simpson, and Balsam, 2016; Cardinal, 2006; Cardinal and Howes, 2005; Cheung and Cardinal, 2005; Floresco, St Onge, Ghods-Sharifi, and Winstanley, 2008; Fobbs and Mizumori, 2017; Frost and McNaughton, 2017; Zeeb, Floresco, and Winstanley, 2010). The anatomical connectivity between the OFC and the hippocampus, however, is indirect with the most significant connecting hubs identified as the nucleus reuniens and perirhinal cortex (PRC). Specifically, the PRC is densely connected to the amygdala, hippocampus, and OFC (Burwell RD, Witter MP, & Amaral DG, 1995), regions implicated in reward-based decision making (van der Meer et al., 2012). In addition, prior research supports the role of the PRC in processing high-level sensory information that is critical for disambiguating between stimuli that share features (Bartko, Winters, Cowell, Saksida, and Bussey, 2007a; b; Bussey, Saksida, and Murray, 2002a; 2005; Forwood, Bartko, Saksida, and Bussey, 2007; Murray and Bussey, 1999). Such computations are likely to be integral for the deliberative and outcome evaluation processes that are critical for decision making (Baxter, Hadfield, and Murray, 1999; Baxter and Murray, 2002; Baxter, Parker, Lindner, Izquierdo, and Murray, 2000). The specific involvement of the PRC in deliberative decision making, however, has not been empirically tested.
Given its position in the network, we hypothesized that the PRC is a critical hub that integrates information regarding reward magnitude, delay contingencies and predicted outcomes to disambiguate between two outcomes that share features. The current study tested this hypothesis by determining the effects of unilateral and bilateral PRC inactivation on intertemporal choice behavior.
MATERIALS & METHODS
Subjects
A total of n=13 young adult (6 months old) male Fischer 344 × Brown Norway F1 hybrid rats from the NIA colony (Charles River) were behaviorally characterized in the spatial delay discounting task. From these, n=7 rats underwent cannulation surgery targeting the PRC and were tested following reversible inactivation. The other 6 rats received cannula in other structures, and those data are not included in the current paper. Rats were housed individually in standard Plexiglas cages and maintained on a 12-h reversed light/dark cycle (lights off at 8:00 am), with all manipulations carried out in the dark phase. Rats were acclimated to the laboratory facility and handled by experimenters for 1-2 weeks following arrival. Following this, they were placed on a restricted feeding protocol in which 20±5 g mash (Teklad Global Diet 2918, Harlan Labs; equal parts chow and water; 38 +/− 9.5 kcal) was provided daily. Drinking water was available ad libitum. Habituation and training began once rats reached ~85% of their initial body weight. In addition to their daily mash, rats received a total of 200 45-mg enriched, unflavored pellets (Research Diets, New Brunswick, N J) over the course of each training or test session. Throughout all experiments, rats were weighed and monitored daily to ensure they maintained an optimal body condition score of 2.5 to 3. The body condition score is assigned based on the presence of palpable fat deposits over the lumbar vertebrae and pelvic bones (Ullman-Culleré and Foltz, 1999; Hickman and Swan, 2010). Procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Florida.
Apparatus
The testing apparatus consisted of a rectangular figure-8 maze built of plastic Duplo blocks (LEGO®, Enfield, CT), recapitulating the design of Papale and colleagues (Fig 2; Papale et al., 2012). Overall dimensions of the maze were 82 cm × 64 cm, with each arm measuring 64 cm × 10 cm and bounded by walls 12 cm in height. Behavior was recorded with a webcam (Logitech; Newark, CA) fixed 55 cm directly above the center stem of the maze. Pellet dispensers (Med Associates, Fairfax, VT) were positioned on each of the two outer arms of the maze, 44 cm from the front wall (Fig. 1). One white light bulb, angled away from the apparatus and onto the wall to provide diffuse low light, and two red bulbs, placed on either side of the camera, were used to illuminate the maze. Auditory cues were played on a single speaker located outside the center of the back wall of the maze. The task was fully automated based on continuous monitoring of the rats’ position via the overhead camera. This consisted of a custom interface written in Python 3.4 integrated computer vision software (OpenCV 3.3) and commands issued by an Arduino UNO microcontroller board (Arduino, Turin, Italy), connected to a dedicated Linux PC. The program tracked the rat’s location at the center arm, right and left reward locations, and the adjacent section as the rat was exiting the reward location (see Supplemental video 1). Activation of the sensors in the correct sequence was necessary for initiation of a new trial and delivery of reward (Fig. 1).
Figure 8.
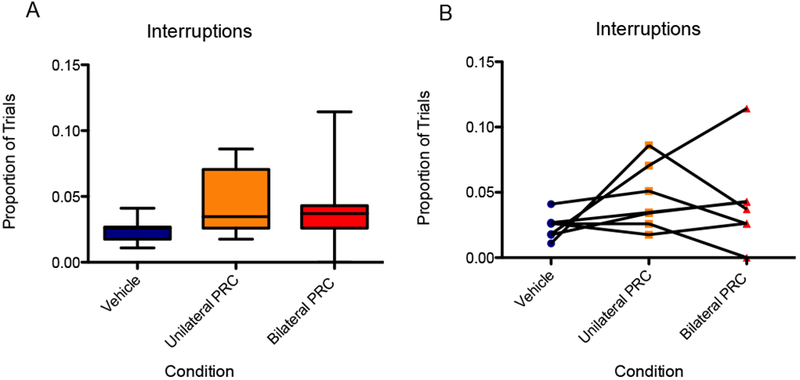
There was no significant main effect of infusion condition on the proportion of trials interrupted. (A) Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (B) Points represent individual rats across conditions.
Figure 2.
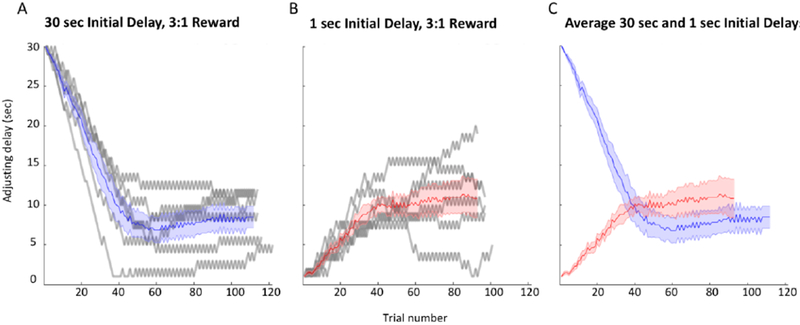
(A) Adjusting delay duration is plotted against trial number for a single control session (30-second initial delay) for each of the 7 rats in the experiment (gray curves). The blue curve represents the mean delay plus SEM. (B) Another control session for each of the 7 rats at a 1-second initial delay is shown (gray curves). The red curve represents the mean delay plus SEM. (C) The mean plus SEM curves for the sample control sessions provide a comparison of performance at the 30-second and 1-second initial delays.
Figure 1.
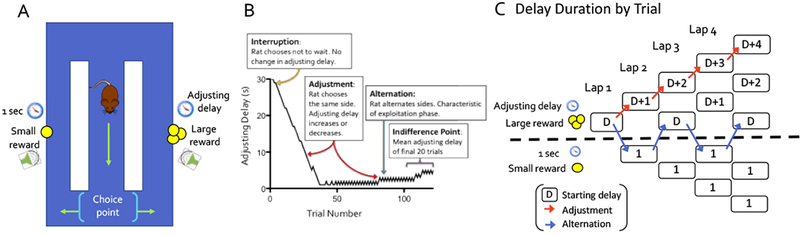
(A) The schematic shows the major features of the spatial delay discounting maze. (B) Adjusting delay duration is plotted against trial number in a single control session. The adjusting delay varies depending on the rat’s choice of the same side (adjustment), the opposite side (alternation, characterizing the exploitation phase of the session), or initiation of a new trial in place of the delay and reward (interruption). (C) In the paradigm, alternating between sides stabilizes the delay, while choosing the same side consecutively adjusts the delay. The paradigm was adapted from Papale, Stott, Powell, Regier, & Redish, 2012.
Behavioral procedures
Habituation and Shaping.
The shaping process followed the procedures of Papale and colleagues (2012). Rats were first allowed to free forage and retrieve pellets scattered throughout the maze in 10-minute daily sessions for 2 days. Rats were then shaped to run laps through a single arm of the maze, with the side (right or left) switched from one day to the next. During this period, rats were initially trained to attend to the pellet dispensers at reward sites by placement of a single pellet on the adjacent section of the maze floor. Rats were next trained to pause at the dispenser to receive a single pellet, then were trained to associate an auditory cue (1 kHz tone) with reward delivery. Each shaping session would end after rats completed 100 laps or a period of 60 min had elapsed. Rats moved on to the next phase when they readily completed 100 laps within 30 minutes.
Spatial delay discounting.
Procedures for the spatial delay discounting task followed those of Papale and colleagues (2012). In the task, rats titrate the duration of a variable delay preceding reward delivery by alternating between arms on the maze. Specifically, choice of one maze arm always results in a fixed delay-reward ratio of 1 sec:1 pellet (small reward arm), whereas choice of the opposite arm results in a variable delay-reward ratio of 1 to 30 seconds and 1-4 pellets (large reward arm). During initial characterization, delay and reward parameters for the large reward arm differed across sessions, such that the magnitude of the large reward was held constant at 1,2, 3, or 4 pellets, and the starting delay was 30, 20, 10, or 1 sec. For experimental testing following infusions into the PRC, all sessions used 3 pellets for the large reward (3:1 reward ratio) and 30 sec or 1 sec for the starting delay. In the large reward arm, the delay duration was signaled via a countdown tone, beginning at 8.5 kHz at 30 seconds and decreasing by 250 Hz for each second of delay. In the short reward arm, the tone was 1 kHz, as at the end of the large reward arm countdown. Pellets were delivered at the end of the delay when the tone reached 1 kHz. During the delay, rats could choose to interrupt the trial by moving past the feeder, causing the tone to stop and no pellets to be delivered.
Throughout all sessions, choice of the small reward arm decreased the delay for the large reward by 1 sec on the following trial, while choice of the large reward arm had the opposite effect. This allowed rats to titrate the delay preceding the large reward to a value that was optimal for them. Behaviorally, this value is reached when rats alternate between arms to maintain a consistent delay. Consecutive choices of the small reward arm or large reward arm can therefore be considered ‘adjustment’ laps, in which the rats’ choice shortens or lengthens the delay, respectively. Spatial position of the small versus large reward arm on the maze (left versus right) was counterbalanced across test sessions for each rat to avoid perpetuating inherent response biases and ensure that the rats’ choices were guided by the delay contingencies in effect for that session.
Surgery
Following initial training and behavioral characterization, rats were surgically implanted with guide cannulae bilaterally targeting the perirhinal cortex. While maintained on isoflurane anesthesia (Isothesia, Henry Schein, Dublin, OH), a longitudinal incision was made to expose and clean the skull surface. Four anchoring bone screws were placed and guide cannulae (22G, Plastics One, Roanoke, VA) were lowered to appropriate coordinates. Cannulae were placed relative to Bregma at AP - 6.5 mm, ML +/−6.8 mm, approximately DV −3 mm from brain surface. The infusion cannulae then protruded 1 mm below such that infusate was delivered approximately 4 mm below brain surface. Cannulae were secured to the skull and anchor screws with dental acrylic (Teets, Patterson Dental, Tampa, FL). Dummy stylets (Plastics One) were kept in each cannula to prevent contamination and maintain patency. Metacam (1-2 mg/kg s.c., Boehringer Ingelheim, Vetmedica Inc., St. Joseph, MO) was given for pre- and post-operative analgesia, and a post-operative recovery period of 7 days was provided with daily oral administration of 0.5 mL antibiotic. Of note, there was a systematic deviation in the placement of left versus right cannulae such that left cannulae were placed slightly more anteriorly. However, since there was no effect of lateralization, the diffusion of muscimol in left versus right hemispheres was likely overlapping.
Intracerebral Infusions and experimental tests
Following surgery and recovery, rats were given at least 7 days of behavioral training to confirm baseline performance similar to that observed before cannulation. Rats were then infused every 3 days, with 2 days of behavioral training between infusions to re-establish baseline performance and allow full wash-out of infusate. The PRC was reversibly inactivated with local microinfusions of the GABA(A) receptor agonist muscimol (1 mg/mL; Sigma-Aldrich, St. Louis, MO). Drug or vehicle (0.9% sterile physiological saline) were infused at a volume of 0.5 μL at a rate of 0.25 μL/min. Internal microinjectors (28G, Plastics One) protruding 1 mm below the guide cannulae were connected via polyethylene tubing (PE50, Plastics One) to 10 μL syringes (Hamilton, Franklin, MA) mounted in a microinfusion pump (Harvard Apparatus, Holliston, MA). Tubing was backfilled with sterile water and a 1-μL bubble was aspirated to create a barrier between backfill and infusate, and permit confirmation by visual inspection that the correct volume of infusate had been delivered. Needles were left in place for 2 min after infusions to allow dispersion of the drug. Rats were returned to their home cage for 30 min prior to beginning testing.
Seven rats were tested with four infusion conditions: 1) bilateral PRC inactivation (0.5 μg muscimol bilaterally); 2) right PRC inactivation (0.5 μg muscimol unilaterally); 3) left PRC inactivation (0.5 μg muscimol unilaterally); and 4) vehicle control (0.5 μL 0.9% saline bilaterally). The order of infusion conditions was randomized for each rat. Separate tests were carried out with an initial delay of either 30 sec or 1 sec on the large reward arm, all with a reward ratio of 3:1.
Histology.
After behavioral testing, the rats were sacrificed, and tissue was collected to assess accurate placement of the cannulae in the PRC. A lethal dose of sodium pentobarbital (Vortech Pharmaceuticals, Dearborn, Ml) was administered, followed by transcardial perfusion with 4% paraformaldehyde. Brains were stored in 4% paraformaldehyde with 30% sucrose at 4°C for 72 hours, then sectioned at 40 μm on a cryostat (Microm HM550; Thermo Scientific, Waltham, MA) and thaw-mounted on Superfrost Plus slides (Fisher Scientific, Waltham, MA). The tissue was Nissl stained and imaged using bright-field microscopy (Keyence; Osaka, Osaka Prefecture, Japan), confirming bilateral cannula placement in the PRC for all 7 rats (Fig. 3).
Figure 3.
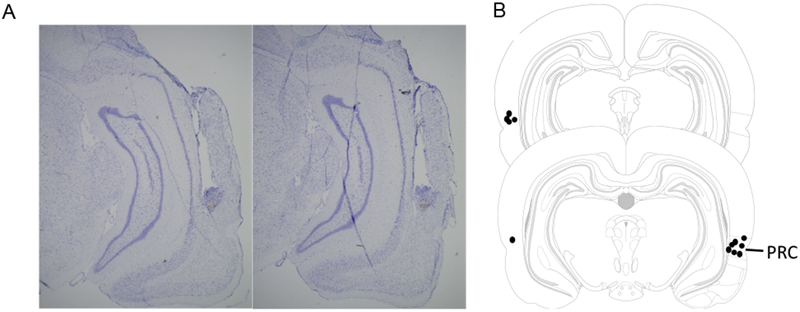
(A) Representative examples of histological images showing the cannula placements and locations of injector needles for muscimol infusions. (B) Schematic showing the approximate sites of cannula placement for the seven rats in the experiment based on histological images.
Deliberation behavior.
Deliberation behavior was characterized by reaction time and vicarious trial and error (VTE). Videos of experimental sessions were scored frame-by-frame (~24 frames/sec) by research assistants blind to the experimental condition and rat identity. Prior to analyzing experimental videos, scorers analyzed a training video for which reaction time and VTE had been determined by consensus; a score within 10% of the consensus score was required to proceed to analyze experimental videos. To measure reaction time at the choice point, scorers marked the rat’s entry into and exit from a defined area at the top of the center arm, and computer software measured the time difference to the nearest millisecond. VTE was defined as the presence of any head turn behavior to an angle of at least 20 degrees while the rat was within the choice area (supplemental video 1). Proportions were calculated by dividing the number of trials with any amount of VTE by the total number of trials in that session. Proportions of VTE were calculated by dividing the number of trials in which the rat exhibited any VTE behavior by the total number of trials in that session.
Statistical analyses.
All sessions continued until the rat consumed 200 pellets; the number of trials in a session varied based on the rat’s choices. Thus, adjustments, alternations, interruptions, and deliberation behavior were analyzed as proportions, or the number of trials in which the rat exhibited the behavior divided by the total number of trials for that session for that rat. Although, overall, the total number of trials did not differ between vehicle (114.8 trials, SEM = 1.6) and bilateral inactivation (115.3 trials, SEM = 1.7). Because rats show more adjustments early in testing and more exploitation (alternations) in the latter portion, performance metrics were also calculated for the first and second half of trials. There were no a priori expectations of lateralization effects of inactivation on performance. During the review process, a Bayesian repeated measure ANOVA was suggested. Therefore, a Bayesian repeated measure ANOVA conducted between left and right hemispheres with default priors set to 0.5 yielded a Bayes factor of 0.634 (Love et al., 2015). This suggests that the data are 1.58 times more likely under the null hypothesis (no hemisphere difference) relative to alternative (there is a difference between hemispheres); however, this effect magnitude is marginal. As there were no other statistical or qualitative performance differences in other measures, data from these sessions were collapsed for analyses. Repeated measure ANOVAs were conducted to statistically compare the bilateral inactivation, unilateral inactivation, and vehicle control conditions for experimental parameters. Significance was set to an alpha of p < 0.05. Further, confidence intervals on the difference between means of the data for bilateral inactivation and vehicle were reported along with the effect size (d, or the difference between means divided by the standard deviation). In addition, because there is a potential for proportion and reaction time data to be nonparametric and skewed, we also performed statistical analyses on logit-transformed proportion data and log-transformed reaction time data and reported these statistics alongside the original analyses. The logit transformations could not be performed on the proportion of trials with VTE in the second half of the session, though, because three of four rats did not exhibit any VTE in the vehicle condition.
RESULTS
Baseline temporal discounting behavior
In initial testing in which 13 rats completed each of the 16 unique sessions combining one of four reward ratios (1, 2, 3, or 4 pellets for the adjusting delay to 1 pellet for the 1-second delay) and one of four initial delays (1, 10, 20, or 30 seconds), increasing reward ratios mediated an approximately linear increase in indifference point (mean adjusting delay of the last 20 trials of a session). A repeated measures ANOVA showed a significant main effect of reward ratio on indifference point [F(3,18) = 13.74, p < 0.01], and a difference contrast indicated that for the 2:1, 3:1, and 4:1 reward ratios, the indifference point was significantly higher than that for the reward ratio below: 2:1 vs. 1:1 [F(1,12) = 47.87, p < 0.01], 3:1 vs. 2:1 [F(1,12) = 52.7, p < 0.01], and 4:1 vs. 3:1 [F(1,12) = 17.51, p < 0.01]. Further, the indifference point plotted against the four reward ratios followed the linear trend y = 3.99× - 0.693, and the reward ratio explained the majority of variance in indifference point according to R2 = 0.9679, p < 0.01 (Fig. 4A).
Figure 4.
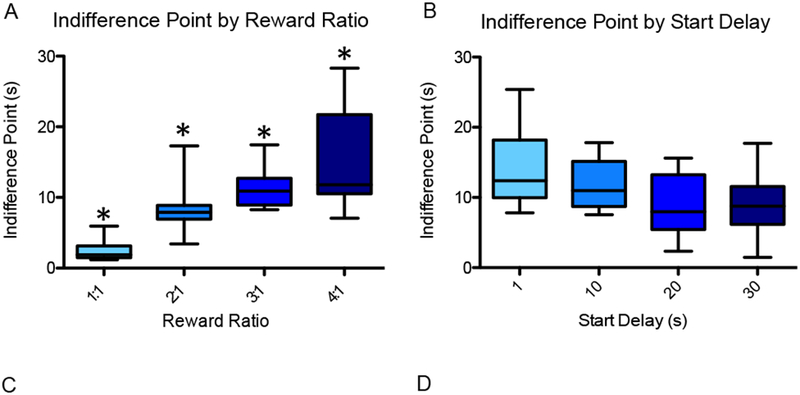
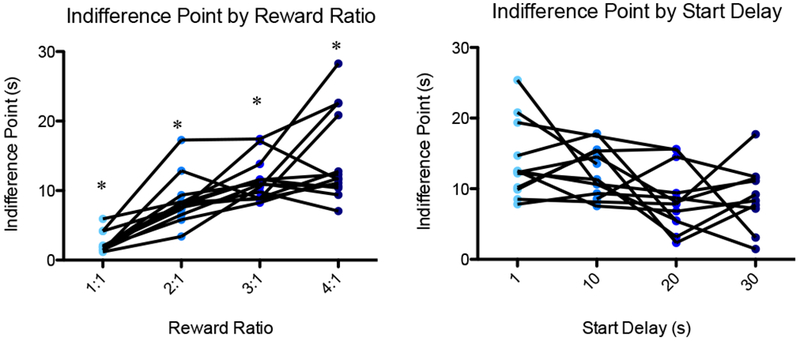
To test baseline temporal discounting behavior, 13 rats (including the 7 used for experimental testing) each completed 16 sessions combining one of four reward ratios (1:1, 2:1, 3:1, 4:1) with one of four initial delays (30, 20, 10, 1 sec). (A) Indifference point (mean adjusting delay duration of the final 20 trials of the session) is plotted as a function of reward ratio. There was a significant main effect of reward ratio on indifference point, and for the 2:1, 3:1, and 4:1 reward ratios, the indifference point was significantly higher than that for the reward ratio below. (B) Indifference point is plotted as a function of start delay for the 3:1 reward condition. The effect of start delay on indifference point was insignificant. An asterisk represents a statistically significant difference from the other conditions. Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (C and D) Points represent individual rats across conditions.
For consistency of analysis, the 3:1 reward condition was chosen for all subsequent experimental testing because experimental effects (increases or decreases) on the middle-range indifference point would be apparent, and because the rat can complete a large number of trials before becoming satiated. For the 3:1 reward condition, the indifference point appeared to trend downward with increasing duration of the start delay (Fig. 4B); however, a repeated measures ANOVA revealed no significant main effect of start delay on indifference point [F(3,24) = 2.593, p > 0.07]. In addition, a paired-samples t-test indicated no significant difference between the indifference points for the 30-second (mean = 8.58) and 1-second (mean = 11.18) start delays at the 3:1 reward condition [t(8) = 1.305, p = 0.228]. Following this initial testing, since all 7 rats that were included in the PRC inactivation were tested at the 30-second initial delay, all comparisons are of the 30-second initial delay, 3:1 reward condition.
Effects of perirhinal cortical inactivation on behavior in the spatial delay discounting task
At a 30-second initial delay and 3:1 reward ratio, inactivation of the PRC (bilateral and unilateral) had no significant effect on the indifference point during spatial intertemporal choice testing [F(2,12) = 0.51, p = .62], 95% CI [−1.3, 0.77], d = −0.28 (Fig. 5). Data for left and right were collapsed for data analysis. This suggests that the perirhinal cortex is not critical for the overall valuation of the reward relative to the delay.
Figure 5.
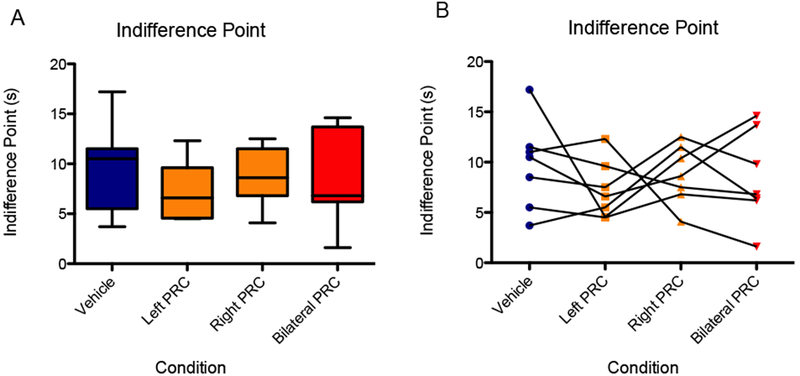
There was no significant effect of infusion condition on indifference point during spatial intertemporal choice testing (30-second initial delay, 3:1 reward ratio). (A) Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (B) Points represent individual rats across conditions.
Figure 6 shows the mean proportion of trials in which rats selected the same side over consecutive trials, that is, made adjustments (Y-axis) for the different infusion conditions (X-axis). A repeated-measures ANOVA with the within-subjects factor of infusion condition detected a significant main effect of inactivation on the proportion of adjustments in the session [F(2,12) = 6.613, p < 0.05]. Planned orthogonal comparisons indicated that bilateral PRC inactivation condition resulted in significantly more adjustments compared to the vehicle control condition [F(1,6) = 7.913, p < 0.05], 95% CI [0.27, 2.62], d = 1.44. In contrast, unilateral PRC inactivation was not significantly different from the vehicle condition [F(1,6) = 1.714, p = 0.24]. These results were upheld when the data were logit-transformed and analyzed: main effect of infusion condition [F(2,12) = 7.31, p < 0.01] and orthogonal comparison of bilateral inactivation to vehicle [F(1,6) = 9.68, p < 0.05].
Figure 6.
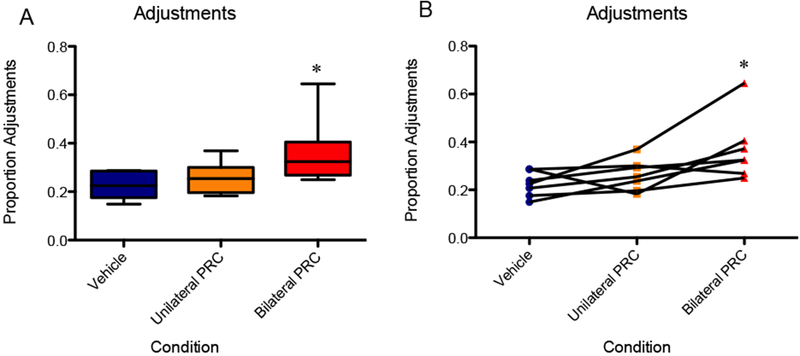
The bilateral PRC inactivation condition resulted in significantly more adjustments compared to the vehicle control. In contrast, unilateral PRC inactivation was not significantly different from the vehicle infusion. An asterisk represents a statistically significant difference from the other conditions. (A) Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (B) Points represent individual rats across conditions.
Because many rats are not willing to wait 30 seconds for the larger reward, the proportion of adjusting responses tends to be greater in the first half of the sessions (in order to rapidly reduce the waiting time for the large reward), whereas the latter half of the sessions are characterized by more exploitation behavior and fewer adjustments (Fig. 2). The proportion of trials adjusted overall was significantly greater in the first half of the session compared to the second half [F(1,24) = 59.278, p < .01]. Therefore, adjustments in the first and second halves of the testing sessions were analyzed separately.
During the first half of the session, there was no main effect of infusion condition on the proportion of adjustments [F(2,12) = 0.45, p = 0.65], 95% CI [−0.62, 1.5], d = 0.44. The logit-transformed data likewise did not show an effect [F(2,12) = 0.40, p = 0.68]. During the second half of the session, however, a repeated measures ANOVA showed a significant main effect of infusion condition on adjustments [F(2,12) = 9.665, p < 0.01]. Specifically, mean adjustments reached 32% for bilateral inactivation versus 8% for vehicle in the second half. Planned orthogonal contrasts indicated that bilateral inactivation resulted in a significantly higher proportion adjustments in the second half compared to vehicle [F(1,6) = 10.67, p < 0.05)], 95% CI [0.49, 2.94], d = 1.72. Although for the unilateral inactivation condition, this measure did not significantly differ from vehicle [F(1,6) = 3.69, p = 0.10] (Fig. 7). These results were upheld when the data were logit-transformed and analyzed: main effect of infusion condition [F(2,12) = 14.1, p < 0.01] and orthogonal comparison of bilateral inactivation to vehicle [F(1,6) = 20.7, p < 0.01].
Figure 7.
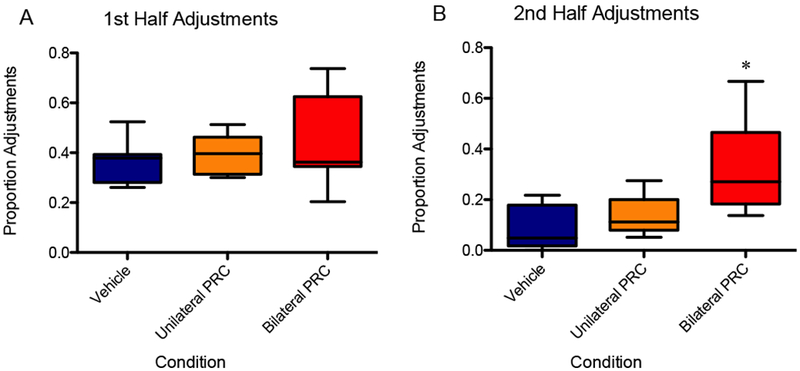
(A) There was not a significant effect of infusion condition on adjustments in the first half of the session. (B) The bilateral inactivation resulted in a significantly higher proportion adjustments in the second half compared to vehicle, although for the unilateral inactivation condition, this measure did not significantly differ from vehicle. An asterisk represents a statistically significant difference from the other conditions. Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (C and D) Points represent individual rats across conditions.
Inactivation of the PRC did not have a significant effect on the proportion of trials in which the rat interrupted; i.e., bypassed the delayed reward in favor of initiating the next trials [F(2,12) = 1.63, p = 0.24], 95% CI [−0.40, 1.75], d = 0.67 (Fig. 8). The logit-transformed data likewise did not show an effect [F(2,12) = 1.73, p = 0.22].
Finally, we were interested in the effect of perirhinal inactivation on reaction time and vicarious trial and error events at the choice point. To maintain a within-subjects analysis, reaction time and VTE data were considered for only the rats for which video recordings were complete for all conditions. Of the seven rats, four had complete recordings, so these four rats are compared in the following section. In response to bilateral PRC inactivation, the average reaction time at the choice point appeared to increase. Specifically, the mean reaction time was 1.08 seconds for the vehicle condition versus 1.82 seconds for bilateral inactivation. The difference did not reach statistical significance [F(2,6) = 3.39, p = 0.10], 95% CI [−0.12, 2.98], d = 1.43 (Fig. 9), nor when the data were logit-transformed and analyzed [F(2,6) = 3.87, p = 0.08].
Figure 9.
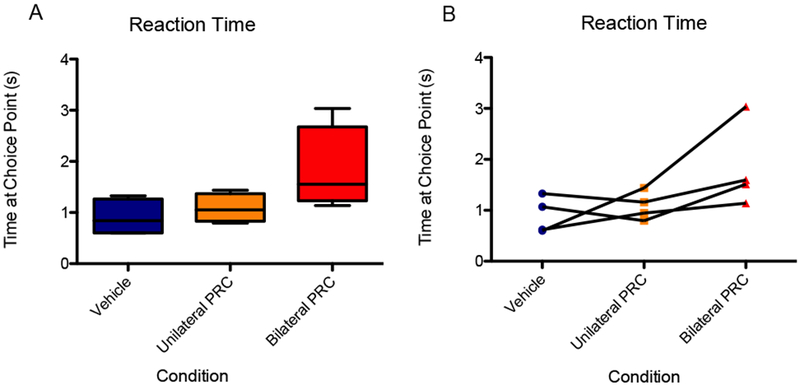
The average reaction time at the choice point appeared higher for the bilateral PRC inactivation condition compared to the vehicle and unilateral PRC inactivation conditions, although the effect of infusion condition was not statistically significant. (A) Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (B) Points represent individual rats across conditions.
Figure 10 shows the proportion of trials in which the rat exhibited VTE behavior as a function of PRC inactivation condition. A repeated measures ANOVA showed a significant main effect of PRC inactivation on VTE behavior [F(2,6) = 5.30, p < 0.05]; although in a planned orthogonal contrast, VTE for the bilateral inactivation was not significantly higher than VTE for vehicle [F(1,3) = 6.52, p = 0.084], 95% CI [0.04, 3.24], d = 1.64. However, upon logit transformation, the data revealed a significant main effect of PRC inactivation condition on VTE [F(2,6) = 6.36, p < 0.05] as well as significantly more VTE in the bilateral condition compared to vehicle [F(1,3) = 12.69, p < 0.05]. Considering the second half of the session, a repeated measures ANOVA showed a significant main effect of PRC inactivation on VTE behavior [F(2,6) = 31.41, p < 0.01], and a planned orthogonal contrast confirmed that VTE for the bilateral inactivation was significantly higher than VTE for vehicle [F(1,3) = 46.56, p < 0.01], 95% CI [0.57, 4.20], d = 2.38. The effect was not observed, however, in the first half of the session [F(2,6) = 2.19, p = 0.19], 95% CI [−0.46, 2.48], d = 1.0, nor upon logit transformation of the VTE data of the first half [F(2,6) = 1.18, p = 0.37].
Figure 10.
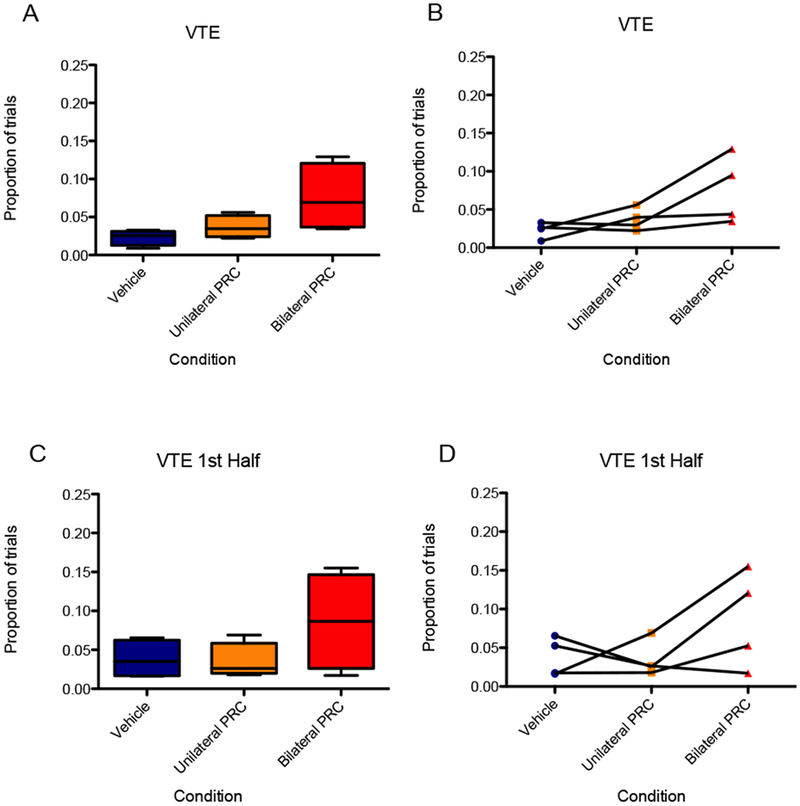
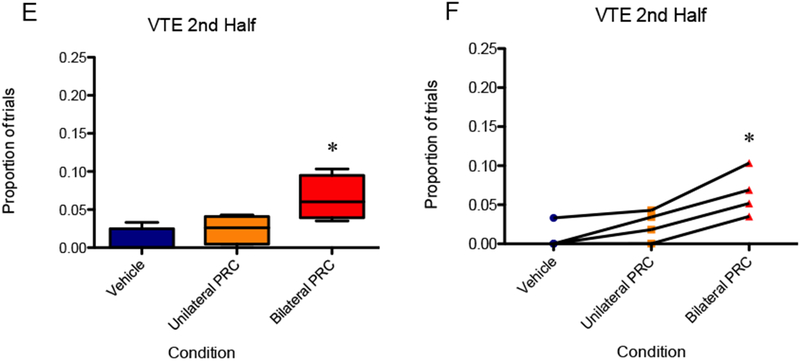
(A and B) VTE for the bilateral inactivation in the 30-second initial delay, 3:1 reward condition was not significantly higher than VTE for vehicle. (C and D) There was no significant effect of PRC inactivation on VTE behavior in the first half of the session. (E and F) VTE for the bilateral inactivation was significantly higher than VTE for vehicle in the second half of the session. An asterisk represents a statistically significant difference from the other conditions. (A, C, and E) Boxes represent the interquartile range; lines in the boxes and error bars represent the median and minimum/maximum values. (B, D, and F) Points represent individual rats across conditions.
DISCUSSION
In discussing the nature of the nervous system, Hebb (1958) noted that no psychological function exists in a specific segment of cortex although certain regions can be essential (such as the visual cortex for visual imagery; Nadel and Maurer, 2018). In light of this, it stands to reason that decision making occurs across the anatomical loops of the brain (Buzsáki, 2013; van der Meer et al., 2012). The prefrontal cortex (PFC) has been assigned the role of contributing to value assessment (Baxter et al., 2000; Denburg, Cole, Hernandez, Yamada, Tranel, Bechara, and Wallace, 2007; Gallagher, McMahan, and Schoenbaum, 1999; Izquierdo, Suda, and Murray, 2004; McAlonan and Brown, 2003; Rudebeck, Bannerman, and Rushworth, 2008; Rudebeck and Murray, 2011a; b; Rudebeck et al., 2006) while hippocampal integrity has been assigned to the role of outcome prediction (Buckner, 2010; Davachi and DuBrow, 2015; Johnson, Fenton, Kentros, and Redish, 2009; Johnson, van der Meer, and Redish, 2007; Lisman and Redish, 2009; Wikenheiser and Redish, 2012; 2015; Wikenheiser and Schoenbaum, 2016) as well as spatial navigation (Buzsáki, 2005; Nadel and Hardt, 2004; O’Keefe and Nadel, 1978). Given previous data regarding the roles of hippocampus and PFC in prediction and outcome evaluation, it stands to reason that hippocampal-prefrontal communication would be critical for supporting the deliberative processes involved in spatial inter-temporal choice. While little attention has been given to the perirhinal cortex (PRC) in regards to decision making, this brain structure provides a direct anatomical link between the PFC and hippocampus (Agster and Burwell, 2009; 2013; Burwell and Amaral, 1998). Moreover, sensory input that is integrated in the PRC (Burwell and Amaral, 1998; Bussey et al., 2005; Kent, Hvoslef-Eide, Saksida, and Bussey, 2016) could be critical for integrating and updating information about temporal delay and reward outcomes together with current behavioral state. The results from the present study are supportive of a role for the PRC in decision making. Moreover, it is conceivable that the well-described involvement of the PRC in enabling discriminations between stimuli that share features, that is, resolving perceptual ambiguity (Bartko et al., 2007a; b; Bussey et al., 2002a; 2005; Forwood et al., 2007; Murray and Bussey, 1999) is critical for stabilizing choices between outcomes that share features.
The ability to disambiguate stimuli that share perceptual features is more often tested in the visual domain, in which, once a subject can successfully discriminate between two stimuli, ambiguity is added by including “morphed” stimuli with varying degrees of overlap (Bussey, Saksida, and Murray, 2003). A similar manipulation has been employed using LEGO block objects that can be constructed to have varying degrees of feature overlap (Bartko et al., 2007a; b; Forwood et al., 2007) or odorants of the same chemical class with varying differences in the length of carbon change backbone (Yoder, Gaynor, Burke, Setlow, Smith, and Bizon, 2017; Yoder, Setlow, Bizon, and Smith, 2014). In the current experiment, the analog ambiguity that was manipulated was the temporal delay associated with a large quantity food reward, which needed to be discriminated from a small immediate food reward. In this analogous description, should the delay versus large reward be advantageous (e.g., wait 1 second for 3 pellets), the ambiguity is low, and the animal should optimize for the large reward outcome. The reverse is true when the delay to the large reward is long (the unambiguous optimal choice is the 1 second:1 pellet). Unlike the visual discrimination tasks, however, the optimal behavior is not a binary choice, but rather a balance between delay and reward (e.g., the “ambiguous” situation of 9 seconds for 3 pellets).
Notably, PRC inactivation did not affect the indifference point during spatial intertemporal choice testing (30-second initial delay, 3:1 reward ratio) suggesting that, overall, rats could still relate reward magnitude to delay when given the opportunity to titrate and iteratively correct their behavior. However, there was a significant effect of bilateral PRC inactivation on the number of adjustments (i.e., choosing the same side across consecutive trials) and the proportion of VTE events in the second half of the trials. As PRC lesions result in an inability to discriminate between stimuli that share features (Barense, Bussey, Lee, Rogers, Davies, Saksida, Murray, and Graham, 2005; Bartko et al., 2007a; Bussey, Saksida, and Murray, 2002b), our results suggest that PRC inactivation impairs the animals ability to disambiguate between the delay-reward associations at the choice point, resulting in less stable behavior. Both the increased time at the choice point and the number of VTEs are indicative of increased deliberation (Blumenthal, Steiner, Seeland, and Redish, 2011; Breton, Seeland, and Redish, 2015; Hasz and Redish, 2018; Papale et al., 2012; Redish, 2016; Schmidt, Papale, Redish, and Markus, 2013), suggesting that the animal is unable to clearly choose the optimal delay-to-reward ratio on a trial-by-trial basis, although errors are corrected throughout the entire session. This conclusion is supported by the number of increased adjustments in the second half in the bilateral PRC inactivation conditions relative to control conditions. While this interpretation of the present data are consistent with literature regarding PRC function, it is also conceivable that when the PRC was inactivated the animals were still able resolve ambiguity, but could not make a choice based upon that resolution. In other words, their choice behavior just became less stable without PRC activity.
Importantly, the observation that the PRC may be critical for stable deliberative decision making speaks to the larger phenomenon of exaptation. Specifically, although the PRC may have evolved against one background, it has an inherent trait that makes it optimal to be co-opted for a different task (Gould and Vrba, 1982). In terms of the perirhinal cortex, evaluation of equivalences and prediction of future outcomes likely takes precedence over “object representation”. The principal challenge in behavior is the solution to the “stimulus equivalence” problem (the primary topic of consideration in Hebb, 1949). Simply, stimulus equivalence is the ability of the nervous system to perceive an object as the same despite changing position and orientation when conveyed to the retina. This concept complements the theory of perirhinal view invariant object representation (Devlin and Price, 2007). However, the concept of stimulus equivalence is not so narrow as to only be discussed in terms of vision and objects. Rather, equivalences can be evaluated across sensory modalities (e.g., Holdstock, Hocking, Notley, Devlin, and Price, 2009). For example, while two glasses of milk may look the same, should one glass smell sour, the adaptive outcome would be to alter the representation. In terms of optimal decision making, the concept of equivalences becomes slightly more abstract. At a derivative, it may be simply described as comparing the outcome of a previous trial to what may occur in the future, with the perirhinal cortex fundamentally involved in answering the question, “Does the same behavior yield equivalent results although the contingencies have changed?” When presented with a choice of a small reward with a short delay versus a large reward at an 11-second delay (ambiguous choice for our rats in the present experiment), the comparison comes in the domain of potential delay-reward associations. This comparison of potential future events necessitates the use of a prediction mechanism to evaluate the options, such as vicarious trial and error (for review, see Redish, 2016). While VTE is a neurophysiological event, it is the reentrant circuits that anatomically support the ability to anticipate the future (Buzsáki, 2013; Nadel and Maurer, 2018). Should the activity in the well-trained loops move at a time-step faster than what occurs in the real world, the animal inherently has the ability to anticipate the future. As the perirhinal cortex resides at the nexus of recurrent loops, it becomes essential to any cognitive process by which potential outcomes need to be disambiguated in support of deliberation (Murray and Bussey, 1999). Should the behavior be visual in nature, then PRC neural activity will most strongly correlate with objects and visual features. If the task is olfactory based, then the neurons will exhibit firing patterns to distinct odorants. However, these features – as Hebb warned (1958) – do not define the function of the region. Rather, it is necessary to move away from a functional specialization model of the nervous system towards one that describes how regions act in concert to optimize behavior. This path forward will move towards an understanding of how neurons work in concert to support higher cognition and how these processes go awry in mental health disorders (Allen and Collins, 2013).
Supplementary Material
Highlights:
The perirhinal cortex supports recognition memory and perception
The Anatomical connectivity of perirhinal cortex point to a role in decision making
Spatial delay discounting task assesses dynamic reward valuation
Perirhinal inactivation results in less stable choices on temporal discounting
The Perirhinal cortex may be critical for stabilizing choice and reward outcome
Acknowledgements:
This work was supported by NIH grants AG055544 and MH109548 and the McKnight Brain Research Foundation (MBRF), including a MBRF Fellowship to SAJ. We thank Wendy Mandell, John Bateh, Nicole Williamson, Huzaifa Wasanwala, Sarah Rukmini Garcia-Sosa, Ethan Wildes, Patreis Young, Shiela Chancoco, Courtney Desrosiers, Kaeli Fertal, Sean Turner, Zoe Pointer, and Julia Silliman for help running and scoring behavioral sessions and Justin Bausch, Charly Elvira-Martin, Jack Kennedy, and Nick Topper for help with maze design and implementation.
Footnotes
These authors contributed equally to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- Abela AR, & Chudasama Y (2013). Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci, 37, 640–647. [DOI] [PubMed] [Google Scholar]
- Abela AR, & Chudasama Y (2014). Noradrenergic α2A-receptor stimulation in the ventral hippocampus reduces impulsive decision-making. Psychopharmacology (Berl), 231, 521–531. [DOI] [PubMed] [Google Scholar]
- Agster KL, & Burwell RD (2009). Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus, 19, 1159–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, & Burwell RD (2013). Hippocampal and subicular efferents and afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Behav Brain Res, 254, 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G (1975). Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull, 82, 463–496. [DOI] [PubMed] [Google Scholar]
- Allen PG, & Collins FS (2013). Toward the final frontier: the human brain, The Wall Street Journal [Google Scholar]
- Bailey MR, Simpson EH, & Balsam PD (2016). Neural substrates underlying effort, time, and riskbased decision making in motivated behavior. Neurobiol Learn Mem, 133, 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, & Graham KS (2005). Functional specialization in the human medial temporal lobe. Journal of Neuroscience, 25, 10239–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, & Bussey TJ (2007a). Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci, 27, 2548–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, & Bussey TJ (2007b). Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem, 14, 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Hadfield WS, & Murray EA (1999). Rhinal cortex lesions produce mild deficits in visual discrimination learning for an auditory secondary reinforcer in rhesus monkeys. Behav Neurosci, 113, 243–252. [DOI] [PubMed] [Google Scholar]
- Baxter MG, & Murray EA (2002). The amygdala and reward. Nat Rev Neurosci, 3, 563–573. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, & Murray EA (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci, 20, 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal A, Steiner A, Seeland K, & Redish AD (2011). Effects of pharmacological manipulations of NMDA-receptors on deliberation in the Multiple-T task. Neurobiol Learn Mem, 95, 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton YA, Seeland KD, & Redish AD (2015). Aging impairs deliberation and behavioral flexibility in inter-temporal choice. Front Aging Neurosci, 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2010). The role of the hippocampus in prediction and imagination. Annu Rev Psychol, 61, 27–48, C21–28. [DOI] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol, 398, 179–205. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2002a). Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci, 15, 365–374. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2002b). Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience, 15, 365–374. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2003). Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’vs.‘perceptual-mnemonic’views of perirhinal cortex function European Journal of Neuroscience, 17, 649–660. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2005). The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Q J Exp Psychol B, 58, 269–282. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2005). Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus, 15, 827–840. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2013). Cognitive neuroscience: Time, space and memory. Nature, 497, 568–569. [DOI] [PubMed] [Google Scholar]
- Cardinal RN (2006). Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw, 19, 1277–1301. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, & Howes NJ (2005). Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, & Cardinal RN (2005). Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, & DuBrow S (2015). How the hippocampus preserves order: the role of prediction and context. Trends Cogn Sci, 19, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, & Wallace RB (2007). The orbitofrontal cortex, real-world decision making, and normal aging. Ann N Y Acad Sci, 1121, 480–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, & Price CJ (2007). Perirhinal contributions to human visual perception. Curr Biol, 17, 1484–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, & Ryan CN (1996). The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl), 128, 161–170. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, & Winstanley CA (2008). Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, affective & behavioral neuroscience, 8, 375–389. [DOI] [PubMed] [Google Scholar]
- Fobbs WC, & Mizumori SJ (2017). A framework for understanding and advancing intertemporal choice research using rodent models. Neurobiol Learn Mem, 139, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Bartko SJ, Saksida LM, & Bussey TJ (2007). Rats spontaneously discriminate purely visual, two-dimensional stimuli in tests of recognition memory and perceptual oddity. Behav Neurosci, 121, 1032–1042. [DOI] [PubMed] [Google Scholar]
- Frost R, & McNaughton N (2017). The neural basis of delay discounting: A review and preliminary model. Neurosci Biobehav Rev, 79, 48–65. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, & Schoenbaum G (1999). Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci, 19, 6610–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, & Vrba ES (1982). Exaptation—a missing term in the science of form. Paleobiology, 8, 4–15. [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, & Fry A (1996). Temporal discounting in choice between delayed rewards: the role of age and income. Psychol Aging, 11, 79–84. [DOI] [PubMed] [Google Scholar]
- Hasz BM, & Redish AD (2018). Deliberation and Procedural Automation on a Two-Step Task for Rats. Frontiers in integrative neuroscience, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D (1949). The organization of behavior. 1949 New York: Wiely. [Google Scholar]
- Hebb DO (1958). A textbook of psychology. Philadelphia, PA: W. B. Saunders. [Google Scholar]
- Holdstock JS, Hocking J, Notley P, Devlin JT, & Price CJ (2009). Integrating visual and tactile information in the perirhinal cortex. Cereb Cortex, 19, 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, & Murray EA (2004). Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci, 24, 7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Fenton AA, Kentros C, & Redish AD (2009). Looking for cognition in the structure within the noise. Trends Cogn Sci, 13, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, & Redish AD (2007). Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol, 17, 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Hvoslef-Eide M, Saksida LM, & Bussey TJ (2016). The representational-hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiol Learn Mem, 129, 99–106. [DOI] [PubMed] [Google Scholar]
- Lisman J, & Redish AD (2009). Prediction, sequences and the hippocampus. Philos Trans R Soc Lond B Biol Sci, 364, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, & Robbins TW (2011). Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci, 31, 6398–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, & Brown VJ (2003). Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res, 146, 97–103. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, & Setlow B (2010). Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral neuroscience, 124, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB, & Zeiss AR (1972). Cognitive and attentional mechanisms in delay of gratification. J Pers Soc Psychol, 21, 204–218. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, & Anderson IM (2002). Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl), 160, 290–298. [DOI] [PubMed] [Google Scholar]
- Murray EA, & Bussey TJ (1999). Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci, 3, 142–151. [DOI] [PubMed] [Google Scholar]
- Nadel L, & Hardt O (2004). The spatial brain. Neuropsychology, 18, 473–476. [DOI] [PubMed] [Google Scholar]
- Nadel L, & Maurer AP (2018). Recalling Lashley and Reconsolidating Hebb. Hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The Hippocampus as a Cognitive Map. Oxford: Claredon Press. [Google Scholar]
- Papale AE, Stott JJ, Powell NJ, Regier PS, & Redish AD (2012). Interactions between deliberation and delay-discounting in rats. Cogn Affect Behav Neurosci, 12, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, Redish AD, Smith KS, & Voorn P (2009). Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. J Neurosci, 29, 12831–12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Curr Biol, 23, R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, & Green L (1972). Commitment, choice and self-control. J Exp Anal Behav, 17, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, & Montague PR (2008). A framework for studying the neurobiology of valuebased decision making. Nat Rev Neurosci, 9, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD (2016). Vicarious trial and error. Nat Rev Neurosci, 17, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, & Rushworth MF (2008). The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci, 8, 485–497. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, & Rushworth MF (2008). Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci, 28, 13775–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, & Murray EA (2011a). Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values. Ann N Y Acad Sci, 1239, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, & Murray EA (2011b). Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci, 31, 10569–10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, & Rushworth MF (2006). Separate neural pathways process different decision costs. Nat Neurosci, 9, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Papale A, Redish AD, & Markus EJ (2013). Conflict between place and response navigation strategies: effects on vicarious trial and error (VTE) behaviors. Learn Mem, 20, 130–138. [DOI] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, & Bizon JL (2010). Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging, 31, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M, Kurth-Nelson Z, & Redish AD (2012). Information processing in decision-making systems. Neuroscientist, 18, 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, & Redish AD (2012). Hippocampal sequences link past, present, and future. Trends Cogn Sci, 16, 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, & Redish AD (2015). Hippocampal theta sequences reflect current goals. Nat Neurosci, 18, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, & Schoenbaum G (2016). Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci, 17, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Gaynor LS, Burke SN, Setlow B, Smith DW, & Bizon JL (2017). Interaction between age and perceptual similarity in olfactory discrimination learning in F344 rats: relationships with spatial learning. Neurobiol Aging, 53, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder WM, Setlow B, Bizon JL, & Smith DW (2014). Characterizing olfactory perceptual similarit using carbon chain discrimination in Fischer 344 rats. Chem Senses, 39, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, & Winstanley CA (2010). Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology, 211, 87–98. [DOI] [PubMed] [Google Scholar]
- Hickman DL, Swan M (2010) Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci JAALAS 49:155–159. [PMC free article] [PubMed] [Google Scholar]
- Papale AE, Stott JJ, Powell NJ, Regier PS, Redish AD (2012) Interactions between deliberation and delaydiscounting in rats. Cogn Affect Behav Neurosci 12:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman-Culleré MH, Foltz CJ (1999) Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


