Abstract
Background
Viscosupplementation of the synovial fluid with intra-articular hyaluronic acid (IA HA) is a well-known symptomatic treatment of knee osteoarthritis. The question arises whether a monoinjection (ie, single injection) could be as efficient as multi-injection (ie, 3–5 injections) regimens.
Methods
A meta-analysis of published studies relating to IA HA monoinjection trials was performed. The efficacy criterion was the Western Ontario and MacMaster Universities pain subscore. Any study design was accepted, from randomized control trials to single-arm observational open-label studies. An extensive search was performed using PubMed, Google, Google Scholar, and references found in recent meta-analyses, for all articles published before end of April 2018. Population profiles were analyzed in terms of age, sex, body mass index (BMI), and Kellgren-Lawrence (KL) radiology grades. Results of intra-articular single injection of placebo were collected to create a database allowing post hoc comparisons. Each IA HA study arm was compared to an IA placebo arm (either pooled or not), to present a similar KL profile controlled with the χ2 test. The effect size (ES) (95% CI) and P values were calculated and synthesized for each follow-up visit at 1, 2, 3, and 6 months. In parallel, a global approach was used to represent the variations from baseline for each group or subgroup studied.
Results
From 1547 citations, 28 studies were included in the meta-analysis, representing 4129 patients treated with monoinjection: 3360 received IA HA and 769 patients received IA placebo. The mean (SD) IA HA patient was 61.2 (9.6) years, 63% women, BMI 28.0 (4.1), 47% KL III, and 3% KL IV. A good placebo KL profile matching was obtained for 26 of the 31 IA HA arms. For the whole IA HA population, ES = 0.30 (95% CI, 0.25–0.35) at 3 months and ES = 0.39 (95% CI, 0.33–0.44) at 6 months. In a restricted analysis, after removal of outliers, poorly KL matched and active arms <30 patients, results remained unchanged, ES = 0.29 (95% CI, 0.23–0.34) and ES = 0.40 (95% CI, 0.34–0.45) at 3 and 6 months respectively, whilst heterogeneity was improved.
Conclusions
There are certainly limits to the post hoc placebo comparison method, for individual studies. But for each synthesis per subgroup or group, the results were properly confirmed using multiple statistical approaches and weighing methods. This meta-analysis suggests that monoinjections produce results similar to multi-injections of IA HA in terms of pain relief in the treatment of knee osteoarthritis. (Curr Ther Res Clin Exp. 2019; 80:XXX–XXX)
Introduction
Viscosupplementation (VS) of the synovial fluid by intra-articular (IA) injections of hyaluronic acid (HA) is a well-known symptomatic treatment of knee osteoarthritis (OA), in use for more than 30 years. Typically, the treatment consisted of 3 to 5 injections at 1-week intervals, but more recently—in the past 10 to 15 years—alternative regimens have been proposed, consisting of 1 injection only. The question therefore arises whether a single injection (ie, monoinjection) of IA HA has the same level of efficacy as multi-injection regimens, particularly in comparison to injected placebo.
Objectives
To evaluate the efficacy of a single IA injection of HA, in the symptomatic treatment of knee OA, by comparing clinical results obtained through various trials using the Western Ontario and MacMaster universities pain subscore (WOMAC A), to those obtained with a single injection of placebo.
A meta-analysis of published studies was performed, to collect the largest quantity of clinical results. No systematic review was anticipated.
Methods
The methods were adapted from the preferred reporting items for systematic reviews and meta-analyses statement1 recommendations.
Protocol and ethic statements
The protocol was to extract and explore all data results for the WOMAC A, from published studies without limiting the investigation to randomized controlled trials (RCTs). Consequently, with inclusion of all types of study, a high level of evidence is not claimed for this meta-analysis, and no registration was needed. Institutional review board/ethics committee approval was not required for this retrospective meta-analysis that was based solely on published statistical results, without need of any individual patient results.
Eligibility criteria
Inclusion of articles was done after passing the following criteria:
-
•
Clinical prospective studies on human patients with knee OA, with all designs permitted, from double-blind RCT to open-label single arm studies. Any kind of comparator was allowed, including injected placebo (ie, saline solution), another IA HA (single or multi-injections), or any other alternative treatment (preferably injected). At minimum, a comparison to baseline was required.
-
•
At least 1 population arm treated with a single injection of HA, positioned as the product analyzed or as the control.
-
•
The WOMAC A—the single criterion used for this meta-analysis—with at least 2 measures made: 1 at inclusion (ie, baseline) and 1 at follow-up.
-
•
Known patient profile for each population, with data documented for age, body mass index (BMI), distribution of Kellgren-Lawrence (KL) radiology grades, and OA anteriority to allow the assessment of comparability between groups.
-
•
Quantitative results allowing analysis, preferably given as mean, standard deviation (SD) and number of patients, for each measure.
-
•
Qualitative results provided as frequency (percentage or population).
Placebo-injected comparators were analyzed similarly, and their results were pooled in various combinations to match the patient profile of each trial. Other comparator arms present in these studies, such as IA steroids, multi-injection HA, or alternative treatments (eg, plasma rich in growth factors), were not considered for this analysis.
Information sources
A systematic research was done to select proper results, all published before end of April 2018. No limitation to the past was set, because single-injection of IA HA is a relatively recent regimen for the VS in knee OA. There was no restriction placed on the country in each study was done, but only articles published in English were considered. Articles were selected among references found in PubMed, Google, Google Scholar, and from recent meta-analyses.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 We used the following key words, sequentially associated, to get limited lists of properly oriented citations: single, hyaluronic acid, sodium hyaluronate, intra-articular, injection, knee, osteoarthritis, viscosupplementation, cross-linked, G-F 20, Hylan, Synvisc-One, Durolane, NASHA, Monovisc, Gel-200, and clinical trial.
Search and study selection
A first selection was done after screening the titles and abstracts, then full-text articles were assessed for their eligibility. Special care was taken to eliminate duplicate publications at different levels of the search. Animal or laboratory testing, general articles, reviews, meta-analyses, recommendations (ie, guidelines), or author's opinions were also eliminated.
At the end, articles were also not included if they described:
-
•
A specific context different from the current practice of VS of knee OA: other joint, surgery context, or other pathology associated;
-
•
A multi-injection regimen (more than 1 IA HA injection per treatment);
-
•
A clinical trial done with a nonmarket-approved mono-injection IA HA (European Community or United States);
-
•
A planned combination of treatments, without use of IA HA alone; or
-
•
Insufficient data for the WOMAC A.
Data process
Data extracted from the articles were taken as published and no question has been addressed to any of the authors. A strong effort was made to include all possible studies; when needed and justified, the standard error (SE) was calculated from the P value, or data was measured on the available graphs. No alternative pain assessment was considered to compensate or complete any missing WOMAC A data.
Data were collected and processed using Excel (Microsoft Corp, Redmond, Washington). Interpretation was done following the description made within each study. Data was recorded per study arm either for the IA HA mono-injection or, when present, the injected placebo comparator. These data included the arm size (ie, population), the average patient profile at inclusion (ie, number, sex, age, BMI, OA anteriority), and the WOMAC A subscore (SD) at each observation time from the inclusion to the last control visit. For a comprehensive assessment, all these scores were recalculated on a 0 to 100 scale, and the observation times were classified at months M1 (2–5 weeks), M2 (6–9 weeks), M3 (10–18 weeks), and M6 (22–26 weeks).
Many studies did not have their own IA placebo control arms, so our approach consisted of using the available IA placebo results as an independent database, and subsequently selecting the best placebo comparator for each study by matching the KL profile. Proper matching was controlled with the χ2 test and considered as satisfactory if P > 0.05. Each RCT versus placebo kept its own control arm.
Summary measures
In the first phase, each individual study and its matching placebo were analyzed. The variations from baseline (or inclusion) were calculated with the SD for each population seen at each visit (M1, M2, M3, and M6). Then, the comparison to placebo was done, calculating the difference of variation from baseline, the pooled SD and SE, the effect size (ES)—which is defined here as Cohen's d13 with the attached 95% CI—and P value.
Synthesis of results
In the second phase, different pooling methods were tested. Using MIX 2.0 software (BiostatXL, Frederick, Maryland) (fixed effect, inverse-variance pooling), a synthesis at each visit time was proposed, representing the results as forest plots. The clinical efficacy was therefore assessed for each individual study, from the absolute difference with placebo (relevant or not) and the statistical significance (P value). Also, ES = 0.2 was a priori considered small, 0.5 was considered medium, and 0.8 was considered large. Heterogeneity was assessed from funnel plots and from the indexes I2 and τ2.
Additional analysis
In a separate approach, single-injection HAs were pooled per product to form subgroups >500 patients. Alternatively, they were pooled together into a subgroup called “other IA HA.” Intergroup comparisons were performed between each subgroup (product) and its matched placebo group. Finally, a synthesis was done for all single IA HA products. Graphs were made to illustrate these results, representing the score variations from baseline and ES as a function of time that follows the concept of a therapeutic trajectory.2
Risk of bias within or across studies
There was no systematic review of each characteristic of the studies. Our intention was to explore widely existing data, so we included all types of studies. Consequently, factors like the presence of a control arm, the allocation technique, or the quality of the blinding could not apply. To assess the risk of bias, we first used the funnel plots to detect outlying results and potential publication bias, demonstrated by asymmetry of the funnel plot. Then, to limit the risk of selection bias, special care was given to the quality of the placebo matching for each active study arm. In summary, for the included studies, outlying results, other potential bias, heterogeneity assessment results, and other abnormalities were analyzed. This led us to discard several studies that are further detailed in the Results.
Results
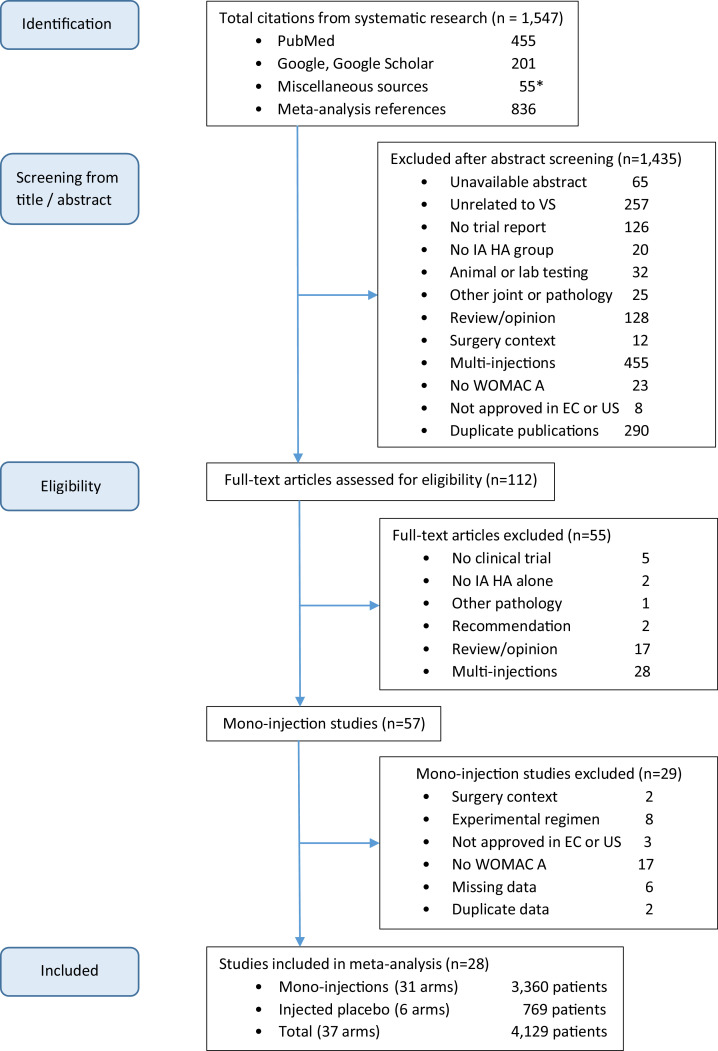
Study selection
Study selection results are described by the flow chart (Figure 1). The research was initiated via PubMed, proceeded with Google, and subsequently with Google Scholar. As complement, 55 miscellaneous citations (35 abstracts and 20 full-text articles) were added from various sources. A final search of the lists of references generated numerous duplicates that were eliminated during our selection process. A total of 1547 citations were identified. After screening from title and/or abstract and removal of the duplicate publications, 112 full-text articles of interest were assessed for eligibility. Of these, 57 included data for IA HA mono-injection.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 At the end, 28 studies were included,43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 whereas 29 studies were removed for 1 or several of the following reasons:
-
•
Two studies were in combination with surgery: anterior cruciate ligament reconstruction,26 or arthroscopy27;
-
•
Eight with experimental regimens from a multi-injection product: single-shot,26, 27, 28 or large shot grouping several syringes,29, 30, 31 or smaller shot,32 or retreatment33;
-
•
Three nonapproved products for VS in European Community or United States16, 17, 34;
-
•
Seventeen did not include a WOMAC A result14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30;
-
•
Six with missing data: no SD or SE,35, 36, 37, 38 no score at inclusion (set at 100% = major bias),39 or inappropriate data (population ratio for gain >15 mm or 40%)40; and
-
•
Two had duplicate datasets: the dataset used by Frampton41 was the same as that used by Chevalier et al43 and the dataset used by Belzile et al42 was the same as that used by Hangody et al.60
Figure 1.
Flow chart. EC = European community; IA HA = intra-articular hyaluronic acid; US = United States; VS = viscosupplementation; WOMAC A = Western Ontario and MacMaster Universities pain subscore. *Thirty-five abstracts and 20 full-text articles.
In summary, 25 of the 28 included studies were successively identified from PubMed (19 during the first search), 3 from Google, and none additional from the other sources.
Study characteristics and placebo matching
Among the 28 included studies, 8 were single-arm, observational studies and 20 were RCTs. Of those RCTs, 14 were described as double-blind. In the 6 remaining RCTs,49, 55, 57, 59, 64, 65 there were numerous differences in treatments (eg the number of injections) and, although it seemed possible to maintain study blinding for the patient, it was unclear whether the assessment was made by a blinded investigator. The comparators within the 20 RCTs were:
-
•
IA saline solution (ie, placebo) for 6 studies;43, 51, 52,60, 61, 62
-
•
IA autologous preparation for 4 studies:56, 57, 64, 65 platelet rich plasma (PRP), plasma rich in growth factors (PRGF), or mesenchymal stem cells (MSC);
-
•
IA ozone for 1 study64;
- •
-
•
Another IA HA monoinjection for 3 studies, here analyzed as separate arms,45, 46, 48 or ignored for 2 other experimental, nonapproved products45; and
-
•
IA HA multi-injection for 4 studies.54, 55, 59, 70 To maintain the same number of injections per patient in both trial arms, 1 study used sham injections from empty syringes54 and another used IA saline injections.70
In Table 1, studies have been organized per subgroups (Synvisc-One [Sanofi-Aventis, Bridgewater, New Jersey], Durolane [Bioventus LLC, Durham, North Carolina], or other products), allowing intermediate and global syntheses. The active arms were identified from #1 to #31. The reference index of each citation is given with the principal author's name and later in the document, we attached the year of publication, as given in the citation.
Table 1.
Selected studies and patient profiles.
| ID | Author | RCT | Follow-up, wk | IA HA mono-injection | N per arm | Sex, W% | Age (SD), y | BMI (SD) | KL % |
Anteriority, y | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| III | IV | ||||||||||
| 1 | Chevalier, et al43 | Yes | 26 | Synvisc-One* | 124 | 74 | 63.6 (12.6) | 29.1 (4.8) | 49 | 0 | 6.5 (6.4) |
| 2 | Pal, et al44 | No | 52 | Synvisc-One | 394 | 72 | 57.6 (9.8) | 27.7 (4.5) | 57 | 0 | 1.4 (2.8) |
| 3 | Petrella, et al45 | Yes | 26 | Synvisc-One | 32 | 50 | 59.0 (12.0) | 29.0 (3.8) | 44 | 0 | 5.8 (4.7) |
| 4 | Dreiser, et al46 | Yes | 26 | Synvisc-One | 147 | 61 | 66.6 (10.7) | 26.3 (2.8) | 24 | 0 | 6.9 (6.7) |
| 5 | Tammachote, et al47 | Yes | 26 | Synvisc-One | 50 | 86 | 62.6 (10.0) | 26.3 | 44 | 14 | NA |
| 6 | Sun, et al48 | Yes | 26 | Synvisc-One | 59 | 71 | 62.5 (10.0) | 25.2 (4.2) | 34 | 0 | 5.2 (4.6) |
| 7 | De Campos, et al49 | Yes | 26 | Synvisc-One | 52 | 75 | 61.0 (12.0) | 30.0 (5.2) | 34 | 25 | NA |
| 8 | Kearey, et al50 | No | 52 | Synvisc-One | 119 | 66 | 60.2 (11.3) | 30.9 (6.4) | 55 | 0 | 5.3 (6.2) |
| Subgroup | 26 | Synvisc-One | 977 | 70 | 60.8 (9.5) | 28.0 (4.0) | 47 | 2 | 4.0 | ||
| 9 | Altman, et al51 | Yes | 26 | Durolane† | 172 | 46 | 62.9 (10) | 30.3 (5.0) | 53 | 23 | 5.0 |
| 10 | Arden, et al52 | Yes | 6 | Durolane | 108 | 55 | 64.5 (15.9) | 27.2 (5.6) | 67 | 0 | 2.2 (2.2) |
| 11 | Leighton, et al53 | Yes | 26 | Durolane | 218 | 51 | 61.9 (9.6) | 28.2 (4.2) | 69 | 0 | 4.7 (5.4) |
| 12 | Zhang, et al54 | Yes | 26 | Durolane | 161 | 74 | 60.2 (8.1) | NA | 42 | 0 | 3.9 (5.3) |
| 13 | Estades-Rubio, et al55 | Yes | 26 | Durolane | 27 | 52 | 52.9 (13.9) | 30.0 (4.5) | 19 | 0 | 2.1 (1.2) |
| 14 | Louis, et al56 | Yes | 13 | Durolane | 24 | 54 | 48.5 (11.5) | 27.0 (2.9) | NA | NA | 8.4 (8.4) |
| 15 | Vaquerizo, et al57 | Yes | 48 | Durolane | 48 | 54 | 64.8 (7.7) | 31.0 (4.6) | 44 | 19 | NA |
| Subgroup | 26 | Durolane | 758 | 56 | 61.6 (8.9) | 28.9 (4.2) | 55 | 7 | 4.4 | ||
| 16 | Baron, et al58 | No | 26 | Arthrum 75‡ | 218 | 56 | 62.9 (12.6) | 27.2 (4.3) | 46 | 0 | 4.1 (5.4) |
| 17 | Diraçoglu, et al59 | Yes | 26 | Monovisc§ | 20 | 80 | 58.0 (7.0) | 30.5 (4.9) | NA | NA | NA |
| 18 | Hangody, et al60 | Yes | 26 | Monovisc | 150 | 66 | 59.2 (8.6) | 28.4 (4.5) | 18 | 1 | NA |
| 19 | Hangody, et al60 | Yes | 26 | Cingal§ | 149 | 65 | 57.5 (8.4) | 28.9 (4.7) | 19 | 0 | NA |
| 20 | Strand, et al61 | Yes | 13 | Gel-One|| | 247 | 60 | 60.9 (10.2) | 28.3 (4.1) | 53 | 0 | NA |
| 21 | Takamura, et al62 | No | 26 | Gel-One | 152 | 58 | 61.0 (9.4) | NA | 44 | 0 | 0.6 (0.6) |
| 22 | Borras-Verdera, et al63 | No | 26 | Ostenil-Plus¶ | 80 | NA | > 40 | NA | 100 | 0 | NA |
| 23 | Dreiser, et al46 | Yes | 26 | Ostenil-Plus | 143 | 73 | 67.1 (9.7) | 26.4 (2.9) | 31 | 0 | 5.4 (5.4) |
| 24 | Duymus, et al64 | Yes | 52 | Ostenil-Plus | 34 | 97 | 60.3 (9.1) | 28.4 (3.6) | 29 | 0 | NA |
| 25 | Lamo-Espinosa, et al65 | Yes | 52 | HyalOne# | 10 | 30 | 60.3 (4.4) | 29.6 (3.4) | 20 | 40 | 6.0 (4.4) |
| 26 | Conrozier, et al66 | No | 26 | HappyCross⁎⁎ | 40 | 73 | 60.7 (13.9) | 28.6 (5.0) | 43 | 25 | NA |
| 27 | Monet, et al67 | No | 28 | HappyCross | 53 | 66 | 62.6 (12.3) | 27.5 (5.2) | 43 | 19 | 4.5 (3.0) |
| 28 | Bashaireh, et al68 | No | 39 | Crespine Gel†† | 84 | 37 | 55.8 (9.3) | 30.5 (4.9) | 56 | 1 | NA |
| 29 | Sun, et al48 | Yes | 26 | Hya-Joint Plus‡‡ | 62 | 77 | 62.7 (8.4) | 24.7 (3.3) | 35 | 0 | 5.4 (4.4) |
| 30 | Tuan, et al69 | No | 26 | Hya-Joint-Plus | 46 | 80 | 65.1 (9.3) | 24.0 (3.6) | 41 | 0 | NA |
| 31 | Ha, et al70 | Yes | 15 | Hyruan-One§§ | 137 | 81 | 62.0 (8.6) | 25.1 (2.9) | 43 | 0 | 4.0 (4.0) |
| Subgroup | 26 | other IA HA | 1625 | 65 | 61.3 (10.0) | 27.7 (4.1) | 42 | 2 | 3.2 | ||
| Total monoinjections | All IA HA | 3360 | 63 | 61.2 (9.6) | 28.0 (4.1) | 47 | 3 | 3.7 | |||
BMI, body mass index; HA = hyaluronic acid; IA = intra-articular; KL = Kellgren-Lawrence; NA = not applicable; W% = percentage of women.
Sanofi-Aventis, Bridgewater, New Jersey.
Bioventus LLC, Durham, North Carolina.
LCA Pharmaceutical, Chartres, France.
Anika Therapeutics Inc, Bedford, Massachusetts.
Seikagaku Corporation, Tokyo, Japan.
TRB Chemedica Geneva, Switzerland.
Fidia Farmaceutici, Abano Terme, Italy.
Labrha International, Lyon, France.
Biopolymer, Dummer, Germany.
Hyajoint, SciVision, Taiwan.
LG Life Science Ltd, Seoul, Korea.
Characteristics of each average patient profile are given in Table 1 for the active IA HA monoinjection arms, and in Table 2 for the injected placebo arms. There were great differences of population size, from 10 to 394 (average 108) per active arm. The follow-up period was from 6 to 52 weeks, with most at 26 weeks. Because we were limited to 26 weeks for the placebo arms, it was not possible to make any longer comparison. Patient profiles were quite homogeneous, in terms of age and BMI, but there was more concern for the KL grades, varying from 0% to 81% grades I and II, with presence of grades IV from 0% to 40%, which is very high. This confirms the choice in the data process session to match in priority the KL profiles for the selection of each placebo arm, and therefore limit the risk of bias for each comparison. For the placebo arms, less population was available, but pooling placebo arms together allowed intermediate profiles, as shown in the second part of Table 2. As for the active arms, placebo profiles were homogeneous in terms of age and BMI.
Table 2.
Selected placebo arms and patient profiles.
| Author | Follow-up, wk | IA comparator | N per arm | Sex, % | Age (SD), y | BMI (SD) | KL % |
Anteriority, y | |
|---|---|---|---|---|---|---|---|---|---|
| III | IV | ||||||||
| Altman et al51 | 26 | Saline | 174 | 64 | 63.3 (10.0) | 29.5 (5.0) | 52 | 26 | 6.5 |
| Chevalier et al43 | 26 | Saline | 129 | 68 | 62.5 (9.2) | 29.8 (5.7) | 60 | 1 | 5.8 (5.4) |
| Strand et al61 | 13 | Saline | 128 | 60 | 60.3 (10.0) | 28.7 (3.8) | 49 | 0 | NA |
| Arden et al52 | 6 | Saline | 110 | 46 | 60.9 (20.5) | 27.5 (6.1) | 64 | 0 | 3.1 (3.1) |
| Hangody et al60 | 26 | Saline | 69 | 74 | 58.0 (9.0) | 29.1 (4.5) | 20 | 0 | NA |
| Takamura et al62 | 26 | Saline | 159 | 62 | 62.8 (8.9) | NA | 42 | 0 | 0.7 (0.5) |
| Pooled placebo | |||||||||
| A+C | 26 | Saline | 303 | 66 | 63.0 (9.7) | 29.6 (5.3) | 55 | 15 | 6.2 |
| A+A’ | 26 | Saline | 284 | 57 | 62.4 (15.0) | 28.7 (5.4) | 56 | 16 | 5.2 |
| A+T | 26 | Saline | 333 | 63 | 63.1 (9.5) | 30.3 (5.0) | 47 | 14 | 3.7 |
| C+S | 26 | Saline | 257 | 64 | 61.4 (9.6) | 29.2 (4.9) | 55 | 0 | 5.8 |
| C+A’ | 26 | Saline | 239 | 58 | 61.8 (15.4) | 28.7 (5.9) | 62 | 0 | 4.6 |
| C+T | 26 | Saline | 288 | 65 | 62.7 (9.0) | 29.8 (5.7) | 50 | 0 | 3.0 |
| H+T | 26 | Saline | 228 | 66 | 61.3 (8.9) | 29.1 (4.5) | 36 | 0 | 0.7 |
| A+C+S | 26 | Saline | 276 | 64 | 62.2 (9.8) | 29.3 (4.9) | 53 | 11 | 6.2 |
| C+S+A’ | 26 | Saline | 367 | 59 | 61.3 (13.8) | 28.7 (5.3) | 57 | 0 | 4.6 |
| C+S+H | 26 | Saline | 326 | 66 | 60.7 (9.5) | 29.2 (4.8) | 47 | 0 | 5.8 |
| A+C+S+A’ | 26 | Saline | 541 | 60 | 61.9 (12.7) | 29.0 (5.2) | 56 | 8 | 5.4 |
| C+S+H+T | 26 | Saline | 485 | 65 | 61.4 (9.3) | 29.2 (4.8) | 46 | 0 | 3.0 |
| C+S+H+T+A’ | 26 | Saline | 595 | 62 | 61.3 (12.1) | 28.8 (5.2) | 49 | 0 | 3.0 |
| A+C+S+H+T+A’ | 26 | Saline | 769 | 62 | 61.7 (11.7) | 29.0 (5.1) | 50 | 6 | 4.1 |
Matching placebo arms versus active arms is illustrated in Table 3. KL grade are detailed in numbers of patients for grades I and II, III, and IV. The χ2 test was used to generate the P value by comparing the profiles, study per study. The significant differences were shown by P < 0.05. A good profile concordance was obtained for 26 of 29 arms; among failures, #22 was very atypical with 100% grade III, so it was impossible to match. Two arms—#14 and #17— without known KL profile, were allocated to an average placebo based on presumed similar populations. Obviously, the risk of mismatching between any active arm and its placebo exists, and we took care to minimize the bias for the results.
Table 3.
Matching placebo arms to patient Kellgren-Lawrence (KL) profiles.
| IA HA mono-injection |
IA comparator |
Statistics |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID# | Author | N | KL I–II |
KL III |
KL IV |
Pooled placebo |
N | KL I–II |
KL III |
KL IV |
χ2 |
P value |
| 1 | Chevalier et al43 | 123 | 63 | 60 | 0 | C | 129 | 51 | 78 | 1 | 4.42 | 0.11 |
| 2 | Pal et al44 | 394 | 171 | 223 | 0 | C+S | 258 | 116 | 141 | 1 | 1.72 | 0.42 |
| 3 | Petrella et al45 | 32 | 18 | 14 | 0 | C+S C+S+A’ |
258 368 |
116 156 |
141 211 |
1 1 |
1.54 2.35 |
0.46 0.31 |
| 4 | Dreiser et al46 | 147 | 112 | 35 | 0 | H+T | 228 | 147 | 81 | 0 | 5.74* | 0.017 |
| 5 | Tammachote et al47 | 50 | 21 | 22 | 7 | A+C+S A+C+S+A’ |
432 541 |
155 195 |
231 301 |
46 46 |
1.68 3.13 |
0.43 0.21 |
| 6 | Sun et al48 | 59 | 39 | 20 | 0 | C+T | 289 | 143 | 145 | 1 | 5.53 | 0.063 |
| 7 | De Campos et al49 | 52 | 21 | 18 | 13 | A+T | 333 | 131 | 157 | 45 | 5.51 | 0.064 |
| 8 | Kearey,et al50 | 115 | 50 | 65 | 0 | C+S | 258 | 116 | 141 | 1 | 0.54 | 0.77 |
| Subgroup Synvisc-One† | 972 | 495 | 457 | 20 | C+S+H | 327 | 171 | 155 | 1 | 4.74 | 0.093 | |
| 9 | Altman et al51 | 172 | 40 | 92 | 40 | A | 174 | 39 | 90 | 45 | 0.32 | 0.85 |
| 10 | Arden et al52 | 108 | 33 | 75 | 0 | A’ | 110 | 40 | 70 | 0 | 0.83* | 0.36 |
| 11 | Leighton et al53 | 218 | 71 | 147 | 0 | C C+A’ |
129 239 |
51 91 |
78 148 |
1 1 |
3.40 2.42 |
0.18 0.30 |
| 12 | Zhang et al54 | 161 | 94 | 67 | 0 | C+S+H | 327 | 171 | 155 | 1 | 2.02 | 0.36 |
| 13 | Estades-Rubio et al55 | 27 | 22 | 5 | 0 | C+T | 289 | 143 | 145 | 1 | 10.1 | 0.006 |
| 14 | Louis et al56 | 24 | NA | NA | NA | A+C | 304 | 90 | 168 | 46 | NA | NA |
| 15 | Vaquerizo et al57 | 48 | 18 | 21 | 9 | A | 174 | 39 | 90 | 45 | 4.60 | 0.10 |
| Subgroup Durolane‡ | 734 | 278 | 407 | 49 | A+C+S A+C+S+A’ |
432 542 |
155 195 |
231 301 |
46 46 |
5.75 1.68 |
0.056 0.43 |
|
| 16 | Baron et al58 | 217 | 118 | 99 | 0 | C+S+H+T C+S+H+T+A’ |
486 596 |
263 303 |
222 292 |
1 1 |
0.45 1.12 |
0.80 0.57 |
| 17 | Diraçoglu et al59 | 20 | NA | NA | NA | A+C+S | 431 | 155 | 231 | 46 | NA | NA |
| 18 | Hangody et al60 | 150 | 122 | 27 | 1 | H | 69 | 55 | 14 | 0 | 0.61 | 0.74 |
| 19 | Hangody et al60 | 149 | 120 | 29 | 0 | H | 69 | 55 | 14 | 0 | 0.02* | 0.89 |
| 20 | Strand et al61 | 247 | 115 | 132 | 0 | S | 128 | 65 | 63 | 0 | 0.60* | 0.44 |
| 21 | Takamura et al62 | 152 | 85 | 67 | 0 | T | 159 | 92 | 67 | 0 | 0.12* | 0.73 |
| 22 | Borras-Verdera et al63 | 80 | 0 | 80 | 0 | A A+A’ |
174 284 |
39 79 |
90 160 |
45 45 |
57.7 53.0 |
< 0.001 < 0.001 |
| 23 | Dreiser et al46 | 143 | 98 | 45 | 0 | H+T | 228 | 147 | 81 | 0 | 0.65* | 0.42 |
| 24 | Duymus et al64 | 34 | 24 | 10 | 0 | H | 69 | 55 | 14 | 0 | 1.06* | 0.30 |
| 25 | Lamo-Espinosa et al65 | 10 | 4 | 2 | 4 | A | 174 | 39 | 90 | 45 | 3.86 | 0.14 |
| 26 | Conrozier et al66 | 40 | 13 | 17 | 10 | A | 174 | 39 | 90 | 45 | 1.92 | 0.38 |
| 27 | Monet et al67 | 53 | 20 | 23 | 10 | A | 174 | 39 | 90 | 45 | 5.06 | 0.080 |
| 28 | Bashaireh et al68 | 84 | 36 | 47 | 1 | C+S C+S+A’ |
258 368 |
116 156 |
141 211 |
1 1 |
0.78 1.33 |
0.68 0.51 |
| 29 | Sun et al48 | 62 | 40 | 22 | 0 | C+T | 289 | 143 | 145 | 1 | 4.74 | 0.093 |
| 30 | Tuan et al69 | 46 | 27 | 19 | 0 | C+S | 258 | 116 | 141 | 1 | 3.07 | 0.22 |
| 31 | Ha et al70 | 137 | 78 | 59 | 0 | C+T | 289 | 143 | 145 | 1 | 2.45 | 0.29 |
| Subgroup other IA HA | 1625 | 900 | 678 | 26 | C+S+H | 327 | 171 | 155 | 1 | 5.73 | 0.057 | |
| All monoinjections | 3360 | 1673 | 1542 | 95 | C+S+H+T C+S+H+T+A’ |
486 596 |
263 303 |
222 292 |
1 1 |
2.16* 0.02* |
0.14 0.89 |
|
A = Altman et al51; A’ = Arden et al52; c = Chevalier et al43; H = Hangody et al60; IA HA = intra-articular hyaluronic acid; KL = Kellgren-Lawrence; NA = not applicable; S = Strand et al61; T = Takamura et al62; df = degree of freedom.
Mixing KL III + IV (df = 1).
Sanofi-Aventis, Bridgewater, New Jersey.
Bioventus LLC, Durham, North Carolina.
Results of individual studies and synthesis
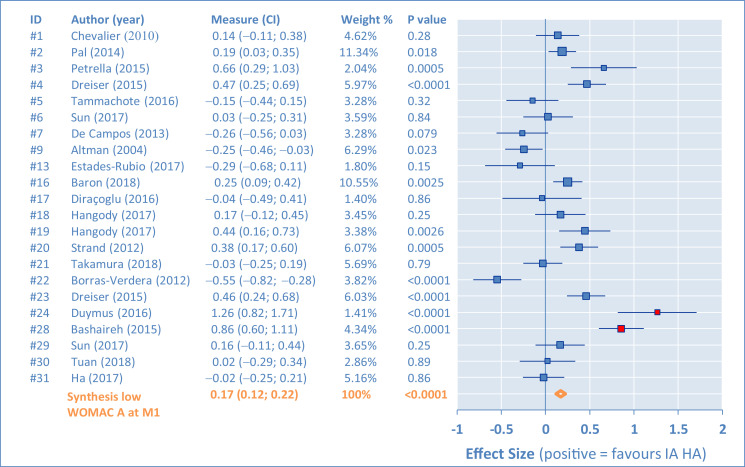
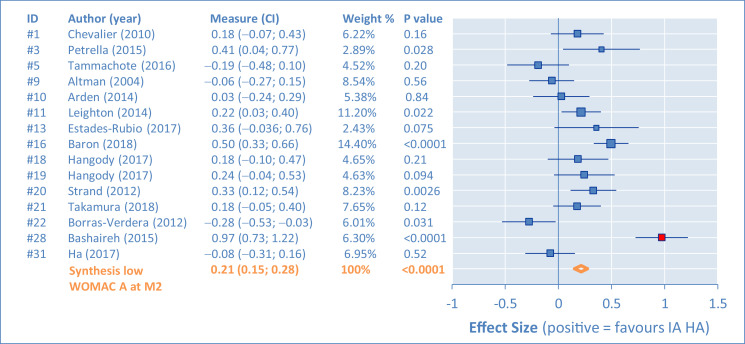
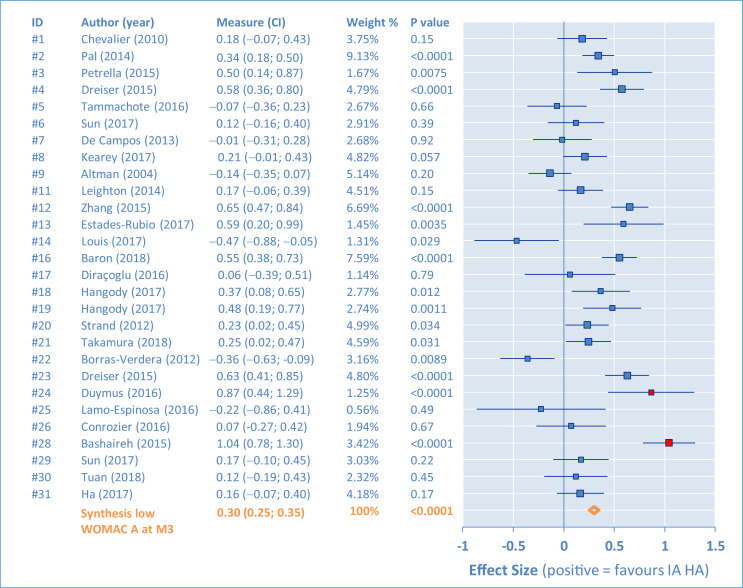
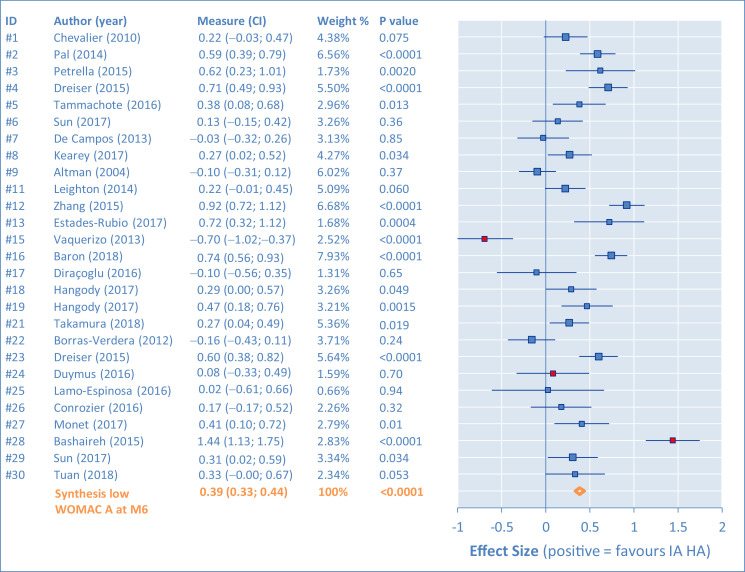
Individual results of the placebo comparisons for the WOMAC A are presented in Figures 2, 3, 4, and 5 as ES in the form of forest plots at M1, M2, M3, and M6, for all studies. The bars represent the 95% CI. Positive results are in favor of the IA HA product. In the tabular details, identification of the study is provided (identifier, author's name, and year) along with ES (95% CI). As complement, the percentage of weight for the synthesis and the P value are also given.
Figure 2.
Western Ontario and MacMaster Universities pain subscore (WOMAC A). Comparison of intra-articular hyaluronic acid (IA HA) monoinjection versus intra-articular placebo at 1-month follow-up visit (M1).
Figure 3.
Western Ontario and MacMaster Universities pain subscore (WOMAC A). Comparison of intra-articular hyaluronic acid (IA HA) monoinjection versus intra-articular placebo at 2-month follow-up visit (M2).
Figure 4.
Western Ontario and MacMaster Universities pain subscore (WOMAC A). Comparison of intra-articular hyaluronic acid (IA HA) monoinjection versus intra-articular placebo at 3-month follow-up visit (M3).
Figure 5.
Western Ontario and MacMaster Universities pain subscore (WOMAC A). Comparison of intra-articular hyaluronic acid (IA HA) monoinjection versus intra-articular placebo at 6-month follow-up visit (M6).
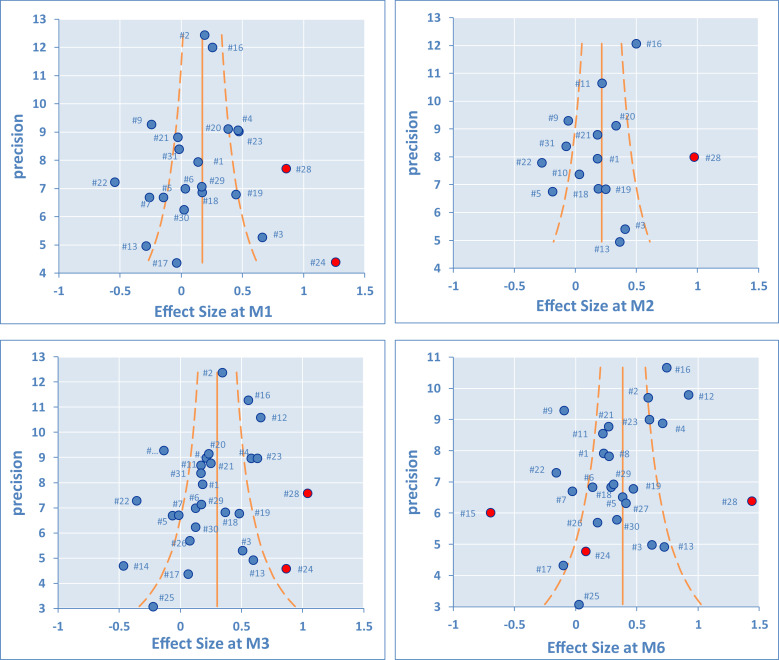
There are important differences between studies, requiring assessment of heterogeneity. This was first done by funnel plots at M1, M2, M3, and M6 (Figure 6). Outlying studies, #15, #24, and #28, were clearly identified and their spots are filled in red on the graphs (both funnel plots and forest plots). No important dissymmetry was found in the funnel plots, and the spots distribution seems balanced around the mean (vertical line).
Figure 6.
Funnel plots.
The synthetic results given with the forest plots were obtained by inverse variance pooling (fixed effect) with MIX 2.0 software. Other synthetic results given in Tables 4 and 5 were calculated for each follow-up time at M1, M2, M3, and M6, to complement the mean difference (MD) of the variation to baseline (0–100 scale), and heterogeneity indicators: I2 is the ratio of the true heterogeneity (moderate at 50% and high at 75%) and τ2 a measure of the heterogeneity between studies, here in dimensionless units (low at 0.04, moderate at 0.09, and high at 0.163).
Table 4.
Selected populations and heterogeneity testing.
| Time | N* | Difference of variation to baseline |
Effect size (95% CI) | Statistics |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | P value | I2, (%) | τ2 | |||
| Extended analysis (all studies) | |||||||
| M1 | 2502 | 3.18 | 19.7 | 0.17 (0.12–0.22) | <0.0001 | 87 | 0.13 |
| M2 | 1887 | 4.27 | 21.0 | 0.21 (0.15–0.28) | <0.0001 | 85 | 0.11 |
| M3 | 3014 | 6.56 | 22.2 | 0.30 (0.25–0.35) | <0.0001 | 84 | 0.11 |
| M6 | 2728 | 8.41 | 21.3 | 0.39 (0.33–0.44) | <0.0001 | 88 | 0.18 |
| Limited analysis (less #15, #24, #28) | |||||||
| M1 | 2384 | 2.13 | 19.7 | 0.12 (0.07–0.18) | 0.0001 | 81 | 0.09 |
| M2 | 1803 | 3.11 | 21.1 | 0.16 (0.10–0.23) | <0.0001 | 76 | 0.06 |
| M3 | 2896 | 5.76 | 22.2 | 0.27 (0.22–0.32) | <0.0001 | 80 | 0.09 |
| M6 | 2562 | 8.27 | 21.4 | 0.39 (0.33–0.44) | <0.0001 | 81 | 0.11 |
| Restricted analysis (less #4, #13, #14, #15, #17, #22, #24, #25, #28) | |||||||
| M1 | 2127 | 2.65 | 19.8 | 0.14 (0.08–0.20) | <0.0001 | 75 | 0.06 |
| M2 | 1698 | 3.86 | 21.5 | 0.19 (0.12–0.26) | <0.0001 | 71 | 0.05 |
| M3 | 2605 | 6.36 | 22.4 | 0.29 (0.23–0.34) | <0.0001 | 74 | 0.06 |
| M6 | 2296 | 8.59 | 21.5 | 0.40 (0.34–0.45) | <0.0001 | 78 | 0.09 |
Intra-articular hyaluronic acid group.
Table 5.
Subgroup analysis and heterogeneity testing.
| Time | N* | Difference of variation to baseline |
Effect size (95% CI) | Statistics |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | P value | I2, % | τ2 | |||
| Subgroup Synvisc-One† | |||||||
| M1 | 843 | 3.10 | 19.5 | 0.16 (0.07 to 0.25) | 0.0007 | 79 | 0.07 |
| M2 | 206 | 1.97 | 20.5 | 0.10 (–0.07 to 0.26) | 0.26 | 72 | 0.08 |
| M3 | 962 | 5.71 | 22.2 | 0.26 (0.17 to 0.34) | <0.0001 | 68 | 0.04 |
| M6 | 959 | 8.17 | 20.8 | 0.39 (0.30 to 0.48) | <0.0001 | 74 | 0.07 |
| Subgroup Durolane‡ (less #15) | |||||||
| M1 | 199 | –4.48 | 18.8 | –0.24 (–0.43 to –0.05) | 0.013 | NS | NS |
| M2 | 525 | 1.87 | 22.0 | 0.09 (–0.04 to 0.21) | 0.17 | 55 | 0.03 |
| M3 | 569 | 5.46 | 22.1 | 0.25 (0.14 to 0.36) | <0.0001 | 91 | 0.22 |
| M6 | 531 | 9.34 | 21.6 | 0.43 (0.31 to 0.55) | <0.0001 | 93 | 0.32 |
| Subgroup other IA HA (less #24, #28) | |||||||
| M1 | 1342 | 2.72 | 20.0 | 0.14 (0.06 to 0.21) | 0.0004 | 81 | 0.10 |
| M2 | 1072 | 4.04 | 21.0 | 0.19 (0.11 to 0.28) | <0.0001 | 84 | 0.09 |
| M3 | 1365 | 5.94 | 22.4 | 0.27 (0.19 to 0.34) | <0.0001 | 79 | 0.10 |
| M6 | 1072 | 7.82 | 21.8 | 0.36 (0.28 to 0.44) | <0.0001 | 78 | 0.09 |
HA = hyaluronic acid; IA = intra-articular; NS = not significant.
IA HA group.
Sanofi-Aventis, Bridgewater, New Jersey.
Bioventus LLC, Durham, North Carolina.
In Table 4, the first group of results are those obtained for the extended analysis that combined all studies. The second group presents the limited analysis by removal of the outlying studies (ie, removing #15, #24, and #28). Finally, the restricted analysis is presented by also removing the studies with active arms having <30 patients and studies with poor KL placebo matching (ie, also removing #4, #13, #14, #17, #22, and #25). This was done to evaluate how the scores changed (MD, ES), and whether heterogeneity could be improved by removal of the most uncertain studies. As evidenced, the results remained stable whilst a visible improvement was noted on both I2 (reduced by –10% to –12%) and τ2 (reduced by –0.05 to –0.09). If the true heterogeneity remains high with I2 at 71% to 78%, heterogeneity between studies has been clearly shown to an acceptable level.
To summarize, IA HA monoinjections were found to be statistically better than the IA placebo (P < 0.001) at any time, for the symptomatic (pain) treatment of knee OA. The ES reached 0.39 or 0.40 at M6, which is clinically relevant.
Risk of bias
There were probably risks of bias for several studies that may raise suspicion. First with the outliers:
-
•
#15: In this autologous preparation study (ie, PRGF),57 Durolane was used as control and, for the first time in our VS experience, there was absolutely no improvement compared with baseline but rather a slight worsening for the IA HA group (confirmed with graph).
-
•
#24: In this alternative treatment study (autologous PRP preparation or IA ozone),64 Ostenil-Plus (TRB Chemedica Ltd, Geneva, Switzerland) was used as control. After an abnormally strong beneficial effect at M1 and M3, the effect was reduced drastically, becoming insignificant at M6, which was surprising.
-
•
#28: In this well-documented open-label study,68 a huge placebo effect was seen and, despite being a good match by KL profile, our comparison placebo arm revealed it to be unsuitable. To us, there was no real bias related to the study, but rather a deficit in our available data.
For the restricted analysis, by removing the poorly KL-placebo-matched studies and the lowest populations (<30 patient/arm), we improved heterogeneity between studies, to confirm a strong average result, unchanged from our extended analysis. We assumed the risk of bias to be acceptable, without incidence on this synthetic result.
Subgroup analyses
Subgroup results are described in Table 5. ES obtained at M3 and M6 for the subgroups Synvisc-One and Durolane are close to those described in the limited analysis presented in Table 4. Synvisc-One was supported by 8 trial arms and Durolane by 5 trial arms, after removal of #15. As shown in the forest plots in Figures 2 through 5, there are differences between trials results inside each subgroup. This synthesis per subgroup allows us to conclude that these 2 products seem to perform similarly, without 1 being better than the other. At M1 and M2, results are less consistent due to reduced populations and more difficulties finding ideal placebo comparators.
There was some improvement for heterogeneity indicators I2 and τ2, with the subgroup Synvisc-One, but none with the subgroup Durolane.
Discussion
Crossing methods
Two approaches were used to reach the final synthetic result of an ES versus injected placebo for the IA HA administrated in monoinjection.
With the first approach, we had to evaluate ES (95% CI) for each trial and select a suitable placebo arm to match the KL profiles. In a second step, the meta-analysis was performed with MIX 2.0, giving results in ES (95% CI), a forest plot representation, and a synthesis based on the fixed effect method.
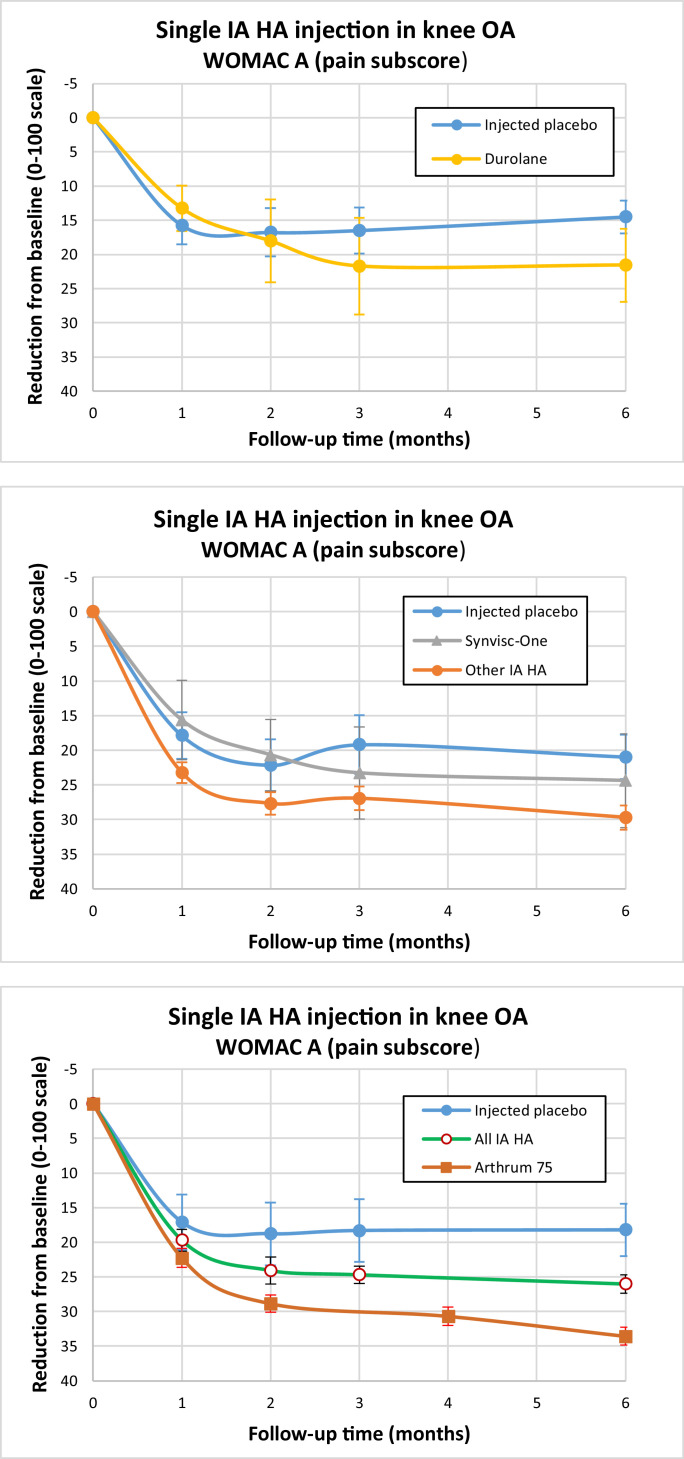
With the second global approach, we first pooled the variations from baseline for each subgroup, weighing population size for each trial. Then, after selection of the pooled placebo comparator with matching KL profile, we evaluated ES (95% CI). Although it gave no individual study result for ES, this method allowed us to represent the WOMAC A variations from baseline, graphically as a function of time (Figure 7), comparing each subgroup with its placebo. The differences were always significantly in favor of IA HA at any time, from M1 to M6. One can see the importance of the IA placebo effect and its contribution to patient improvement, which has been pointed out by Altman et al,10 and Bannuru et al.6, 9, 11
Figure 7.
Variation to baseline per subgroup and group. IA HA = intra-articular hyaluronic acid; OA = osteoarthritis. Synvisc-One (Sanofi-Aventis, Bridgewater, New Jersey). Arthrum 75 (Wellchem Pharmaceuticals, Singapore.).
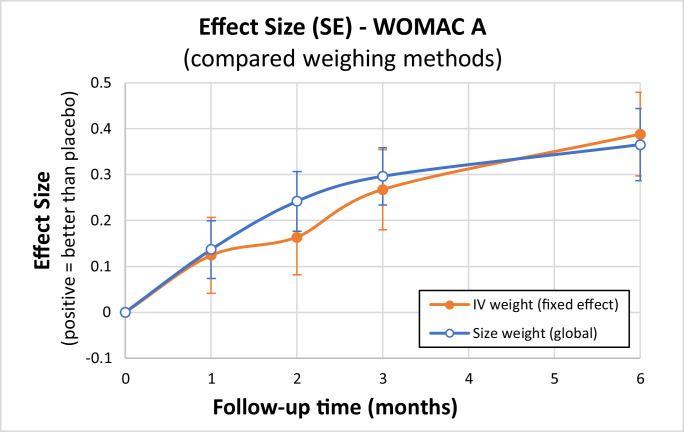
Finally, as illustrated with Figure 8, differences were not so great between these methods, leading to ES from 0.37 to 0.39 (maximum at M6) in the comparison versus IA placebo. In terms of differences in score variations (MD) for the populations (Table 4), we reached MD = 8.27 to 8.59 mm on the 0 to 100 mm scale. This is clinically relevant, being greater than both the minimal clinically important difference (7.5 mm for improvement) and the smallest detectable difference (8.1 mm), as defined by Angst et al.71
Figure 8.
Effect size as time function: Compared weighing methods. IV = inverse variance; WOMAC A = Western Ontario and MacMaster Universities pain subscore; SE = standard error.
Summary of evidence
From an evidence-based medicine point of view, our meta-analysis cannot reach level I or II because all study designs have been accepted, from double-blind RCTs to observational open-label studies. Our objective was to query the largest database and be close to real-world evidence. We believe it was a success to have >4000 patients for this meta-analysis, and therefore a good representation of monoinjection IA HA.
To answer the question about the relative efficacy of IA HA monoinjection compared with multi-injections, we compared our results with those obtained for pain (WOMAC A or/and visual analog scale for pain) in other meta-analyses,2, 3, 4, 5, 6 where most data were obtained from multi-injection regimens:
-
•
Bannuru et al2 found ES = 0.46 (95% CI, 0.28–0.65) with I2=75% at M2, and ES = 0.25 (95% CI, 0.15–0.36) with I2 = 60% at M3.
-
•
Rutjes et al3 found ES = 0.37 (95% CI, 0.28–0.46) with τ2 = 0.09.
-
•
Colen et al4 found MD = 10.20 mm (95% CI, 4.42–15.97 mm) with I2 = 92%.
-
•
Miller and Block5 found SMD = 0.43 (95% CI, 0.36; 0.60) with I2 = 73% at 4 to 13 weeks and SMD = 0.38 (95% CI, 0.21–0.55) with I2 = 75% at 14 to 26 weeks.
-
•
Bannuru et al6 found ES = 0.34 (95% CI, 0.26–0.42).
In terms of ES or SMD, all these results are very close to ours and heterogeneity seems present at a similar level. Differences between products have been studied by Colen et al,4 but these results are limited to a few multi-injection products. More recently, Altman et al8 assessed ES depending on molecular weight (Mw) and found ES = 0.52 (95% CI, 0.48–0.56) for high Mw (>3 MDa (MegaDalton (= 1 million Dalton))) and ES = 0.31 (95% CI, 0.20–0.42) for moderate Mw (1.5–3 MDa). Our results in Table 5 are roughly comparable: a bit smaller with Synvisc-One and Durolane subgroups for the high Mw, and a bit higher with the other IA HA subgroup for the moderate Mw. In other words, we found less difference between high and moderate Mw IA HA products.
In the symptomatic treatment of knee OA with IA HA, the results of monoinjections demonstrate an efficacy similar to the multi-injections in terms of MD, ES (or SMD), and P value, when compared with the IA placebo.
Limitations
There are many limitations to our analysis, including making post-hoc IA placebo comparisons when no placebo control was available. Data were obtained from multiple studies done in many different countries, with different patient populations and doctors. This creates possibilities for differences unrelated to treatments used, with a risk of bias, especially for individual trials; however, for each synthesis per subgroup or group, each with large population sizes, the results were properly compared and confirmed using multiple different statistical approaches and weighing methods.
Conclusions
Results of this meta-analysis suggest that the effects of monoinjections of HA produce results similar to multi-injections of IA HA in terms of pain relief in the treatment of knee OA.
Acknowledgments
Acknowledgments
The author thanks Thierry Thomas, MD, Department Rheumatology, Centre Hospitalier Universitaire (French equivalent of University Hospital) (CHU), St Etienne, France, for providing valuable advice in the conception of this meta-analysis, the interpretation of the results, and the writing of this article. The author also thanks Jo-Ann Elicia West, MSc, an independent contractor in Cartigny L'Epinay, France, for providing editorial support, which was funded by LCA Pharmaceutical, Chartres, France, in accordance with Good Publication Practice guidelines.
Conflicts of Interest
This meta-analysis was entirely sponsored by LCA Pharmaceutical, Chartres, France. P. Vincent is an employee and shareholder of LCA Pharmaceutical. The author has indicated that he has no other conflicts of interest regarding the content of this article.
References
- 1.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. July 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannuru R.R., Natov N.S., Dasi U.R., Schmid C.H., McAlindon T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in the treatment of knee osteoarthritis – meta-analysis. Osteoarthritis Cartilage. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Rutjes A.W.S., Jüni P., da Costa B.R., Trelle S., Nüesch E., Reichenbach S. Viscosupplementation for Osteoarthritis of the Knee. A systematic review and meta-analysis. Annals of Internal Medicine. 2012;157(3):180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 4.Colen S., Van den Bekerom, Mulier M., Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis. A systematic review and meta-analysis wirh emphasis on the efficacy of different products. Biodrugs. 2012;26(4):257–268. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Miller L.E., Block J.E. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized, saline-controlled trials. Clinical medicine insights Arthritis and musculoskeletal disorders. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannuru R.R., Schmid C.H., Kent D.M., Vaysbrot E.E., Wong J.B., McAlindon T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis - A Systematic Review and Network Meta-analysis. Annals of Internal Medicine. 2015;162(1):46–55. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 7.Campbell K.A., Erickson B.J., Saltzman B.M., Mascarenhas R., Bach Jr B.R., Cole B.J., Verma N.N. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: A systematic review of overlapping meta-analyses. J of Arthroscopic and Related Surgery. 2015;10:1–10. doi: 10.1016/j.arthro.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Altman R.D., Bedi A., Karlsson J., Sancheti P., Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. The American Journal of Sports and Medicine. 2015;44(8):2158–2165. doi: 10.1177/0363546515609599. [DOI] [PubMed] [Google Scholar]
- 9.Bannuru R.R., McAlindon T.E., Sullivan M.C., Wong J.B., Kent D.M., Schmid C.H. Effectiveness and implications of alternative placebo treatments. A systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med. 2015;163(5):365–372. doi: 10.7326/M15-0623. [DOI] [PubMed] [Google Scholar]
- 10.Altman R.D., Devji T., Bhandari M., Fierlinger A., Niazi F., Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Seminars in Arthritis and Rheumatism. 2016;46:151–159. doi: 10.1016/j.semarthrit.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Bannuru R.R., Osani M., Vaysbrot E.E., McAlindon T.E. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis and Cartilage. 2016;24:2022–2041. doi: 10.1016/j.joca.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy N., Campbell J., Robinson V., Gee T., Bourne R., Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006 Apr 19;(2) doi: 10.1002/14651858.CD005321.pub2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J. 2nd edition. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 14.Lindqvist U., Tolmachev V., Kairemo K., Astrom G., Jonsson E., Lundqvist H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin Pharmacokinet. 2002;41(8):603–613. doi: 10.2165/00003088-200241080-00004. [DOI] [PubMed] [Google Scholar]
- 15.Skwara A., Ponelis R., Tibesku C.O., Rosenbaum D., Fuchs-Winkelmann S. Gait patterns after intra-articular treatment of patients with osteoarthritis of the knee – Hyaluronan versus triamcinolone: a prospective, randomized, double-blind, monocentric study. Eur J Med Res. 2009;14:157–164. doi: 10.1186/2047-783X-14-4-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. Identifier: NCT00988091. Investigation of 1.2% sodium hyaluronate for treatment of painful chronic osteoarthritis of the knee. https://clinicaltrials.gov/ct2/show/study/NCT00988091?sect=X01256
- 17.Palmieri B., Rottigni V., Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Design, Development & Therapy. 2013;7:7–12. doi: 10.2147/DDDT.S37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath A.F., McGrath A.M., Jessop Z.M., Gandham S., Datta G., Dawson-Bowling S., Cannon S.R. A comparison of intra-articular hyaluronic acid competitors in the mild to moderate knee osteoarthritis. J Arthritis. 2013;2:108. [Google Scholar]
- 19.Yan C.H., Chan W.L., Yuen W.H., Yung P.S., Ip K.Y., Fan J.C., Chiu K.Y. Efficacy and safety of Hylan G-F 20 injection in treatment of knee osteoarthritis in Chinese patients: results of a prospective, multicentre, longitudinal study. Hong Kong Med J. 2015 Aug;21(4):327–332. doi: 10.12809/hkmj144329. Epub 2015 Jun 19. [DOI] [PubMed] [Google Scholar]
- 20.Saturveithan C., Premganesh G., Fakhrizzaki S., Mahathir M., Karuna K., Rauf K., William H., Akmal H., Sivapathasundaram N., Jaspreet K. Intra-articular hyaluronic acid (HA) and platelet rich plasma (PRP) versus hyaluronic acid (HA) injection alone in patients with grade III and IV knee osteoarthritis (OA): a retrospective study on functional outcome. Malaysian Orthopaedic Journal. 2016;10(2):35–40. doi: 10.5704/MOJ.1607.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen J., Sancheti P., Fierlinger A., Niazi F., Johal H., Bedi A. Cost-effectiveness of different forms of intra-articular injections for the treatment of osteoarthritis of the knee. Adv Ther. 2016;33:998–1011. doi: 10.1007/s12325-016-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das Saubhik, Narendran, Saurabh, Singh Navin Kumar. How efficacious are intra-articular viscosupplements in the management of early osteoarthritis? A detailed comparative study with various outcome measures. International Journal of Orthopaedics Sciences. 2017;3(1):426–430. [Google Scholar]
- 23.Hafez M.A., Askar M., Nabeel A., Hassan K.O., Khalifa A.M.S. Comparison between four types of single-dose hyaluronic acid in patients with knee osteoarthritis: a randomized control trial. Remedy Open Access. 2017;2(article 1063):1–4. [Google Scholar]
- 24.Henrotin Y., Berenbaum F., Chevalier X., Marty M., Richette P., Rannou F. Reduction of the serum levels of a specific biomarker of cartilage degradation (Coll2-1) by hyaluronic acid (KARTILAGE® CROSS) compared to placebo in painful knee osteoarthritis patients: the EPIKART study, a pilot prospective comparative randomized double blind trial. BMC Musculoskeletal Disorders. 2017;18:222. doi: 10.1186/s12891-017-1585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrozier T., Jerosch J., Beks P., Kemper F., Euller-Ziegler L., Bailleul F., Chevalier X. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with Hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: a pilot study. Arch Orthop Trauma Surg. 2009 Mar;129(3):417–423. doi: 10.1007/s00402-008-0601-2. Epub 2008 Mar 26. [DOI] [PubMed] [Google Scholar]
- 26.Di Martino A., Tentoni F., Di Matteo B., Cavicchioli A., Lo Presti M., Filardo G., Zaffagnini S., Marcacci M., Kon E. Early viscosupplementation after anterior cruciate ligament reconstruction: A randomized controlled trial. The American Journal of Sports Medicine. 2016;44(10):2572–2578. doi: 10.1177/0363546516654909. [DOI] [PubMed] [Google Scholar]
- 27.Filardo G., Di Matteo B., Tentoni F., Cavicchioli A., Di Martino A., Lo Presti M., Iacono F., Kon E., Marcacci M. No effects of early viscosupplementation after arthroscopic partial menisectomy: A randomized controlled trial. The American Journal of Sports Medicine. 2016;44(12):3119–3125. doi: 10.1177/0363546516660070. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Shimmin A., Ghosh P., Marks P., Linklater J., Connell D., Hall S., Skerrett D., Itescu S., Cicuttini F.M. Safety, tolerability, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: a controlled double-blind randomized trial. Arthritis Research and Therapy. 2017;19:180. doi: 10.1186/s13075-017-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suppan V.K.L., Wei C.Y., Siong T.C., Mei T.M., Chern W.B., Nanta Kumar V.K., Sheng K.R., Rao A.S. Randomized controlled trial comparing efficacy of conventional and new larger dose of intra-articular viscosupplementation in management of knee osteoarthritis. Journal of Orthopaedic Surgery. 2017;25(3):1–6. doi: 10.1177/2309499017731627. [DOI] [PubMed] [Google Scholar]
- 30.Yengkhom J.S., Nongmaithem R.S., Chongrellen Chiru M.S., Thakur K.B., Debnath U. Efficacy of single-dose intra-articular injection of high-molecular-weight hyaluronic acid in patients suffering from primary osteoarthritis of the knee. Indian Journal of Physical Medicine and Rehabilitation. 2017 (Sept);28(3):89–94. [Google Scholar]
- 31.Zoboli A.A.C., de Rezende M.U., de Campos G.C., Pasqualin T., Frucchi R., de Camargo O.P. Prospective randomized clinical trial: single and weekly viscosupplementation. Acta Ortop Bras. 2013;21(5):271–275. doi: 10.1590/S1413-78522013000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozcamdalli M., Misir A., Kizkapan T.B., Uzun E., Duygulu F., Yazici A., Kafadar I.H. Comparison of intra-articular injection of hyaluronic acid and N-acetyl cysteine in the treatment of knee osteoarthritis: A pilot study. Cartilage. 2017;8(4):384–390. doi: 10.1177/1947603516675915. journals.sagepub.com/CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strand V., Lim S., Takamura J. Evidence of safety of retreatment with a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee from double-blind pivotal and open-label retreatment clinical trials. BMC Musculoskeletal Disorders. 2016;17:240. doi: 10.1186/s12891-016-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtori S., Orita S., Yamauchi K., Eguchi Y., Ochiai N., Kishida S., Kuniyoshi K., Aoki Y., Nakamura J., Ishikawa T., Miyagi M., Kamoda H., Suzuki M., Kubota G., Sakuma Y., Oikawa Y., Inage K., Sainoh T., Sato J., Shiga Y., Abe K., Fujimoto K., Kanamoto H., Toyone T., Inoue G., Takahashi K. Efficacy of direct injection of etanercept into knee joints for pain in moderate and severe knee osteoarthritis. Yonsei Med J. 2015 Sep;56(5):1379–1383. doi: 10.3349/ymj.2015.56.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanasuk Y., Dechmaneenin T., Tanavalee A. Prospective randomized trial comparing the efficacy of single 6-mL injection of Hylan G-F 20 and hyaluronic acid for primary knee arthritis: a preliminary study. J Med Assoc Thai. 2012;95(Suppl.10):S92–S97. [PubMed] [Google Scholar]
- 36.Polacco A., Beomonte-Zobel B., Polacco M., Scarlata S., Gasparro F., Del Vescovo R., Scarciolla L. The effect of intra-articular hyaluronic acid (Sinovial One) on knee osteoarthritis: a preliminary study. European Journal of Inflammation. 2013;11(3):847–853. [Google Scholar]
- 37.Hussain S., Rather H., Qayoom A. Efficacy, tolerability and adverse events of single-shot intra-articular hyaluronic acid injection in knee osteoarthritis. J Trauma Treat. 2015;4:3. [Google Scholar]
- 38.Lee J.K., Choi C.H., Oh K.J., Kyung H.S., Yoo J.H., Ha C.W., Bin S.I., Kang S.B., Kim M.K., Lee J.H., Lee M.C. Safety and efficacy of bi-annual intra-articular LBSA0103 injections in patients with knee osteoarthritis. Rheumatology International. 2017 (August) doi: 10.1007/s00296-017-3803-5. [DOI] [PubMed] [Google Scholar]
- 39.Dernek B., Duymus T.M., Koseoglu P.K., Aydin T., Kesiktas F.N., Aksoy C., Mutlu S. Efficacy of single-dose hyaluronic acid products with two different structures with early-stage knee osteoarthritis. J Phys Ther Sci. 2016 Nov;28(11):3036–3040. doi: 10.1589/jpts.28.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MONOVISC™ Summary of safety and effectiveness data – main study: protocol 0702. PMA application number: P090031 February 25, 2014. https://www.accessdata.fda.gov/cdrh_docs/pdf9/P090031B.pdf
- 41.Frampton J.E. Hylan GF-20 single-injection formulation. Drugs Aging. 2010;27(1):77–85. doi: 10.2165/11203900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Belzile E.L., Deakon R.T., Vannabouathong C., Bhandari M., Lamontagne M., McCormack R. Cost-utility of a single-injection combined corticosteroid-hyaluronic acid formulation vs a 2-injection regimen of sequential corticosteroid and hyaluronic acid injections. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2017;10:1–10. doi: 10.1177/1179544117712993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevalier X., Jerosch J., Goupille P., van Dijk N., Luyten F.P., Scott D.L., Bailleul F., Pavelka K. Single, intra-articular treatment with 6 ml Hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010 Jan;69(1):113–119. doi: 10.1136/ard.2008.094623. Online first March 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal S., Thuppal S., Reddy K.J., Avasthi S., Aggarwal A., Bansal H., Mohanasundaram S., Bailleul F. Long-term (1-year) safety and efficacy of a single 6-mL injection of Hylan G-F 20 in Indian patients with symptomatic knee osteoarthritis. Bentham: Open Rheumatology Journal. 2014;8:54–68. doi: 10.2174/1874312901408010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrella R.J., Emans P.J., Alleyne J., Dellaert F., Gill D.P., Maroney M. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: a prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet Disord. 2015 Mar 18;16:57. doi: 10.1186/s12891-015-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreiser R.L., Avouac B., Bardin T. Efficacy of one intra-articular injection of 2% natural sodium hyaluronate is non-inferior to chemically crosslinked Hylan G-F 20 in the treatment of painful tibiofemoral osteoarthritis. P 308, WCO-IOF-ESCEO World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases; Milan, Italy; 26-29 March 2015. [Google Scholar]
- 47.Tammachote N., Kanitnate S., Yakumpor T., Panichkul P. Intra-Articular, Single-Shot Hylan G-F 20 Hyaluronic Acid Injection Compared with Corticosteroid in Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial. J Bone Joint Surg Am. 2016 Jun 1;98(11):885–892. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 48.Sun S.F., Hsu C.W., Lin H.S., Liou I.H., Chen Y.H., Hung C.L. Comparison of single intra-articular injection of novel hyaluronan (Hya-Joint Plus) with Synvisc-One for Knee Osteoarthritis: A randomized, controlled, double-blind trial of efficacy and safety. J Bone Joint Surg Am. 2017 Mar 15;99(6):462–471. doi: 10.2106/JBJS.16.00469. [DOI] [PubMed] [Google Scholar]
- 49.De Campos G.C., Rezende M.U., Pailo A.F., Frucchi R., Camargo O.P. Adding triamcinolone improves viscosupplementation: A randomized clinical trial. Clin Orthop Relat Res. 2013;471:613–620. doi: 10.1007/s11999-012-2659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keary P., Popple A.E., Warren J., Davis T., Bellamy N & for the LOBRAS study group Improvement in condition-specific and generic quality of life outcomes in patients with knee osteoarthritis following Synvisc-One: Results from the LOBRAS study. Current Medical Research and Opinion. 2017 doi: 10.1080/03007995.2016.1260533. [DOI] [PubMed] [Google Scholar]
- 51.Altman R.D., Akermark C., Beaulieu A.D., Schnitzer T., Durolane International Study Group Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage. 2004 Aug;12(8):642–649. doi: 10.1016/j.joca.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Arden N.K., Akermark C., Andersson M., Todman M.G., Altman R.D. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Current Medical Research and Opinion. 2014;30(2):279–286. doi: 10.1185/03007995.2013.855631. [DOI] [PubMed] [Google Scholar]
- 53.Leighton R., Akermark C., Therrien R., Richardson J.B., Andersson M., Todman M.G., Arden N.K., DUROLANE Study Group NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis and Cartilage. 2014 (Jan);22(1):17–25. doi: 10.1016/j.joca.2013.10.009. Epub 2013 Nov 1. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H., Zhang K., Zhang X., Zhu Z., Yan S., Sun T., Guo A., Jones J., Steen R.G., Shan B., Zhang J., Lin J. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: a multicenter, randomized, double-blind, 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Res Ther. 2015 Mar 10;17:51. doi: 10.1186/s13075-015-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Estades-Rubio F.J., Reyes-Martin A., Morales-Marcos V., Garcia-Piriz M., Garcia-Vera J.J., Peran M., Marchal J.A., Montanez-Heredia E. Knee viscosupplementation: Cost-effectiveness analysis between stabilized hyaluronic acid in a single injection versus five injections of standard hyaluronic acid. Int J Mol Sci. 2017 Mar 17;18(3) doi: 10.3390/ijms18030658. pii: E658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louis M.L., Magalon J., Jouve E., Bornet C.E., Mattei J.C., Chagnaud C., Rochwerger A., Veran J., Sabatier F. Growth factors levels determine efficacy of platelets rich plasma injections in knee osteoarthritis: a randomized double blind non-inferiority trial compared with viscosupplementation. Arthroscopy. 2018 Jan 20 doi: 10.1016/j.arthro.2017.11.035. pii: S0749-8063(17)31481-0. [DOI] [PubMed] [Google Scholar]
- 57.Vaquerizo V., Plasencia M.A., Arribas I., Seijas R., Padilla S., Orive G., Anitua E. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013 Oct;29(10):1635–1643. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 58.Baron D., Flin C., Porterie J., Despaux J., Vincent P. Hyaluronic acid single injection in knee osteoarthritis: a multi-center open prospective study (ART-ONE 75) with placebo post-hoc comparison. Current Therapeutic Research. 2018;88C:35–46. doi: 10.1016/j.curtheres.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diraçoglu D., Tunçay T.B., Sahbaz T., Aksoy C. Single versus multiple dose hyaluronic acid: Comparison of the results. J Back Musculoskelet Rehabil. 2016 Nov 21;29(4):881–886. doi: 10.3233/BMR-160714. [DOI] [PubMed] [Google Scholar]
- 60.Hangody L., Szody R., Lukasik P., Zgadzaj W., Lenart E., Dokoupilova E., Bichovsk D., Berta A., Vasarhelyi G., Ficzere, Hangody G., Stevens G., Szendroi M. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: A randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage. 2017:1–8. doi: 10.1177/1947603517703732. journals.sagepub.com/CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strand V., Baraf H.S., Lavin P.T., Lim S., Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012 May;20(5):350–356. doi: 10.1016/j.joca.2012.01.013. Epub 2012 Feb 1. [DOI] [PubMed] [Google Scholar]
- 62.Takamura J., Takayuki S., Strand V. A single intra-articular injection of Gel-200 for treatment of symptomatic osteoarthritis of the knee is more effective than phosphate buffered saline at 6 months; a sub-group analysis of a multicenter, randomized controlled trial. Cartilage. 2018:1–6. doi: 10.1177/1947603518768015. journals.sagepub.com/CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borras-Verdera A., Calcedo-Bernal V., Ojeda-Levenfeld J., Clavel-Sainz C. Efficacy and safety of a single intra-articular injection of 2% hyaluronic acid and mannitol in knee osteoarthritis over a 6-month period. Rev Esp Cir Ortop Tramatol. 2012;56(4):274–280. doi: 10.1016/j.recot.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Duymus T.M., Mutlu S., Dernek B., Komur B., Aydogmus S., Kesiktas F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrose. 2017;25(2):485–492. doi: 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 65.Lamo-Espinosa J.M., Mora G., Blanco J.F., Granero-Molto F., Nunez-Cordoba J.M., Sanchez-Echenique C., Bondia J.M., Damaso Aquerreta J., Andreu E.J., Ornilla E., Villaron E.M., Valenti-Azcarate A., Sanchez-Guijo F., del Canizo M.C., Valenti-Nin J.R., Prosper F. Intra-articular injections of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Translational Medicine. 2016;14:246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conrozier T., Bozgan A.M., Bossert M., Sondag M., Lohse-Walliser A., Balblanc J.C. Standardized follow-up of patients with symptomatic knee osteoarthritis treated with a single intra-articular injection of a combination of cross-linked hyaluronic acid and mannitol. Clin Med Insights Arthritis Musculoskelet Disord. 2016 Sep 25;9:175–179. doi: 10.4137/CMAMD.S39432. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monet M., Bozgan A.M., Conrozier T. Safety and efficacy of single intra-articular injection of a cross-linked hyaluronic acid/mannitol formulation (Happycross™) in knee osteoarthritis results of a prospective observational study in daily practice conditions. Ortho & Rheum Open Access J. 2017;5(3) OROAJ.MS.ID.555664. [Google Scholar]
- 68.Bashaireh K., Naser Z., Hawadya K.A., Sorour S., Al-Khateeb R.N. Efficacy and safety of cross-linked hyaluronic acid single injection on osteoarthritis of the knee: a post-marketing Phase IV study. Drug Des Devel Ther. 2015 Apr 8;9:2063–2072. doi: 10.2147/DDDT.S81524. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuan S., Liou I., Su H., Tsai Y., Chen G., Sun S. Improvement of self-reported functional scores and thickening of quadriceps and femoral intercondylar cartilage under ultrasonography after single intra-articular injection of a novel cross-linked hyaluronic acid in the treatment of knee osteoarthritis. Journal of Back and Musculoskeletal Rehabilitation. 2018;1:1–10. doi: 10.3233/BMR-170950. [DOI] [PubMed] [Google Scholar]
- 70.Ha C.W., Park Y.B., Choi C.H., Kyung H.S., Lee J.H., Doo Yoo J., Yoo J.H., Choi C.H., Kim C.W., Kim H.C., Oh K.J., Bin S.I., Lee M.C. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: a double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskeletal Disorders. 2017;18:223. doi: 10.1186/s12891-017-1591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angst F., Aeschlimann A., Stucki G. Smallest Detectable and Minimal Clinically Important Differences for rehabilitation intervention with their implication for required sample sizes unsing WOMAC and SF-36 Quality of Life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Care & Research. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]