Short abstract
Purpose
Paroxysmal atrial fibrillation is often suspected as a probable cause of cryptogenic stroke. Continuous long-term ECG monitoring using insertable cardiac monitors is a clinically effective technique to screen for atrial fibrillation and superior to conventional follow-up in cryptogenic stroke. However, more studies are needed to identify factors which can help selecting patients with the highest possibility of detecting atrial fibrillation with prolonged rhythm monitoring. The clinical relevance of short-term atrial fibrillation, the need for medical intervention and the evaluation as to whether intervention results in improved clinical outcomes should be assessed.
Method
The Nordic Atrial Fibrillation and Stroke Study is an international, multicentre, prospective, observational trial evaluating the occurrence of occult atrial fibrillation in cryptogenic stroke and transient ischaemic attack. Patients with cryptogenic stroke or transient ischaemic attack from the Nordic countries are included and will have the Reveal LINQ® Insertable cardiac monitor system implanted for 12 months for atrial fibrillation detection. Biomarkers which can be used as predictors for atrial fibrillation and may identify patients, who could derive the most clinical benefit from the detection of atrial fibrillation by prolonged monitoring, are being studied.
Conclusion
The primary endpoint is atrial fibrillation burden within 12 months of continuous rhythm monitoring. Secondary endpoints are atrial fibrillation burden within six months, levels of biomarkers predicting atrial fibrillation, CHA2DS2-VASc score, incidence of recurrent stroke or transient ischaemic attack, use of anticoagulation and antiarrhythmic drugs, and quality of life measurements. The clinical follow-up period is 12 months. The study started in 2017 and the completion is expected at the end of 2020.
Keywords: Cryptogenic stroke, atrial fibrillation, insertable cardiac monitor, biomarkers, arrhythmia monitoring, anticoagulation, Nordic Atrial Fibrillation and Stroke Study
Introduction
One of the important goals of stroke and transient ischaemic attack (TIA) management is to clarify stroke cause to optimise secondary prevention. Up to 30–40% of all ischaemic stroke and TIA cases remain, despite a modern extensive diagnostic work-up, with unknown aetiology and classify as cryptogenic.1–2 Paroxysmal atrial fibrillation (PAF) is suspected to cause one of three cryptogenic strokes3–5 but can easily be missed if the screening is performed only by routine short-term rhythm monitoring.5,6 Current American and European Stroke Guidelines still recommend secondary prevention with antiplatelet agents to prevent stroke recurrence in patients diagnosed with cryptogenic stroke or TIA without known AF.7,8 Oral anticoagulation is recommended only when AF is detected. Further investigation of the cardiac rhythm in cryptogenic stroke or TIA is therefore important,9 as the detection of AF has therapeutic implications on patient care.
The likelihood of revealing AF is increased if ECG monitoring is started early after index event and is performed continuously.5,10–12 Developments in technologies have changed the landscape of long-term AF monitoring and has been useful in reducing the proportion of patients diagnosed with cryptogenic stroke.6,10–12 In western cohorts, insertable cardiac monitors (ICMs) have been shown to be the gold-standard for AF detection.10–12 Performing early long-term AF monitoring with Reveal LINQ®13–15 in large groups of cryptogenic stroke or TIA patients in prospective observational trails will allow a better understanding of the significance of changes in AF burden over time and the risk of stroke among these patients.
Cryptogenic strokes are heterogeneous in origin. Biochemical and genetic analyses may therefore be useful to predict the probable cause.16,17 Levels of cardiac biomarkers have been demonstrated to be increased in AF patients18 and cryptogenic stroke patients with confirmed PAF.19,20 However, these biomarkers are also increased in other cardiac conditions than AF. Markers of inflammation, implicated in the pathogenesis of AF, have so far given mixed results21–23 and this field therefore needs to be assessed further. The identification of reliable biomarkers, which could be used in clinical practice for indicating AF in patients with cryptogenic stroke or TIA would be useful to select patients for prolonged cardiac rhythm monitoring with high likelihood of finding AF.
The main purpose of the Nordic Atrial Fibrillation and Stroke (NOR-FIB) Study is to assess the incidence of AF detection under continuous cardiac rhythm monitoring using the new generation of ICM (Reveal LINQ®) in patients with cryptogenic stroke or cryptogenic TIA and to identify biomarkers which can be used in clinical practice as predictors of incident AF. This article presents the rationale and design of the NOR-FIB Study.
Patients and methods
Study design
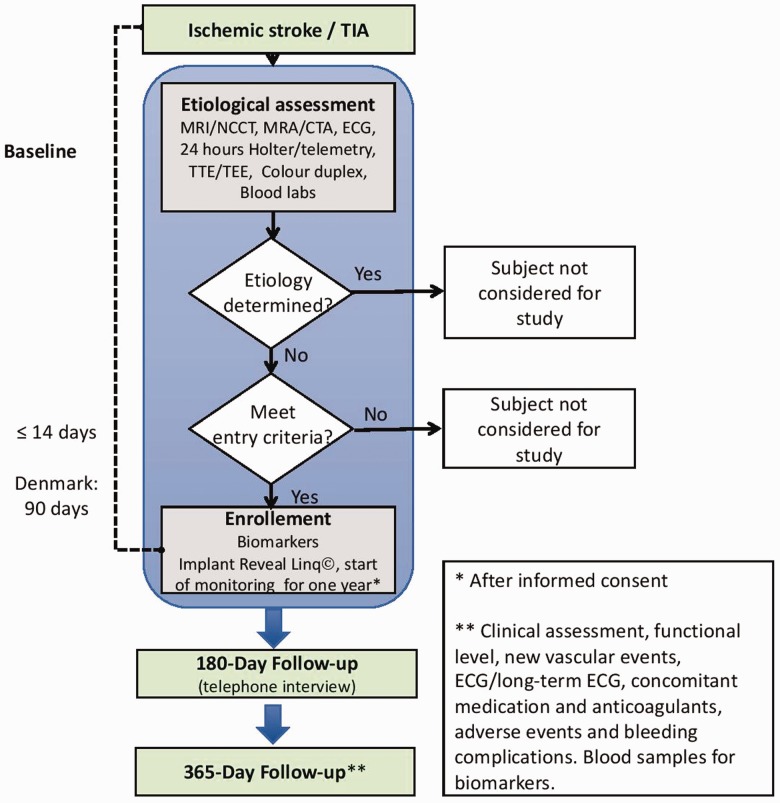
The NOR-FIB Study (ClinicalTrials.gov Identifier: NCT02937077, EudraCT 2018-002298-23) is a prospective, multicentre observational real-life study that will collect data from patients admitted with cryptogenic stroke or TIA without previously documented history of AF (defined below). Cryptogenic stroke is defined as ischaemic stroke where the aetiology is not detected even though all the investigation mentioned in the inclusion criteria are performed and when patent foramen ovale, pregnancy, history of atrial fibrillation or flutter is excluded (Table 1). Among TIA cases only those with acute non-lacunar infarct on magnetic resonance imaging (MRI) diffusion weighted imaging are included. Stroke units in the Nordic countries are participating (up to 30 centres). All patients will undergo continuous cardiac rhythm monitoring using the Reveal LINQ® device, started as soon as possible and within 14 days after the index event (Figure 1). In Danish centres, the time window for inclusion is up to 90 days from symptom start due to delay in echocardiography diagnostics. Continuous ECG monitoring will be carried out for 12 months. Periods of AF ≥2 min will be assessed for the risk of thromboembolism.11 Detection of AF will result in a change of the secondary prevention from antiplatelet drugs to oral anticoagulation unless there are strong contraindications. Blood samples measuring biomarkers will be taken in the acute phase and at 12 months´ follow-up. Estimated number of patients to be included is 500. The inclusion and exclusion criteria are outlined in Table 1.
Table 1.
Study inclusion and exclusion criteria.
| Inclusion criteria: |
| 1. Cryptogenic ischaemic stroke or TIA patients ≤14 days from symptom start. In Denmark, the time window for inclusion is up to 90 days from symptom start due to delay in echocardiography diagnostics. |
| 2. A stroke or TIA is considered to be cryptogenic if no cause can be determined despite an extensive workup according to the standard protocol of the participating centre. Before inclusion to the study, the following tests are required to establish the diagnosis of cryptogenic stroke or TIA:a |
| a. Brain MRI or CT.a |
| b. 12-Lead ECG for AF detection. |
| c. 24-h ECG monitoring for AF detection and premature atrial complex analysis. |
| d. Transthoracic echocardiography. |
| e. Transoesophageal echocardiography in patients aged ≤65 years. |
| f. Colour duplex ultrasound examination of the pre-cerebral arteries. |
| g. CTA or MRA of head and neck to rule out other causes of stroke. |
| h. Screening for thrombophilia <50 years of age. |
| 3. Age ≥18 years at onset of TIA/stroke. |
| 4. A participation consent form signed by the patient or a legally authorised representative. |
| Exclusion criteria: |
| 1. Known aetiology of TIA or stroke. |
| 2. TIA without documented cerebral ischemia on MRI diffusion weighted imaging. |
| 3. Untreated hyperthyroidism. |
| 4. Myocardial infarction <1 month prior to the stroke or TIA. |
| 5. Coronary bypass grafting <1 month prior to the stroke or TIA. |
| 6. Valvular heart disease requiring immediate surgical intervention. |
| 7. History of AF or atrial flutter. |
| 8. Patent foramen ovale |
| 9. Permanent indication for oral anticoagulation treatment at enrolment. |
| 10. Permanent contraindication for oral anticoagulation. |
| 11. Life expectancy <1 year. |
| 12. Pregnancy now or <3 months. |
| 13. An indication for an implantable pulse generator, implantable cardioverter-defibrillator, cardiac resynchronisation therapy or an implantable haemodynamic monitoring system. |
| 14. Patient otherwise not eligible for the study or adherent for follow-up (e.g. non-resident) or patient with concurrent disease which may affect clinical outcome (e.g. multiple sclerosis, cancer). |
CT: computed tomography; MRI: magnetic resonance imaging.
aTIA cases with acute non-lacunar infarct on MRI diffusion weighted imaging are included as TIA events.
Figure 1.
Study design diagram. NCCT non-contrast computed tomography, MRI: magnetic resonance imaging; CTA: computed tomography angiography; MRA: magnetic resonance angiography; ECG: electrocardiography; TTE: transthoracic echocardiography; TEE: transoesophagal echocardiography.
Patients with cryptogenic stroke or TIA with start of symptoms within 14 days and evidence of brain infarction on brain imaging will be considered eligible for the study if they have no previously documented history of AF. Ischaemic stroke or TIA is cryptogenic if no stroke mechanism can be determined after an evaluation according to standards of the American and European Guidelines.7,8 The minimum tests that must be performed before considering a stroke as ‘cryptogenic’ are outlined in Table 1.
Study outcomes
The aims of the study are to assess the incidence of AF detection in cryptogenic stroke or TIA patients using ICM (Reveal LINQ®), and to compare the levels of thrombotic and inflammation biomarkers in cryptogenic stroke or TIA patients with and without AF after one year continuous rhythm monitoring.
The primary endpoint is AF detection rate within 12 months of continuous rhythm monitoring in patients with a recent cryptogenic stroke or TIA without previously documented history of AF.
Secondary endpoints:
AF detection rate within six months.
Levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP), brain natriuretic peptide (BNP), troponin-T and troponin-I.
Levels of cardiovascular biomarkers.
Levels of biomarkers of fibrosis associated with AF.
Levels of inflammatory biomarkers in AF.
Distribution of ischaemic lesions on CT or MRI.
Left atrium diameter and risk of AF in cryptogenic stroke.
CHA2DS2-VASc score prior to ischaemic stroke or TIA.
Incidence of recurrent stroke or TIA within 12 months.
Use and type of oral anticoagulation and antiarrhythmic drugs at the 12-month follow-up visit.
Health outcome as evaluated by an EQ-5D questionnaire.
Insertable cardiac monitor
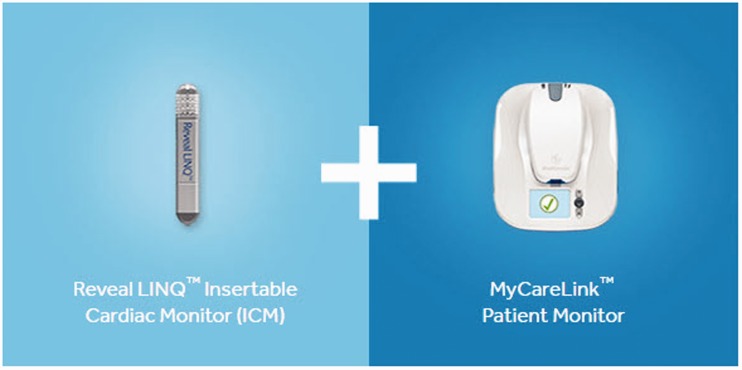
The Reveal LINQ® ICM system consists of an insertion kit, the Reveal LINQ® device, and the MyCareLink® patient monitor to allow remote automatically transmission of device data and diagnostic information through a secure network to the treating physician.13 (Figure 2). Dedicated incision and insertion tools facilitate insertion of the device. Electrodes on the ends of the device record ECG signals and an embedded accelerometer measures patient activity.24 Required programming for the Reveal LINQ® (‘cryptogenic stroke’ mode) is shown in Table 2. The insertion of the ICM is performed bedside in the stroke unit or in the outpatient clinic. All the investigators are trained in the insertion procedure before inclusion of the first patient. Real-world data from the ICM registry validated the detection rates of AF,14,15 the expected rate of unsuccessful insertion and ICM- or insertion-related complications (<2%).25,26 All adverse events are registered prospectively in the study.
Figure 2.
Reveal LINQ® insertable cardiac monitor. The left picture shows the loop recorder that is inserted. The right picture shows the patient monitor that allows remote transmission of device data and diagnostic information through a secure network to the treating physician. Copyright with permission from Medtronic.
Table 2.
Required programming for the Reveal LINQ®.
| Parameter | Current | Lifetime | Parameters |
|---|---|---|---|
| Symptom | On | On | Four 7.5 min episodes |
| Tachycardia | On | On | > x bpm1, ≥16 beats2 |
| Pause | On | On | >3 s |
| Bradycardia | On | On | <30 bpm, ≥4 beats |
| Atrial tachycardia (AT) | Off | Off | Off |
| Atrial fibrillation (AF) | On | On | All episodes |
| % of time in AT/AF | On | On |
Tachy Interval (Rate): Programmable range of 270–520 ms, in 10 ms intervals. (Nominal is the closest value that is ≤ (230 bpm minus the Patient’s age). Patient age is based on the data entered in patient date of birth. Tachy duration: programmable number of beats (5,12,16,24,32,48.)
The AF detection algorithm is based on both an R-R interval and a P-wave evidence score. The P-wave evidence score reduces inappropriate AF detections in the original R-R interval pattern-based algorithm by leveraging the evidence of a single P wave between two R waves using morphologic processing of the ECG signal. The AF detection algorithm makes a rhythm classification every 2 min. This algorithm has been previously validated against simultaneous Holter monitor data in patients with known AF. Sensitivity was 96.1% for identifying patients with AF and a negative predictive value of 97.4% for correctly excluding the absence of AF.27
When an AF episode is detected at the end of a 2-min detection period, the first 2 min of the ECG from that episode are stored in the device. The device can store up to 14 AF episodes with ECG and the earliest episode is overwritten by newer episodes. The longest AF episode detected (≥10 min) is always preserved in memory until a full manual transmission is carried out. Every night, the ICM wirelessly transmits the last 10 s of the 2-min ECG segment for the longest episode of the day. The patient can also manually transmit the full information on all episodes stored in memory at any given time.
The investigators will have access to e-learning programs at www.medtronicacademy.com and access to the CareLink Network pages for monitoring of included patients. In addition, the Executive Working Group will serve as a core lab to follow-up all the results from the ICM.
Biomarkers
NT-proBNP/ BNP and troponins will be analysed in the laboratories of the participating centres as part of routine clinical practice. Biomarkers will be analysed at Oslo University Hospital, Oslo, Norway; Firalis SAS, Huningue, France and Østfold Hospital Trust, Grålum, Norway. Olink Technology with Proseek® multiplex cardiovascular disease (CVD) will be used to search for predictors of incident AF with reagent kit measuring CVD-related human protein biomarkers. Olink Technology used in the pilot study detected relevant biomarkers that will be replicated in the main study.28
The search for potential biomarkers will also include the following approaches:
Measuring inflammatory and related markers in blood collections (using enzyme immunoassays and multiplex).
Assessing lncRNA exosome profile in blood collections using LncProfiler qPCR array.
In depth characterisation of the inflammatory and fibrotic pathways, by RNA sequencing of leukocyte subpopulations from blood samples and supplementary analyses of mRNA levels in leukocyte subfractions using qRT-PCR.
Enrolment and follow-up
The patients will be screened for stroke cause during their admission for acute ischaemic stroke or TIA. When the diagnosis of cryptogenic stroke or TIA is made, they will be included in the study after the consent form is signed. The rhythm monitoring should be started as soon as possible and no later than 14 days after the onset of stroke or TIA symptoms. The local coordinator will implant the Reveal LINQ® according to the description and training provided by Medtronic and the Executive Working Group. Continuous ECG monitoring will then be carried out for 12 months. We plan to include 500 patients. After inclusion the patients will have study visits at six and 12 months (respectively telephonic and clinical interview), as shown in Figure 1 and Table 3. The enrolment period is planned for 18 months and the follow-up period is 12 months. Blood samples for biomarkers investigation are collected in the acute phase and at 12 months follow-up.
Table 3.
Study visits.
| Assessment method | Screening | Inclusion | 180-day follow-up | 365-day follow-up | Unscheduled follow-up | |
|---|---|---|---|---|---|---|
| Range | 0 | 0 | ±20 days | ±20 days | ||
| NIHSS | X | X | ||||
| ECG | X | X | X | X | ||
| MRI or NCCT | X | |||||
| MRA or CTA | X | |||||
| Colour duplex of the precerebral arteries | X | |||||
| 24-h ECG monitoring | X | |||||
| Echocardiography TTE/TEE | X | |||||
| Blood labs | X | X | ||||
| Modified Rankin Scale mRS | X | X | ||||
| Pre-stroke mRS | X | |||||
| Pre-stroke Barthel Index | X | |||||
| Barthel Index | X | |||||
| EuroQol 5D-5L | X | X | ||||
| Concomitant medication | X | X | X | X | X | |
| Recurrent stroke/TIA | X | X | X | X | ||
| Major cardiovascular events | X | X | X | X | ||
| Adverse events | X | X | X | X | X | |
NIHSS: National Institute of Health Stroke Scale; ECG: electrocardiography; MRI: magnetic resonance imaging; CT: computer tomography; TTE: transthoracic echocardiography; TEE: transoesophageal echocardiography; EuroQOL: European Quality of Life Scale.
This study is based on broad collaboration and interaction between neurologists, geriatricians, cardiologists, and biochemists in the Specialized Health Care System. At all participating centres cardiologists are involved in the investigation of the patients with standardised protocols. This will secure an optimal assessment of the patients before inclusion in the study. The cardiologists are also involved during the follow-up in patients where AF is detected. In addition, a core lab will assess the quality of the long-term rhythm monitoring recordings and ensure that patients with newly diagnosed AF receive any necessary adjustments of their secondary prevention treatment.
Data will be registered using the webform in the Services for Sensitive Data (TSD) at the University of Oslo, Norway. Storage and management of the data will be performed at Oslo University Hospital. The centres in the NOR-FIB Study are already participating in the research network NORSTROKE which is using the European Cerebrovascular Research Infrastructure (ECRI, www.ecri.no).
Safety monitoring
The investigators have access to the Care Link Network pages for the monitoring of included patients. In addition, the Executive Working Group serves as core lab to follow-up all the results from the ICM. Medical therapy for stroke prevention is left to the discretion of the treating physician in collaboration with the study cardiologist. If anticoagulation is initiated in any patient, both the intention and the reason will be recorded.
Ethical conduct
This study is conducted in accordance with ISO-14155 as guidance and in compliance with the Declaration of Helsinki and the laws and regulation for the participating countries and approved by local ethical committees. The NOR-FIB Study is supported by Medtronic, Inc which is funding 400 out of 500 devices. The rest of the costs in the study are not supported by the sponsor. The sponsor ensures training of all involved study personnel in terms of use of the device and interpretation of the study data. Devices used in this study have CE mark and FDA approval, are market released, and are being used according to licensed indications. The sponsor has not had any influence on study design or study conduct.
Statistical analysis
In order to achieve 90% power to detect a 9% higher percentage rate of AF using the Reveal LINQ device, a sample size calculation pointed to a sample size of 500 subjects in this population with increased levels of biomarkers relevant for AF, as opposed to patient population with low biomarkers levels.
Timeline
The inclusion of patients was started from the first centre in January 2017 and we are planning to recruit up to 30 centres. One hundred twenty-three patients at 14 centres have been enrolled; study recruitment is expected to be completed within end of 2019. The follow-up period will be finished by the end of 2020.
Discussion
Detection of PAF in patients with cryptogenic stroke or TIA has important therapeutic implications for optimising secondary prevention and reducing the risk of recurrent stroke.7,8 Approximately 30% of the patients with cryptogenic stroke or TIA have episodes of silent AF that remain undiscovered.12 Studies have shown that even subclinical AF strongly predicts clinical AF and is associated with an elevated absolute stroke risk.29 In those patients secondary prevention with antiplatelet drugs, according to current standard of treatment, is insufficient, while the detection of AF and the use of anticoagulation can prevent recurrent stroke. Prolonged cardiac rhythm monitoring with ICMs is an effective tool for diagnosing an underlying AF. In the previous RCTs, ICMs have shown a superior diagnostic yield compared to conventional short-term monitoring and have been significantly more effective than any of the simulated intermittent monitoring strategies for identifying AF in patients with previous cryptogenic stroke.10,11 The vast majority of patients with AF would not have been detected with routine short-term monitoring (24-h or even 7 days ECG). ICMs should therefore be considered more often in the evaluation of cryptogenic ischaemic events.5,30,31 Currently only a minority of cryptogenic stroke patients are screened with long-term ECG because this method is still not routinely available in clinical practice due to high costs.32 Another reason is that the cryptogenic stroke patients group is very heterogenic regarding aetiology and only a minority have cardioembolism due to AF. On the other hand, routine use of oral anticoagulation for secondary prevention in patients with cryptogenic stroke have not been shown to reduce the risk.9 Recently published results from the NAVIGATE ESUS study demonstrated a lack of effect of rivaroxaban compared to aspirin on the stroke recurrence risk, and a higher risk of bleeding, suggesting that embolism from undetected paroxysmal AF was not a major cause of recurrent stroke among patient with cryptogenic stroke as assumed.9
Performing new large studies combining cardiac rhythm monitoring with ICMs and the identification of AF specific biomarkers from a broad spectrum of molecular biomarkers seems to be a novel and better concept in the evaluation of patients with cryptogenic stroke. To our knowledge such studies have not been performed. In our study, we also include patients with clinical TIAs, as these events may as well be cryptogenic, and these patients are generally treated identically to those with stroke and have the same risk factors.
The study's ambition is to find new biomarkers that potentially can improve secondary prevention, by identifying those cryptogenic stroke or TIA patients in whom AF is more likely to be detected by prolonged ECG monitoring (targeted selection). These selected patients will then be screened with continuous ECG for a longer period. The demonstration that known or new cardiovascular biomarkers can be used to identify AF in cryptogenic stroke or TIA patients will have a considerable clinical impact in the further management of these patients. This will result in a new optimised cost-effective AF detection algorithm. Other AF detection algorithms have already been tested and seem to be useful, but in our opinion need improvements.4,33–35 The detection methods for some of these biomarkers are already available and they can potentially be used routinely in the clinical assessment of cryptogenic stroke and TIA patients.22 The results from our pilot study indicate that multiple proteins may potentially be used as biomarkers.28
Conclusion
The NOR-FIB Study will provide more information on the relationship between episodes of PAF and the risk of stroke. Hopefully, the study will contribute to a better understanding of the risk of short-term AF and optimal duration of cardiac rhythm monitoring. Secondary stroke prevention will be considerably improved if a larger proportion of cryptogenic stroke or TIA patients with undetected AF are diagnosed with AF and treated with anticoagulation, instead of antiplatelet drugs. The detection of new biomarkers will allow its use in a new and better screening algorithm and in the future change the guidelines for stroke screening for underlying cause.
Acknowledgements
We would like to thank Thomas von Lueder, Vigdis Bjerkeli, Gudrun Anette Høie, Lise Aagaard Sørby and Waleed Ghanima for assistance and guidance in this research, and all cooperating stroke units in Norway, Denmark and Sweden for their engagement.
Contributorship
AHA, DR, DA and BH researched literature and conceived the study. BRT, ATL, HJ, BH, DR, DA and AHA were involved in protocol development, gaining ethical approval, patient recruitment and data analysis. BRT wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DA has received honoraria from BMS/Pfizer, Boehringer-Ingelheim and MSD, Bayer, Sanofi, Novartis. DA has received research grants to institution from BMS/Pfizer and Medtronic. AHA has received travel funding from Medtronic.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NOR-FIB is an investigator driven academic study. Of the 500 devices, 400 are supported by Medtronic. The study is supported by the research infrastructure of the European Cerebrovascular Research Infrastructure (ECRI).
Ethical approval
Ethical approval for this study was obtained from The Norwegian South Eastern Ethical Committee (REC number: 2013/2371).
Guarantor
BRT.
Informed consent
Written informed consent is obtained from all subjects before including in the study.
References
- 1.Saver JL. Cryptogenic stroke. N Engl J Med 2016; 375: e26. [DOI] [PubMed] [Google Scholar]
- 2.Li LYiin GS, Geraghty OC, et al. . Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015; 14: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glotzer TV, Ziegler PD. Cryptogenic stroke: is silent atrial fibrillation the culprit?. Heart Rhythm 2015; 12: 234–241. [DOI] [PubMed] [Google Scholar]
- 4.Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke 2008; 39: 1901–1910. [DOI] [PubMed] [Google Scholar]
- 5.Sposato LACipriano LE, Saposnik G, et al. . Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 377–387. [DOI] [PubMed] [Google Scholar]
- 6.Andrade JG, Field T, Khairy P. Detection of occult atrial fibrillation in patients with embolic stroke of uncertain source: a work in progress. Front Physiol 2015; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Stroke Organisation Executive Committee and ESOW Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJRabinstein AA, Ackerson T, et al. . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 9.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018; 378: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 10.Sanna TDiener HC, Passman RS, et al. . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 11.Christensen LMKrieger DW, Højberg S, et al. . Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21: 884–889. [DOI] [PubMed] [Google Scholar]
- 12.Carrazco C, Golyan D, Kahen M, et al. Prevalence and risk factors for paroxysmal atrial fibrillation and flutter detection after cryptogenic ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27: 203–209. [DOI] [PubMed] [Google Scholar]
- 13.Tomson TT, Passman R. The reveal LINQ insertable cardiac monitor. Expert Rev Med Devices 2015; 12: 7–18. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler PDRogers JD, Ferreira SW, et al. . Real-world experience with insertable cardiac monitors to find atrial fibrillation in cryptogenic stroke. Cerebrovasc Dis 2015; 40: 175–181. [DOI] [PubMed] [Google Scholar]
- 15.Sanders PSanders P, Purerfellner H, Pokushalov E, et al. . Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ usability study. Heart Rhythm 2016; 13: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 16.Llombart VGarcia-Berrocoso T, Bustamante A, et al. . Cardioembolic stroke diagnosis using blood biomarkers. Curr Cardiol Rev 2013; 9: 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santamarina EPenalba A, Garcia-Berrocoso T, et al. . Biomarker level improves the diagnosis of embolic source in ischemic stroke of unknown origin. J Neurol 2012; 259: 2538–2545. [DOI] [PubMed] [Google Scholar]
- 18.Seegers JZabel M, Gruter T, et al. . Natriuretic peptides for the detection of paroxysmal atrial fibrillation. Open Heart 2015; 2: e000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca ACBrito D, Pinho e Melo T, et al. . N-terminal pro-brain natriuretic peptide shows diagnostic accuracy for detecting atrial fibrillation in cryptogenic stroke patients. Int J Stroke 2014; 9: 419–425. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu-Boire ILeblanc N, Berger L, et al. . Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2013; 22: 978–983. [DOI] [PubMed] [Google Scholar]
- 21.Wu NChen X, Cai T, et al. . Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol 2015; 31: 278–286. [DOI] [PubMed] [Google Scholar]
- 22.Lind LSundström J, Stenemo M, et al. . Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart 2017; 103: 377–382. [DOI] [PubMed] [Google Scholar]
- 23.Wallentin LHijazi Z, Andersson U, et al. . Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014; 130: 1847–1858. [DOI] [PubMed] [Google Scholar]
- 24.Mittal SSanders P, Pokushalov E, et al. . Safety profile of a miniaturized insertable cardiac monitor: results from two prospective trials. Pacing Clin Electrophysiol 2015; 38: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 25.Rogers JDSanders P, Piorkowski C, et al. . In-office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In-Office 2 randomized study. Heart Rhythm 2017; 14: 218–224. [DOI] [PubMed] [Google Scholar]
- 26.Wong GRLau DH, Middeldorp ME, et al. . Feasibility and safety of Reveal LINQ insertion in a sterile procedure room versus electrophysiology laboratory. Int J Cardiol 2016; 15: 13–17. [DOI] [PubMed] [Google Scholar]
- 27.Mittal SRogers J, Sarkar S, et al. . Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm 2016; 13: 1624–1630. [DOI] [PubMed] [Google Scholar]
- 28.Lambert TA, Ratajczak-Tretel B, Halvorsen BE, et al. Bomarkers for ischemic strokes due to atrial fibrillation – the Nordic Atrial Fibrillation and Stroke Study (NOR-FIB). In: The 3rd European Stroke Organisation conference, 2017; Prague, Czech Republic, 16th–18th May, 2017.
- 29.Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018; 39: 1407–1415. [DOI] [PubMed]
- 30.Carmona-Puerta R, Castro-Torres Y. . Atrial fibrillation and cryptogenic stroke. What is the current evidence? Role of electrocardiographic monitoring. J Arrhythmia 2018; 34: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conti SReiffel JA, Gersh BJ, et al. . Baseline demographics, safety, and patient acceptance of an insertable cardiac monitor for atrial fibrillation screening: the REVEAL-AF Study. J Atr Fibrillation 2017; 9: 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler PDRogers JD, Ferreira SW, et al. . Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol 2017; 244: 175–179. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen KBMadsen C, Sandgaard NCF, et al. . Subclinical atrial fibrillation in patients with recent transient ischemic attack. J Cardiovasc Electrophysiol 2018; 29: 707–714. [DOI] [PubMed] [Google Scholar]
- 34.Poli SDiedler J, Härtig F, et al. . Insertable cardiac monitors after cryptogenic stroke – a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol 2016; 23: 375–381. [DOI] [PubMed] [Google Scholar]
- 35.Reinke FBettin M, Ross LS, et al. . Refinement of detecting atrial fibrillation in stroke patients: results from the TRACK-AF Study. Eur J Neurol 2018; 25: 631–636. [DOI] [PubMed] [Google Scholar]