Abstract
Background
The cuticle is an indispensable structure that protects the mosquito against adverse environmental conditions and prevents pathogen entry. While most cuticles are hard and rigid, some parts of cuticle are soft and flexible to allow movement and blood-feeding. It has been reported that 3, 4-dihydroxyphenylacetaldehyde (DOPAL) synthase is associated with flexible cuticle formation in Aedes aegypti. However, the molecular function of DOPAL synthase in the ontogenesis of mosquito remains largely unknown. In this study, we characterized gene expression profiles of DOPAL synthase and investigated its functions in larvae and female adults of Aedes agypti by RNAi.
Results
Our results suggest that the expression of DOPAL synthase is different during development and the transcriptional level reached its peak at the female white pupal stage, and DOPAL synthase was more highly expressed in the cuticle and midgut than other tissues in the adult. The development process from larva to pupa was slowed down strikingly by feeding the first-instar larvae with chitosan/DOPAL synthase dsRNA nanoparticles. A qRT-PCR analysis confirmed that the dsRNA-mediated transcription of the DOPAL synthase was reduced > 50% in fourth-instar larvae. Meanwhile, larval molt was abnormal during development. Transmission electron microscopy results indicated that the formation of endocuticle and exocuticle was blocked. In addition, we detected that the dsDOPAL synthase RNA caused significant mortality when injected into the female adult mosquitoes.
Conclusions
Our findings demonstrate that DOPAL synthase plays a critical role in mosquito larval development and adult survival and suggest that DOPAL synthase could be a good candidate gene in RNAi intervention strategies in mosquito control.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3568-7) contains supplementary material, which is available to authorized users.
Keywords: DOPAL synthase, Aedes aegypti, RNA interference, Development, Mortality
Background
The insect cuticle is a complex exoskeleton which covers all the exposed tissues and protects insects against adverse environmental conditions, forms a physical barrier against the intrusion of pathogens and maintains their movement [1–4]. It is also the main route of insecticide penetration, and the intake of insecticide can be reduced by thickening or changing the chemical composition of the cuticle [5]. The cuticle is mainly composed of chitin and cuticular proteins (CPs) [6–9]. Insects undergo molting several times throughout their lives. The new cuticle is soft and fragile, and it needs sclerotization, melanization and other physiological processes to play a protective role [8, 10, 11]. Cuticle sclerotization and melanization are important parts of the cuticle formation and hardening process. Tyrosine-mediated cuticle tanning is a vital step to achieve rapid formation of the protective cuticle. Tyrosine is first hydroxylated to 3,4-dihydroxyphenylalanine (DOPA), then DOPA is decarboxylated to dopamine by DOPA decarboxylase (DDC) [12]. Dopamine not only can be acetylated by arylalkylamine N-acetyltransferase (aaNAT) [13] or combined with N-β-alanine to form sclerotization precursors [14], but can also be oxidized by phenoloxidases to form melanization precursors [4, 10].
3,4-dihydroxyphenylacetaldehyde (DOPAL) synthase, which exists in most insect species according to sequence identity [11], can catalyze the conversion of L-DOPA to DOPAL which is involved in flexible cuticle formation. DOPAL synthase is a member of the insect DDC-like protein family. Although DOPAL synthase and DDC share high sequence similarity, they catalyze rather different chemical reactions. Structural and biochemical comparisons between DOPAL synthase and DDC in Drosophila identified that the key active site residue, Asn192, in insect DOPAL synthase can be used to distinguish all available insect DOPAL synthases from DDC sequences [15]. DOPAL has a high biological activity, which is directly linked to the CPs cross-linking through interaction of free amino acid groups on the CPs [11, 16, 17]. Early studies reported that DOPAL synthase-like protein, alpha methyldopa resistant (AMD-r), in Drosophila is resistant to the toxic compound, AMD. Homozygous mutations of amd-r resulted in embryo death during early development stages [18, 19]. Electron microscopic evidence revealed that loss of the gene function leads to cuticle defects in a region of amd-r expression [18]. These reports implied the role of the amd-r gene in flexible cuticle formation rather than the role of DDC in rigid cuticle melanization and sclerotization [20, 21]. However, the function of DOPAL synthase or AMD-r in the postembryonic development of insects is unknown.
Mosquitoes are the most dangerous disease vectors because they transmit deadly pathogens such as Plasmodium spp., chikungunya virus, yellow fever virus, dengue virus, Japanese encephalitis virus and West Nile virus [22]. Chemical insecticides have been widely used across the world for a long time. However, many classes of chemical insecticide have caused serious resistance in hundreds of mosquito species across sixty countries [23]. To address the problem of mosquito control, RNA interference (RNAi) has been increasingly used to conduct genetic studies in mosquito vectors and to study the evolution of insecticide resistance in mosquitoes [24–27].
RNAi is caused by double-stranded RNA (dsRNA), which degrades the homologous mRNA with high efficiency and specificity [28–30]. RNAi technology can be used to eliminate or knock down the specific expression of specific genes, so this technique has been widely used to explore gene function in physiological processes of different insect species. Improved administration and formulation of dsRNAs for insects coupled with the current progress in RNAi technology enables RNAi to be an alternative strategy for insect pest control [31–34]. Several methods of RNAi have been successfully used by researchers with their own advantages and limitations [24, 35, 36]. Microinjection of dsRNAs into adult insects is considered to be the most reliable delivery of the dsRNAs and has achieved high knockdown efficiency; however, this method is not applicable to larvae, especially the first- and second-instar larvae, since it demands a high level of skill from the operators [24, 36]. Oral delivery of dsRNAs can be facilitated by using a lipid-based transfection reagent in the larvae [37, 38]. However, it is susceptible to the intestinal environment. dsRNA is not stable, and the induction effect is inferior to the microinjection [36, 38]. Thus, dsRNA formulation of nanoparticles for larvae has attracted much attention. Chitosan encapsulates dsRNA to form nanoparticles which can make dsRNA release slowly and continuously, keep dsRNA to specific tissues outside the gut, and protect from digestion of intestinal nuclease and damage of pH change [39–42].
In the present study, we fed nanoparticles containing dsDOPAL synthase RNA to larvae and microinjected the dsRNA into adults to investigate the role of DOPAL synthase in cuticle information in Aedes aegypti mosquitoes. Our data demonstrated that DOPAL synthase plays a key role during the larvae development and adult survival in Ae. aegypti, and could be a good candidate gene in RNAi intervention strategies in mosquito control.
Methods
Mosquito rearing
Aedes aegypti mosquitoes (Rockefeller strain, provided by Beijing Institute of Microbiology and Epidemiology, Beijing, China) were used in the investigation. Mosquitoes were reared based on the previously reported procedure [43].
Collection and dissection of mosquito tissues
To examine the temporal expression of DOPAL synthase, mosquito eggs, first- to fourth-instar larvae, white and black female pupae, white and black male pupae, female and male adults were collected for investigation. To examine the spatial expression of DOPAL synthase, different tissues of non-blood-fed female adults, including the head, thorax, cuticle and midgut were derived for the experiment. Tissues were dissected from around 30 mosquitoes, which were cold anesthetized on ice. The cuticles of male and blood-fed female adults were also collected. Around 10 individuals were used for each test and 3 tests were performed for each stage and tissue sample. A total of 30–50 first- and second instar-larvae were collected in each tube and 5–10 larvae of the other stages were collected in each tube.
The expression profiles of DOPAL synthase
The collected samples were homogenized in Trizol reagent (Invitrogen, CA, USA) and total RNA was isolated according to the manufacturer’s instructions. Total RNA was treated with a PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) to remove DNA contamination from the samples and cDNA was synthesized for real-time PCR. Real-time PCR amplification and analysis were performed on a LightCycler® 96 (Thermo Fisher Scientific, Massachusetts, USA) using a Power SYBR Green Supermix kit (TaKaRa). Primers used for real-time PCR (forward: 5′-CAT TGG CAA ATT CAG CTC GG-3′ and reverse: 5′-CAT TGG CAA ATT CAG CTC GG-3′) were designed to amplify fragments of DOPAL synthase (XM_001661007). gapdh (glyceraldehyde 3 phosphate dehydrogenase) (XM_011494724) was used as an internal reference gene with primers (forward: 5′-TTG GAC TAC ACC GAA GAG GA-3′ and reverse: 5′-TGT CGT ACC AGG AGA TGA GC-3′). Amplifications were performed in a final volume of 10 μl containing 1 μl of cDNA, 1 μl of each primer, 4 μl of 2× SYBR Green Supermix, and 3 μl ddH2O. The thermal cycle conditions used for the real-time PCR were: 95 °C for 10 min, followed by 45 cycles of 95 °C for 10 s, 55 °C for 10 s and 72 °C for 10 s. Following the real-time PCR cycles, the specificity of the SYBR green PCR signal was confirmed by melting curve analysis or agarose gel electrophoresis. The mRNA expression was quantified using the comparative CT method (cross threshold, the PCR cycle number that crosses the signal threshold). The relative gene expression data were analyzed using the relative 2−ΔΔct method [44].
dsRNA preparation
Total RNA was extracted from Ae. aegypti adults with Trizol reagent (Invitrogen) and was then handled with a PrimeScript™ RT reagent kit with gDNA Eraser Kit (TaKaRa). A fragment of DOPAL synthase was PCR-amplified from the cDNA using the primers (forward: 5′-CCT GGA GGA AAT TGG TC-3′ and reverse: 5′-AGT AGG TGT CCT CTC CTT TCA-3′). The β-glucuronidase (gus) gene (KY848224), a bacterial gene specific to Escherichia coli, as a negative control, was amplified by PCR from the pBI121 plasmid using the primers (forward: 5′-GCG GCC GCC CCT TAC GCT GAA GAG ATG C-3′ and reverse: 5′-CTC GAG GGC ACA GCA CAT CAA AGA GA-3′) as reported [28]. Then, the PCR products were detected and purified using gel electrophoresis. The gene fragments were subcloned into the cloning vector PMD18-T (TaKaRa), and later excised from PMD18-T using SmaI and XbaI restriction enzymes, then ligated into plasmid pL4440, a vector possessing convergent T7 promoters. The recombinant plasmid were transformed into competent cells of HT115 (DE3) following a previously described method [28], which is an RNase-III-deficient E. coli strain, and the cells were grown in 2× LB medium (10 g/l of tryptone, 5 g/l of yeast extract, 10 g/l of NaCl) containing ampicillin and tetracycline at 37 °C for 12–14 h. The bacterial solution was diluted 100× with 2× LB medium and grown until OD600 = 0.5. IPTG was added in a final concentration of 0.6 mM to induce T7 polymerase activity. The expressed dsRNA was extracted and confirmed by electrophoresis on 1% agarose gel.
Preparation of chitosan/dsRNA nanoparticles
Medium range chitosan (90–95% deacetylated) was purchased from BBI Life Sciences (Shanghai, China). Chitosan (1 mg/ml) was dissolved in sodium acetate buffer to obtain a 0.02% w/v working solution and the solution was kept at room temperature before use. A total of 32 μg of dsRNA was added to 1 μl of 50 mM sodium sulfate buffer, diethyl pyrocarbonate (DEPC) water was made up to 100 μl and then mixed with an equal volume of chitosan solution. The mixture was heated at 50 °C for 1 min, as vortexed for 30 s, and centrifuged at 13,000×g for 10 min to allow the formation of the nanoparticles. The percentage of dsRNA embedded in the nanoparticles was calculated using the starting amounts of dsRNA and amounts remaining in the supernatants by the absorbance measurement at 260 nm.
Delivery of dsRNA to mosquito larvae
Chitosan/dsRNA nanoparticles were mixed with mosquito larval food consisting of agar (Biowest, Shanghai, China). The gel was cut into 6 equal slices using a clean razor blade or toothpick. One hundred age-synchronized first-instar larvae, 24 h after egg hatching, were placed into 10 Petri dishes in about 10 ml of deionized water. Larvae were fed one slice per Petri dish each day. The procedure was repeated daily until the larvae were raised to the fourth instar. The transcript levels were tested and other phenotypic changes were observed in different larval stages. The negative control gel with dsgus was prepared in the same way. The black control was directly fed with food without using nanoparticles.
Quantitative RT-PCR to measure gene knockdown
Quantitative RT-PCR (qRT-PCR) assays were performed with three biological replicates on at least 10 pooled control vs experimental larvae as described. Larvae from each treatment were collected and pooled together each instar after dsRNA feedings. RNA extractions and cDNA syntheses were performed as mentioned above. The cDNA from each replicate treatment was then used to assess the extent of RNAi by measuring levels of gene expression using qRT-PCR. Reactions were performed in triplicate using the primers listed as above. The gapdh gene was used as an internal reference to compare levels of RNAi. Melt curve analyses were also performed and confirmed that only a single product was amplified with each primer pair in every sample. Analysis of gene expression was performed using the 2−ΔΔCT method, comparing expression in specific dsRNA treated samples to gus-dsRNA treated samples.
Transmission electron microscope (TEM) analysis
The cuticle of Ae. aegypti in the abdomen were dissected from the native larvae of the first to fourth instar (sampling immediately after molting) (n = 10), and RNAi-treated fourth-instar larvae (n = 10). Normal cuticles of the wild larvae groups in the same instar were used as a blank control. Specimens were fixed in 2.5% glutaraldehyde (Kemiou, Tianjin, China) in phosphate buffer (0.1 M, pH 7.0) overnight, and samples were washed 3 times for 5 min with phosphate buffer. Specimens were further fixed with 1% osmium tetroxide buffer in phosphate buffer (0.1 M, pH 7.0) at 4 °C for 1–3 h. After fixation, specimens were washed 3 times with phosphate buffer (0.1 M, pH 7.0) for 5 min. The samples were gradually dehydrated with 30, 50, 70, 90, 95 and 100% ethanol for 5 to 10 min at each step; the 100% ethanol step was repeated 2 to 3 times to ensure complete dehydration. After dehydration, the samples were embedded in epoxy resin (Sigma-Aldrich, St. Louis, USA). The specimens were sliced with an ultramicrotome (Leica UC6; Leica, Vienna, Austria). The sections were stained with uranyl acetate (Syntechem, Changzhou, China) and lead citrate (Acros, Shanghai, China) and observed using an electron microscope (JEM1200EX; Jeol, Tokyo, Japan).
Injection of dsRNA into adult mosquitoes
Aedes aegypti adults were collected 3–5 d after hatching and in 4–7 h after they had fed on sugar water. The number of mosquitoes were controlled at around 300 at a time, and put into a refrigerator at − 20 °C for 4 min. The frozen mosquitoes were put on ice and purified dsRNA was injected into the side of their chests using an oil pressure manual microinjection device (Eppendorf AG, Hamburg, Germany). Meanwhile, three groups were injected with the dsRNA of gus, DEPC water and native treatment, respectively, as controls. Each time, 100 female and 100 male were injected and all experiments were repeated for three times. Each sample was injected with 3 μl into the thorax.
Statistical analyses
A one-way ANOVA test was performed to evaluate the significance of the difference between the groups. The threshold level of significance was P < 0.05.
Results
Spatiotemporal expression profiles of the DOPAL synthase gene
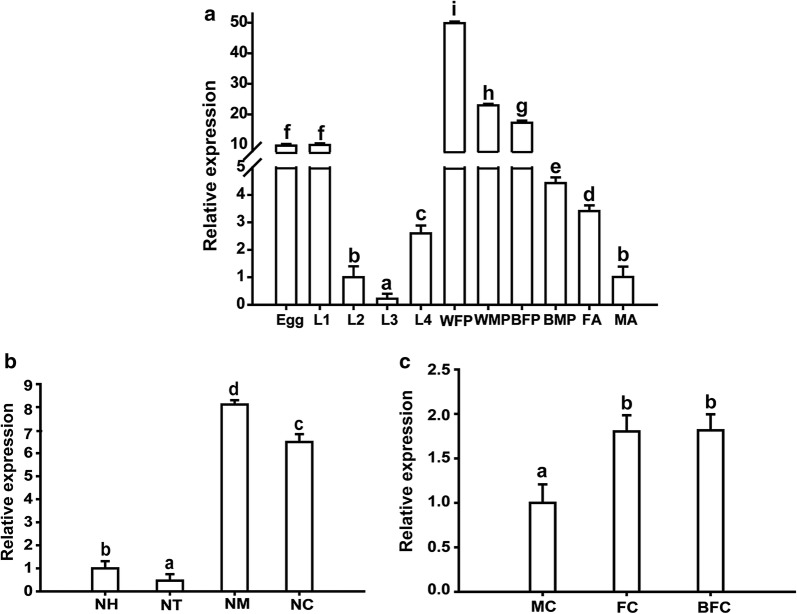
Data showed that the DOPAL synthase gene is differently expressed throughout the life of Ae. aegypti. The temporal expression profile was determined in the larval, pupal, male and female adult stages. Our test showed that the gapdh gene has a similar expression trend with the rps17 gene (40S ribosomal protein s17) (Additional file 1: Figure S1), which is a reference gene, during qPCR for late embryos, all four larval-instars and pupae samples in Aedes albopictus [45]. In addition, gapdh in this study as reference gene was deemed sufficient, as the PCR efficiencies of the primer sets were greater than 95%, calculated using the method of Pfaffl [46]. The expression levels of DOPAL synthase were higher in the first-instar than in the other three instars; expression then peaked in the white female pupae and reduced in black pupae and adults. In addition, female adults had significantly higher expression of DOPAL synthase than male adults (Fig. 1a).
Fig. 1.
The expression profiles of DOPAL synthase gene with gapdh as the reference gene. Bars represent the standard deviation. a Temporal expression of DOPAL synthase in different developmental stages. b Spatial expression of DOPAL synthase in different tissues of Ae. aegypti. c Sex-different expression of DOPAL synthase in the cuticle of female and male adults. Abbreviations: L1, first-instar larvae; L2, second-instar larvae; L3, third-instar larvae; L4, fourth-instar larvae; WFP, white female pupae; WMP, white male pupae; BFP, black female pupae; BMP, black male pupae; FA, female adults; MA, male adults; NH, head; NT, thorax; NM, midgut; NC, cuticle; MC, cuticle of male adults; FC, cuticle of female adults; BFC, cuticle of blood-fed female adults
qRT-PCR showed that DOPAL synthase expression in different tissues of Ae. aegypti adults was also different. It was highly expressed in the cuticle and midgut of non-blood-fed mosquitoes. The expressions in the head and thorax were relatively low (Fig. 1b). The expression of DOPAL synthase in the male mosquito cuticle was the lowest, and there was no difference between non-blood-fed and blood-fed females (Fig. 1c).
RNAi and phenotypes in mosquito larvae
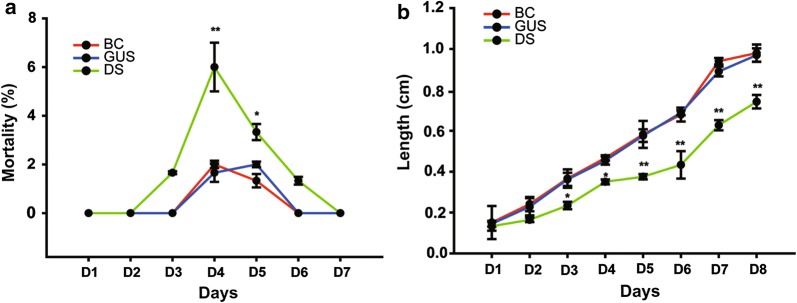
In larvae fed on DOPAL synthase dsRNA, the gene silencing led to high mortality compared with the negative controls. The mortality rate of larvae fed on DOPAL synthase dsRNA was nearly three times higher than that of the two control groups (ANOVA, F(3,9) = 9.81, P = 0.013) (Fig. 2a). Most larvae in the experimental group grew slowly compared with the control groups (Fig. 2b). The length of DOPAL synthase dsRNA-treated larvae was significantly less than that of the control groups from the third day after gene silencing (ANOVA, F(3,9) = 326.38, P < 0.0001) and the disparity was about 1–2 times (Fig. 2b). Many larvae died from DOPAL synthase dsRNA treatment, with incomplete molting as observed under a microscope (Fig. 3a). At the same time, the silence of DOPAL synthase arrested the emergence of larvae into pupae compared with dsgus and black control (Table 1). As shown in Table 1, the larvae stopped growing and development, 10% of the larvae in the experimental group stopped at the second-instar compared with the two control groups (Fig. 3b).
Fig. 2.
Treatment of mosquito larvae with DOPAL synthase dsRNA increased mortality of larvae and slowed in larval development. Bars represent the standard deviation. a The mortality of larvae after treatment with DOPAL synthase dsRNA. b The length of larvae after treatments with DOPAL synthase dsRNA. *P < 0.05, **P < 0.01. Abbreviations: DS, treatment of larvae with dsDOPAL synthase; GUS, treatment of larvae with dsgus RNAs; BC, black control
Fig. 3.
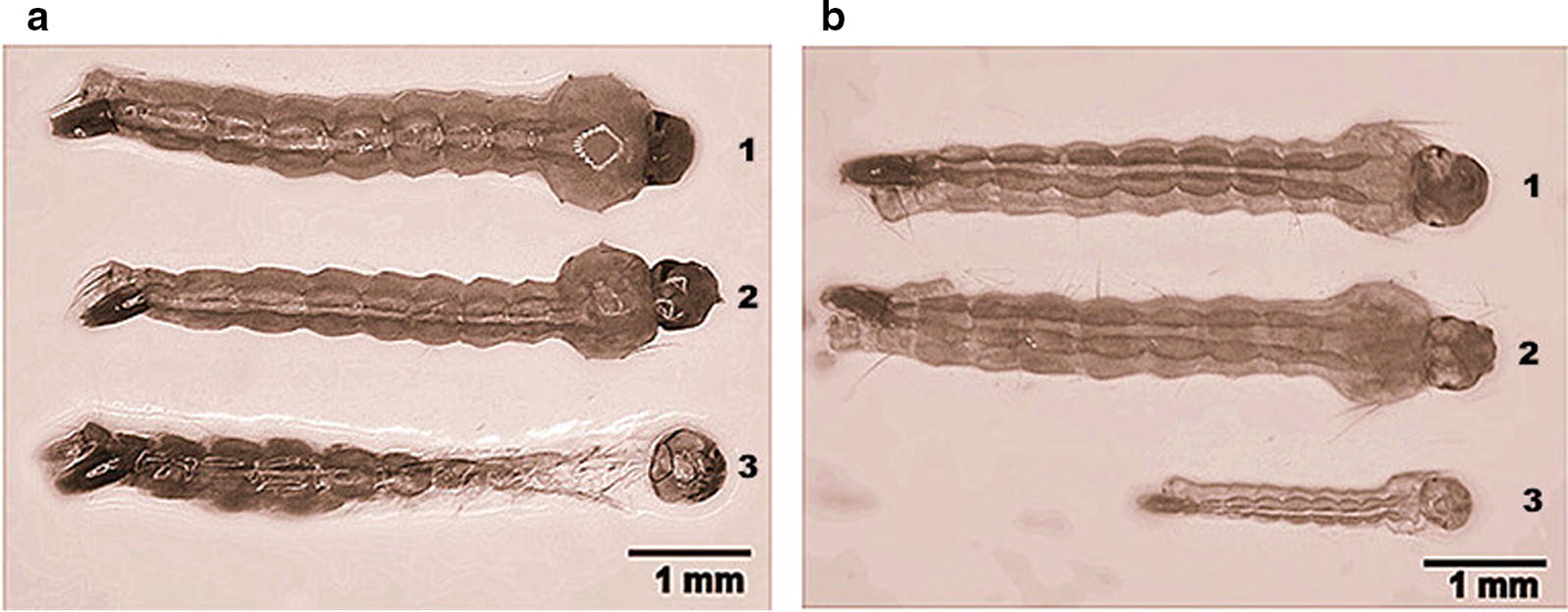
Morphological changes of the larvae fed on nanoparticles of the dsRNAs. a Compared with the third-instar larvae of the blank control (1) and those fed on dsgus (2), the dead larva which had fed on DOPAL synthase dsRNA were unable to detach from the exuvia (3). b Compared with the fourth-instar larva of the blank control (1) and those fed on dsgus (2), the larva which had fed on DOPAL synthase dsRNAs showed a much smaller size (3)
Table 1.
Statistics on the number of abnormal larvae
| 1a | 2a | 3a | Mean ± SD | |
|---|---|---|---|---|
| DS | 13 | 9 | 8 | 10 ± 2.65b |
| GUS | 3 | 0 | 2 | 1.67 ± 1.53 |
| BC | 1 | 1 | 2 | 1.33 ± 0.577 |
a1, 2, 3, three biological replicates
bThe number of abnormal larvae in DOPAL synthase-dsRNA-treated group was much higher compared with gus-dsRNA treated larvae and blank control (one-way ANOVA, P < 0.01)
Abbreviations: DS, treatment of larvae with dsDOPAL synthase; GUS, treatment of larvae with dsgus RNAs; BC, blank control
The efficiency of RNAi in larvae
Our results showed that feeding the mosquito larvae with DOPAL synthase dsRNA nanoparticles effectively triggered RNAi in the larvae. The expression shown in Table 2 demonstrates that the transcript levels of DOPAL synthase are repressed about 80% by DOPAL synthase dsRNAs and there is no significant difference between the dsgus fed group and black control (Fig. 4).
Table 2.
Quantitation of the expressions of DOPAL synthasea after RNAi of larvae
| 1b | 2b | 3b | Mean ± SD | |
|---|---|---|---|---|
| DS | 0.23 | 0.24 | 0.31 | 0.26 ± 0.043c |
| GUS | 0.90 | 0.95 | 0.97 | 0.95 ± 0.036 |
| BC | 1 | 1 | 1 | 1 ± 0.047 |
aLevel of mRNAs relative to blank control transcripts with 2-△△ct method
b1, 2, 3, three biological replicates
cThe DOPAL synthase gene expression in DOPAL synthase-dsRNA treated group was higher compared with gus-dsRNA treated larvae and blank control (one-way ANOVA, P < 0.01)
Abbreviations: DS, treatment of larvae with dsDOPAL synthase; GUS, treatment of larvae with dsgus RNAs; BC, blank control
Fig. 4.
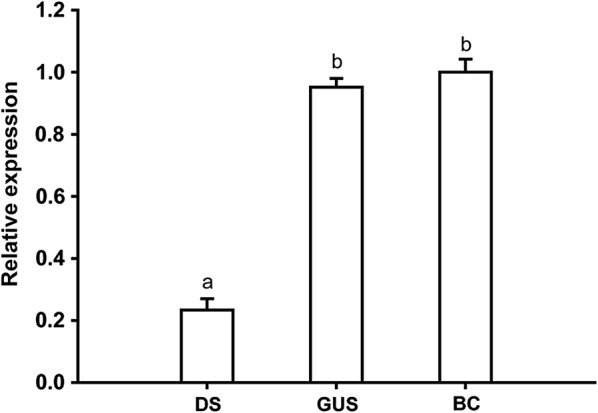
Quantitation of the expressions of DOPAL synthase after RNAi of larvae. Relative transcript levels of DOPAL synthase in the larvae after being continuously fed on DOPAL synthase dsRNA or gus dsRNA (dsgus as a control). The data are presented as means ± SD of three replicate samples. Bars represent the standard deviation. Different letters indicate statistically significant differences. Abbreviations: DS, treatment of larvae with dsDOPAL synthase; GUS, treatment of larvae with dsgus RNAs; BC, black control
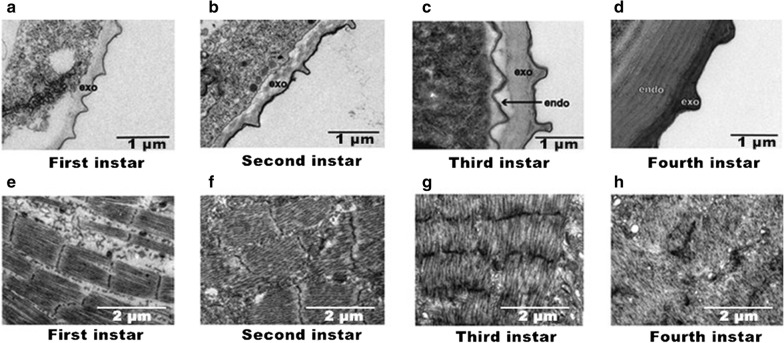
Transmission electron microscope (TEM) analysis
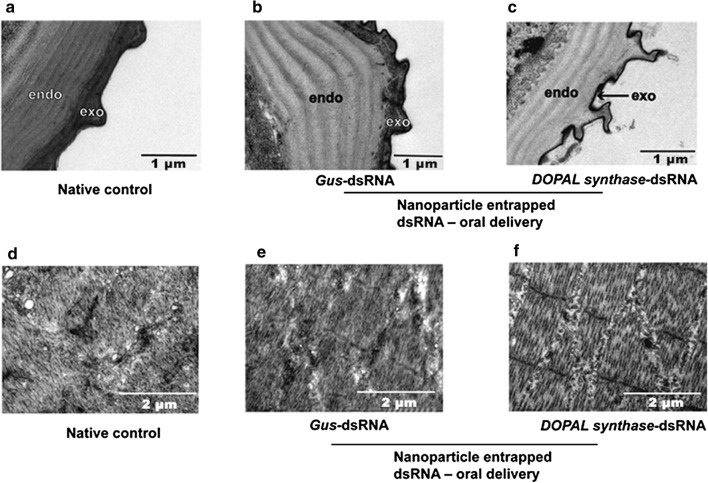
TEM detection of the larval abdomen collected from first-instar to fourth-instar larvae showed the RNAi effect on the development of cuticle. The insect cuticle is composed of the epicuticle and procuticle, and the latter is made up of the exocuticle and endocuticle [4]. The vertical section results showed that the endocuticle is deposited at the third-instar stage and becomes thicker at the fourth instar stage (Fig. 5a–d). In addition, the horizontal section results indicated that the surface texture of the larvae gradually becomes irregular and chaotic from the first- to the fourth-instar larvae (Fig. 5e–h). In order to understand the role of DOPAL synthase on the development of the larval cuticle, microstructural analysis of the abdomen integuments of the fourth-instar larvae was performed by TEM (Fig. 6). The integuments of larvae untreated (Fig. 6a) and treated with dsgus (Fig. 6b) were normal. However, the mosquitoes treated with DOPAL synthase dsRNA showed that the cuticle microstructure had only four endocuticle lamellae without the exocuticular deposited (Fig. 6c) compared with the mosquitoes in dsgus-treated (Fig. 6b) and untreated (Fig. 6a) groups. The interference of DOPAL synthase can also result in an abnormal surface texture of larval integument at the second-instar larvae, as illustrated in Fig. 6f, compared with the larvae in the dsgus-treated (Fig. 6e) and untreated (Fig. 6d) groups. These results reveal that Ae. aegypti DOPAL synthase is indispensable for the normal formation of the endocuticle and the larval development.
Fig. 5.
Morphology of vertical and horizontal sections of larval abdominal integument of different larval stages by TEM. a–d Vertical sections of integument from the first- to the fourth-instar larvae, respectively. e–h Horizontal sections of integument of the first- to fourth-instar larvae, respectively. Abbreviations: exo, exocuticle; endo, endocuticle
Fig. 6.
Morphology of vertical and horizontal sections of larval abdominal integument after treatment with DOPAL synthase dsRNA. Larvae treated with dsgus and without treatment were used as controls. a, d Vertical and horizontal sections of integuments of the control without treatment, respectively. b, e Vertical and horizontal sections of integuments of larvae which were treated with dsgus, respectively. c, f Vertical and horizontal sections of integument of larvae which were treated with DOPAL synthase-dsRNA, respectively
RNAi leads to high mortality of adults
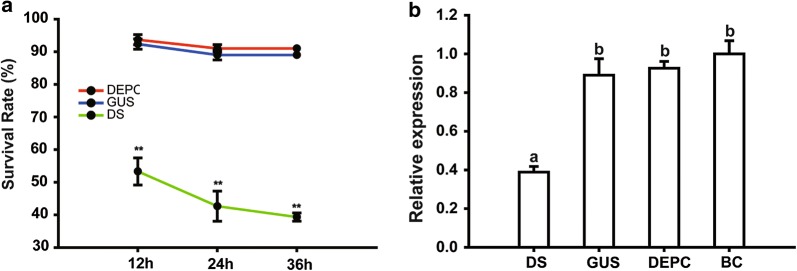
Most male mosquitoes died after they were injected with water or dsRNA. Therefore, we performed injection on the female mosquitoes to elucidate the function of DOPAL synthase. As shown in Fig. 7, the results of the dsRNA injection showed that DOPAL synthase RNAi can cause mortality in female adults, and the mortality rate was significantly higher than that of the control groups (Fig. 7a). At 12 h post-inoculation, the mortality was 46.6 ± 4.2% in dsRNA-inoculated mosquitoes, but mortality was 7.66 ± 1.6% and 6.3 ± 1.5% in the gus-dsRNA-inoculated and DEPC water-inoculated mosquitoes, respectively. At 24 h post-inoculation, the cumulative mortality of DOPAL synthase dsRNA-inoculated mosquitoes progressively increased to 57.2 ± 4.6%; gus-dsRNA -inoculated mosquitoes and DEPC water-inoculated were only 10.0 ± 2.7% and 8.9 ± 1.2%, respectively. In summary, the mortality of adult mosquitoes treated with DOPAL synthase-dsRNA was over 50%, while those in the control groups were only about 10% each (Fig. 7a).
Fig. 7.
The survival curve and interference of DOPAL synthase transcription after injection of DOPAL synthase dsRNA. Bars represent the standard deviation. Different letters indicate statistically significant differences, **P < 0.01. a The survival rate of mosquitoes at 12, 24 and 36 h after injection. b Knockdown of DOPAL synthase transcription by injection. Abbreviations: DS, treatment of adult mosquitoes with DOPAL synthase-dsRNA; GUS, treatment of adult mosquitoes with gus-dsRNA; DEPC, treatment of adult mosquitoes with DEPC water; BC, black control
At 12 h post-injection, RNA was extracted from adult mosquitoes for testing DOPAL synthase expression using qRT-PCR. As shown in Fig. 7b, the levels of DOPAL synthase transcript in DOPAL synthase dsRNA-inoculated mosquitoes were significantly reduced as compared with those in dsgus-inoculated, DEPC water-inoculated and non-treated mosquitoes. The results indicated > 60% mRNA knockdown with DOPAL synthase dsRNA.
Discussion
The insect cuticle has the function of protection and defense against pathogen infection and environmental stress factors, and plays an important role in the life of insects [3, 8]. The insect cuticle undergoes sclerotization, melanization and other physiological processes to play a protective role [8, 10, 11]. DOPAL synthase can catalyze the reaction from L-dopa to DOPAL, which can interact with free amino acids of CPs and directly participate in surface protein cross-linking [15–17]. However, there is no known biological effect of DOPAL in the postembryonic development of mosquitoes. In this study, we demonstrated the function of DOPAL synthase in larvae and adults of Ae. aegypti mosquitoes by RNAi and in order to better understand the roles of DOPAL synthase during post-embryonic development.
In the present study, it was found that the expression of DOPAL synthase was the highest in white female pupae and mainly distributed in the cuticle (Fig. 5). The results suggest that DOPAL synthase may be involved in soft and flexible cuticle formation in the developmental stages from pupa to female adult. The exocuticle is the main new cuticle that is deposited in each instar post-ecdysis, and the endocuticle is accumulated from the third-instar larvae, then becomes thicker with each layer deposition in the fourth-instar larvae. During the early development of Drosophila melanogaster, homozygous mutations of DOPAL synthase-like gene, amd-r, resulted in embryo death [18, 19]. By reducing DOPAL synthase expression through RNAi, we found that the mortality rate of larvae was increased, and some larvae died due to failure of molting. It is worth noticing that there are obvious flaws in the abdominal cuticle of the arrested developmental larvae, as seen with transmission electron microscopy. The procuticle, composed of the exocuticle and the endocuticle, was thinner in the DOPAL synthase RNAi-injected group than in the controls. In addition, DOPAL synthase RNAi can also lead to changes in integument structure, whereby its surface texture becomes more regular and ordered, which may lead to poor flexibility of the integument. Based our results, it can be proved that DOPAL synthase is associated with the formation of cuticle structure, particularly of the endocuticle, and that larval development could be stopped at any of the larval stages if DOPAL synthase is totally knocked out in mosquitoes.
To identify if the dsRNA delivery was by feeding or by absorption through the larval cuticle, the dsRNA was mixed with a visible dye to identify those individuals that absorb the solution to assess the extent of ingestion of the dsRNA solution. The results showed that the dye, bromophenol blue, was mostly found in the alimentary tract, and little found in other tissues (Additional file 2: Figure S2), suggesting that the dsRNA delivery was mainly through feeding by larvae.
We know that juvenile hormones are the major hormonal regulator during the development of insects, which also can regulate cuticle formation [47–50]. Whether DOPAL synthase, juvenile hormone and ecdysone have any interactions via metabolism pathway cross-talk or any other interactions in regulating cuticle formation should be further studied. Moreover, RNAi of DOPAL synthase also leads to the massive death of female adult mosquitoes treated with DOPAL synthase dsRNA. The mortality rate is up to 50% compared with the controls. The levels of DOPAL synthase transcript in the dsRNA-inoculated mosquitoes were also significantly reduced 12 h after inoculation (Fig. 7a). Microinjection of the dsRNA into adult insects is considered to be the most reliable dsRNA administration and has achieved high knockout efficiency [51]. In this study, such rapid interference of DOPAL synthase is surprising. Although the mechanism underlying the mortality in adult resulted by the DOPAL synthase dsRNA treatment remains to be determined, we speculate that DOPAL synthase plays a more critical role in adults than in larval stages, which may explain the fact that interference of the gene expression has more significant effects on adults than on larvae. The previous hypothesis proposed that the gene may be associated with the feeding ability of adults, particularly blood-feeding of female adults by forming more a flexible cuticle [17, 52], which allows the mosquito to enlarge its body to have a large enough meal. Adults have less chance to access food than larvae; they need to eat enough food once they have a host or a food source.
Our findings demonstrate that RNAi of DOPAL synthase gene expression results in larval development stagnation, abnormality of cuticle structure, adult death, and other molting behavioral alterations. It may, therefore, be inferred that DOPAL synthase is an essential gene for mosquito development and survival and thus implies its worth as a target for mosquito control.
Conclusions
This study provides evidence for the functions of DOPAL synthase during larvae development and adult survival in mosquitoes. Interference of DOPAL synthase can affect the molting and normal development of larvae, and leads to the death of adult mosquitoes. Our results suggest that DOPAL synthase is a potential target for mosquito control using RNAi technology.
Additional files
Additional file 1: Figure S1. The expression profiles of DOPAL synthase gene with rpf17 as the reference gene. Bars represent the standard deviation. Different letters indicate statistically significant differences (P < 0.05). a Temporal expression of DOPAL synthase in different developmental stages. b Spatial expression of DOPAL synthase in different tissues of Ae. aegypti. c Sex-different expression of DOPAL synthase in the cuticle of female and male adults. Abbreviations: L1, first-instar larvae; L2, second-instar larvae; L3, third-instar larvae; L4, fourth-instar larvae; WFP, white female pupae; WMP, white male pupae; BFP, black female pupae; BMP, black male pupae; FA, female adults; MA, male adults; NH, head; NT, thorax; NM, midgut; NC, cuticle; MC, cuticle of male adults; FC, cuticle of female adults; BFC, cuticle of blood-fed female adults.
Additional file 2: Figure S2. Double-stranded RNA mediated RNA interference with a dye by soaking. a The fourth-instar larvae after being soaked in dsRNAs with bromophenol blue, and without the dye and dsRNA (blank control). 1, blank control; 2, the dye and dsDOPAL synthase treated; 3, the dye and dsgus treated. b The dye distribution in alimentary tract and other tissues. 1, the midgut of dsDOPAL synthase and the dye treated larva; 2, the other extraintestinal tissues of dsDOPAL synthase and the dye treated larva; 3, the midgut of dsgus and the dye treated larva; 4, the other extraintestinal tissues of dsgus treated larva and the dye; 5, the midgut of the blank control (without being given dsRNA and dye) larva; 6, the other extraintestinal tissues of the blank control larva.
Acknowledgements
We are grateful to Professor Tongyuan Zhao of the Beijing Institute of Microbiology and Epidemiology for kindly providing the Aedes agypti strain.
Abbreviations
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- DDC
DOPA decarboxylase
- RT-PCR
real-time polymerase chain reaction
- RNAi
RNA interference
- TEM
transmission electron microscope
Authors’ contributions
QH and CHL conceived and designed the study, and critically revised the manuscript. JC and HRL performed the experiment and analysis data and drafted the manuscript. CHL and LZ helped in data analysis and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Research and Development Program of Hainan Province (ZDYF2019073), the National Natural Science Foundation of China (31860702) and Hainan University Research Fund (hdkytg201702).
Availability of data and materials
The data supporting the findings of this article are included within the article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Chen and Hao-Ran Lu contributed equally to this work
Contributor Information
Jing Chen, Email: 1101173833@qq.com.
Hao-Ran Lu, Email: 1048322442@qq.com.
Lei Zhang, Email: 386863728@qq.com.
Cheng-Hong Liao, Email: liaochh77@163.com.
Qian Han, Email: qianhan@hainanu.edu.cn.
References
- 1.Gallot A, Rispe C, Leterme N, Gauthier JP, Jaubert-Possamai S, Tagu D. Cuticular proteins and seasonal photoperiodism in aphids. Insect Biochem Mol Biol. 2010;40:235–240. doi: 10.1016/j.ibmb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Xiong G, Tong X, Gai T, Li C, Qiao L, Monteiro A, et al. Body shape and coloration of silkworm larvae are influenced by a novel cuticular protein. Genetics. 2017;207:1053–1066. doi: 10.1534/genetics.117.300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornman RS, Willis JH. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol Biol. 2009;18:607–622. doi: 10.1111/j.1365-2583.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moussian B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol. 2010;40:363–375. doi: 10.1016/j.ibmb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Duan B, Sun Y, Ma L, Zhu C, Shen B. Preliminary characterization of putative structural cuticular proteins in the malaria vector Anopheles sinensis. Pest Manag Sci. 2017;73:2519–2528. doi: 10.1002/ps.4649. [DOI] [PubMed] [Google Scholar]
- 6.Jasrapuria S, Specht CA, Kramer KJ, Beeman RW, Muthukrishnan S. Gene families of cuticular proteins analogous to peritrophins (CPAPs) in Tribolium castaneum have diverse functions. PLoS ONE. 2012;7:e49844. doi: 10.1371/journal.pone.0049844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao L, Xiong G, Wang RX, He SZ, Chen J, Tong XL, et al. Mutation of a cuticular protein, BmorCPR2, alters larval body shape and adaptability in silkworm, Bombyx mori. Genetics. 2014;196:1103–1115. doi: 10.1534/genetics.113.158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles JP. The regulation of expression of insect cuticle protein genes. Insect Biochem Mol Biol. 2010;40:205–213. doi: 10.1016/j.ibmb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Jasrapuria S, Arakane Y, Osman G, Kramer KJ, Beeman RW, Muthukrishnan S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem Mol Biol. 2010;40:214–227. doi: 10.1016/j.ibmb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem Mol Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Vavricka CJ, Han Q, Huang Y, Erickson SM, Harich K, Christensen BM, et al. From L-dopa to dihydroxyphenylacetaldehyde: a toxic biochemical pathway plays a vital physiological function in insects. PLoS ONE. 2011;6:e16124. doi: 10.1371/journal.pone.0016124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Q, Ding H, Robinson H, Christensen BM, Li J. Crystal structure and substrate specificity of Drosophila 3,4-dihydroxyphenylalanine decarboxylase. PLoS ONE. 2010;5:e8826. doi: 10.1371/journal.pone.0008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Q, Robinson H, Ding H, Christensen BM, Li J. Evolution of insect arylalkylamine N-acetyltransferases: structural evidence from the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:11669–11674. doi: 10.1073/pnas.1206828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.True JR, Yeh SD, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, et al. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 2005;1:e63. doi: 10.1371/journal.pgen.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang J, Han Q, Ding H, Li J. Biochemical identification of residues that discriminate between 3,4-dihydroxyphenylalanine decarboxylase and 3,4-dihydroxyphenylacetaldehyde synthase-mediated reactions. Insect Biochem Mol Biol. 2017;91:34–43. doi: 10.1016/j.ibmb.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Plotegher N, Berti G, Ferrari E, Tessari I, Zanetti M, Lunelli L, et al. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci Rep. 2017;7:40699. doi: 10.1038/srep40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao C, Upadhyay A, Liang J, Han Q, Li J. 3,4-dihydroxyphenylacetaldehyde synthase and cuticle formation in insects. Dev Comp Immunol. 2018;83:44–50. doi: 10.1016/j.dci.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Marsh JL, Wright TR. Evidence for regulatory variants of the dopa decarboxylase and alpha-methyldopa hypersensitive loci in Drosophila. Genetics. 1986;112:249–265. doi: 10.1093/genetics/112.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Marsh JL. Developmental regulation of the alpha-methyldopa hypersensitive gene of Drosophila melanogaster. Dev Biol. 1995;168:598–612. doi: 10.1006/dbio.1995.1105. [DOI] [PubMed] [Google Scholar]
- 20.Black BC, Pentz ES, Wright TR. The alpha methyl dopa hypersensitive gene, 1(2)amd, and two adjacent genes in Drosophila melanogaster: physical location and direct effects of amd on catecholamine metabolism. Mol Gen Genet. 1987;209:306–312. doi: 10.1007/BF00329658. [DOI] [PubMed] [Google Scholar]
- 21.Wright TR. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987;24:127–222. doi: 10.1016/S0065-2660(08)60008-5. [DOI] [PubMed] [Google Scholar]
- 22.Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 23.Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishna Pillai A, Nagarajan U, Mitra A, Krishnan U, Rajendran S, Hoti SL, et al. RNA interference in mosquito: understanding immune responses, double-stranded RNA delivery systems and potential applications in vector control. Insect Mol Biol. 2017;26:127–139. doi: 10.1111/imb.12282. [DOI] [PubMed] [Google Scholar]
- 25.Airs PM, Bartholomay LC. RNA Interference for mosquito and mosquito-borne disease control. Insects. 2017;8:4. doi: 10.3390/insects8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalla Bona AC, Chitolina RF, Fermino ML, de Castro Poncio L, Weiss A, Pereira Lima JB, et al. Larval application of sodium channel homologous dsRNA restores pyrethroid insecticide susceptibility in a resistant adult mosquito population. Parasit Vectors. 2016;9:397. doi: 10.1186/s13071-016-1634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu F, Xu P, Barbosa RM, Choo YM, Leal WS. RNAi-based demonstration of direct link between specific odorant receptors and mosquito oviposition behavior. Insect Biochem Mol Biol. 2013;43:916–923. doi: 10.1016/j.ibmb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whyard S, Erdelyan CNG, Partridge AL, Singh AD, Beebe NW, Capina R. Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit Vectors. 2015;8:96. doi: 10.1186/s13071-015-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice K, Christiaens O, Pertry I, Bailey A, Niblett C, Ghislain M, et al. RNAi-based gene silencing through dsRNA injection or ingestion against the African sweet potato weevil Cylas puncticollis (Coleoptera: Brentidae) Pest Manag Sci. 2017;73:44–52. doi: 10.1002/ps.4337. [DOI] [PubMed] [Google Scholar]
- 30.Shen ZJ, Zhang SD, Liu YJ, Liu XM, Li Z, Zhang QW, et al. Functional analysis by RNAi of an glutaredoxin gene in Helicoverpa armigera. J Insect Physiol. 2018;106:98–105. doi: 10.1016/j.jinsphys.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhai Y, Fan X, Yin Z, Yue X, Men X, Zheng L, et al. Identification and functional analysis of chitin synthase A in oriental armyworm, Mythimna separata. Proteomics. 2017;17:1700165. doi: 10.1002/pmic.201700165. [DOI] [PubMed] [Google Scholar]
- 32.Bingsohn L, Knorr E, Billion A, Narva KE, Vilcinskas A. Knockdown of genes in the Toll pathway reveals new lethal RNA interference targets for insect pest control. Insect Mol Biol. 2017;26:92–102. doi: 10.1111/imb.12273. [DOI] [PubMed] [Google Scholar]
- 33.Camargo RA, Barbosa GO, Possignolo IP, Peres LE, Lam E, Lima JE, et al. RNA interference as a gene silencing tool to control Tuta absoluta in tomato (Solanum lycopersicum) PeerJ. 2016;4:e2673. doi: 10.7717/peerj.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu R, Xu X, Liang Y, Tian H, Pan Z, Jin S, et al. The insect ecdysone receptor is a good potential target for RNAi-based pest control. Int J Biol Sci. 2014;10:1171–1180. doi: 10.7150/ijbs.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J Insect Physiol. 2010;56:227–235. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S, et al. Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci. 2013;20:4–14. doi: 10.1111/j.1744-7917.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 37.Whyard S, Singh AD, Wong S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol. 2009;39:824–832. doi: 10.1016/j.ibmb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Singh AD, Wong S, Ryan CP, Whyard S. Oral delivery of double-stranded RNA in larvae of the yellow fever mosquito, Aedes aegypti: implications for pest mosquito control. J Insect Sci. 2013;13:69. doi: 10.1673/031.013.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taning CN, Andrade EC, Hunter WB, Christiaens O, Smagghe G. Asian citrus psyllid RNAi pathway—RNAi evidence. Sci Rep. 2016;6:38082. doi: 10.1038/srep38082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavez-Pena C, Kamen AA. RNA interference technology to improve the baculovirus-insect cell expression system. Biotechnol Adv. 2018;36:443–451. doi: 10.1016/j.biotechadv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed AMA, Diab MR, Abdelsattar M, Khalil SMS. Characterization and RNAi-mediated knockdown of Chitin Synthase A in the potato tuber moth, Phthorimaea operculella. Sci Rep. 2017;7:9502. doi: 10.1038/s41598-017-09858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramesh KD, Saravana KP, Gandhi MR, Al-Dhabi NA, Paulraj MG, Ignacimuthu S. Delivery of chitosan/dsRNA nanoparticles for silencing of wing development vestigial (vg) gene in Aedes aegypti mosquitoes. Int J Biol Macromol. 2016;86:89–95. doi: 10.1016/j.ijbiomac.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Mysore K, Andrews E, Li P, Duman-Scheel M. Chitosan/siRNA nanoparticle targeting demonstrates a requirement for single-minded during larval and pupal olfactory system development of the vector mosquito Aedes aegypti. BMC Dev Biol. 2014;14:9. doi: 10.1186/1471-213X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Dzaki N, Azzam G. Assessment of Aedes albopictus reference genes for quantitative PCR at different stages of development. PLoS ONE. 2018;13:e0194664. doi: 10.1371/journal.pone.0194664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng P, Xu QY, Fu KY, Guo WC, Li GQ. RNA interference against the putative insulin receptor substrate gene chico affects metamorphosis in Leptinotarsa decemlineata. Insect Biochem Mol Biol. 2018;103:1–11. doi: 10.1016/j.ibmb.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Nouzova M, Michalkova V, Hernandez-Martinez S, Rivera-Perez C, Ramirez CE, Fernandez-Lima F, et al. JH biosynthesis and hemolymph titers in adult male Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2018;95:10–16. doi: 10.1016/j.ibmb.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soares MP, Elias-Neto M, Simoes ZL, Bitondi MM. A cuticle protein gene in the honeybee: expression during development and in relation to the ecdysteroid titer. Insect Biochem Mol Biol. 2007;37:1272–1282. doi: 10.1016/j.ibmb.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Andersen SO. Studies on proteins in post-ecdysial nymphal cuticle of locust, Locusta migratoria, and cockroach, Blaberus craniifer. Insect Biochem Mol Biol. 2000;30:569–577. doi: 10.1016/S0965-1748(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 51.Kumar SS, Puttaraju HP. Improvised microinjection technique for mosquito vectors. Indian J Med Res. 2012;136:971–978. [PMC free article] [PubMed] [Google Scholar]
- 52.Vavricka CJ, Han Q, Mehere P, Ding H, Christensen BM, Li J. Tyrosine metabolic enzymes from insects and mammals: a comparative perspective. Insect Sci. 2014;21:13–19. doi: 10.1111/1744-7917.12038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The expression profiles of DOPAL synthase gene with rpf17 as the reference gene. Bars represent the standard deviation. Different letters indicate statistically significant differences (P < 0.05). a Temporal expression of DOPAL synthase in different developmental stages. b Spatial expression of DOPAL synthase in different tissues of Ae. aegypti. c Sex-different expression of DOPAL synthase in the cuticle of female and male adults. Abbreviations: L1, first-instar larvae; L2, second-instar larvae; L3, third-instar larvae; L4, fourth-instar larvae; WFP, white female pupae; WMP, white male pupae; BFP, black female pupae; BMP, black male pupae; FA, female adults; MA, male adults; NH, head; NT, thorax; NM, midgut; NC, cuticle; MC, cuticle of male adults; FC, cuticle of female adults; BFC, cuticle of blood-fed female adults.
Additional file 2: Figure S2. Double-stranded RNA mediated RNA interference with a dye by soaking. a The fourth-instar larvae after being soaked in dsRNAs with bromophenol blue, and without the dye and dsRNA (blank control). 1, blank control; 2, the dye and dsDOPAL synthase treated; 3, the dye and dsgus treated. b The dye distribution in alimentary tract and other tissues. 1, the midgut of dsDOPAL synthase and the dye treated larva; 2, the other extraintestinal tissues of dsDOPAL synthase and the dye treated larva; 3, the midgut of dsgus and the dye treated larva; 4, the other extraintestinal tissues of dsgus treated larva and the dye; 5, the midgut of the blank control (without being given dsRNA and dye) larva; 6, the other extraintestinal tissues of the blank control larva.
Data Availability Statement
The data supporting the findings of this article are included within the article and its additional files.