Abstract
Background: BAY 94-9027 is an extended half-life recombinant factor VIII (rFVIII) that prevents bleeding in persons with hemophilia A at twice-weekly, 5-day, and 7-day dosing intervals. In rare diseases such as hemophilia, where small populations preclude head-to-head comparisons in randomized controlled trials, outcomes from different studies can be compared by matching-adjusted indirect treatment comparisons (MAICs) via matched summary statistics of individual patient data. This study compared MAIC-adjusted outcomes of BAY 94-9027 with other FVIII agents for prophylaxis of hemophilia.
Methods: Weighted patient data from the BAY 94-9027 PROTECT VIII trial were used to compare annualized bleeding rates (ABRs), percentage of patients with zero bleeds, and factor utilization against published data on rFVIII–Fc fusion protein (rFVIIIFc), BAX 855, and recombinant antihemophilic factor/plasma/albumin-free method (rAHF-PFM).
Results: After matching BAY 94-9027 and comparators, the mean BAY 94-9027 utilization was significantly lower than rFVIIIFc pre- and post-matching (66.2 vs 82.2 IU/kg/week; 66.5 vs 82.2 IU/kg/week; both P<0.001). Median BAY 94-9027 utilization (IU/kg/week) trended lower than BAX 855 (64.3 vs 87.4) and rAHF-PFM (2004 study: 64.0 vs 107.5; 2012 study: 63.6 vs 109.9). Mean ABRs and percentages of patients with zero bleeds were similar post-matching between BAY 94-9027 and comparators.
Conclusion: BAY 94-9027 demonstrated similar MAIC-adjusted ABR with lower utilization than rFVIIIFc, BAX 855, and rAHF-PFM.
Keywords: rFVIII, annualized bleeding rate, utilization
Plain language summary
Physicians rely on comparative studies to make treatment decisions for their patients. Clinical research in rare diseases, such as hemophilia, are often limited by their small population sizes, making it unrealistic to conduct large, statistically-powered, randomized, controlled trials. Matching-adjusted indirect comparison (MAIC) is a validated methodology that is used to compare outcomes of clinical trials when direct head-to-head comparisons are not feasible. Applying MAIC to comparisons between studies of the long-acting recombinant factor VIII (rFVIII) agent, BAY 94-9027, vs two other long-acting agents (rFVIII-Fc fusion protein and BAX 855) and a standard-acting agent (rAHF-PFM), our study demonstrated that BAY 94-9027 required lower utilization, with no differences in annual bleed rate or percentage of patients who had no bleeds.
Introduction
Major advances have been achieved in hemophilia healthcare over the past several decades, owing to the discovery that a blood clotting factor VIII (FVIII) deficiency could be replaced with intravenous injections of recombinant or virally inactivated FVIII. These findings led to a notable shift in life expectancy from the early-to-mid teens to near that of the general population.1,2 Prophylaxis is the gold standard for preventing bleeding episodes and their sequelae, ie, joint damage, arthropathy, pain, loss of mobility, and reduced quality of life.3 However, despite significant clinical improvements experienced by persons with hemophilia (PWH) on prophylaxis, frequent injections or high doses of replacement factor are often required to maintain adequate protection levels because of the short half-lives of most current therapies. Most treatment regimens require injections every 2 or 3 days, representing a treatment burden for a lifelong disease that is compounded by additional issues of venous access, fear of pain, and high costs.4
Recent research efforts have led to the introduction of extended half-life (EHL) agents as new long-acting treatment options that alleviate the treatment burden of frequent injections. Currently available EHL treatments include a recombinant FVIII–Fc fusion protein (rFVIIIFc) and a PEGylated full-length rFVIII (BAX 855). rFVIIIFc and BAX 855 have approximately 1.5-fold higher half-lives than standard-acting rFVIII, without compromised efficacy.5,6 Increased half-life is anticipated to have a positive impact on patient compliance owing to flexible dosing or reduced treatment burden.5,6
BAY 94-9027, a site-specific PEGylated rFVIII, is an EHL treatment with approximately 1.6-fold higher half-life than standard rFVIII (about 19 vs 12 hours).7 In the pivotal phase II/III PROTECT VIII trial, treatment of severe hemophilia A with BAY 94-9027 on-demand or prophylactic (every 7 days, every 5 days, and twice weekly) was associated with favorable efficacy results8 BAY 94-9027 was generally well tolerated, and no patients developed inhibitors.8
While head-to-head comparisons in randomized controlled trials (RCTs) are the preferred method for evaluating differences between treatments, such approaches are not often feasible in rare diseases such as hemophilia owing to the small population sizes available for trial recruitment. In those cases, indirect treatment comparisons provide comparative evidence to inform treatment decisions.9–11 Matching-adjusted indirect comparison (MAIC) is a widely used, validated method for comparing outcomes of interventions in the absence of head-to-head trials.12–16 Since 2017, MAIC has been recognized by the UK’s National Institute for Health and Care Excellence as a valid population-adjusted indirect comparison approach, and is accepted across various health technology assessment bodies.17,18 MAIC takes into account differences in trial design and baseline characteristics between treatments when common comparators are absent. Such population-adjusted analyses ensure that comparisons are conducted within a more balanced patient population. In brief, MAIC uses individual patient data from a trial (or trials) of one treatment to match baseline summary statistics from a comparator’s trial(s);9,19 after adjusting (or “balancing”) for heterogeneity in baseline characteristics between patient populations; confounding factors caused by divergent population characteristics are largely eliminated.
We report here the application of MAIC to compare outcomes of annualized bleeding rate (ABR) and rFVIII utilization of BAY 94-9027 compared with EHL therapies, rFVIIIFc and BAX 855, as well as with a standard-acting rFVIII, recombinant antihemophilic factor plasma/albumin-free method (rAHF-PFM). rFVIIIFc and BAX 855 were selected as comparators of already approved EHL prophylactic treatments currently used in clinical practice in Europe and the USA. rAHF-PFM was selected as a comparative standard half-life treatment that is considered standard treatment in many countries. MAIC was used to compare those agents in the prophylactic setting, using data from published clinical trials and patient-level BAY 94-9027 data.
Methods
Data sources
Patient-level data were collected from PROTECT VIII, a phase II/III, open-label, partially randomized trial of BAY 94-9027 in adults and adolescents with severe hemophilia A. For MAIC-based comparisons, clinical studies were identified in a systematic literature review (SLR) for published pivotal trials of rFVIIIFc, BAX 855, and rAHF-PFM in adults and adolescents with severe hemophilia A. The screening criteria and the PRISMA flow diagram for the SLR are described in Table S1 and Figure S1. Aggregate data from the prophylaxis arms of each study were extracted from the published trials for the analysis.
Table S1.
Inclusion and exclusion criteria for the SLR
| Inclusion criteria | Exclusion criteria |
|---|---|
| Language | |
|
|
| Study design and publication type | |
|
|
|
|
|
|
|
|
|
|
| Study population | |
|
|
| |
| |
| |
| Study interventions | |
|
|
| Outcomes of interest | |
|
|
Note: aStudies with mixed severe and moderately severe hemophilia A patients may be included.
Abbreviations: rAHF-PFM, recombinant antihemophilic factor/plasma/albumin-free method; RCT, randomized controlled trial; rFVIIIFc, recombinant factor VIII–Fc fusion protein; SLR, systematic literature review.
Figure S1.
PRISMA flow diagram.
As this was a post hoc analysis of previously published data, no institutional board review was required.
Sample selection
For BAY 94-9027, data from three prophylaxis arms (twice weekly, every 5 days, and every 7 days) of PROTECT VIII were used to accommodate the variability of treatment frequencies across comparators.
For rFVIIIFc, since the approved US Food and Drug Administration (FDA) label allows adjustable dosing,20 individualized and weekly prophylaxis arms from the A-LONG trial were pooled, along with a sensitivity analysis using only data from the individualized rFVIIIFc treatment arm.6 For BAX 855, the prophylaxis arm in the PROLONG-ATE trial was used,5 and for rAHF-PFM, analyses of two rAHF-PFM trials published in 200421 and 201222 were used, but analyses were conducted separately because of differences in ABR calculation. The prophylaxis arm in the 2004 trial was used for the first comparison, and standard and pharmacokinetic (PK)-tailored prophylaxis arms in the 2012 trial were pooled for the second comparison. Details on the studies are provided in Table S2.
Table S2.
Summary of clinical trials used in the analysis
| Study | Study drug | Design | Patients | N | Regimen | Outcome Endpoint |
|---|---|---|---|---|---|---|
| PROTECT VIIIa | BAY 94-9027 | Phase II/III, multinational, partially randomized, open-label trial | Men aged 12–65 years with FVIII <1% and ≥150 ED to FVIII | 132 | 10-week run-in period: 25 IU/kg twice weekly (on-demand or prophylaxis) Randomization period: twice weekly (30–40 IU/kg), every 5 days (45–60 IU/kg), or every 7 days (60 IU/kg) prophylaxis for 36 weeks |
ABR (primary) |
| A-LONGb | rFVIIIFc | Phase III open-label, multicenter, partially randomized trial | Previously treated males aged ≥12 years with severe hemophilia A (FVIII <1%) | 165 | Three treatment arms: 1) individualized prophylaxis (25–65 IU/kg every 3–5 days); 2) weekly prophylaxis (65 IU/kg); 3) episodic (10–50 IU/kg)f | ABR (primary) |
| PROLONG-ATEc | BAX 855 | Phase II/III multicenter, open-label study | Subjects aged 12–65 years with severe hemophilia A (FVIII <1%) and ≥150 ED to FVIII | 138 | Prophylaxis regimen: 45±5 IU/kg twice weekly for ≥50 ED On-demand regimen: 10–60±5 IU/kg for 6 months±2 days |
ABR (primary) |
| rAHF-PFM-2004d | rAHF-PFM | Three-part study; open-label, uncontrolled, prophylactic study (part 2) and two PK studies | Subjects aged ≥10 years with FVIII ≤2%, weight >35 kg, and ≥150 ED to FVIII | 111 | Prophylactic regimen: 25–40 IU/kg three times per week or every other day for ≥75 ED (PK regimens: 50±5 IU/kg, two infusions 72 h to 4 weeks apart) | ABR (post hoc) |
| rAHF-PFM-2012e | rAHF-PFM | Open-label, multicenter study | Subjects aged 7–65 years with FVIII ≤2% and ≥150 ED to FVIII | 66 | Standard prophylaxis: 20–40 IU/kg every 48±6 h for 12 monthsg PK-tailored prophylaxis: 20–80 IU/kg every 72±6 h for 12 monthsg |
ABR (primary) |
Notes: aReding et al (2017)1; bMahlangu et al (2014)2; cKonkle et al (2015)3; dTarantino et al (2004)4; dValentino et al (2012)5. fTreatment was terminated upon completion of pharmacokinetic assessments and achievement of prespecified rFVIIIFc exposure to ensure acceptable inhibitor detection. Median duration of treatment was 32.1 and 28.0 days for prophylaxis arms 1 and 2, respectively. gRandomization to standard or PK-tailored prophylaxis occurred after 6 months of on-demand treatment at 50±6 IU/kg.
Abbreviations: ABR, annualized bleeding rate; ED, exposure days; FVIII, factor VIII; PK, pharmacokinetic; rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; rFVIIIFc, recombinant factor VIII–Fc fusion protein.
Outcome assessments
Outcomes evaluated in this study included ABR, percentage of patients with zero bleeds, and weekly rFVIII utilization (IU/kg/week). For PROTECT VIII, the duration of assessment was from randomization to the end of prophylaxis. For the comparator trials, duration of assessment included the prophylaxis exposure period. To accommodate comparisons of outcomes calculated by different methodologies (ie, negative binomial regression for ABR in A-LONG and square root transformation of ABR for rAHF-PFM-2012), identical methodologies were applied to the patient-level ABR data from PROTECT VIII for those comparisons.
Statistical methods
MAIC methodology9,19 was used to compare BAY 94-9027 versus rFVIIIFc, BAX 855, and rAHF-PFM for prophylactic treatment of severe hemophilia A. Individual patient data from the PROTECT VIII trial were weighted to match the mean baseline characteristics reported in each comparator trial. The matched baseline characteristics were selected based on data availability in both PROTECT VIII and the comparator trials. Individual patient weights were estimated from a logistic regression model, similar to a propensity score weighting. However, without patient-level data from comparator trials, the usual maximum likelihood approach to estimate parameters in a logistic regression model was not feasible. Method of moments, which only requires aggregated data for the comparators, was used instead.19
The weighted ABR outcomes in PROTECT VIII were statistically compared to the observed values for rFVIIIFc, BAX 855, and rAHF-PFM in the balanced trial populations. ABR was compared using unweighted Wald tests before matching and weighted Wald tests after matching. The percentage of patients with zero bleeds was compared using chi-squared tests before matching and weighted Wald tests after matching. Median weekly rFVIII utilization was described for the weighted BAY 94-9027 population in the comparisons with BAX 855 and rAHF-PFM. Mean weekly rFVIII utilization was statistically compared between the balanced BAY 94-9027 and rFVIIIFc populations using unweighted Wald tests before matching and weighted Wald tests after matching.
Statistical comparisons were conducted in R 3.4.3 and statistical significance was set at P<0.05.
Results
Selection and comparison between clinical trials
For BAY 94-9027, individual patient data from the phase II/III, partially randomized, open-label, PROTECT VIII trial were used. Four published clinical trials were identified for comparison: the A-LONG trial for rFVIIIFc,6 the PROLONG-ATE trial for BAX 855,5 and two rAHF-PFM trials published in 200421 and in 2012.22
To ensure comparability between patient populations, key inclusion and exclusion criteria for the comparator trials were applied to the BAY 94-9027 population. In general, the study populations of the PROTECT VIII trial and the comparator trials were similar, inclusive of males aged 12–65 years with severe hemophilia A (≤1% FVIII) and previously treated with FVIII concentrates. Patients enrolled in the A-LONG trial weighed more than 40 kg, therefore this criterion was applied to the BAY 94-9027 population for the comparison of BAY 94-9027 versus rFVIIIFc.
The length of treatment varied across the trials: patients in PROTECT VIII and PROLONG-ATE received prophylaxis regimens for 6 months; median durations of rFVIIIFc in the individualized and weekly prophylaxis arms in A-LONG were 32.1 and 28.0 weeks, respectively; median duration of exposure days to rAHF-PFM was 117 in the 2004 study; and mean duration of rAHF-PFM treatment was 362 days for standard prophylaxis and 344 days for PK-tailored prophylaxis in the 2012 study.
Comparison of baseline characteristics between trials
A total of 104 patients treated with BAY 94-9027 prophylaxis were included in the analysis. For the comparison to rFVIIIFc, one patient was excluded from the BAY 94-9027 population because of the weight criterion >40 kg.
rFVIIIFc
Prior to matching, the BAY 94-9027 population had an older median age and included a higher proportion of patients on prior prophylactic treatments compared to the rFVIIIFc population (81% vs 61%) (Table 1). The BAY 94-9027 population also included a higher proportion of patients on prior prophylactic treatment with target joints (59% vs 33%, respectively), but a lower proportion of patients on prior on-demand treatment with target joints (16% vs 34%, respectively). Similar proportions of patients in the BAY 94-9027 and rFVIIIFc populations had had more than six bleeding events in the prior year despite being on prior prophylactic treatment (30% and 31%); 14% of patients previously on on-demand treatment in the BAY 94-9027 population had at least 28 bleeding events, compared with 19% of the patients on prior on-demand treatment in the rFVIIIFc population.
Table 1.
Observed baseline characteristics of patients treated with BAY 94-9027 and rFVIIIFc, before and after matching
| Baseline characteristicsb | Before matching | After matchinga | ||||
|---|---|---|---|---|---|---|
| BAY 94-9027 | rFVIIIFc | P | BAY 94-9027 | rFVIIIFc | P | |
| N=103 | N=140 | N=103 | N=140 | |||
| Age ≤29.4 years | 33 (32.0%) | 70 (50.0%) | <0.01* | 50.0% | 50.0% | 1.00 |
| Weight ≤72.4 kg | 49 (47.6%) | 70 (50.0%) | 0.81 | 50.0% | 50.0% | 1.00 |
| Ethnicity | ||||||
| White | 73 (70.9%) | 90 (64.1%) | 0.35 | 64.1% | 64.1% | 1.00 |
| Asian | 26 (25.2%) | 38 (26.8%) | 0.85 | 26.8% | 26.8% | 1.00 |
| Region | ||||||
| Europe | 37 (35.9%) | 37 (26.1%) | 0.15 | 26.1% | 26.1% | 1.00 |
| North America | 23 (22.3%) | 48 (34.5%) | 0.06 | 34.5% | 34.5% | 1.00 |
| Prior treatment type | ||||||
| Prophylactic | 83 (80.6%) | 86 (61.3%) | <0.01* | 61.3% | 61.3% | 1.00 |
| On-demand | 20 (19.4%) | 54 (38.7%) | <0.01* | 38.7% | 38.7% | 1.00 |
| Number of bleeding events in the prior year | ||||||
| Prior prophylactic: >6.0 | 31 (30.1%) | 43 (30.6%) | 1.00 | 30.6% | 30.6% | 1.00 |
| Prior on-demand: >27.4 | 14 (13.6%) | 27 (19.4%) | 0.32 | 19.4% | 19.4% | 1.00 |
| Presence of target joint(s) | ||||||
| Prior prophylactic | 61 (59.2%) | 46 (33.1%) | <0.001* | 33.1% | 33.1% | 1.00 |
| Prior on-demand | 16 (15.5%) | 47 (33.8%) | <0.01* | 33.8% | 33.8% | 1.00 |
Notes: aThe effective sample size of the BAY 94-9027 overall group after matching was 44.60. bThe median of age, weight, and estimated bleeding events in the prior year in the pooled rFVIIIFc group was approximated using the weighted sum of corresponding medians in the individualized and the weekly prophylaxis arms in A-LONG. *P<0.05.
Abbreviation: rFVIIIFc, recombinant human factor VIII–Fc fusion protein.
BAX 855
Compared to the BAX 855 population, the BAY 94-9027 population was older and had more target joints (Table 2). The distribution of prior treatment type was similar between the two populations.
Table 2.
Observed baseline characteristics of patients treated with BAY 94-9027 and BAX 855, before and after matching
| Baseline characteristics | Before matching | After matchinga | ||||
|---|---|---|---|---|---|---|
| BAY 94-9027 | BAX 855 | P | BAY 94-9027 | BAX 855 | P | |
| N=104 | N=101 | N=104 | N=101 | |||
| Age ≤28 years | 34 (32.7%) | 50 (50.0%) | <0.05* | 50.0% | 50.0% | 1.00 |
| Weight ≤73 kg | 51 (49.0%) | 50 (50.0%) | 1.00 | 50.0% | 50.0% | 1.00 |
| Ethnicity | ||||||
| White | 73 (70.2%) | 77 (76.7%) | 0.41 | 76.7% | 76.7% | 1.00 |
| Asian | 27 (26.0%) | 23 (22.5%) | 0.71 | 22.5% | 22.5% | 1.00 |
| Prior treatment type | ||||||
| Prophylactic | 84 (80.8%) | 83 (82.5%) | 0.94 | 82.5% | 82.5% | 1.00 |
| On-demand | 20 (19.2%) | 18 (17.5%) | 0.94 | 17.5% | 17.5% | 1.00 |
| Presence of target joint(s) | 78 (75.0%) | 66 (65.0%) | 0.17 | 65.0% | 65.0% | 1.00 |
Notes: aThe effective sample size of the BAY 94-9027 overall group after matching was 78.30. *P<0.05.
rAHF-PFM
Compared to the two rAHF-PFM populations, the BAY 94-9027 population was older and included more Asians (Table 3). The BAY 94-9027 population included more patients treated with prior prophylactic treatment than the rAHF-PFM population in the 2004 trial (81% vs 73%), and fewer patients with target joints than the rAHF-PFM population in the 2012 trial (75% vs 96%).
Table 3.
Observed baseline characteristics of patients treated with BAY 94-9027 and rAHF-PFM
| Baseline characteristicsb | Before matching | After matchinga | ||||
|---|---|---|---|---|---|---|
| BAY 94-9027 | rAHF-PFM (2004 study)a |
P | BAY94-9027 | rAHF-PFM (2004 study)a |
P | |
| N=104 | N=107 | N=104 | N=107 | |||
| Age ≤18 years | 13 (12.5%) | 54 (50.0%) | <0.001* | 50.0% | 50.0% | 1.00 |
| Ethnicity | ||||||
| White | 73 (70.2%) | 99 (92.8%) | <0.001* | 92.8% | 92.8% | 1.00 |
| Asian | 27 (26.0%) | 1 (0.9%) | <0.001* | 0.9% | 0.9% | 1.00 |
| Prior treatment typea | ||||||
| Prophylactic | 84 (80.8%) | 78 (73.0%) | 0.23 | 73.0% | 73.0% | 1.00 |
| On-demand | 20 (19.2%) | 29 (27.0%) | 0.23 | 27.0% | 27.0% | 1.00 |
| Baseline characteristicsb | BAY 94-9027 | rAHF-PFM (2012 study) | P | BAY 94-9027 | rAHF-PFM (2012 study) | P |
| N=104 | N=66 | N=104 | N=66 | |||
| Age <16 years | 9 (8.7%) | 9 (13.6%) | 0.44 | 13.6% | 13.6% | 1.00 |
| Ethnicity | ||||||
| White | 73 (70.2%) | 58 (87.9%) | <0.05* | 87.9% | 87.9% | 1.00 |
| Asian | 27 (26.0%) | 1 (1.5%) | <0.001* | 1.5% | 1.5% | 1.00 |
| Presence of target joint(s) | 78 (75.0%) | 63 (95.5%) | <0.001* | 95.5% | 95.5% | 1.00 |
Notes: aThe effective sample size of the BAY 94-9027 overall group was 25.32 after matching with the 2004 study and 55.33 after matching with the 2012 study. bPatients on both prior prophylactic and on-demand treatment in the 2004 study were categorized as being on “prior prophylactic treatment” to be consistent with the definition in the PROTECT VIII trial. *P<0.05.
Abbreviation: rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method.
After matching, the summary statistics of all matched baseline characteristics were exactly balanced for each comparison.
Comparison of outcomes
Annualized bleeding rate
Before matching, BAY 94-9027 was associated with a slightly higher mean ABR compared to rFVIIIFc, BAX 855, and rAHF-PFM-2012; the differences were not statistically significant (left panels, Figure 1A, B and D, respectively). BAY 94-9027 was associated with a significantly lower mean ABR compared to rAHF-PFM-2004 (difference: −2.25, P<0.01; left panel of Figure 1C). After matching, the mean ABR was similar between BAY 94-9027 and the comparators: 3.77 vs 3.90 (rFVIIIFc), 4.25 vs 2.91 (individualized rFVIIIFc), 3.95 vs 3.70 (BAX 855), 4.28 vs 6.30 (rAHF-PFM-2004), and 1.87 vs 1.80 (rAHF-PFM-2012, square root-transformed ABR [see Methods]). None of these differences reached statistical significance (P>0.05).
Figure 1.

Comparison of ABR and proportion of patients with zero bleeds between BAY 94-9027 and (A) rFVIIIFc; (B) BAX 855; (C) rAHF-PFM-2004; (D) rAHF-PFM-2012. *P<0.05.
Abbreviations: ABR, annualized bleeding rate; rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; rFVIIIFc, recombinant factor VIII–Fc fusion protein.
BAY 94-9027 was associated with a slightly higher median ABR compared to rFVIIIFc (before and after: 2.09 vs 1.93), BAX 855 (before and after: 2.1 vs 1.9), and rAHF-PFM-2012 (before: 1.61 vs 1.10; after: 1.56 vs 1.10). Median ABR was not reported in the rAHF-PFM-2004 study for rAHF-PFM. The P-values for comparing the medians were incalculable owing to the unavailability of standard errors for the median ABR of comparators.
Percentage of patients with zero bleeds
Before matching, BAY 94-9027 was associated with fewer patients with zero bleeds compared to rFVIIIFc and BAX 855, and slightly more zero-bleed patients compared to rAHF-PFM (right panels of Figure 1).
The after matching comparisons were similar to before matching: 34.1% vs 40.7% (rFVIIIFc), 35.2% vs 45.3% (individualized rFVIIIFc), 38.9% vs 39.6% (BAX 855), 41.5% vs 29.9% (rAHF-PFM-2004), and 40.0% vs 33.3% (rAHF-PFM-2012). None of the differences was statistically significant either before or after matching.
Comparison of weekly rFVIII utilization
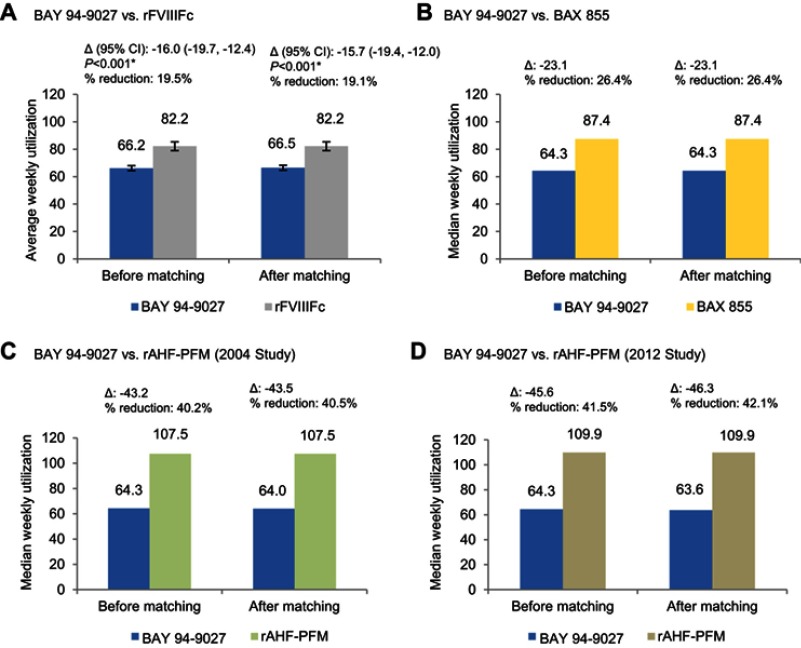
After balancing the baseline characteristics between trial populations, the mean weekly rFVIII utilization of patients treated with BAY 94-9027 was significantly lower than for those treated with rFVIIIFc (66.5 vs 82.2 IU/kg/week; P<0.001) (Figure 2A). The results of the sensitivity analysis of BAY 94-9027 versus individualized rFVIIIFc were consistent with these results (66.4 vs 85.4 IU/kg/week; P<0.001).
Figure 2.
Comparison of weekly rFVIII utilization between BAY 94-9027 and (A) rFVIIIFc; (B) BAX 855; (C) rAHF-PFM-2004; (D) rAHF-PFM-2012. Median weekly rFVIII utilization of prophylactic BAX 855 was estimated using the median dose per infusion (44.6 IU/kg) multiplied by the median of prophylaxis infusions per week (1.96). Median weekly rFVIII utilization of rAHF-PFM-2004 was estimated using the median annualized consumption (5,733.3 IU/kg/year) multiplied by 7/365.25. Median weekly rFVIII utilization of rAHF-PFM-2012 was estimated using the median dose per infusion (30.7 IU/kg) multiplied by the protocol-specified number of prophylaxis infusions per week (ie, every other day). *P<0.05.
Abbreviations: rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; rFVIIIFc, recombinant human factor VIII–Fc fusion protein.
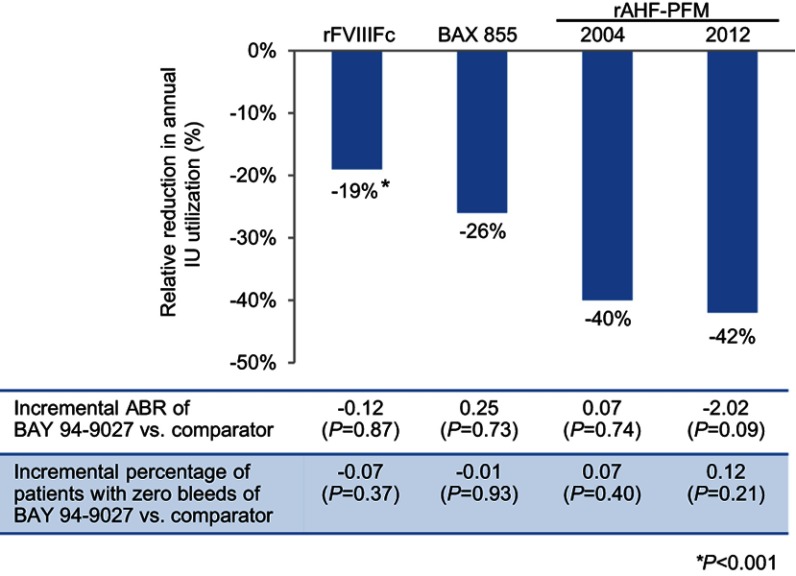
Median weekly rFVIII utilization for BAY 94-9027 was also lower than for BAX 855 (64.3 vs 87.4 IU/kg/week) and rAHF-PFM (64.0 vs 107.5 [rAHF-PFM-2004] and 63.6 vs 109.9 [rAHF-PFM-2012]) (Figure 2B, C and D). However, the P-values were incalculable owing to the unavailability of standard errors for the median utilizations of BAX 855 and rAHF-PFM. Figure 3 shows the relative reductions in annual utilization of BAY 94-9027 compared with each comparator in the context of ABR and zero bleed comparisons.
Figure 3.
Relative reduction in annual IU utilization and incremental ABR and percentage of patients with zero bleeds, BAY 94-9027 vs rFVIIIFc, BAX 855, rAHF-PFM-2004, or rAHF-PFM-2012. *P<0.05.
Abbreviations: ABR, annualized bleeding rate; rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; rFVIIIFc, recombinant human factor VIII–Fc fusion protein.
Discussion
Advances in healthcare for hemophilia have allowed the personalization of treatment based on individual patient priorities, physical attributes, and medical needs. New and emerging therapies have contributed to a wealth of options for healthcare providers and patients alike. The Medical and Scientific Advisory Council of the National Hemophilia Foundation recommends rFVIII as treatment for patients with severe hemophilia A, but does not recommend one product over another.23 However, the hemophilia community faces the challenge of often having insufficient information about differentiating features that are needed to drive treatment decision-making.
For rare diseases such as hemophilia, the small population of afflicted individuals makes it difficult to design RCTs powered enough to conclusively compare different treatments in direct head-to-head trials. The absence of such trials can often result in a knowledge gap in understanding relative treatment outcomes of ABR and utilization in comparison to other therapies. Intertrial comparisons have, in the past, been considered inferior to RCTs because of inherent differences in patient characteristics, study designs, and methodologies between studies, thus limiting the interpretability of such comparisons. While RCTs continue to be the gold standard in clinical trial design, MAIC represents a validated approach that allows balanced comparisons between studies when RCTs are not feasible.
BAY 94-9027 is an EHL rFVIII agent that has demonstrated effective bleed prevention when dosed twice-weekly, every 5 days, and every 7 days, with no inhibitor development.8 Although findings from PROTECT VIII have shown similar outcomes, compared to published data from other EHL and standard-acting FVIII agents, interpretation of these findings is limited to comparisons against other studies. We show that, using MAIC, outcome measures of ABR and percentage of patients with zero bleeds of BAY 94-9027 are similar to those for other available rFVIII products, but with lower utilization.
The current findings have potential implications for real-world practice. This analysis provided valuable evidence on ABR outcomes and rFVIII utilization that should be considered when choosing therapies. The observation of lower BAY 94-9027 utilization compared to other agents also highlights the differences between the newer EHL agents beyond half-life alone. The currently favored endpoint of ABR serves as a clinically meaningful and objective target, but as new therapies emerge, treatment decisions driven by differentiation between similar agents become more challenging when only one outcome measure is considered. Attributes other than ABR can be particularly meaningful to healthcare providers and patients who seek individualized treatment based on personal treatment goals; the value of a healthcare intervention increases when measures beyond clinical outcomes are considered, such as quality of life, school and work participation, and leisure activities.24
From a payer standpoint, alternative payment models that incentivize optimal treatment while maintaining cost control have been created for EHL products to enable their entry into the market,4 and the availability of an rFVIII agent that requires less utilization to reach the same protection levels opens the door to allowing patients to dictate their preference for increased health benefit or less burdensome treatment regimens, or a combination of the two.4
This analysis is subject to the following limitations. First, while MAIC methodology accounted for the imbalance in observed baseline characteristics, undocumented differences in baseline characteristics, either in publications or in online data repositories, could not be eliminated. With implementation of the FDA Amendments Act of 2007,25 the regulatory requirement for clinical trials registration and open access to results has enabled researchers to make more comprehensive evaluations of clinical trial data, a welcome change that facilitates reasonable comparative analyses between different studies. However, such comparisons are limited when data collection is not uniform or when incomplete data sets are made accessible. Future development of clinical trial guidelines for hemophilia drugs, including recommended baseline characteristic and outcomes data collection, and greater access to data would thus be beneficial to the healthcare community. Second, owing to ethical concerns, hemophilia studies have generally not included a common reference arm (eg, randomized placebo arm) that can be used to assess or adjust for residual confounding. Third, some differences between trials could not be eliminated by matching due to a lack of overlap in the patient population, eg, the small percentage of moderately severe hemophilia patients and requirement for prior on-demand treatment in the rAHF-PFM trials. This point is also illustrated by the differences seen between the two rAHF-PFM studies, which reflect different baseline characteristics of two different patient populations. Lastly, exposure duration to prophylaxis varied between studies, but, with the exception of rAHF-PFM-2004, exposure was shorter for BAY 94-9027 than for the comparators, and is therefore unlikely to skew findings in its favor.
Conclusion
MAIC is a suitable method for comparing ABR outcomes and weekly rFVIII utilization between BAY 94-9027 and other prophylactic rFVIII agents for hemophilia A. Prophylaxis with BAY 94-9027 showed lower weekly rFVIII utilization compared to rFVIIIFc (−19.1%), BAX 855 (−26.4%), and rAHF-PFM (−40.5% with 2004 trial; −42.1% with 2012 trial), with similar ABR outcomes.
Acknowledgments
This study was funded by Bayer. Ingrid Koo, PhD, provided writing support during manuscript development.
Abbreviation list
ABR, annualized bleeding rate; EHL, extended half-life; FDA, US Food and Drug Administration; FVIII, factor VIII; MAIC, matching-adjusted indirect comparison; PK, pharmacokinetic; PWH, person with hemophilia; rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; RCT, randomized controlled trial; rFVIII, recombinant factor VIII; rFVIIIFc, recombinant factor VIII–Fc fusion protein; SLR, systematic literature review.
Supplementary materials
Author contributions
Rajeev Ayyagari, Wei Gao, Zhiwen Yao, and Yao Wang performed the research and wrote the paper. Céline Deschaseaux and Parth B Vashi designed the research study. Katharine Batt and Robert Klamroth interpreted the data and provided clinical context and conclusions from the study. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
KB acted as a paid consultant to Bayer Pharmaceuticals in the interpretation of data for this manuscript and is also a scientific advisor to the health consulting firm, Precision Health Economics. WG, RA, ZY, and YW are employees of Analysis Group, Inc., which received funding from Bayer to conduct this analysis. CD, PBV, and SK are employees of Bayer. RK received research funding from Bayer, CSL Behring, Sobi, Novo Nordisk, Shire, and Pfizer, and has acted as a paid consultant for Bayer, Shire, Pfizer, Octapharma, CSL Behring, SOBI, and Novo Nordisk. The authors report no other conflicts of interest in this work.
References
- 1.Mejia-Carvajal C, Czapek EE, Valentino LA. Life expectancy in hemophilia outcome. J Thromb Haemost. 2006;4(3):507–509. doi: 10.1111/j.1538-7836.2006.01776.x [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg J, Dolan G, Lemm G. Haemophilia care then, now and in the future. Haemophilia. 2009;15(Suppl 1):2–7. doi: 10.1111/j.1365-2516.2008.01946.x [DOI] [PubMed] [Google Scholar]
- 3.Srinivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1––47.. [DOI] [PubMed] [Google Scholar]
- 4.Persson U, Steen Carlsson K, Jönsson B. Alternative payment models in haemophilia treatment. IHE Report. 2016;10 Lund: IHE. [Google Scholar]
- 5.Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085. doi: 10.1182/blood-2015-03-630897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII FC fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325. doi: 10.1182/blood-2013-10-529974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyle TE, Reding MT, Lin JC, Michaels LA, Shah A, Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12(4):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reding MT, Ng HJ, Poulsen LH, et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost. 2017;15(3):411–419. doi: 10.1111/jth.13597 [DOI] [PubMed] [Google Scholar]
- 9.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Veroniki AA, Straus SE, Soobiah C, Elliott MJ, Tricco AC. A scoping review of indirect comparison methods and applications using individual patient data. BMC Med Res Methodol. 2016;16:47. doi: 10.1186/s12874-016-0146-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching-based approaches for indirect comparison of treatments. Pharmacoeconomics. 2015;33(6):537–549. doi: 10.1007/s40273-015-0271-1 [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo G, Casciano R, Malangone E, et al. An adjusted indirect comparison of everolimus and sorafenib therapy in sunitinib-refractory metastatic renal cell carcinoma patients using repeated matched samples. Expert Opin Pharmacother. 2011;12(10):1491–1497. doi: 10.1517/14656566.2011.587119 [DOI] [PubMed] [Google Scholar]
- 13.Signorovitch JE, Wu EQ, Swallow E, Kantor E, Fan L, Gruenberger JB. Comparative efficacy of vildagliptin and sitagliptin in Japanese patients with type 2 diabetes mellitus: a matching-adjusted indirect comparison of randomized trials. Clin Drug Investig. 2011;31(9):665–674. doi: 10.2165/11592490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Strand V, Betts KA, Mittal M, Song J, Skup M, Joshi A. Comparative effectiveness of adalimumab versus secukinumab for the treatment of psoriatic arthritis: a matching-adjusted indirect comparison. Rheumatol Ther. 2017;4(2):349–362. doi: 10.1007/s40744-017-0070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):130–137. doi: 10.1002/pds.3246 [DOI] [PubMed] [Google Scholar]
- 16.Pocoski J, Li N, Ayyagari R, et al. Matching-adjusted indirect comparisons of efficacy of BAY 81-8973 vs two recombinant factor VIII for the prophylactic treatment of severe hemophilia A. J Blood Med. 2016;7:129–137. doi: 10.2147/JBM.S104074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decision Support Unit- National Institute for Healthcare Excellence (NICE). Methods for population-adjusted indirect comparisons in submissions to NICE; 2017. Available from: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/05/Population-adjustment-TSD-FINAL.pdf. Accessed April24, 2018.
- 18.Thom H, Jugl S, Palaka E, Jawla S Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals. Paper presented at: ISPOR 21st Annual International Meeting May 2016; 2016; Washington, DC. doi: 10.1016/j.jval.2016.03.1723 [DOI] [Google Scholar]
- 19.Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945. doi: 10.2165/11538370-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 20.United States Food and Drug Administration (FDA). Highlights of prescribing information: ELOCTATE (antihemophilic factor [recombinant], FC fusion protein); 2014. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM400192.pdf. Accessed June15, 2018.
- 21.Tarantino MD, Collins PW, Hay CR, et al. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia. 2004;10(5):428–437. doi: 10.1111/j.1365-2516.2004.00932.x [DOI] [PubMed] [Google Scholar]
- 22.Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359–367. doi: 10.1111/j.1538-7836.2011.04611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Hemophilia Foundation. National hemophilia foundation: MASAC recommendations; 2017. Available from: https://www.hemophilia.org/Researchers-Healthcare-Providers/Medical-and-Scientific-Advisory-Council-MASAC/MASAC-Recommendations. Accessed June6, 2018.
- 24.O’Mahony B, Dolan G, Nugent D, Goodman C; International Haemophilia Access Strategy C. Patient-centred value framework for haemophilia. Haemophilia. 2018;24:873–879. doi: 10.1111/hae.13456 [DOI] [PubMed] [Google Scholar]
- 25.US National Library of Medicine. FDAAA 801 and the final rule; 2018. Available from: https://clinicaltrials.gov/ct2/manage-recs/fdaaa. Accessed January23, 2019.