Short abstract
Background
Anticoagulation with vitamin K antagonists and non-vitamin K antagonists oral anticoagulants (NOAC) is effective in stroke prevention in patients with atrial fibrillation. However, anticoagulation also poses a major challenge for emergency treatment of patients suffering ischaemic stroke or intracerebral haemorrhage.
Aim
The registry RASUNOA-prime is designed to describe current patterns of emergency management, clinical course and outcome of patients with atrial fibrillation experiencing an acute ischaemic stroke or intracerebral haemorrhage under different anticoagulation schemes prior to stroke (NOAC, vitamin K antagonists or no anticoagulation).
Methods and design
RASUNOA-prime (ClinicalTrials.gov, NCT02533960) is a prospective, investigator-initiated, multicentre, observational cohort study aiming to recruit 3000 patients with acute ischaemic stroke and atrial fibrillation, and 1000 patients with acute intracerebral haemorrhage and atrial fibrillation with different anticoagulation schemes pre-stroke. It is a non-interventional triple-armed study aiming at a balanced inclusion of ischaemic stroke and intracerebral haemorrhage patients according to the different anticoagulation schemes. Patients will be followed up for clinical course, management and outcome up to three months after the event. Findings in ischaemic stroke and intracerebral haemorrhage patients on NOAC will be compared with patients taking vitamin K antagonists or no anticoagulant pre-stroke.
Study outcomes
Primary endpoint for ischaemic stroke patients: occurrence of symptomatic intracerebral haemorrhage, for intracerebral haemorrhage patients: occurrence of secondary haematoma expansion. Secondary endpoints include assessment of coagulation, use of thrombolysis and/or mechanical thrombectomy, occurrence of complications, implementation of secondary prevention.
Summary
Describing the current patterns of early management as well as outcome of stroke patients with atrial fibrillation will help guide physicians to develop recommendations for emergency treatment of stroke patients under different anticoagulation schemes.
Keywords: Acute stroke, intracerebral haemorrhage, anticoagulants, vitamin K antagonists, non-vitamin K antagonists
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is responsible for at least 15–20% of all strokes.1,2 Anticoagulant therapy in patients with AF for primary and secondary prevention of stroke is approved in clinical guidelines.1 Non-vitamin K antagonists (VKA) oral anticoagulant drugs (NOAC) have been approved for stroke prevention in non-valvular AF patients as an effective and safe alternative to VKA.3–5 The number of patients receiving long-term treatment with NOAC for stroke prevention in non-valvular AF is increasing.6 Although NOAC offer some advantages over VKA, therapy with NOAC currently poses substantial challenges for management of different emergency situations including stroke. For example, surgical procedures and interventions requiring an intact coagulation system (i.e. thrombolysis for ischaemic (IS) stroke) may not be carried out in patients who are anticoagulated with NOAC.7 While routine laboratory tests such as the prothrombin time may be used for rapid assessment of anticoagulant effects of VKA in emergency situations, coagulation tests for NOACs are less widely available and more time-consuming. In addition, in case of major bleeding, including intracerebral haemorrhage (ICH), rapid anticoagulation reversal is desirable.8,9 Specific reversal agents for particular NOACs have just become available (i.e. idarucizumab for dabigatran)10 or are in various stages of clinical development.11 However, their haemostatic effectiveness in ICH, as measured by haematoma growth and their effect on longer-term clinical outcome, is unknown.
There is a need of systematically collected observational data from routine clinical practice to inform the design of future clinical trials addressing the management and outcome of neurological emergencies in patients with AF and anticoagulation. Therefore, the Registry of Acute Stroke Under Novel Oral Anticoagulants-prime (RASUNOA-prime) will describe the emergency management of stroke patients (IS, ICH) with AF in clinical routine including early diagnostic, therapeutic and preventive procedures and will assess variations in the emergency management of patients with AF by anticoagulation schemes pre-stroke. We will also identify the variations in risk of early complications such as symptomatic secondary intracerebral haemorrhage (sICH) in IS patients with AF by different anticoagulation schemes. Finally, we will determine the factors influencing outcome of stroke patients with AF at three months and clarifying the potential influence of different anticoagulation schemes pre-stroke.
Methods
Design
RASUNOA-prime is a prospective, investigator-initiated, multicentre, observational cohort study of patients with stroke and AF. It is designed as a non-interventional triple-armed study (VKA, NOAC, no anticoagulation) including IS and ICH patients. A standardised central telephone or postal follow-up visit is carried out three months after the index stroke. The registry is not limited to a specific NOAC or VKA but will examine acute stroke management and its outcome during use of any of the approved NOACs and VKAs. The study is not designed to perform comparisons among different NOACs or VKAs. Findings in IS and ICH patients on NOAC will be compared with patients taking VKA or no anticoagulant at the time of the event.
About 40 neurology departments with certified stroke units throughout Germany take part in the study. Certification of a Stroke Unit is defined according to the criteria of the German Stroke Society and the German Stroke Foundation.12
In total, RASUNOA-prime will enrol 4000 consecutive patients, thereof 3000 patients with IS and 1000 patients with ICH equally distributed to the three study arms (NOAC, VKA and no oral anticoagulants (OAC)) in each substudy. The study enrolment will take place over a period of approximately four years.
The trial is registered at ClinicalTrials.gov, identifier NCT02533960.
Patient population – Inclusion and exclusion criteria
Patients eligible for the study are ≥18 years and have an acute stroke with either symptoms lasting ≥24 h attributable to IS or evidence of acute IS/ICH on brain imaging. Patients must also have a previous or present diagnosis of AF. Only patients under anticoagulation therapy with either NOAC or VKA at the time of the stroke or without anticoagulation are eligible (i.e. patients treated with parenteral anticoagulation are excluded). Key exclusion criteria include symptom-onset >24 h before hospital admission and lack of written informed consent by the patient or his legal representative (Table 1).
Table 1.
In-/exclusion criteria.
| Inclusion criteria: |
| – Age≥18 years |
| – Anticoagulation at index stroke: NOAC, VKA or no anticoagulant (no drug-intake or paused for >3 (NOAC) or >7 days (VKA)) |
| – Previous or present diagnosis of AF, verified by |
| – Documented history of AF |
| – Unequivocal 12-channel ECG or rhythm strip |
| – Pacemaker reading |
| – Holter ECG, ECG telemetry |
| – Acute IS with either symptoms lasting≥24 h or evidence of acute infarction in brain imaging |
| or |
| – Acute primary intracerebral haemorrhage |
| Exclusion criteria: |
| – Symptom-onset>24 h before presentation |
| – No informed consent |
NOAC: non-vitamin K antagonists oral anticoagulants; VKA: vitamin K antagonists; AF: atrial fibrillation; ECG: electrocardiography; IS: ischaemic stroke.
Procedures
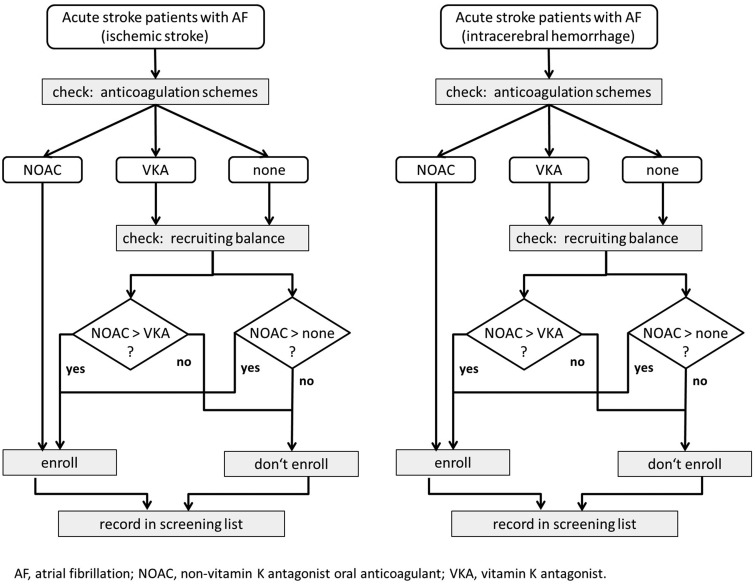
It is ensured that all three treatment arms are equally distributed at any time of recruitment by a standardised enrolment algorithm (Figure 1). Due to specific challenges described above, the main focus of RASUNOA-prime was to investigate acute management of stroke patients with NOAC and to compare emergency management, clinical course and outcome with patients on VKA or no anticoagulation. Thus, IS and ICH patients on NOACs were defined as cases and on VKA or no anticoagulation as controls. The dynamics of the study population is considered by obtaining controls (VKA or no anticoagulation) each time a case (NOAC) is enrolled, and thus a representative and valid sampling of controls from the entire study base during the whole study period is ensured.13 Each participating centre will document consecutively admitted patients who are deemed eligible by in-/exclusion criteria. According to the algorithm, patients are included as follows: only if a patient under NOAC is included in the study, the next eligible patient with VKA and the next patient without anticoagulation can be enrolled. Due to this procedure, patients under different anticoagulation schemes are recruited at a similar point in time and the number of patient is balanced at any given time during the recruitment process. Additionally, this design ensures that patient-triplets originate from the same stroke services.13 The enrolment algorithm is applied for each substudy (IS, ICH) separately.
Figure 1.
Enrolment algorithm.
NOAC: non-vitamin K antagonists oral anticoagulants; VKA: vitamin K antagonists; AF: atrial fibrillation.
The screening and recruitment will be undertaken by physicians of the participating stroke services. All patients fulfilling the eligibility criteria that present to the study sites are documented in an anonymous screening log file irrespective of their participation in the study. Eligible patients or their legal representatives will be asked for participation in the study. Written informed consent must be provided. Participants can withdraw consent at any time.
Treatment
RASUNOA-prime is a strictly observational study, thus all diagnostic and treatment decisions are left at the discretion of the treating physicians.
Primary outcome
The primary outcome for patients suffering acute IS is the occurrence of sICH according to the NINDS criteria (here defined as: occurrence of haemorrhage not seen in previous CT/MRI scan performed after admission associated with any clinical worsening (NIHSS score at the time the bleeding became apparent compared to the lowest value previously registered)).14 All images will undergo central imaging analysis with readers masked to the clinical course. We hypothesise that neither anticoagulation with NOAC nor with VKA pre-stroke leads to an increase of sICH compared to non-anticoagulated patients’ pre-stroke. For patients with ICH, the occurrence of secondary haematoma expansion (HE) will be the primary outcome. Secondary HE is defined as either an increase of haematoma volume by ≥6.5 ml or by ≥33%,15 a new intraventricular haemorrhage not evident on baseline imaging or an increase of intraventricular haematoma on the modified Graeb score by ≥2 points. We will use haematoma volumetry on cranial CT/MRI scans at baseline and at follow-up neuroimaging within 72 h after ICH onset. We hypothesise that neither anticoagulation with NOAC nor with VKA leads to an increase of relevant secondary HE compared to no anticoagulation.
Secondary outcomes
The secondary aims of the study are to describe the differences in acute stroke management regarding the different anticoagulation therapies, including the assessment of coagulation-intensity and anticoagulant concentration, the performance of thrombolysis and endovascular recanalisation, determination of severe complications and usage of anticoagulants for secondary prevention. Complications of stroke were prospectively collected by the on-site study coordinators or trained study nurses.
Safety outcome (reporting of adverse reactions)
The participating centres will report potential adverse drug reactions to a specific drug to the Drug Commission of the German Medical Association according to the rules for professional conduct of the German medical profession. To facilitate the reporting, a set of potential adverse reactions were defined (death, IS, ICH, major haemorrhage (according to the International Society on Thrombosis and Haemostasis criteria), pregnancy, serious liver injury).
Assessments
Data on patient demographics, medical history, anticoagulation status, clinical characteristics, clinical course and laboratory parameters are prospectively collected. Laboratory parameters might differ between centres; hence, each hospital will provide their reference range for the individually used assays. Central core neuroradiological imaging assessment will be performed, with readers blinded to the patient details.
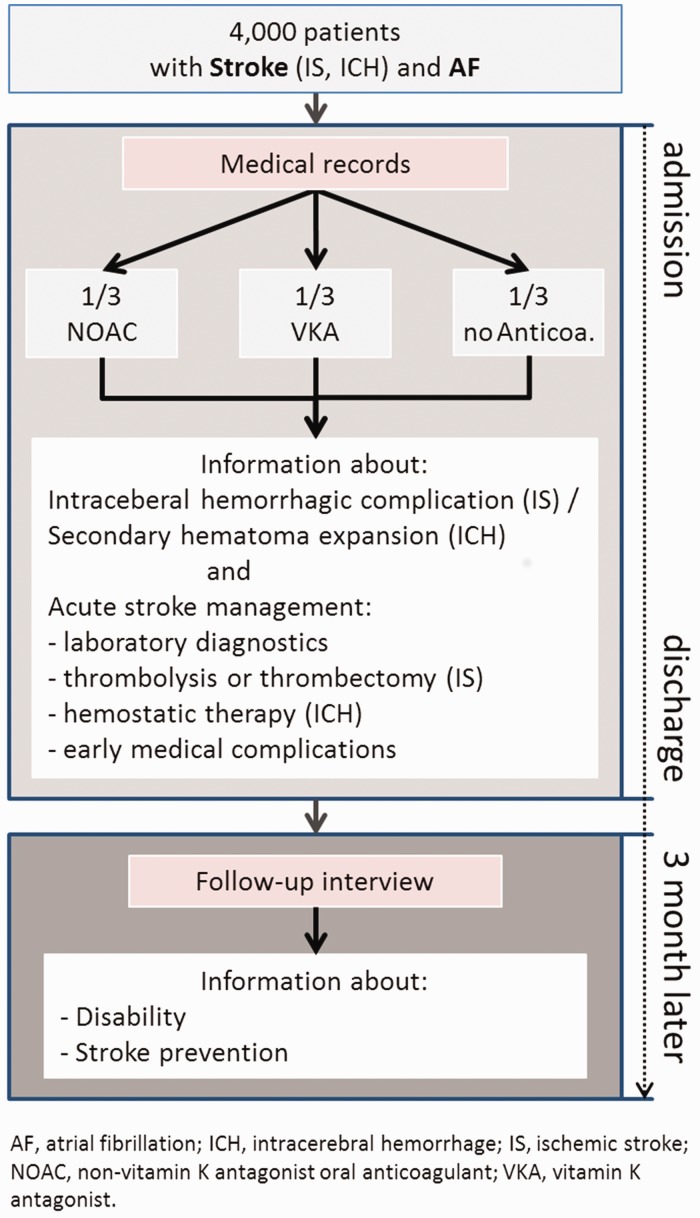
In a central telephone or postal follow-up visit at day 90, data on vital status, stroke recurrence rates, living conditions, secondary prevention and functional outcome (Barthel Index, modified Rankin Scale) will be collected. In addition, information on in-patient and outpatient rehabilitation as well as new hospitalisations after hospital discharge will be documented (Figure 2).
Figure 2.
Assessments and workflow of RASUNOA-prime.
NOAC: non-vitamin K antagonists oral anticoagulants; VKA: vitamin K antagonists; AF: atrial fibrillation; IS: ischaemic stroke; ICH: intracerebral haemorrhage.
Sample size estimates
The primary endpoint of this study is the sICH rate for IS patients with AF under different anticoagulation regimes pre-stroke (NOAC, VKA and no anticoagulation). The data will be analysed in a hierarchical approach: primarily, we will compare the sICH rate for acute stroke patients with NOAC prior to stroke with the sICH rate of no anticoagulation. Only in the case of no statistical significance differences, we will also compare VKA scheme pre-stroke in AF stroke patients with no anticoagulation for the endpoint sICH rate. We assume an sICH rate of patients without anticoagulation pre-stroke of 1.3%, derived from the findings, that the proportion of patients receiving vs. not receiving thrombolytic therapy is 15% vs. 85%,16 and the sICH rate of patients with thrombolytic therapy is 5% and without 0.6%.17 We consider at least a doubling of this sICH rate in the group of stroke patients with NOAC compared to patients without OAC as clinically relevant. To analyse this margin by a test of non-inferiority for two proportions at significance level 0.05, a sample size of 1000 stroke patients per group (NOAC, VKA and no anticoagulation) is needed.
For ICH patients, the main endpoint is secondary HE. For non-anticoagulated patients suffering an ICH, the rate of relevant HE is expected to be 28%.18 It is judged as clinically relevant if the rate of HE in the group of patient under NOAC and receiving haemostatic factors is more than 10% above this rate. To analyse this deviation by a test of non-inferiority with a one-sided alpha of 0.025% and assuming a drop-out rate of 5%, we will need 333 patients per group.
Statistical analyses
The planned data analysis will comprise appropriate descriptive, univariate and multivariable analyses for assessing emergency management of stroke patients with AF in clinical routine, identifying risk of early complications after the index stroke, determining factors influencing long-term outcome after stroke and describing potential variations in these outcomes by different anticoagulation schemes pre-stroke. Additionally, a series of subgroup analysis for primary and secondary outcomes (e.g. variations in emergency management) are planned. The data will be stratified by factors known or suspected to influence treatment decision and the outcomes including: age group (< >75 years), sex, severity of stroke (NIHSS <15 vs. NIHSS ≥15), infarct size (ASPECTS; classification by Paciaroni et al.19) and type of AF (permanent versus paroxysmal AF).
Preliminary results
Enrolment started in June 2015 and until September 2017, 52.3% (n = 2090) of the envisaged number of patients were enrolled (n = 1812 IS, n = 278 ICH). So far, follow-up data could be obtained in 81% of patients eligible for follow up (n = 1523).
Discussion
Anticoagulation is increasingly recognised as a major challenge for effective and safe emergency management of IS and ICH. The RASUNOA-prime study will provide prospectively collected data on the emergency management of acute IS and ICH in patients with AF. It particularly focuses on the association of different schemes of anticoagulation before the event on clinical course, haemorrhagic complications and outcome.
Recanalisation therapy using intravenous thrombolysis or mechanical thrombectomy is a cornerstone of IS management.20,21 An important concern is an increase of the risk of sICH after recanalisation especially under oral anticoagulation. The effect of anticoagulation on the risk of secondary haemorrhage in the ischaemic brain with and without recanalisation therapy is only partially understood. While patients on anticoagulants are not included in the label for i.v. thrombolysis with alteplase, thrombolytic therapy can be considered for patients with VKA within subtherapeutic INR,20,22 as the occurrence of haemorrhagic transformation appears to be unaffected by oral anticoagulation with VKA with a baseline INR of ≤1.7.23 Whether and to what extend patients under NOAC are eligible for recanalisation is currently uncertain.7 Limited evidence from a single-armed study using historical controls suggests that there is no increased risk of sICH for patients under NOAC.24 RASUNOA-prime will be the first study with simultaneously recruited NOAC, VKA and a non-anticoagulated control group to address the issue whether anticoagulation of patients with AF leads to an increase of secondary haemorrhage after IS.
The chances of achieving a favourable outcome with recanalisation therapy depends on the time from symptom onset to start of recanalisation therapies.20 In case of patients under VKA, if the INR is measured rapidly in the emergency room using point-of-care coagulometry, no delays for initiation of thrombolysis are to be expected.25 In contrast, measuring the anticoagulant effects or plasma concentration of NOACs requires less widely established specific assays and thresholds below which thrombolysis is safe remain to be identified empirically.9,26 Specific point-of-care assays are currently not routinely available for the emergency setting.26,27 RASUNOA-prime will provide information regarding the availability and results of emergency laboratory data and their impact on acute stroke management, which may help refine clinical management.
HE is an important prognostic factor in ICH, and its prevention has become a major therapeutic target for emergency management. Patients with ICH related to VKA are at an increased risk of HE compared to non-anticoagulated ICH patients.28 A recent trial suggested a benefit of anticoagulation reversal with prothrombin complex concentrate over Fresh Frozen Plasma in terms of early HE and mortality.8,29 The effects of NOACs on HE in ICH are controversial. Initial small case series suggested no HE, but larger series suggest a similar course for ICH related to NOAC as in VKA-related ICH.30,31 As the available studies are of limited size and mainly retrospective, a key question of the RASUNOA-prime ICH-substudy is whether secondary HE in AF-patients experiencing ICH during oral anticoagulation occurs more often compared to non-anticoagulated patients. We will also examine the potential effects of anticoagulation reversal using unspecific reversal agents including PCC as well as specific antidotes. There are concerns that stroke under NOAC may be more severe and more complications might occur. In a study comparing the clinical presentation and outcome after IS under VKA and NOAC, both VKA within a therapeutic range and the intake of NOAC lowered the risk of worse functional outcome at discharge compared to no anticoagulation.32 No differences of functional outcome at discharge and 90-day mortality were found between patients anticoagulated with VKA or NOAC.30,31 In previous reports, ICH related to anticoagulation carried a higher mortality than other ICH, but this may be the result of different comorbidities in the non-anticoagulated groups. Our study goes beyond these observations and will obtain longitudinal functional outcome to explore if the use of NOAC before IS and ICH and different treatment option will be translated into poor functional outcome in the long term compared to non-anticoagulated patients.
RASUNOA-prime has strengths and limitations. It is unique in combining the three different anticoagulation schemes, a large sample size and information on acute management of stroke in different sites of care. It will be able to give further insights on optimal treatment and its effect on functional outcome after stroke. Due to the elaborated enrolment algorithm, variations in treatment during the study period will affect all arms of the study equally. However, the study has also limitations. First, the study is conducted in Europe, and therefore the vast majority of participants are expected to be of Caucasian ethnicity. Second, patients with ICH might be incompletely enrolled as mainly neurology departments with stroke units are recruiting and despite encouraged local collaboration with neurosurgical departments, some patients may be missed. Third, due to the necessity of obtaining written informed consent from a patient or his legal representative for use of observational data, most severely affected patients might not participate in the study. We attempt to reduce this bias by an additional consent process in which a physician, independent of the study can provide informed preliminary consent. We cannot exclude that younger patients with less comorbidities will be recruited within the non-anticoagulation group, e.g. due to lower CHA2DS2VASc score prior to stroke, that might influence comparability of the study groups regarding prognostic factors. However, we will perform multivariable analysis considering known confounder like age, sex, comorbidities or stroke severity to adjust for potential imbalance in prognostic factors between the groups. Although our sampling strategy for identifying patients with VKA or no anticoagulation as controls for patients with NOACs followed recent epidemiological recommendations,13 we cannot exclude that our study population might not be completely comparable to the entire population of stroke patients with AF. Finally, due the increasing trend of prescribing NOAC instead of VKA or no anticoagulation in AF, the enrolment into the non-NOAC groups may become increasingly challenging particularly in the ICH substudy.
Conclusion
RASUNOA-prime allows a comprehensive and systematic report of current patterns of emergency management in stroke patients with different anticoagulation regimes before the stroke in routine clinical care and the evaluation of its short- and long-term outcomes. It will identify factors to inform future treatment recommendations and interventional trials regarding reperfusion therapies and anticoagulation reversal, respectively.
Supplemental Material
Supplemental Material for Rationale and design of the Registry of Acute Stroke Under Novel Oral Anticoagulants-prime (RASUNOA-prime) by Kirsten Haas, Jan C Purrucker, Timolaos Rizos, Peter U Heuschmann and Roland Veltkamp in European Stroke Journal
Acknowledgments
We thank the study teams of all participating centres represented by the RASUNOA-prime investigators (details see online supplement). We explicitly thank the project management (Perdita Beck) and the data management (Anna Grau, Timo Ludwig, Udo Selig) for their precious support in data acquisition.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCP travel and congress participation support from Pfizer, and speakers’ honoraria from Boehringer-Ingelheim; TR consulting honoraria, speakers’ honoraria and travel support from Bristol-Myers Squibb/Pfizer, Boehringer-Ingelheim, Bayer HealthCare and Daichii Sankyo; PUH grants from BMBF, EU, Charité, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert-Koch-Institute, German Heart Foundation, Charité–Universitätsmedizin Berlin (within MonDAFIS; supported by an unrestricted research grant to the Charité from Bayer Vital GmbH), University Göttingen (within FIND-AFrandomised, supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim); RV is an investigator in the NIHR Imperial Biomedical Research Center and received speaker honoraria and research support from Bayer Vital GmbH, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, consulting honoraria from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Portola, AZT Therapies, Medtronic. KH reports no competing interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is supported by an unrestricted research grant to the University of Heidelberg, Heidelberg, Germany, from Bayer Vital GmbH, Bristol-Myers Squibb/Pfizer, Boehringer-Ingelheim Pharma GmbH &Co.KG, and Daiichi Sankyo Europe GmbH.
Ethical approval
The study was approved by the ethics committee of the Medical Faculty of the University of Heidelberg (S-088/2015). The data protection concept was approved by the data protection officer of the University of Würzburg (J-117.605–37/13). All recruiting centres have obtained approvals by the locally responsible ethics committees before study initiation.
Informed consent
All patients or their legal representatives provided written informed consent before enrolment in the study.
Guarantor
RV.
Contributorship
All authors were involved in designing the study, editing the study protocol, gaining ethical approval and also in the coordination of the study. KH and PUH created the first draft of the article. All authors carried out a critical revision of the article, contributed with comments and approved the final version.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas PL, Wiedmann S, Weingärtner M, et al. Time trends in incidence of pathological and etiological stroke subtypes during 16 years: the Erlangen Stroke project. Neuroepidemiology 2015; 44: 24–29. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 6.Staerk L, Fosbol EL, Gadsboll K, et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: temporal trends 2011–2015 in Denmark. Sci Rep 2016; 6: 31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diener HC, Foerch C, Riess H, et al. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti-thrombotic treatment. Lancet Neurol 2013; 12: 677–688. [DOI] [PubMed] [Google Scholar]
- 8.Steiner T, Weitz JI, Veltkamp R. Anticoagulant-associated intracranial hemorrhage in the era of reversal agents. Stroke 2017; 48: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 9.Drouet L, Bal Dit Sollier C, Steiner T, et al. Measuring non-vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used? Int J Stroke 2016; 11: 748–758. [DOI] [PubMed] [Google Scholar]
- 10.Pollack CV, Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med 2017; 377: 431–441. [DOI] [PubMed] [Google Scholar]
- 11.Veltkamp R, Purrucker J. Management of spontaneous intracerebral hemorrhage. Curr Neurol Neurosci Rep 2017; 17: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabavi DG, Ossenbrink M, Schinkel M, et al. Aktualisierte zertifizierungskriterien für regionale und überregionale stroke-units in Deutschland. Nervenarzt 2015; 86: 978–988. [DOI] [PubMed] [Google Scholar]
- 13.Grobbee DE, Hoes AW. Clinical epidemiology: principles, methods, and applications for clinical research. Sudbury, MA, USA: Jones and Bartlett Publishers, 2009. [Google Scholar]
- 14.Gumbinger C, Gruschka P, Bottinger M, et al. Improved prediction of poor outcome after thrombolysis using conservative definitions of symptomatic hemorrhage. Stroke 2012; 43: 240–242. [DOI] [PubMed] [Google Scholar]
- 15.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011; 76: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedmann S, Heuschmann PU, Hillmann S, et al. The quality of acute stroke care – an analysis of evidence-based indicators in 260 000 patients. Deutsches Arzteblatt Int 2014; 111: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balami JS, Chen R-L, Grunwald IQ, et al. Neurological complications of acute ischaemic stroke. Lancet Neurol 2011; 10: 357–371. [DOI] [PubMed] [Google Scholar]
- 18.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008; 358: 2127–2137. [DOI] [PubMed] [Google Scholar]
- 19.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome. Results Prospective Multicenter Study 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 21. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis (Basel, Switzerland) 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N, Steiner T, Caso V, et al. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13–15 November 2016. Eur Stroke J 2017; 2: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xian Y, Liang L, Smith EE, et al. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA 2012; 307: 2600–2608. [DOI] [PubMed] [Google Scholar]
- 24.Purrucker JC, Haas K, Wolf M, et al. Haemorrhagic transformation after ischaemic stroke in patients taking non-vitamin K antagonist oral anticoagulants. J Stroke 2017; 19: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizos T, Jenetzky E, Herweh C, et al. Fast point-of-care coagulometer guided reversal of oral anticoagulation at the bedside hastens management of acute subdural hemorrhage. Neurocrit Care 2010; 13: 321–325. [DOI] [PubMed] [Google Scholar]
- 26.Ebner M, Birschmann I, Peter A, et al. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Critical Care 2017; 21: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purrucker JC, Haas K, Rizos T, et al. Coagulation testing in acute ischemic stroke patients taking non-vitamin K antagonist oral anticoagulants. Stroke 2017; 48: 152–158. [DOI] [PubMed] [Google Scholar]
- 28.Flibotte JJ, Hagan N, O'Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 29.Steiner T, Poli S, Griebe M, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016; 15: 566–573. [DOI] [PubMed] [Google Scholar]
- 30.Wilson D, Seiffge DJ, Traenka C, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology 2017; 88: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purrucker JC, Haas K, Rizos T, et al. Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol 2016; 73: 169–177. [DOI] [PubMed] [Google Scholar]
- 32.Hellwig S, Grittner U, Audebert H, et al. Non-vitamin K-dependent oral anticoagulants have a positive impact on ischaemic stroke severity in patients with atrial fibrillation. Europace 2018; 20: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Rationale and design of the Registry of Acute Stroke Under Novel Oral Anticoagulants-prime (RASUNOA-prime) by Kirsten Haas, Jan C Purrucker, Timolaos Rizos, Peter U Heuschmann and Roland Veltkamp in European Stroke Journal