Abstract
Atypical hemolytic uremic syndrome and thrombotic thrombocytopenic purpura have traditionally been considered separate entities. Defects in the regulation of the complement alternative pathway occur in atypical hemolytic uremic syndrome, and defects in the cleavage of von Willebrand factor (VWF)-multimers arise in thrombotic thrombocytopenic purpura. However, recent studies suggest that both entities are related as defects in the disease-causing pathways overlap or show functional interactions. Here we investigate the possible functional link of VWF-multimers and the complement system on endothelial cells. Blood outgrowth endothelial cells (BOECs) were obtained from 3 healthy individuals and 2 patients with Type 3 von Willebrand disease lacking VWF. Cells were exposed to a standardized complement challenge via the combination of classical and alternative pathway activation and 50% normal human serum resulting in complement fixation to the endothelial surface. Under these conditions we found the expected release of VWF-multimers causing platelet adhesion onto BOECs from healthy individuals. Importantly, in BOECs derived from patients with von Willebrand disease complement C3c deposition and cytotoxicity were more pronounced than on BOECs derived from normal individuals. This is of particular importance as primary glomerular endothelial cells display a heterogeneous expression pattern of VWF with overall reduced VWF abundance. Thus, our results support a mechanistic link between VWF-multimers and the complement system. However, our findings also identify VWF as a new complement regulator on vascular endothelial cells and suggest that VWF has a protective effect on endothelial cells and complement-mediated injury.
Keywords: atypical hemolytic uremic syndrome, blood outgrowth endothelial cells, complement, thrombotic microangiopathy, thrombotic thrombocytopenic purpura, von Willebrand factor
Atypical hemolytic uremic syndrome (aHUS) and thrombotic thrombocytopenic purpura (TTP) define variants of the extending spectrum of thrombotic microangiopathies (TMAs). aHUS occurs due to the dysfunctional regulation of the complement alternative pathway (AP) caused by mutations or inhibiting autoantibodies affecting the function of complement activators or regulators with subsequent microvascular endothelial cell (EC) injury and the formation of platelet microthrombi.1 TTP, by contrast, is caused by either a deficiency of a dis- integrin and metalloproteinase with a thrombospondin type 1 motif member 13 (ADAMTS13) or autoantibodies inhibiting its function, allowing ultra-large VWF-multimers to be released from ECs into the bloodstream, bind platelets, and produce occlusive thrombi.2,3
Traditionally aHUS and TTP were considered 2 distinct pathologies, although both ultimately lead to a microangiopathic hemolytic anemia.4 However, recognizing their significant clinical overlap, the concept of a disease spectrum with shared underlying pathogenetic mechanisms, for instance, complement AP defects, was also proposed.5,6 The finding of decreased C3 levels7 and increased complement AP activity in TTP patients,8 along with the experimental observation that serum from TTP patients can result in complement deposition on ECs,9 further supports such an overlap. A recent study found that 80% of an aHUS patient cohort carried at least 1 non-synonymous change in ADAMTS13.10 Recent studies further linked the complement AP and its principal regulator, complement factor H (CFH), with VWF and its cleaving protease, ADAMTS13.11–14 Most recently, the assembly and activation of complement AP components was shown on ultra-large VWF-multimer strings secreted from and anchored to ECs (human umbilical vein ECs [HUVECs]).11
We aimed to explore the possible functional link between VWF-multimers and the complement system using BOECs from patients with Type 3 von Willebrand disease (VWD) lacking functional VWF.15 We utilized a standardized strategy of exposing BOECs to a complement challenge,16–18 which allowed us to study EC responses ultimately leading to a TMA phenotype (i.e., complement deposition, VWF release, platelet adhesion, and EC injury). Unexpectedly, we found that endothelial complement fixation was increased on VWD BOECs, and those pathologic consequences of complement fixation such as EC injury and cytotoxicity as well as platelet adhesion were enhanced, findings in keeping with VWF acting to downregulate complement-mediated EC injury. This has physiological implications since (primary) glomerular ECs (GECs) show heterogeneous VWF expression both in vitro and in vivo.
RESULTS
BOECs demonstrate a stable EC phenotype and express the key membrane-anchored complement regulators
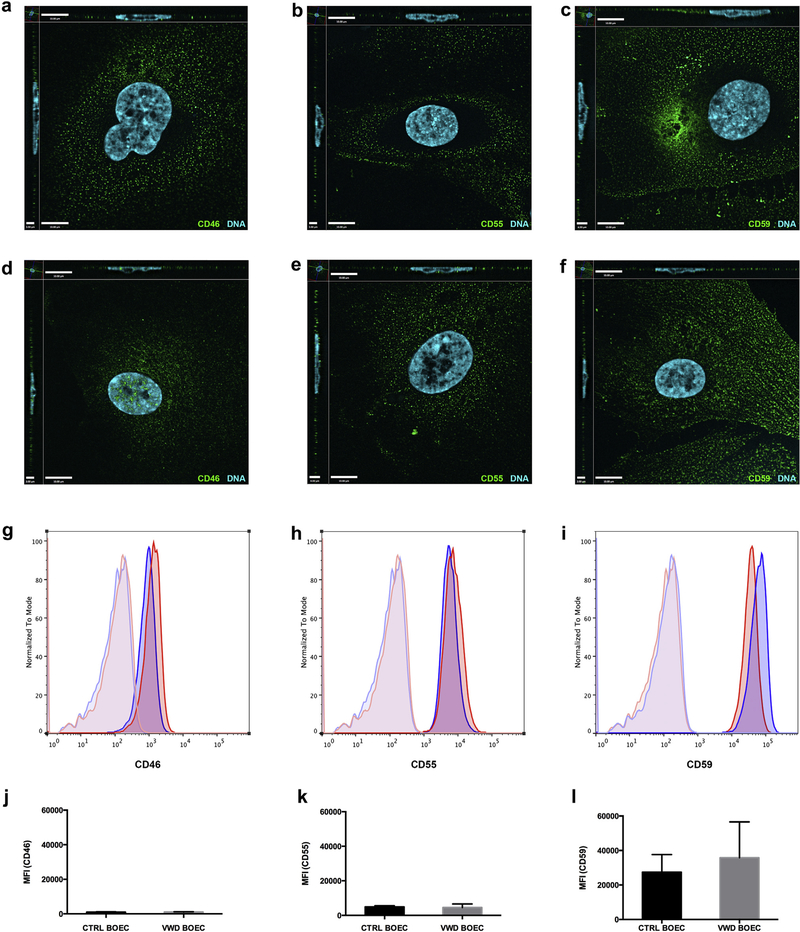
BOECs are late outgrowth endothelial colony-forming cells derived from circulating endothelial progenitor cells isolated from peripheral blood.19,20 For this study we generated BOECs from 3 healthy adult donors and 2 patients with VWD and confirmed their EC phenotype and stability in an early and late passage (Supplementary Figure S1A–D). Using flow cytometry, we determined that the expression level of the membrane-anchored complement regulators CD46, CD55, and CD59 was similar in control and VWD BOECs (Figure 1). Using reverse transcription quantitative real-time PCR (RT-qPCR), we also confirmed that the baseline gene expression of these complement regulators was similar between controls and HUVECs (Supplementary Figure S1E). Of note, in both BOECs and HUVECs the expression level of CD59 was about 10-fold higher as compared to CD46 and CD55.
Figure 1|. Complement regulators are present on control and VWD BOECs.
By immunofluorescence (a–f) and flow cytometry (g–l) the surface expression of complement regulators CD46, CD55, and CD59 was detected. (a–c) Control blood outgrowth endothelial cells (BOECs) and (d–f) von Willebrand disease (VWD) BOECs were seeded on cover slips, stained for CD46 (a,d), CD55 (b,e), and CD59 (c,f) and the representative secondary antibody (Alexa Fluor 488, green), and they were imaged using a fluorescence microscope. Cell nuclei were stained using Hoechst stain (blue). (g–l) For flow cytometry, cells were trypsinized off a 6-well plate, and incubated with primary antibody (CD46, CD55, or CD59) and respective secondary Alexa Fluor 488. Surface expression of complement regulators was acquired using an Attune Acoustic Focusing Cytometer (Invitrogen) and analyzed using FlowJo software. A similar surface expression of complement regulators on VWD BOECs (blue) compared to control BOECs (red) was observed, as shown in representative images (g–i). The unstained controls are displayed in light blue for VWD BOECs and light red for control BOECs. (j–l) Comparison of the median fluorescence intensity (MFI) of 3 experiments (n = 3 for control BOECs and n = 2 for VWD BOECs) did not show a significant difference in surface expression of CD46, CD55, and CD59. (P = 0.7; 2-way analysis of variance.)
Type 3 VWD BOECs lack VWF expression
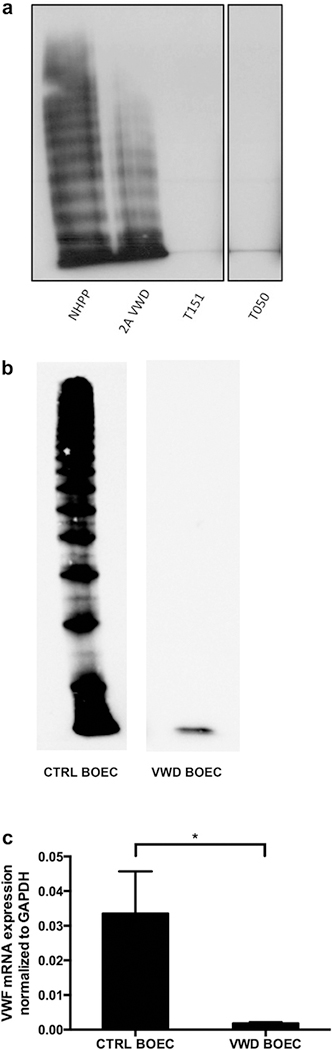
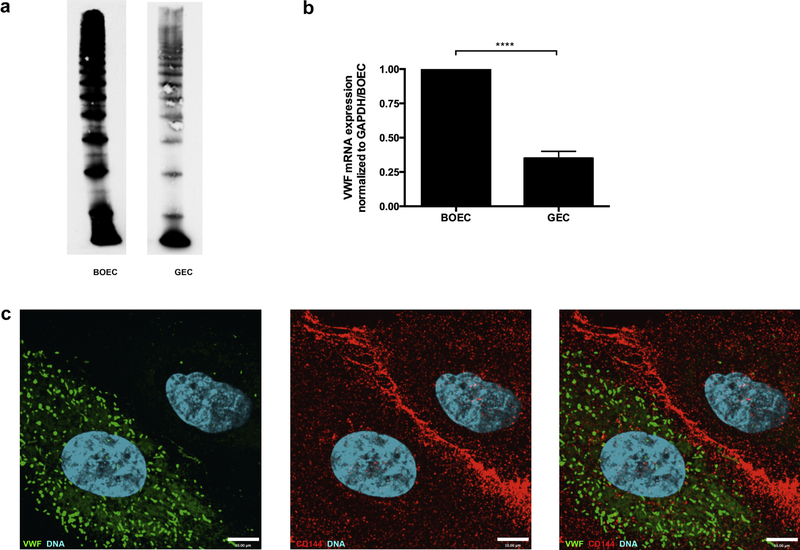
VWF was lacking from plasma of Type 3 VWD patients (Figure 2a, lanes 3 and 4). In addition, VWF was found in cell lysates of control BOECs but lacking from VWF BOECs (Figure 2b), and VWF mRNA expression in Type 3 VWD BOECs was only 5.6% of that in controls (Figure 2c).
Figure 2|. VWD BOECs lack VWF.
(a) Multimer analysis of the Type 3 von Willebrand disease (VWD) patient plasma (T151 and T050), compared with normal human pooled plasma (NHPP) and Type 2A VWD plasma. Both Type 3 VWD plasma samples lack von Willebrand factor (VWF)-multimers. Images are taken from the same gel with the black lines indicating separation from lanes that were not included in this study. (b) Detection of VWF in control and VWD blood outgrowth endothelial cell (BOECs) showed a decreased amount of protein in lysates of VWD patient BOECs. (c) mRNA expression levels of VWF were determined by RT-qPCR. VWD BOECs showed minimal VWF mRNA expression (*P < 0.05; paired t-test). VWF mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Complement fixation on ECs is achieved by recruitment of both the classical and alternative pathways
We established a method of complement fixation on ECs using (blocking) antibodies specific to the membrane-anchored complement regulators CD46, CD55, and CD59 followed by exposure to 50% normal human serum (NHS) in serum-free media (SFM). Of note, the use of anti-CD46 or -CD55 alone was not sufficient to enhance C3c deposition. The use of anti-CD59 antibody, however, was alone able to significantly increase C3c deposition, and the use of all 3 antibodies was additive (Supplementary Figure S2A, B). Using 50% NHS was more effective than 25% or 10% NHS (Supplementary Figure S2E). This method combines the effects of complement induction via sensitization (via the classical pathway) and complement amplification (via the alternative pathway [AP])—a strategy that has previously been utilized.16–18 The contribution of the classical pathway was confirmed using C1q-deficient NHS (CompTech, Tyler, TX), which reduced EC complement (C3c) fixation by ~ 80% (Supplementary Figure S2C, D). C5b-9 deposition on ECs was determined by immunofluorescence (Supplementary Figure S2F), which confirmed full activation of the complement cascade.
Given the ~ 10-fold higher surface expression of CD59 on BOECs (confirmed by both RT-qPCR and flow cytometry) (Supplementary Figure S1E; data not shown for flow cytometry), the fact that the monoclonal anti-CD59 antibody is an IgG2b—an isotype known to activate complement via the classical pathway—and the fact that the mouse monoclonal antibodies to CD46 and CD55 are IgG1 and typically non-complement-activating, complement fixation to BOECs in our model is likely due to anti-CD59 antibody-initiated classical pathway activation.21 Of note, complement fixation was also achieved by incubating BOECs with a non–complement-specific, rabbit polyclonal anti-β2-micro-globulin antibody (Supplementary Figure S3). In keeping with their lower expression level, antibodies to PECAM/CD31 (mouse monoclonal IgG1) and endoglin/CD107a (mouse monoclonal IgG2a) were not able to induce complement fixation (data not shown).
Taken together, we have established a reliable and reproducible method of complement fixation on ECs.
Complement fixation on ECs results in VWF release
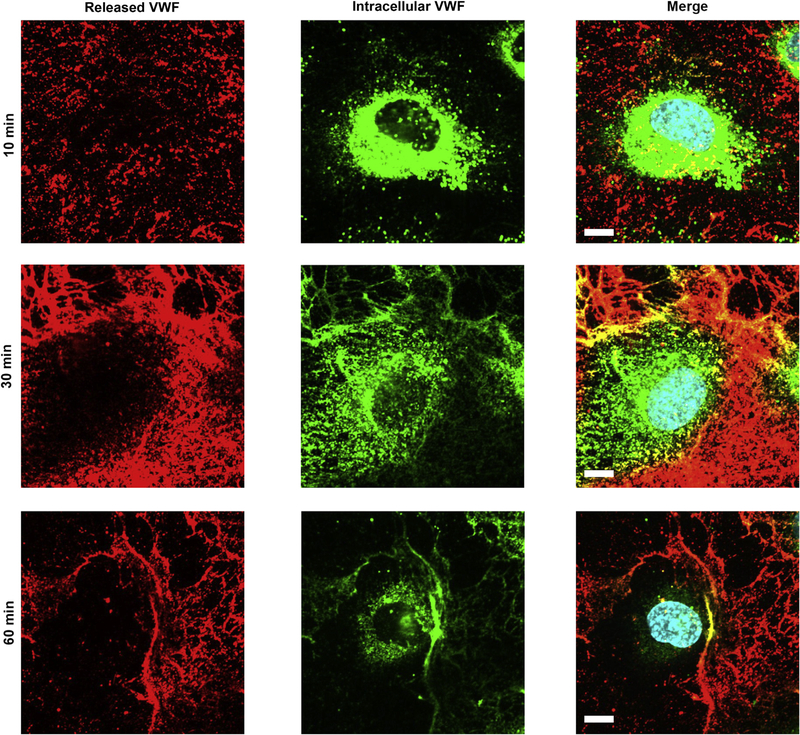
VWF, stored in Weibel–Palade bodies, was released upon complement fixation. By using 2 different antibodies against VWF we were able to identify stored VWF (cells permeabilized) and released VWF (cells non-permeabilized). We observed that over time VWF was released, and after 60 minutes control BOECs were almost completely depleted of (intracellular) VWF (Figure 3).
Figure 3|. Complement fixation results in VWF release.
Von Willebrand factor (VWF) release from control blood outgrowth endothelial cells (BOECs) was detected via immunofluorescence. Control BOECs were treated with CD46, CD55, and CD59 antibody for 20 minutes, followed by 50% normal human serum for 10, 30, and 60 minutes. Cells were fixed with 4% paraformaldehyde, followed by sheep anti-VWF (red). Subsequently, cells were permeabilized with 0.2% Triton in phosphate-buffered saline and incubated with rabbit anti-VWF (green). VWF staining before permeabilization identifies secreted VWF, VWF staining after permeabilization intracellular VWF. Images were taken using an IX81 inverted fluorescence microscope (Olympus Corp., Tokyo, Japan) with a 60/1.35 oil immersion objective and a C9100–13 back-thinned EM-CCD camera (Hamamatsu Photonics, Hamamatsu City, Shizuoka Pref., Japan) with a CSU X1 spinning disk confocal scan head (Yogokawa, Yokogawa Canada, Inc., Alberta, Canada). Bar = 10 μm.
Complement fixation on BOECs results in platelet adhesion via VWF
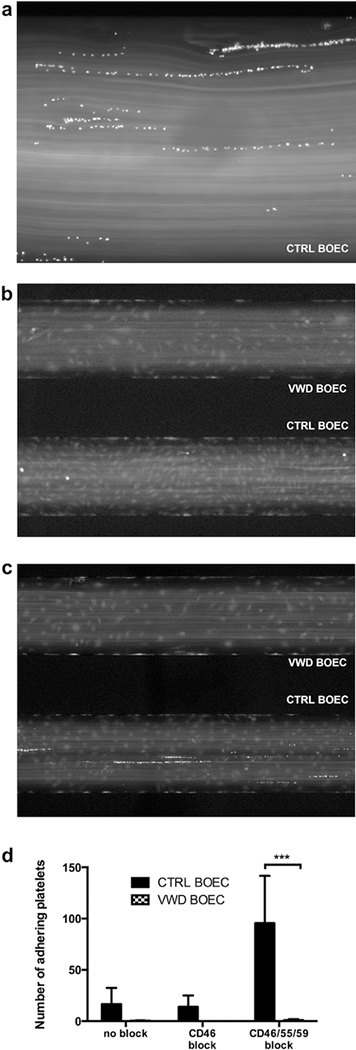
In aHUS dysregulation of the complement AP is hypothesized to ultimately lead to EC activation, a procoagulant endothelium, and platelet aggregation. To investigate the functional role of VWF in this context, we quantified platelet adhesion to complement-challenged BOECs grown in a microfluidic flow chamber comparing control to VWD BOECs. Calcein-labeled platelets (15 × 107 per ml) were perfused at 2 dynes/cm2 for 10 to 20 minutes over control and VWD BOECs. Platelet adhesion was observed on VWF strings (Figure 4a) when BOECs were treated with 50% NHS, a phenomenon that was enhanced after EC preincubation with CD46, CD55, and CD59 antibodies (20 platelets per high-power field vs. 84 platelets per high-power field at X4 magnification; P < 0.05). Of note, platelets adhered only to control BOECs and not to VWD BOECs devoid of VWF (P < 0.001) (Figure 4b–d). We conclude from these results that platelet adhesion to BOECs in response to complement fixation is VWF dependent.
Figure 4|. Complement-induced platelet adhesion.
(a) In a fluidic system platelet adhesion on control blood outgrowth endothelial cells (BOECs) exposed to complement (treatment as in c) occurred on von Willebrand factor (VWF) strings (original magnification X20). (b,c) Von Willebrand disease (VWD) (upper channel) and control BOECs (lower channel) were exposed to 50% normal human serum for 1 hour without (b) or with (c) CD46, CD55, and CD59 treatment; platelets (15 × 107 per ml, 100 μl per well, in Tyrodes buffer) were perfused at 2 dynes/cm2. No platelet adhesion was observed on VWD BOECs. (d) Platelet adhesion was analyzed by counting adherent platelets of 4 random pictures per channel using ImageJ software. Significantly more platelets were seen to adhere in control BOECs as compared to VWD BOECs devoid of VWF. (N = 3; ***P < 0.001; 2-tailed analysis of variance; Sidak's multiple comparisons test.) Pictures were taken with a Nikon Eclipse Ti camera at X4 (b,c) and X20 (a) original magnification after 5 minutes.
Complement fixation on BOECs is increased in the absence of VWF
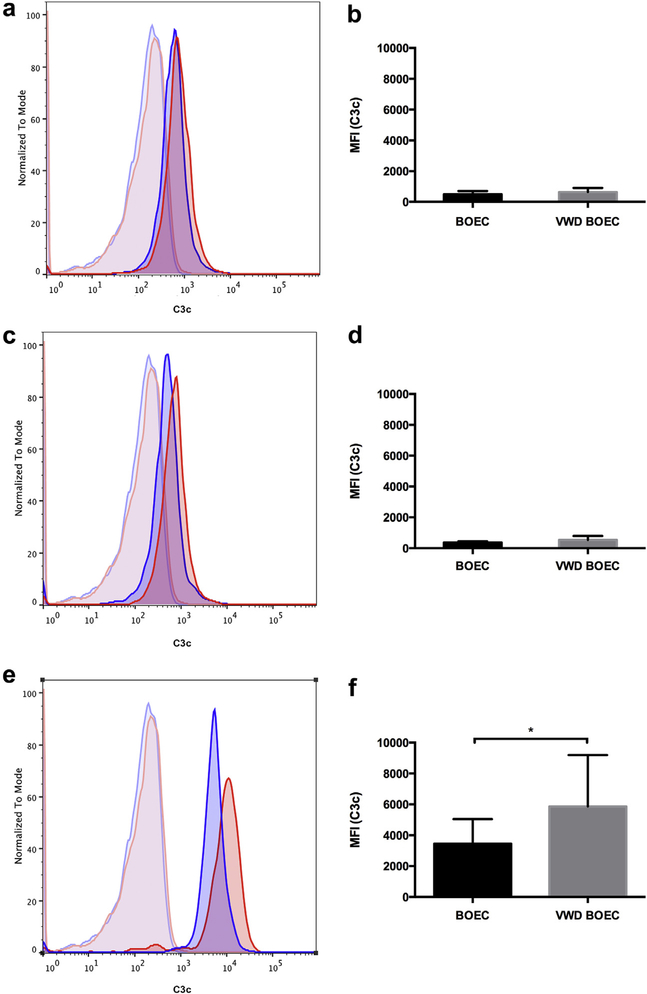
To investigate the functional relevance of VWF for complement fixation on ECs, we performed flow cytometry detecting C3c deposition on control and VWD BOECs as follows: (i) 50% NHS, (ii) functional blockade of 1 membrane-anchored complement regulator (CD46) with 50% NHS, and (iii) combined sensitization and functional blockade using monoclonal antibodies to the 3 main complement regulators (CD46, CD55, and CD59) with 50% NHS. We observed an increase of C3c deposition on VWD BOECs after preincubation with CD46, CD55, and CD59 antibodies as compared to control BOECs (i.e., increase in median fluorescence intensity from 3450 ± 1599 to 5861 ± 3332, P < 0.05, N = 5; Figure 5). Thus, under complement stress, the absence of VWF resulted in increased complement fixation.
Figure 5|. Complement deposition on VWD BOECs.
Surface deposition of C3b detected via a polyclonal rabbit anti-C3c antibody was acquired using an Attune Acoustic Focusing Cytometer (Invitrogen) after gating for live and single cells. Experiments were performed using 2 different controls and 2 different von Willebrand disease (VWD) blood outgrowth endothelial cells (BOECs). (a,c,e) Representative figures for control (blue) and VWD BOECs (red) when treated (a) with 50% normal human serum (NHS) alone, (c) after CD46 block, and (e) after CD46, CD55, and CD59 block. Unstained controls for control (light blue) and VWD BOECs (light red). (b,d,f) The median fluorescence intensity (MFI) was calculated using FlowJo software after subtraction of MFI of the unstained sample. No difference was observed when control and VWD BOECs were treated (b) with 50% NHS alone or (d) after CD46 block. (f) A significant increase of C3c deposition was seen in VWD BOECs compared to control BOECs after functional blockade of complement regulators CD46, CD55, CD59, and 50% NHS for 1 hour in both. (N = 5; *P < 0.05; paired t-test.)
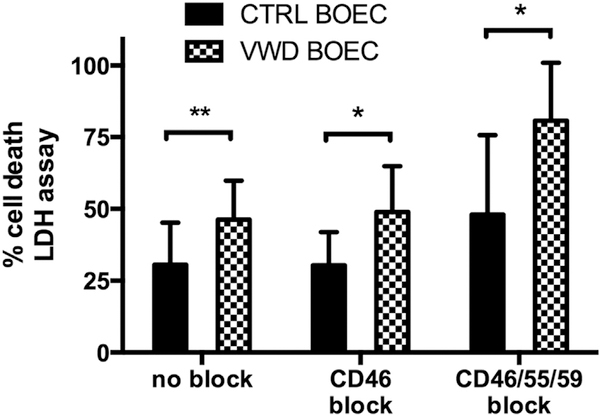
BOEC cytotoxicity is increased in the absence of VWF
Cytotoxicity was quantified by the measurement of lactate dehydrogenase (LDH) released from BOECs into the supernatant. LDH release, reflective of cell damage, correlated with incremental complement fixation. LDH release of VWD BOECs was 46% ± 14% when treated with 10% NHS, 49% ± 16% when treated with 10% NHS after treatment with the anti-CD46 antibody, and 81% ± 20% when treated with 10% NHS after preincubation with the CD46, CD55, and CD59 antibodies, as compared to 31% ± 15%, 30% ± 12%, and 48% ± 28%, respectively, in control BOECs (P < 0.01, P < 0.05, P < 0.05, N = 5; Figure 6).
Figure 6|. Complement-mediated cytotoxicity in VWD BOECs.
Cell death was measured by detection of lactate dehydrogenase (LDH) release in supernatant of control and von Willebrand disease (VWD) blood outgrowth endothelial cells (BOECs) after incubation with 10% normal human serum in serum-free media (SFM) for 4 hours of pretreatment with none, only CD46, or a combination of CD46, CD55, and CD59 blocking antibodies. Cell death was calculated using a standard curve and normalized to positive control (100%), obtained adding lysis buffer 45 minutes prior to incubation end. Data were gathered from 3 different experiments (mean of 4–8 wells per plate) using 2 different control BOECs and 2 different VWD BOECs. A more profound increase of cytotoxicity was observed in VWD BOECs compared to control BOECs in all conditions. (*P < 0.05; **P < 0.01; paired t-test.)
VWF demonstrates a heterogeneous expression pattern in glomerular ECs
As previously described, VWF expression is not homogeneous but demonstrates a heterogeneous expression pattern in ECs.22,23 Studying primary GECs we found decreased VWF gene expression and decreased protein levels (Figure 7a and b). By immunofluorescence, we confirmed that these findings resulted from VWF heterogeneity. VWF was positive in 61.5% of the fixed and stained GECs, only minimally present in 7.7%, but completely absent in 31%. GECs were costained for CD144, CD31, or both, in order to ensure retention of their EC phenotype (Figure 7c).
Figure 7|. Glomerular endothelial cells express less VWF.
(a) Control and glomerular endothelial cell (GEC) lysates were resolved by 1.6% LGT agarose gel and probed for von Willebrand factor (VWF). A lower expression of VWF was seen in GECs. (b) mRNA levels of VWF were measured by RT-qPCR in control BOECs and GECs, and VWF mRNA levels of GECs were only 35% of levels found in control BOECs (****P < 0.0001, 2-tailed t-test, N = 3). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (c) Immunofluorescence labeling of GECs with antibodies detecting VWF (1:1000, green) and VE-cadherin as endothelial cell marker (1:200, red) revealed partial expression of VWF in GECs. Image was taken using an Olympus IX81 inverted fluorescence microscope with a 60/1.35 oil immersion objective and a Hamamatsu C9100–13 back-thinned EM-CCD camera with a Yokogawa CSU X1 spinning disk confocal scan head.
DISCUSSION
Thrombotic microangiopathy (TMA) defines a growing spectrum of diseases sharing the pathologic consequences of the disruption of the integrity of the vascular endothelium. A variety of insults can be responsible for the EC injury, which subsequently recruits the inflammatory and coagulatory systems and leads to transient or permanent impairment or loss of organ function.1,24 Complement-mediated TMA has been defined as atypical hemolytic uremic syndrome (aHUS) and is caused by loss- or gain-of-function mutations in complement factors participating in maintaining the balance between complement activation and regulation, or the presence of autoantibodies with similar functional consequences.1,25
We developed an ex vivo model utilizing blood outgrowth endothelial cells (BOECs), which allows us to study the functional consequences of complement activation on ECs under static and microfluidic conditions. The synchronous blockade of the 3 surface regulators CD46, CD55, and CD59 via specific inhibiting antibodies allowed us to achieve complement fixation on BOECs recruiting the classical and alternative pathways. EC complement fixation resulted in release of VWF as previously also reported by others,26–28 and enhanced platelet adhesion and cell injury. Under fluidic conditions we found platelets adhering to VWF-multimers.29–31
By contrast, until recently, complement involvement in the pathogenesis of TTP was categorically excluded, and genetic or autoimmune defects in the cleavage of VWF-multimers via ADAMTS13 were claimed to be the sole cause.2,4 Evidence for the functional interactions of the 2 systems as recently collected in aHUS and TTP patients10,32,33 and in vitro12–14,34 fundamentally challenges the proposed dichotomy. It was first reported in static conditions that components of the complement cascade, including C3 and C5, were present on EC-secreted VWF-multimers.11 Tati et al. subsequently showed that, under shear conditions, and in the absence of ADAMTS13, C3 bound to histamine-induced VWF-multimers, to VWF adherent platelets, and to ECs secreting VWF.35 While the functional implications remained unclear,35 a complement amplifying effect of the association of the complement AP and VWF was recently proposed.36
We aimed to study the functional interactions between complement and VWF in TMA pathogenesis utilizing BOECs from Type 3 VWD patients. This approach allowed us to take advantage of a naturally occurring EC line expressing essentially no VWF, equivalent to a VWF-null EC, to study the functional interactions of VWF and the complement system.
It was expected that VWF-multimers act as a complement amplifier on ECs, thus less complement activation would be observed on VWD BOECs (cells devoid of VWF). Contrary to our expectation, we found that VWD BOECs demonstrated increased C3c deposition when compared to control BOECs even though the expression level of membrane-anchored complement regulators was not different from that in BOECs. Importantly, VWD BOECs exhibited decreased survival of complement-mediated cytotoxicity. Taken together, our data suggest that VWF is secreted upon complement fixation and serves as a complement regulator on EC surfaces, protecting ECs from complement deposition. This adds to the recent finding that secreted VWF might act as a cofactor for complement factor I-mediated cleavage and inactivation of C3b.37
The vascular endothelium is diverse, including VWF expression.22 In the pulmonary vascular endothelium, VWF expression is strongest in veins and very weak in capillaries.38,39 By contrast, immunohistochemical studies of normal human kidney tissue obtained from biopsies and autopsy specimens showed that the fenestrated glomerular endothelium has patchy positivity for VWF.23 Testing primary GECs, we confirmed less VWF by both RT-qPCR and Western blot. In addition, immunofluorescence revealed that the overall reduced VWF expression level was caused by the complete absence of VWF from about one-third of GECs rather than an overall reduced expression level. As the capacity of VWF to act as endothelial complement regulator will depend on its abundance, heterogeneous VWF expression may explain the susceptibility of the glomerular endothelium to the complement-mediated injury in aHUS. The elevated VWF expression level of the brain microvasculature,40,41 on the other hand, could explain minimal central nervous system involvement during aHUS and predominant central nervous system manifestation during TTP.
In this study we determined that VWF-deficient ECs (Type 3 VWD BOECs) resulted in the upregulation of complement-mediated EC injury, contrary to previous suggestions and recent publications. We were able to demonstrate that VWF-multimers released by ECs contribute to EC protection by acting as complement regulator. The lack of VWF resulted in increased complement deposition and cytotoxicity. Our results provide evidence for a functional link between complement and VWF. Further work is needed to elucidate the exact mechanism of TMA pathogenesis.
MATERIALS AND METHODS
Ethics
Ethics approval was obtained from the Research Ethics Boards of The Hospital for Sick Children, Toronto, Ontario, and Queen’s University, Kingston, Ontario, Canada. Written informed signed consent was obtained from all patients whose samples were used in this study. The study was executed in keeping with the regulations of the Declaration of Helsinki.
Patients
BOECs were isolated from the peripheral blood of 3 healthy adult volunteers (control BOECs) and from 2 patients with Type 3 VWD (VWD BOECs). The VWD patients have been previously reported and lack functional VWF with undetectable plasma VWF antigen levels due to compound heterozygous mutations (c. 876delC, c. 1255C>T; c. 3939G>A, c. 5842+1 G>C) in both patients.15
Blood outgrowth ECs
Control and VWD BOECs were isolated by a standard protocol (Supplementary Materials and Methods)42 and grown in EC growth medium (cEGM-2: Endothelial Basal Medium 2 [EBM-2] supplemented with growth factors [EGM-2 BulletKit]) (Lonza, Walkersville, MD), 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), and 1% Antibiotic-Antimycotic (Gibco, Invitrogen, Life Technologies, Carlsbad, CA). T75 flasks (Sarstedt, Numbrech, Germany) were collagen-coated with 0.05 mg/ml of rat tail collagen type I (Becton Dickinson, Franklin Lakes, NJ) in 0.02 M glacial acetic acid overnight. Cells were kept at 37 °C and in an environment with 5% CO2. BOEC characterization is provided in Supplementary Figure S1. Passages 3 to 14 were used for experiments, and stable expression of VWF across passages was confirmed by RT-qPCR.
Glomerular ECs
Human renal glomerular ECs (GECs) obtained from ScienCell Research Laboratories (San Diego, CA)43,44 were cultured in T75 flasks coated with collagen in proprietary EC medium (ScienCell Research Laboratories, Carlsbad, CA) supplemented with Endothelial Cell Growth Supplement (ScienCell Research Laboratories), 5% fetal bovine serum, and 1% Antibiotic-Antimycotic (Gibco, Invitrogen, Life Technologies). Passages 4 to 7 were used for experiments.
Characterization of membrane-anchored complement regulators
For determination of surface expression of the membrane-anchored complement regulators CD46 (membrane cofactor protein [MCP]), CD55 (decay accelerating factor [DAF]), and CD59 on control BOECs, VWD BOECs, and GECs, we performed both immunofluorescence imaging and flow cytometry. Gene expression was quantified via RT-qPCR on a StepOne System from Life Technologies (Supplementary Materials and Methods). For 1 experiment, HUVECs were used.
Characterization of Type 3 VWD patients
VWF-multimers were analyzed in normal human pooled plasma (NHPP; CRYOcheck, Canada) and compared to Type 3 VWD (absent VWF) and Type 2A VWD (reduced VWF) patients as described previously.45 Briefly, VWF-multimers were separated by sodium dodecylsulfate–agarose electrophoresis, transferred onto a nitrocellulose membrane, detected with anti-human VWF antibody–horseradish peroxidase (DAKO, Glostrup, Denmark), and visualized by luminescence.
In addition, cell lysates of BOECs from Type 3 VWD were examined for VWF-multimers (Figure 2b). Multimer analysis was performed as described before46 in gels of medium resolution (1.6% LGT agarose type VII, Sigma-Aldrich, Munich, Germany). Gel analysis was performed using software provided with the video detection system (AlphaEaseFC Stand Alone software, Alpha Innotech Corp., San Leandro, CA). Samples with the same quantity of VWF:Ag were applied to the gels. The sensitivity of the assay is high compared to that of the VWF:Ag enzyme-linked immunosorbent assay (detection limit 0.038 U/ml = ~2 pg compared to 0.08 U/ml = ~4 pg).
Complement fixation on BOECs
BOECs were exposed to 50% (in exceptional experiments 10%) NHS with AP buffer [7 mM MgCl2, 10 mM EGTA, 144 mM NaCl, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, pH 7.4] or serum-free media (SFM) in the presence of antibodies specifically blocking the membrane-anchored complement regulators CD46 (with monoclonal mouse anti-human anti-CD46 antibody: GB24, IgG116), CD55 (BRIC216, IgG1), and CD59 (BRIC229, IgG2b) (International Blood Group Reference Laboratory, NHS Blood and Transplant, Bristol, UK). Antibodies were used at a concentration of 5 μg/ml and diluted in serum-free cEGM-2 media for 30 minutes in all experiments. The concentration of blocking antibodies used was derived from titration and saturation experiments performed by FACS (Supplementary Figure S4).
C3b deposition was demonstrated by FACS using an antibody to C3c detecting the C3c portion of native C3 and C3b (ab15980 and ab4212, Abcam). Cells were seeded overnight in 6-well plates and incubated with blocking antibodies followed by 50% NHS in AP buffer for 1 hour. Cells were trypsinized for 1 minute and washed twice in flow buffer (1% fetal bovine serum/phosphate-buffered saline). The primary antibody (rabbit anti-C3c, 1:100 dilution, Abcam) in flow buffer and the secondary antibody [R-Phycoerythrin–conjugated AffiniPure F(ab')2 Fragment donkey anti-rabbit IgG (H + L), 1:200 dilution, Jackson ImmunoResearch, West Grove, PA) together with Fixable Viability Dye eFluor780 (1:1000 dilution, eBioscience, San Diego, CA) in phosphate-buffered saline were incubated at 4 °C for 20 minutes. For experiments with C1q-depleted serum we used a C3c-FITC-conjugated antibody from Abcam. C1q-depleted serum was purchased from CompTech.
Assessing functional consequences of complement fixation on BOECs
VWF secretion
Cells were seeded overnight in a 6-well plate and treated with anti-human CD46, CD55, and CD59 blocking antibodies for 20 minutes, followed by 50% NHS in SFM. Primary (sheep anti-human VWF) and secondary antibody were incubated each for 1 hour at room temperature. Cells were then permeabilized with 0.2% Triton in PBS for 5 minutes before rabbit anti-human VWF and the secondary antibody were added for 1 hour each at room temperature.
LDH cytotoxicity assay
BOECs from 3 healthy controls and 2 Type 3 VWD patients were seeded overnight in a 96-well enzyme-linked immunosorbent assay plate (Sarstedt) and grown to confluence. After incubation with antibodies, 10% NHS in serum-free media was added for 4 hours. A Pierce LDH cytotoxicity assay kit (Thermo Fisher Scientific, Waltham, MA) was used according to the manufacturer’s instructions. Optical density was determined using a standard curve, normalized to the positive control (maximal release achieved by solubilizing cells with lysis buffer), and displayed in percent increase over the negative control (10% NHS).
Platelet adhesion assay
Platelets were isolated from healthy controls as described elsewhere.47 Isolated, washed platelets were incubated with 2.5 μM calcein (Life Technologies) for 30 minutes at 37 °C, pelleted at 950g, and resuspended at a concentration of 15 × 107 per ml in Tyrode’s buffer.47 For BOEC–platelet adhesion experiments, control BOECs and VWD BOECs were grown in collagen-coated microfluidic channels of the BioFlux system (Fluxion Biosciences, South San Francisco, CA) as described before.47 For complement challenge experiments, the membrane complement regulators were first blocked with anti-human CD46, CD55, and CD59 functional blocking antibodies diluted in serum-free media and perfused through the channels at 1 to 2 dynes/cm2 for 30 minutes. Fifty percent NHS in AP buffer was subsequently perfused through the BioFlux chambers at a shear rate of 2 dynes/cm2 for 60 minutes. For platelet adhesion assays, 15 × 107 per ml calcein-labeled platelets in Tyrode’s buffer were flowed through the chamber at 2 dynes/cm2 for 10 minutes after BOEC exposure to serum. In each experiment 3 pictures of the individual channels of the flow chamber were taken with a Leitz DM IL microscope (Leica, Wetzlar, Germany) equipped with an Eclipse Ti camera (Nikon, Tokyo, Japan) at X4 magnification, and platelets adhering were manually counted using ImageJ software.
Statistical analysis
Figures were generated using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA) and displayed as mean and SD. Statistical analysis was performed using either 2-way analysis of variance with post hoc analysis or paired t-test. A P value of <0.05 was considered statistically significant. In the figure legends, P values are presented as follows: *≤0.05, **≤0.01, ***≤0.001, ****≤0.0001, not significant (NS) >0.05.
Supplementary Material
Figure S1. Blood outgrowth endothelial cells (BOECs) demonstrate a stable endothelial cell phenotype. (A) The mononuclear layer of cells was isolated from the peripheral blood of healthy adult donors by density gradient centrifugation, resuspended in complete endothelial growth medium (Endothelial Basal Medium 2 [EBM-2] supplemented with growth factors [EGM-2 BulletKit], 10% fetal bovine serum, and 1% Antibiotic-Antimycotic), and seeded onto collagen-coated 6-well tissue culture plates at a concentration of 3 × 107 to 5 × 107 cells per well. During week 1 early outgrowth cell populations predominate. After 2 to 3 weeks typically, a BOEC monolayer was identifiable by their endothelial cobblestone morphology and ready for passaging. (B) An endothelial phenotype was confirmed by demonstrating the presence of Weibel–Palade bodies containing fluorescently labeled von Willebrand factor (VWF, Alexa Fluor 555; red) and P-selectin (CD62P, Alexa Fluor 488; green). (C) RT-qPCR confirms that BOECs maintain their endothelial characteristics when comparing an early (passage 3) and late passage (passage 10) for VWF. N = 3. (D) Flow cytometry confirms their endothelial phenotype, as BOECs are positive for PECAM/CD31 and VE-cadherin/CD144 and negative for the hemangioblast marker CD45 and the immature EPC marker CD14. (E) mRNA expression of von Willebrand factor (VWF) and complement regulators is similar on blood outgrowth endothelial cells (BOECs) and HUVECs. RT-qPCR shows the relative expression of complement regulators on control BOECs as compared to VWF (VWF expression level = 1). Results were compared to HUVECs. No significant difference was observed (2-way analysis of variance with Sidak's multiple comparison test). N = 6 for HUVECs and N = 5 for BOECs.
Figure S2. Complement fixation on endothelial cells (ECs) was achieved by combining the effects of the classical (i.e., initiation via sensitization) and alternative (i.e., amplification) pathways via blocking antibodies specific for the principal membrane-anchored complement regulators CD46, CD55, and CD59 in the presence of 50% normal human serum (NHS). Complement fixation on ECs was quantified via flow cytometric assessment of the deposition of complement activation products on ECs. (A,B) Blockade of CD46 did not result in a significant increase in C3c deposition (detected via rabbit polyclonal anti-C3c and PE) over incubation with serum alone. Combined blockade of CD46, CD55, and CD59, however, resulted in a significant (7-fold) increase of C3 deposition on blood outgrowth endothelial cells (BOECs) (355 ± 193 vs. 2488 ± 2120). (A) A representative image with the following conditions: unstained (gray), 50% NHS (green), CD46 blocked prior to 50% NHS (blue), and CD46, CD55, and CD59 blocked prior to adding 50% NHS (red) for 1 hour. (B) The median fluorescence intensity (MFI) of 7 to 8 different experiments is shown (*P < 0.05; analysis of variance). (C,D) Using a FITC-conjugated C3c antibody we detected that C3c deposition was both classical and alternative pathway dependent, as the use of C1q-depleted serum reduced (but did not abolish) C3c deposition. (C) A representative image with unstained control (gray), 50% C1q-depleted serum (orange), and 50% NHS (red) after CD46, CD55, and CD59 block. (D) Analysis of 3 independent experiments (***P < 0.001; paired t-test). (E) Increased C3c deposition was seen with an increasing percentage of serum used after the blockade of CD46, CD55, and CD59. (*P < 0.05; analysis of variance). (F) Progression of complement activation beyond C3 deposition was confirmed by immunofluorescence microscopy confirming the presence of the terminal complement complex C5b-9 on BOECs in both static (not shown) and fluidic conditions. The image is shown in extended focus and was taken using an Olympus IX81 inverted fluorescence microscope with a 60/1.35 oil immersion objective and a Hamamatsu C9100-13 back-thinned EM-CCD camera with a Yokogawa CSU X1 spinning disk confocal scan head. Confocal images were taken with an Improvision Piezo Focus Drive. In summary, these results indicate that complement deposition on ECs can be achieved as a result of the combined blockade of the principal membrane-anchored complement regulators.
Figure S3. Treatment with a polyclonal rabbit anti–β2-microglobulin antibody induces complement fixation. C3 deposition was detected when blood outgrowth endothelial cells (BOECs) (early passage) were incubated with a polyclonal rabbit anti–β2-microglobulin antibody (M8523, Sigma-Aldrich) for 20 minutes followed by 50% normal human serum (NHS) for 1 hour. C3 was detected using a polyclonal goat anti-C3 antibody. (A) Antibody saturation experiment was performed using flow cytometry, and the following conditions are shown: unstained control (red), rabbit serum (1:10), anti–β2-microglobulin 1:10, anti–β2 microglobulin 1:20, anti–β2 microglobulin 1:40, and anti–β2 microglobulin 1:80. For the next experiment, we used a 1:40 dilution of the antibody. (B) Cells were treated either with 50% NHS (blue) or with anti–β2-microglobulin (1:40) followed by 50% NHS for 1 hour. Significant C3 deposition was seen upon sensitization with anti–β2-microglobulin antibody.
Figure S4. Functional blocking antibody binding and saturation. (A–C) Using flow cytometry, the antibody saturation of anti-CD46 antibody (GB24, A), anti-CD55 antibody (BRIC216, B), and anti-CD59 antibody (BRIC229, C) was determined. Antibody concentrations of 2 μg/ml (blue), 5 μg/ml (orange), and 10 μg/ml (green) were compared to unstained control (red). Subsequently, 5 μg/ml was used for all blocking antibodies in all experiments. (D–F) Colocalization of blocking antibody with epitope was determined using immunofluorescence. The blocking antibodies were detected with secondary donkey anti-mouse labeled with A555 (red) and staining antibody with representative Alexa Fluor 488 (green). Hoechst dye (0.12 μg/ml) was used to stain DNA (blue). Pictures were taken with a spinning disk confocal microscope as described in Materials and Methods. Supplementary material is linked to the online version of the paper at www.kidney-international.org.
ACKNOWLEDGMENTS
DGN was funded in part by Restracomp and a Transplant Center Fellowship at The Hospital for Sick Children, Toronto, Ontario, Canada. MR received a Marietta Blau Stipend of the Österreichischer Austauschdienst (OeAD) GmbH, funded by the Austrian Federal Ministry for Science and Research. CL was funded by the American Society of Nephrology (2009 Norman Siegel Research Scholar Grant), the Heart and Stroke Foundation of Ontario (NA 6716), the Kidney Foundation of Canada (KFOC120001), and SickKids intramural grants. WHAK was supported by operating grants from the Canadian Institutes of Health Research (MOP-81208 and MOP-259952). MKL and JPA were supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM099111 and the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number U54HL112303. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. [DOI] [PubMed] [Google Scholar]
- 2.Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George JN. Measuring ADAMTS13 activity in patients with suspected thrombotic thrombocytopenic purpura: when, how, and why? Transfusion. 2015;55:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai HM. Untying the knot of thrombotic thrombocytopenic purpura and atypical hemolytic uremic syndrome. Am J Med. 2013;126:200–209. [DOI] [PubMed] [Google Scholar]
- 5.Chapin J, Eyler S, Smith R, et al. Complement factor H mutations are present in ADAMTS13-deficient, ticlopidine-associated thrombotic microangiopathies. Blood. 2013;121:4012–4013. [DOI] [PubMed] [Google Scholar]
- 6.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. [DOI] [PubMed] [Google Scholar]
- 7.Noris M, Ruggenenti P, Perna A, et al. Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: role of factor H abnormalities. Italian Registry of Familial and Recurrent Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura. J Am Soc Nephrol. 1999;10:281–293. [DOI] [PubMed] [Google Scholar]
- 8.Feng S, Kroll MH, Nolasco L, et al. Complement activation in thrombotic microangiopathies. Br J Haematol. 2013;160:404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Torres MP, Casiraghi F, Galbusera M, et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost. 2005;93: 443–452. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Eyler SJ, Zhang Y, et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood. 2013;122:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner NA, Moake J. Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasis-thrombosis. PLoS One. 2013;8: e59372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolasco L, Nolasco J, Feng S, et al. Human complement factor H is a reductase for large soluble von Willebrand factor multimers—brief report. Arterioscler Thromb Vasc Biol. 2013;33:2524–2528. [DOI] [PubMed] [Google Scholar]
- 13.Feng S, Liang X, Cruz MA, et al. The interaction between factor H and Von Willebrand factor. PLoS One. 2013;8:e73715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayes J, Roumenina LT, Dimitrov JD, et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood. 2014;123:121–125. [DOI] [PubMed] [Google Scholar]
- 15.Bowman M, Tuttle A, Notley C, et al. The genetics of Canadian type 3 von Willebrand disease: further evidence for co-dominant inheritance of mutant alleles. J Thromb Haemost. 2013;11:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liszewski MK, Atkinson JP. Membrane cofactor protein (MCP; CD46). Isoforms differ in protection against the classical pathway of complement. J Immunol. 1996;156:4415–4421. [PubMed] [Google Scholar]
- 17.Barilla-LaBarca ML, Liszewski MK, Lambris JD, et al. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol. 2002;168:6298–6304. [DOI] [PubMed] [Google Scholar]
- 18.Triantafilou K, Hughes TR, Triantafilou M, et al. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. [DOI] [PubMed] [Google Scholar]
- 19.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 20.Timmermans F, Plum J, Yoder MC, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seino J, Eveleigh P, Warnaar S, et al. Activation of human complement by mouse and mouse/human chimeric monoclonal antibodies. Clin Exp Immunol. 1993;94:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. [DOI] [PubMed] [Google Scholar]
- 23.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54: 385–395. [DOI] [PubMed] [Google Scholar]
- 24.Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846. [DOI] [PubMed] [Google Scholar]
- 25.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:1847–1848. [DOI] [PubMed] [Google Scholar]
- 26.Hattori R, Hamilton KK, McEver RP, et al. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- 27.Nakashima S, Qian Z, Rahimi S, et al. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620–4627. [DOI] [PubMed] [Google Scholar]
- 28.Ota H, Fox-Talbot K, Hu W, et al. Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005;79:276–281. [DOI] [PubMed] [Google Scholar]
- 29.Bryckaert M, Rosa JP, Denis CV, et al. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72:307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121:270–277. [DOI] [PubMed] [Google Scholar]
- 32.Reti M, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10:791–798. [DOI] [PubMed] [Google Scholar]
- 33.Choi HS, Cheong HI, Kim NK, et al. ADAMTS13 gene mutations in children with hemolytic uremic syndrome. Yonsei Med J. 2011;52:530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu TC, Yang S, Haven S, et al. Complement activation and mortality during an acute episode of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2013;11:1925–1927. [DOI] [PubMed] [Google Scholar]
- 35.Tati R, Kristoffersson AC, Stahl AL, et al. Complement activation associated with ADAMTS13 deficiency in human and murine thrombotic microangiopathy. J Immunol. 2013;191:2184–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner N, Nolasco L, Nolasco J, et al. Thrombotic microangiopathies and the linkage between von Willebrand factor and the alternative complement pathway. Semin Thromb Hemost. 2014;40:544–550. [DOI] [PubMed] [Google Scholar]
- 37.Feng S, Liang X, Kroll MH, et al. von Willebrand factor is a cofactor in complement regulation. Blood. 2015;125:1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawanami O, Jin E, Ghazizadeh M, et al. Heterogeneous distribution of thrombomodulin and von Willebrand factor in endothelial cells in the human pulmonary microvessels. J Nippon Med Sch. 2000;67:118–125. [DOI] [PubMed] [Google Scholar]
- 39.Muller AM, Skrzynski C, Skipka G, et al. Expression of von Willebrand factor by human pulmonary endothelial cells in vivo. Respiration. 2002;69:526–533. [DOI] [PubMed] [Google Scholar]
- 40.Dorovini-Zis K, Prameya R, Bowman PD. Culture and characterization of microvascular endothelial cells derived from human brain. Lab Invest. 1991;64:425–436. [PubMed] [Google Scholar]
- 41.Bernas MJ, Cardoso FL, Daley SK, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Ramirez J, Hofman M, van den Biggelaar M, et al. Establishment of outgrowth endothelial cells from peripheral blood. Nat Protoc. 2012;7: 1709–1715. [DOI] [PubMed] [Google Scholar]
- 43.Advani A, Kelly DJ, Advani SL, et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A. 2007;104:14448–14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuen DA, Stead BE, Zhang Y, et al. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23:1810–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budde U, Schneppenheim R, Plendl H, et al. Luminographic detection of von Willebrand factor multimers in agarose gels and on nitrocellulose membranes. Thromb Haemost. 1990;63:312–315. [PubMed] [Google Scholar]
- 46.Budde U, Schneppenheim R, Eikenboom J, et al. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD). J Thromb Haemost. 2008;6:762–771. [DOI] [PubMed] [Google Scholar]
- 47.Patel S, Huang YW, Reheman A, et al. The cell motility modulator Slit2 is a potent inhibitor of platelet function. Circulation. 2012;126:1385–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Blood outgrowth endothelial cells (BOECs) demonstrate a stable endothelial cell phenotype. (A) The mononuclear layer of cells was isolated from the peripheral blood of healthy adult donors by density gradient centrifugation, resuspended in complete endothelial growth medium (Endothelial Basal Medium 2 [EBM-2] supplemented with growth factors [EGM-2 BulletKit], 10% fetal bovine serum, and 1% Antibiotic-Antimycotic), and seeded onto collagen-coated 6-well tissue culture plates at a concentration of 3 × 107 to 5 × 107 cells per well. During week 1 early outgrowth cell populations predominate. After 2 to 3 weeks typically, a BOEC monolayer was identifiable by their endothelial cobblestone morphology and ready for passaging. (B) An endothelial phenotype was confirmed by demonstrating the presence of Weibel–Palade bodies containing fluorescently labeled von Willebrand factor (VWF, Alexa Fluor 555; red) and P-selectin (CD62P, Alexa Fluor 488; green). (C) RT-qPCR confirms that BOECs maintain their endothelial characteristics when comparing an early (passage 3) and late passage (passage 10) for VWF. N = 3. (D) Flow cytometry confirms their endothelial phenotype, as BOECs are positive for PECAM/CD31 and VE-cadherin/CD144 and negative for the hemangioblast marker CD45 and the immature EPC marker CD14. (E) mRNA expression of von Willebrand factor (VWF) and complement regulators is similar on blood outgrowth endothelial cells (BOECs) and HUVECs. RT-qPCR shows the relative expression of complement regulators on control BOECs as compared to VWF (VWF expression level = 1). Results were compared to HUVECs. No significant difference was observed (2-way analysis of variance with Sidak's multiple comparison test). N = 6 for HUVECs and N = 5 for BOECs.
Figure S2. Complement fixation on endothelial cells (ECs) was achieved by combining the effects of the classical (i.e., initiation via sensitization) and alternative (i.e., amplification) pathways via blocking antibodies specific for the principal membrane-anchored complement regulators CD46, CD55, and CD59 in the presence of 50% normal human serum (NHS). Complement fixation on ECs was quantified via flow cytometric assessment of the deposition of complement activation products on ECs. (A,B) Blockade of CD46 did not result in a significant increase in C3c deposition (detected via rabbit polyclonal anti-C3c and PE) over incubation with serum alone. Combined blockade of CD46, CD55, and CD59, however, resulted in a significant (7-fold) increase of C3 deposition on blood outgrowth endothelial cells (BOECs) (355 ± 193 vs. 2488 ± 2120). (A) A representative image with the following conditions: unstained (gray), 50% NHS (green), CD46 blocked prior to 50% NHS (blue), and CD46, CD55, and CD59 blocked prior to adding 50% NHS (red) for 1 hour. (B) The median fluorescence intensity (MFI) of 7 to 8 different experiments is shown (*P < 0.05; analysis of variance). (C,D) Using a FITC-conjugated C3c antibody we detected that C3c deposition was both classical and alternative pathway dependent, as the use of C1q-depleted serum reduced (but did not abolish) C3c deposition. (C) A representative image with unstained control (gray), 50% C1q-depleted serum (orange), and 50% NHS (red) after CD46, CD55, and CD59 block. (D) Analysis of 3 independent experiments (***P < 0.001; paired t-test). (E) Increased C3c deposition was seen with an increasing percentage of serum used after the blockade of CD46, CD55, and CD59. (*P < 0.05; analysis of variance). (F) Progression of complement activation beyond C3 deposition was confirmed by immunofluorescence microscopy confirming the presence of the terminal complement complex C5b-9 on BOECs in both static (not shown) and fluidic conditions. The image is shown in extended focus and was taken using an Olympus IX81 inverted fluorescence microscope with a 60/1.35 oil immersion objective and a Hamamatsu C9100-13 back-thinned EM-CCD camera with a Yokogawa CSU X1 spinning disk confocal scan head. Confocal images were taken with an Improvision Piezo Focus Drive. In summary, these results indicate that complement deposition on ECs can be achieved as a result of the combined blockade of the principal membrane-anchored complement regulators.
Figure S3. Treatment with a polyclonal rabbit anti–β2-microglobulin antibody induces complement fixation. C3 deposition was detected when blood outgrowth endothelial cells (BOECs) (early passage) were incubated with a polyclonal rabbit anti–β2-microglobulin antibody (M8523, Sigma-Aldrich) for 20 minutes followed by 50% normal human serum (NHS) for 1 hour. C3 was detected using a polyclonal goat anti-C3 antibody. (A) Antibody saturation experiment was performed using flow cytometry, and the following conditions are shown: unstained control (red), rabbit serum (1:10), anti–β2-microglobulin 1:10, anti–β2 microglobulin 1:20, anti–β2 microglobulin 1:40, and anti–β2 microglobulin 1:80. For the next experiment, we used a 1:40 dilution of the antibody. (B) Cells were treated either with 50% NHS (blue) or with anti–β2-microglobulin (1:40) followed by 50% NHS for 1 hour. Significant C3 deposition was seen upon sensitization with anti–β2-microglobulin antibody.
Figure S4. Functional blocking antibody binding and saturation. (A–C) Using flow cytometry, the antibody saturation of anti-CD46 antibody (GB24, A), anti-CD55 antibody (BRIC216, B), and anti-CD59 antibody (BRIC229, C) was determined. Antibody concentrations of 2 μg/ml (blue), 5 μg/ml (orange), and 10 μg/ml (green) were compared to unstained control (red). Subsequently, 5 μg/ml was used for all blocking antibodies in all experiments. (D–F) Colocalization of blocking antibody with epitope was determined using immunofluorescence. The blocking antibodies were detected with secondary donkey anti-mouse labeled with A555 (red) and staining antibody with representative Alexa Fluor 488 (green). Hoechst dye (0.12 μg/ml) was used to stain DNA (blue). Pictures were taken with a spinning disk confocal microscope as described in Materials and Methods. Supplementary material is linked to the online version of the paper at www.kidney-international.org.