Abstract
Red blood cell (RBC) transfusion is common in criticallyill, postsurgical, and posttrauma patients in whom both systemic inflammation and immune suppression are associated with adverse outcomes. RBC products contain a multitude of immunomodulatory mediators that interact with and alter immune cell function. These interactions can lead to both proinflammatory and immunosuppressive effects. Defining clinical outcomes related to immunomodulatory effects of RBCs in transfused patients remains a challenge, likely due to complex interactions between individual blood product characteristics and patient-specific risk factors. Unpacking these complexities requires an in-depth understanding of the mechanisms of immunomodulatory effects of RBC products. In this review, we outline and classify potential mediators of RBC transfusion-related immunomodulation and provide suggestions for future research directions.
In the United States, 11 to 16 million red blood cell (RBC) units were administered annually during the past decade, equating to a RBC transfusion every 2 seconds.1–5 RBC transfusion is particularly commonplace in emergency departments, intensive care units, and operating suites, with 37% to 60% of intensive care unit patients receiving a transfusion during hospitalization.6–12 Nonetheless, RBC transfusion may have deleterious immunologic effects, particularly for critically ill patients.13,14 Mounting evidence from predominantly observational studies demonstrate independent associations between RBC transfusion, dysregulated immunity, and increased mortality and morbidity, mechanisms of which are only partly understood.15–26 The following review will summarize current literature on mechanisms of RBC transfusion-related immunomodulation (TRIM), classify potential mediators, and propose a research agenda to fill critical knowledge.
RBC TRIM
Beginning in 1973, Opelz and colleagues27 provided initial evidence for RBC TRIM with the observation that the survival rate of transplanted kidneys was significantly higher in cadaveric renal transplant patients who received RBC transfusion.13 These findings strongly suggested immunosuppressive effects of nonleukoreduced allogeneic RBC transfusion. More recent findings suggest both proinflammatory and immunosuppressive effects of RBC product exposure, including prestorage leukoreduced blood products. Clinically, RBC transfusion is associated with new or worsening organ dysfunction, the development of nosocomial infection, and cancer recurrence, suggesting dysregulated recipient immune responses.13,14,21,28–32 The extent to which RBC transfusion directly contributes to immuno-logic dysregulation in transfused patients remains unclear, although a wealth of preclinical evidence demonstrates that RBC products can directly modulate immune cell function. In a variety of preclinical models, RBC product exposure results in inflammatory effects including white blood cell (WBC) priming, enhanced neutrophil chemo-taxis, monocyte/macrophage activation, and inflammatory cytokine release.13,17,21,31,33–35 Immunosuppressive effects include impaired natural killer (NK) cell function, alterations in T lymphocyte ratios, defective antigen presentation, suppression of lymphocyte proliferation, and decreased macrophage phagocytic function.14,36–40 While evidence supporting both proinflammatory and immunosuppressive effects of RBC transfusion may seem contradictory, given the complex nature of transfused blood products and the multitude of potentially immunomodulatory mediators contained therein, mixed effects are not surprising. Indeed, mixed immunomodulatory potential of RBC transfusion may be particularly relevant for critically ill patients in whom both excess inflammation and immune suppression are significantly associated with adverse outcomes.14 Overall, defining the sum total immunomodulatory effects of particular RBC products in individual patients remains challenging. Future research to determine the effects of individual blood products on individual patients and to mitigate potential risks depends on understanding mechanisms of RBC TRIM.
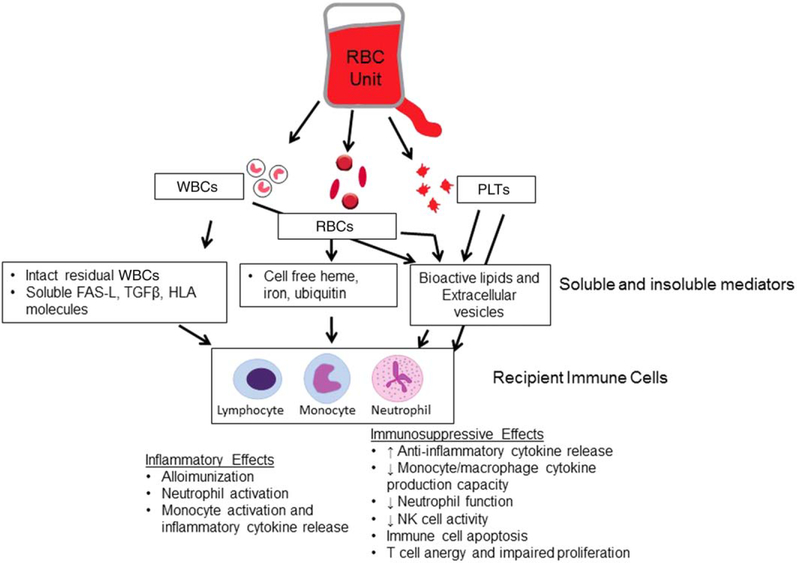
While mechanisms for RBC TRIM are not yet fully characterized, many potential mediators have been identified. These include WBC-derived mediators, component hemolytic contents (heme, iron release), platelet (PLT)-derived factors, and extracellular vesicles (EVs; Fig. 1).
Fig. 1.
Proposed mechanisms of RBC TRIM. RBC units contain multiple immunomodulatory mediators, including WBC-derived, RBC-derived, PLT-derived, and lipid and microvesicle–derived factors. Effects of these mediators on immune cell function vary and include both inflammatory and immunosuppressive changes. As such, the sum total immunomodulatory effects of RBC transfusion on recipient immune function will likely vary based on individual unit and recipient characteristics
PROPOSED MECHANISMS
WBCs and WBC-derived mediators
The observation that prestorage leukoreduction may mitigate TRIM suggests that either intact WBCs and/or soluble WBC-derived mediators play a role in its development.41–44 Leukoreduction removes most residual WBCs from stored blood components and appears to improve clinical outcomes. Randomized trials in surgical patients receiving leukoreduced versus nonleukoreduced RBCs, autologous versus allogeneic RBC transfusions, or restrictive versus liberal RBC transfusion thresholds demonstrate that in each case, subjects in the leukoreduced, autologous, or restricted transfusion arms developed fewer nosocomial infections.15,45–47 Likewise, meta-analyses demonstrate that leukoreduction, autologous RBC transfusions (which prevent exposure to allogeneic WBCs), and restrictive transfusion thresholds (which decrease exposure to residual allogeneic WBCs) are each associated with decreased risk of postoperative infection.15,45,47 RBC unit leukoreduction may also attenuate the systemic inflammatory response after cardiac surgery, with a dose-dependent increase in survival when leukoreduced RBCs are utilized.48 Finally, animal models demonstrate that leukoreduction may reduce transfusion-associated cancer metastasis and T-cell apoptosis.29,49 Taken together, these data suggest that residual WBCs or WBC-derived mediators in RBC products may be harmful via immunomodulatory mechanisms. Although in the United States, 75% to 80% of RBC units transfused are leukoreduced before storage to mitigate these risks, it is worth noting that a substantial number of residual WBCs (approx. 5000 to approx. 5 × 106 WBCs/unit) remain despite current leukoreduction technologies.50–52
Residual WBCs
Antigen-presenting cells (APCs; i.e., monocytes and dendritic cells) carry major histocompatibility complex (MHC) II molecules (i.e., HLA-DR) on their cell surfaces. MHC II molecules function to present processed antigens and activate lymphocytes. After transfusion, interactions between donor MHC II molecules on residual WBCs and recipient lymphocytes may result in either alloimmunization or immune suppression.53–56 Features such as the degree of HLA compatibility, the functionality of donor APCs, and the inflammatory state of the recipient likely determine whether residual allogeneic WBCs induce immune tolerance or alloimmunization.21 In the case of immune suppression, residual allogeneic APCs, which engage recipient T cells without necessary secondary or costimulatory signals, would be expected to produce antigen-specific T-cell anergy.21 The resulting immune tolerance is a proposed mechanism for allogeneic RBC transfusion-related adaptive immune cell (T-cell) suppression.21 T-cell immune tolerance may also be responsible for development of microchimerism in allogeneic blood transfusion recipients, whereby donor WBCs fail to elicit an immune response and become “accepted” by the recipient.57 Microchimerism may be common in trauma patients and may persist for up to 2 years after transfusion.57,58 Moreover, immune tolerance and associated microchimerism may explain the observed shift to immunosuppressive TH2 responses after blood transfusion.38,59–62 However, clear demonstration of direct causal links between HLA molecules on residual allogeneic APCs and posttransfusion immune suppression is currently lacking.
In addition to residual functional allogeneic WBCs, it is possible that apoptotic WBCs in RBC products may also induce immune suppression.63 During collection and storage, WBCs undergo apoptosis.64 One of the early steps in apoptosis involves exposure of phosphatidylserine on the outer leaflet of the cell membrane. Interaction between immune cells and phosphatidylserine has been shown to induce immunosuppressive signals, including release of anti-inflammatory cytokines interleukin (IL)-10 and transforming growth factor (TGF)-β, inhibition of proinflammatory cytokine release, inhibition of APC activation, and predominance of immunosuppressive regulatory T cells.63,65 The degree to which apoptotic residual WBCs in RBC units contribute to recipient immune suppression in the clinical setting remains unknown. However, it is worth noting that similar responses may also be seen in response to phosphatidylserine-containing membrane fragments or microparticles.
Soluble WBC-derived mediators
Removal of supernatant from stored RBC units by washing reduces the inflammatory response in pediatric cardiac surgery patients and preclinical studies suggest that RBC-induced immunomodulation can be recapitulated using RBC unit supernatants.24,25,66,67 Thus, it seems likely that soluble mediators also play a role in TRIM pathogenesis.
There are multiple soluble WBC-derived factors, including cytokines, WBC degranulation products, soluble FAS-L, and soluble HLA molecules, which directly inhibit the immune response.68,69 Of these, sFAS-L and the anti-inflammatory cytokine TGF-β have the strongest evidence suggesting that they may promote TRIM, particularly in nonleukoreduced blood products.36,68 In vitro studies indicate that sFAS-L and TGF-β found in blood components may directly induce innate immune cell apoptosis, impair neutrophil chemotaxis, and decrease NK cell activity.36,69,70 Immunosuppressive effects may not be limited to these, as TGF-β is a known anti-inflammatory cytokine with broad immunosuppressive effects.
In addition to anti-inflammatory cytokines, proinflammatory cytokines may also accumulate in blood products during storage.71–74 However, in some reports prestorage leukoreduction appears to substantially decrease the accumulation of proinflammatory cytokines in RBC products such that levels are undetectable.72,74 When cytokines are detected, it is unclear whether their concentrations are high enough to strongly influence recipient immune function.73,74 In addition to cytokines, WBC degranulation products such as histamine and eosinophil cationic protein have been detected in RBC components.75 Each of these mediators has immunomodulatory potential. For example, histamine has been shown to inhibit neutrophil chemotaxis and decrease T-cell proliferation, while eosinophilic cationic protein may also reduce T-cell proliferation.76,77
While WBCs and WBC-derived soluble mediators appear to promote TRIM, such effects are likely reduced by prestorage leukoreduction. Because evidence for TRIM remains in the postleukoreduction era, it is likely that non–WBC-derived factors are also involved.14
RBC storage lesion and decompartmentalized RBC contents
Another potential mechanism for TRIM arises from the RBC itself. As RBC units age under refrigerated conditions, a well-described “storage lesion(s)” develops. The RBC storage lesions are characterized by altered RBC morphology, rheologic changes, metabolic derangements, changes in oxygen affinity, changes in osmotic regulation, and changes in the ability to vasoregulate.78–85 In addition, RBC hemolysis (both during storage and after transfusion) can lead to reduced pH, increased lactate and other metabolic wastes, and release of microparticles as well as accumulation of cell-free hemoglobin (Hb), heme, and iron.26,78,86–90 Iron content can be in the form of transferrin-bound iron, non–transferrin-bound iron (NTBI), or labile plasma iron. Given the well-described bioactivities of these species, RBC hemolysis can disturb plasma redox balance and broadly disrupt normal signaling in coagulation, vascular, and immune systems.4,22,23,78,86,91,92
In normal physiology, plasma haptoglobin sequesters cell-free Hb, forming a complex for removal by macrophages via CD163.18,22,23,93 However, in critical illness, even moderate intravascular hemolysis may overwhelm plasma-binding capacity resulting in unbound extracellular Hb. When extracellular Hb is unbound, it becomes oxidized to methemoglobin, releasing free heme. Free heme can then undergo the Fenton reaction to cause further release of iron.67,93–97 Accumulation of uncomplexed heme and iron in plasma is associated with significant tissue damage, presumably by iron-catalyzed generation of reactive oxygen species, promotion of other radical chains, increases in WBC activation and migration, up regulation of adhesion molecules, and subsequent deleterious effects to tissue barriers and to immunity.22,93,98–104 In murine models, transfusion of long-stored RBCs led to increased iron in the form of NTBI and augmented circulating proinflammatory cytokine release.22,23,105,106 How ever, in human healthy volunteers, while transfusion with older versus fresher RBCs significantly increased circulating NTBI levels, a proinflammatory cytokine response was not observed.91,105,107 The lack of observed inflammatory response in the human studies may relate to differences between mice and humans or relative transfusion dose, or the inflammatory response to RBC transfusion may not be apparent in healthy subjects (without underlying inflammation). That said, in a study of 33 premature neonates, while levels of NTBI were increased after transfusion, NTBI levels were not associated with increases in plasma inflammatory cytokines.108 These data suggest that proinflammatory effects of NTBI may be minimal.
Red blood cell transfusion may also burden the mononuclear phagocyte system, delivering large amounts of Hb and RBC contents to monocytes and macrophages.93 Phagocytosis of RBCs by macrophages (i.e., extravascular hemolysis) increases macrophage intracellular heme and iron to a degree that can trigger inflamma-some activation and proinflammatory cytokine release via NLRP3 and NF-κB signaling; this process is further exacerbated by generation of iron-related reactive oxygen species.93 Conversely, macrophage exposure to high concentrations of heme may also bias macrophage phenotype from the activated/inflammatory (M1) phenotype toward an immunosuppressive (M2) profile via up regulation of heme oxygenase 1 and release of the anti-inflammatory cytokine IL-10.109 Similarly, macrophage iron loading may promote immune suppression by inhibiting interferon (IFN)-γ–mediated secretion of proinflammatory cytokines, reducing expression of MHC II and impairing nitric oxide synthesis. Cumulatively, these effects compromise phagocytic and microbicidal macrophage activity.110 Iron overload may also further promote immune suppression by impairing proliferation and activation of T, B, and NK cells.111 Additionally, independent of direct effects on immune cells, uncomplexed heme and iron may directly promote bacterial growth.78,93,105
Finally, an additional compound of interest is ubiquitin, an intracellular regulatory protein present in a variety of cell types. RBCs carry large amounts of ubiquitin relative to other cell types, and extracellular ubiquitin has been found to accumulate in RBC unit supernatants during storage.112 Extracellular ubiquitin has varied effects on immune cell function, including blunting lipopolysaccha-ride (LPS)-induced tumor necrosis factor-α production while augmenting LPS-induced IL-8 production.112–114 Additionally, extracellular ubiquitin found in RBC units may skew helper T-cell function toward an immunosuppressive Th2 phenotype, as evidenced by increased IL-4 production and decreased IFN-γ production by LPS-stimulated PBMCs exposed to 35-day-old stored RBC supernatant or ubiquitin.112,114 The mix of proinflamma-tory and immunosuppressive effects of extracellular ubiquitin mirrors immunomodulatory effects observed in response to RBC supernatants in vitro and may explain mixed responses reported in vivo.
In summary, soluble mediators resulting from RBC aging and breakdown are varied, and individual mediators likely have pleiotropic effects on recipient immune response. Although animal studies show worsened survival and increased inflammation from transfusion with longer stored RBCs, these findings have not been demonstrated in recently published human randomized controlled trials.4,16,78,87,115 This may be because animal studies can carefully delineate “fresh versus old” RBC cutoffs (i.e., >21 days) which has proven difficult in human randomized controlled trials, where a mean duration of RBC storage in the United States of 17.9 days results in comparisons between “fresh” versus “middle-age.”87,116 Additionally, storage duration effects may be more robust if transfusion occurs in the setting of more significant baseline inflammation, although to date this question has not been adequately evaluated. The relative impact of inflammatory and immunosuppressive effects of RBC-derived mediators for individual patients, particularly in the setting of baseline inflammation or immune suppression, remains largely unknown. It is likely that a complex interplay between decompartmentalized RBC contents and underlying host immune response contributes to patient-specific immune modulation, a topic of active ongoing research.
Residual PLTs and PLT-derived factors
While less is known about PLT-derived factors as TRIM mediators, emerging data strongly suggest that PLTs and PLT-derived factors have important immunomodulatory potential.117–119 For instance, PLT-derived microparticles are capable of inducing both immune cell suppression and activation.120,121 PLTs themselves may play important roles in modulating immune cell response in both health and disease, suggesting that residual PLTs found in RBC products likely contribute to immunomodulation. Nonleukoreduced RBC units have been shown to accumulate PLT-WBC aggregates over time, which correlate with immune cell apoptosis and monocyte tissue factor expression.122 These changes are expected to be immunomodulatory; however, effects of PLT-WBC aggregates on recipient immune cells were not evaluated. Likewise, the immunomodulatory potential of residual PLTs within leukoreduced RBC products is unknown.
Bioactive lipids and EVs
Bioactive lipids
Bioactive lipids with proinflammatory and procoagulant activity accumulate during storage in RBC units and may contribute to inflammatory complications of RBC transfusion, including transfusion-related acute lung injury (TRALI).83,123 Accumulation of some bioactive lipids, such as lysophosphatidylcholines, appears to be reduced by leukoreduction.124 However, a variety of polyunsaturated fatty acids, including arachidonic acid, linoleic acid, docosahexaenoic acid, and their metabolites, accumulate in RBC units despite leukoreduction.123,125 Arachidonic acid and its oxidized metabolites, when isolated from older stored RBC supernatants, are capable of priming neutrophils in vitro. Further, infusion of these bioactive lipids in rats that are primed by LPS induces acute lung injury—providing evidence that bioactive lipids may provide the second-hit in the two-hit model of non–antibody-mediated TRALI.125,126 Observational studies demonstrating the presence of lipids with neutrophil priming activity in the plasma of TRALI patients provide additional supportive evidence of the link between bioactive lipids and non–antibody-mediated TRALI.127 The extent to which bioactive lipids may contribute to systemic inflammation or modulation of immune function outside of TRALI remains unclear and is a topic deserving of further study.
EVs
EV count and profile in blood products.
The term “extracellular vesicle” broadly encompasses larger micro-vesicles (200–1200 nm), exosomes (30–150 nm), and apoptotic bodies (50–500 nm).128–130 For more than a decade, it has been appreciated that plasma from healthy subjects contains EVs, including exosomes, derived from WBCs, PLTs, RBCs, and endothelial cells.131–133
EV counts in RBC products increase with storage duration.86,134 Storage-related morphologic changes to RBCs are accompanied by shedding and release of RBC-derived EVs, while residual PLTs and WBCs contribute to PLT-derived and WBC-derived EVs.135–138 Tracking EV cell of origin reveals that RBC-derived EVs increase continuously during storage, while PLT-derived EV counts peak at 3 to 4 weeks of storage.86,139 EV release and accumulation are significantly influenced by component manufacture processes and storage conditions such that individual products may have very different EV profiles despite similar storage duration.140,141
In vitro evidence for EV TRIM effects.
Although once considered debris without bioactivity and discounted as artifact, EVs are increasingly recognized as playing a central role in the body’s complex network of intercellular signaling, both in normal physiology and in disease.142 EVs derived from stored PLTs bind to and activate neutrophils in vitro and have anti-inflammatory or proinflammatory effects on monocytes and macrophages.135,143,144 Neutrophil- and RBC-derived EVs are also capable of suppressing inflammatory responses.130,145 Similar to the variability in effects of EVs from various cell types, EVs isolated from plasma have dual proinflamma-tory and immunosuppressive effects.139,146 The proposed mechanism of action of blood-derived EVs varies, with immunosuppressive effects potentially mediated by FasL expression by EVs and inflammatory effects resulting from direct activation of monocytes and other APCs after EV uptake by these cells.139,146
In vivo evidence for EV TRIM effects.
Given the incomplete understanding of how EVs from different cells of origin might act, it is not surprising that in vivo evidence of an EV-based role in TRIM is scant. The circulating half-life of EVs appears to be fairly short, less than 15 to 20 minutes in a rat model.86 However, the biologic activity of EVs is likely related to EV uptake by target cells rather than plasma concentration. For example, injected EVs are rapidly and widely distributed to the spleen, liver, kidneys, and lungs in mice.147 Donor dendritic cell–derived EV uptake by dendritic cells in a recipient mouse can activate responding T cells in an antigen-specific manner.148 This property has been exploited by several groups as a potential vaccine delivery approach.149–151 Additionally, adoptive transfer of CD154 (CD40L)-expressing PLT-derived EVs is sufficient to stimulate immunoglobulin G production and germinal center formation in mice after adenovirus vaccination, indicating that exogenous EVs can modulate a nascent immune response.152 The significance of the immunomodulatory effects of EVs found in blood products transfusion recipients remains an open question and an area of active research. Better understanding EV interaction with the human immune system would allow manipulation of this pathway, both in the context of TRIM and in the context of immune perturbation seen in many hospitalized patients.
FUTURE DIRECTIONS
Ample evidence exists that RBC products are capable of interacting with and modulating immune cell function through a variety of mechanisms and mediators; however, conclusive clinical evidence of TRIM effects in transfused patients remains elusive. Given recent clinical studies that fail to demonstrate benefit to fresh RBC transfusion compared to longer stored products, one might conclude that RBC TRIM does not exist in the era of prestorage leukoreduced blood products or that RBC storage duration does not contribute to TRIM mechanisms.87,115,153,154 However, emerging evidence suggests that the concentrations of potentially immunomodulatory mediators vary not only with storage duration, but also with donor characteristics, manufacturer, storage solution, and other processing factors.88,155–158 We are only beginning to understand the complex interplay between storage duration, processing methods, RBC unit contents, and subsequent potential TRIM effects. Similarly, a patient’s underlying state of inflammation and/or immune suppression at the time of transfusion likely influences the immunologic response to transfusion. Critically ill patients, in particular, exhibit both exaggerated systemic inflammation and immune suppression that fluctuate over time.159–164 In this context, one would expect that immunologic effects of RBC trans-fusion might vary widely based on the underlying state of the recipient’s immunologic response. However, most studies to date have failed to sufficiently characterize or account for individual differences in pretransfusion immune function. Additionally, patients who are trans-fused with RBCs often also receive other blood products, which may have different or additive TRIM effects.14,165 Overall, much work remains to understand interactions between individual blood product characteristics and patient-specific risk factors with respect to clinical consequences of TRIM.
Defining immunomodulatory mediators found within blood products, and understanding how these mediators may modulate recipient immunity, is essential to identify potential TRIM effects at the bedside. A bench-to-bedside approach must carefully attempt to define these mediators in context of host immune function. Next, guided by an enhanced understanding of TRIM biology, observational studies will be necessary to determine patient-specific risk factors for specific TRIM effects and related clinical consequences. Moreover, delineation of the effects of RBC donor, product processing, and storage conditions upon accumulation of immunomodulatory mediators can then inform future prospective and interventional trials aimed at defining and ameliorating TRIM effects for those patients most at risk.
ACKNOWLEDGMENT
The authors thank Lisa Feurer for her assistance with figure preparation.
ABBREVIATIONS:
- APC(s)
antigen-presenting cell(s)
- EV(s)
extracellular vesicle(s)
- LPS
lipopolysaccharide
- NTBI
non–transferrin-bound iron
- TRIM
transfusion-related immunomodulation
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Lacroix J, Heberté PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609–19. [DOI] [PubMed] [Google Scholar]
- 2.Wald ML. Blood industry shrinks as transfusions decline. The New York Times; 2014: A1. [Google Scholar]
- 3.Hébert PC. Transfusion requirements in critical care (TRICC): a multicentre, randomized, controlled clinical study. Transfusion Requirements in Critical Care Investigators and the Canadian Critical Care Trials Group. Br J Anaesth 1998;81Suppl1:25–33. [PubMed] [Google Scholar]
- 4.Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol 2014;165:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker BI, Hinkins S. The 2011 National Blood Collection and Utilization Survey Report. Washington (DC): US Department of Health and Human Services; 2011. [Google Scholar]
- 6.Armano R, Gauvin F, Ducruet T, et al. Determinants of red blood cell transfusions in a pediatric critical care unit: a prospective, descriptive epidemiological study. Crit Care Med 2005;33:2637–44. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med 2008;178:26–33. [DOI] [PubMed] [Google Scholar]
- 8.Demaret P, Tucci M, Ducruet T, et al. Red blood cell transfusion in critically ill children (CME). Transfusion 2014;54: 365–75. [DOI] [PubMed] [Google Scholar]
- 9.Lacroix J, Tucci M, Du Pont-Thibodeau G. Red blood cell transfusion decision making in critically ill children. Curr Opin Pediatr 2015;27:286–91. [DOI] [PubMed] [Google Scholar]
- 10.Corwin HL. Anemia and red blood cell transfusion in the critically ill. Semin Dial 2006;19:513–6. [DOI] [PubMed] [Google Scholar]
- 11.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med 2004; 32:39–52. [DOI] [PubMed] [Google Scholar]
- 12.Hébert PC, Tinmouth A, Corwin H. Anemia and red cell transfusion in critically ill patients. Crit Care Med 2003;31: S672–7. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood 2001;97:1180–95. [DOI] [PubMed] [Google Scholar]
- 14.Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017; 57:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 2012;52:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilgin YM, Brand A. Transfusion-related immunomodulation: a second hit in an inflammatory cascade? Vox Sang 2008;95:261–71. [DOI] [PubMed] [Google Scholar]
- 18.Ozment CP, Mamo LB, Campbell ML, et al. Transfusion-related biologic effects and free hemoglobin, heme, and iron. Transfusion 2013;53:732–40. [DOI] [PubMed] [Google Scholar]
- 19.Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus 2010;8Suppl3:s26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal MD, Raval JS, Triulzi DJ, et al. Innate immune activation after transfusion of stored red blood cells. Transfus Med Rev 2013;27:113–8. [DOI] [PubMed] [Google Scholar]
- 21.Vamvakas EC, Blajchman MA. Transfusion-related immuno-modulation (TRIM): an update. Blood Rev 2007;21:327–48. [DOI] [PubMed] [Google Scholar]
- 22.Hod EA, Spitalnik SL. Stored red blood cell transfusions: iron, inflammation, immunity, and infection. Transfus Clin Biol 2012;19:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010;115: 4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Piknova B, Solomon SB, et al. In vivo reduction of cell-free methemoglobin to oxyhemoglobin results in vasoconstriction in canines. Transfusion 2013;53:3149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes-Puché I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 2014; 123:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Cortes-Puché I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion 2014; 54:1712–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med 1978;299:799–803. [DOI] [PubMed] [Google Scholar]
- 28.Blajchman MA. Immunomodulatory effects of allogeneic blood transfusions: clinical manifestations and mechanisms. Vox Sang 1998;74Suppl2: 315–9. [DOI] [PubMed] [Google Scholar]
- 29.Blajchman MA, Bardossy L, Carmen R, et al. Allogeneic blood transfusion-induced enhancement of tumor growth: two animal models showing amelioration by leukodepletion and passive transfer using spleen cells. Blood 1993;81: 1880–2. [PubMed] [Google Scholar]
- 30.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol 1994; 1:457–61. [PubMed] [Google Scholar]
- 31.Blajchman MA, Dzik S, Vamvakas EC, et al. Clinical and molecular basis of transfusion-induced immunomodulation: summary of the proceedings of a state-of-the-art conference. Transfus Med Rev 2001;15:108–35. [DOI] [PubMed] [Google Scholar]
- 32.Dzik S, Blajchman MA, Blumberg N, et al. Current research on the immunomodulatory effect of allogeneic blood trans-fusion. Vox Sang 1996;70:187–94. [DOI] [PubMed] [Google Scholar]
- 33.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apher Sci 2008;38:117–25. [DOI] [PubMed] [Google Scholar]
- 34.Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 2012;73(2Suppl1):S128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrickson JE, Hod EA, Hudson KE, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion 2011;51:2695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghio M, Contini P, Negrini S, et al. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-b(1), soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion 2011;51:1567–73. [DOI] [PubMed] [Google Scholar]
- 37.Long K, Meier C, Bernard A, et al. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion 2014;54:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long K, Meier C, Ward M, et al. Immunologic profiles of red blood cells using in vitro models of transfusion. J Surg Res 2013;184:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muszynski J, Nateri J, Nicol K, et al. Immunosuppressive effects of red blood cells on monocytes are related to both storage time and storage solution. Transfusion 2012;52:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ottonello L, Ghio M, Contini P, et al. Nonleukoreduced red blood cell transfusion induces a sustained inhibition of neutrophil chemotaxis by stimulating in vivo production of transforming growth factor-beta1 by neutrophils: role of the immunoglobulinlike transcript 1, sFasL, and sHLA-I. Transfusion 2007;47:1395–404. [DOI] [PubMed] [Google Scholar]
- 41.Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oncol Stem Cell Ther 2008;1:106–23. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg N, Fine L, Gettings KF, et al. Decreased sepsis related to indwelling venous access devices coincident with implementation of universal leukoreduction of blood transfusions. Transfusion 2005;45:1632–9. [DOI] [PubMed] [Google Scholar]
- 43.Hébert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA 2003;289:1941–9. [DOI] [PubMed] [Google Scholar]
- 44.Lannan KL, Sahler J, Spinelli SL, et al. Transfusion immunomodulation—the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis 2013;50:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumberg N, Zhao H, Wang H, et al. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion 2007;47:573–81. [DOI] [PubMed] [Google Scholar]
- 46.Fergusson D, Khanna MP, Tinmouth A, et al. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth 2004;51:417–24. [DOI] [PubMed] [Google Scholar]
- 47.Vanderlinde ES, Heal JM, Blumberg N. Autologous transfusion. BMJ 2002;324:772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation 1998;97: 562–8. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto MN, Kimura EY, Yamamoto M, et al. Expression of Fas and Fas ligand on spleen T cells of experimental animals after unmodified or leukoreduced allogeneic blood transfusions. Transfusion 2004;44:158–63. [DOI] [PubMed] [Google Scholar]
- 50.Sharma RR, Marwaha N. Leukoreduced blood components: advantages and strategies for its implementation in developing countries. Asian J Transfus Sci 2010;4:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sut C, Tariket S, Chou ML, et al. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus 2017;15:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shapiro MJ. To filter blood or universal leukoreduction: what is the answer? Crit Care 2004;8Suppl2:S27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storb R, Rudolph RH, Graham TC, et al. The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol 1971; 107:409–13. [PubMed] [Google Scholar]
- 54.Storb R, Epstein RB, Rudolph RH, et al. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol 1970;105:627–33. [PubMed] [Google Scholar]
- 55.Desmarets M, Cadwell CM, Peterson KR, et al. Minor histo-compatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood 2009;114:2315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SR, Zimring JC. Transfusion-induced bone marrow transplant rejection due to minor histocompatibility antigens. Transfus Med Rev 2013;27:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed W, Lee TH, Norris PJ, et al. Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients. Semin Hematol 2007;44:24–31. [DOI] [PubMed] [Google Scholar]
- 58.Lee TH, Paglieroni T, Ohto H, et al. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood 1999;93:3127–39. [PubMed] [Google Scholar]
- 59.Bernard A, Meier C, Ward M, et al. Packed red blood cells suppress T-cell proliferation through a process involving cell-cell contact. J Trauma 2010;69:320–9. [DOI] [PubMed] [Google Scholar]
- 60.Fragkou PC, Torrance HD, Pearse RM, et al. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: a prospective cohort study. Crit Care 2014;18:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gafter U, Kalechman Y, Sredni B. Blood transfusion enhances production of T-helper-2 cytokines and transforming growth factor beta in humans. Clin Sci (Lond) 1996;91:519–23. [DOI] [PubMed] [Google Scholar]
- 62.Leal-Noval SR, Muñoz-Gómez M, Arellano V, et al. Influence of red blood cell transfusion on CD41 T-helper cells immune response in patients undergoing cardiac surgery. J Surg Res 2010;164:43–9. [DOI] [PubMed] [Google Scholar]
- 63.Saas P, Angelot F, Bardiaux L, et al. Phosphatidylserine-expressing cell by-products in transfusion: a pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol 2012;19:90–7. [DOI] [PubMed] [Google Scholar]
- 64.Frabetti F, Musiani D, Marini M, et al. White cell apoptosis in packed red cells. Transfusion 1998;38:1082–9. [DOI] [PubMed] [Google Scholar]
- 65.Doffek K, Chen X, Sugg SL, et al. Phosphatidylserine inhibits NFjB and p38 MAPK activation in human monocyte derived dendritic cells. Mol Immunol 2011;48:1771–7. [DOI] [PubMed] [Google Scholar]
- 66.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med 2012;13:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muszynski JA, Bale J, Nateri J, et al. Supernatants from stored red blood cell (RBC) units, but not RBC-derived microvesicles, suppress monocyte function in vitro. Trans-fusion 2015;55:1937–45. [DOI] [PubMed] [Google Scholar]
- 68.Ghio M, Contini P, Ubezio G, et al. Blood transfusions with high levels of contaminating soluble HLA-I correlate with levels of soluble CD8 in recipients’ plasma; a new control factor in soluble HLA-I-mediated transfusion-modulated immunomodulation? Blood Transfus 2014;12Suppl1:s105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion 2001;41:988–96. [DOI] [PubMed] [Google Scholar]
- 70.Vallion R, Bonnefoy F, Daoui A, et al. Transforming growth factor-β released by apoptotic white blood cells during red blood cell storage promotes transfusion-induced alloimmunomodulation. Transfusion 2015;55:1721–35. [DOI] [PubMed] [Google Scholar]
- 71.Benson DD, Beck AW, Burdine MS, et al. Accumulation of pro-cancer cytokines in the plasma fraction of stored packed red cells. J Gastrointest Surg 2012;16:460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karam O, Tucci M, Toledano BJ, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion 2009;49:2326–34. [DOI] [PubMed] [Google Scholar]
- 73.Keir AK, McPhee AJ, Andersen CC, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res 2013;73:75–9. [DOI] [PubMed] [Google Scholar]
- 74.Nagura Y, Tsuno NH, Tanaka M, et al. The effect of pre-storage whole-blood leukocyte reduction on cytokines/chemokines levels in autologous CPDA-1 whole blood. Transfus Apher Sci 2013;49:223–30. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion 1996;36:960–5. [DOI] [PubMed] [Google Scholar]
- 76.Bury TB, Corhay JL, Radermecker MF. Histamine-induced inhibition of neutrophil chemotaxis and T-lymphocyte proliferation in man. Allergy 1992;47:624–9. [DOI] [PubMed] [Google Scholar]
- 77.Peterson CG, Skoog V, Venge P. Human eosinophil cationic proteins (ECP and EPX) and their suppressive effects on lymphocyte proliferation. Immunobiology 1986;171:1–13. [DOI] [PubMed] [Google Scholar]
- 78.Remy KE, Natanson C, Klein HG. The influence of the storage lesion(s) on pediatric red cell transfusion. Curr Opin Pediatr 2015;27:277–85. [DOI] [PubMed] [Google Scholar]
- 79.Alshalani A, Acker JP. Red blood cell membrane water permeability increases with length of ex vivo storage. Cryobiology 2017;76:51–8. [DOI] [PubMed] [Google Scholar]
- 80.D’Alessandro A, Gray AD, Szczepiorkowski ZM, et al. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion 2017;57:1019–30. [DOI] [PubMed] [Google Scholar]
- 81.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015;55:205–19. [DOI] [PubMed] [Google Scholar]
- 82.Spinella PC, Sparrow RL, Hess JR, et al. Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion 2011;51:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang 2009;96:93–103. [DOI] [PubMed] [Google Scholar]
- 84.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units inadditive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion 2015;55:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteom 2012; 76SpecNo:181–93. [DOI] [PubMed] [Google Scholar]
- 86.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011;124:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Remy KE, Sun J, Wang D, et al. Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: a meta-analysis. Vox Sang 2016;111:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Remy KE, Spinella PC. Red blood cell storage age—what we know from clinical trials. Expert Rev Hematol 2016;9:1011–3. [DOI] [PubMed] [Google Scholar]
- 89.Baek JH, Yalamanoglu A, Gao Y, et al. Iron accelerates hemoglobin oxidation increasing mortality in vascular diseased guinea pigs following transfusion of stored blood. JCI Insight 2017;2:e93577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 2012;122:1444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating nontransferrin-bound iron. Blood 2011;118:6675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.L’Acqua C, Bandyopadhyay S, Francis RO, et al. Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children. Am J Hematol 2015;90:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spitalnik SL. Stored red blood cell transfusions: iron, inflammation, immunity, and infection. Transfusion 2014; 54:2365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cherayil BJ. The role of iron in the immune response to bacterial infection. Immunol Res 2011;50:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang X, Lin T, Sun G, et al. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol 2009;86:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin T, Sammy F, Yang H, et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol 2012;189:2017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rifkind JM, Mohanty JG, Nagababu E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol 2014;5:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganz T Systemic iron homeostasis. Physiol Rev 2013;93: 1721–41. [DOI] [PubMed] [Google Scholar]
- 99.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015;15:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 2012;2:a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012;1823:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Macciò A, Madeddu C, Gramignano G, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica 2015;100:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Porto BN, Alves LS, Fernández PL, et al. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem 2007;282:24430–6. [DOI] [PubMed] [Google Scholar]
- 104.Graça-Souza AV, Arruda MA, de Freitas MS, et al. Neutro-phil activation by heme: implications for inflammatory processes. Blood 2002;99:4160–5. [DOI] [PubMed] [Google Scholar]
- 105.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Trans-fusion 2011;51:881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hod EA, Brittenham GM, Spitalnik SL. The role of iron in toxicity of stored red blood cell units. Blood 2012;120:SCI–46. [Google Scholar]
- 107.Berra L, Coppadoro A, Yu BL, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology 2012;117:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child Fetal Neonatal Ed 2013;98:F424–9. [DOI] [PubMed] [Google Scholar]
- 109.Yazdanbakhsh K, Bao W, Zhong H. Immunoregulatory effects of stored red blood cells. Hematology Am Soc Hematol Educ Program 2011;2011:466–9. [DOI] [PubMed] [Google Scholar]
- 110.Theurl I, Fritsche G, Ludwiczek S, et al. The macrophage: a cellular factory at the interphase between iron and immunity for the control of infections. Biometals 2005; 18:359–67. [DOI] [PubMed] [Google Scholar]
- 111.Walker EM Jr, Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci 2000;30:354–65. [PubMed] [Google Scholar]
- 112.Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res 2006;135:226–32. [DOI] [PubMed] [Google Scholar]
- 113.Majetschak M, Krehmeier U, Bardenheuer M, et al. Extra-cellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 2003;101:1882–90. [DOI] [PubMed] [Google Scholar]
- 114.Zhu X, Yu B, You P, et al. Ubiquitin released in the plasma of whole blood during storage promotes mRNA expression of Th2 cytokines and Th2-inducing transcription factors. Transfus Apher Sci 2012;47:305–11. [DOI] [PubMed] [Google Scholar]
- 115.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aubron C, Bailey M, McQuilten Z, et al. Duration of red blood cells storage and outcome in critically ill patients. J Crit Care 2014;29:476.e1–8. [DOI] [PubMed] [Google Scholar]
- 117.Cognasse F, Nguyen KA, Damien P, et al. The inflammatory role of platelets via their TLRs and Siglec receptors. Front Immunol 2015;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamzeh-Cognasse H, Damien P, Chabert A, et al. Platelets and infections—complex interactions with bacteria. Front Immunol 2015;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stolla M, Refaai MA, Heal JM, et al. Platelet transfusion—the new immunology of an old therapy. Front Immunol 2015;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin HC, Chang HW, Hsiao SH, et al. Platelet-derived microparticles trigger THP-1 monocytic cell aggregation and release of pro-coagulant tissue factor-expressing microparticles in vitro. Transfus Apher Sci 2015;53:246–52. [DOI] [PubMed] [Google Scholar]
- 121.Sadallah S, Schmied L, Eken C, et al. Platelet-derived ectosomes reduce NK cell function. J Immunol 2016;197:1663–71. [DOI] [PubMed] [Google Scholar]
- 122.Keating FK, Butenas S, Fung MK, et al. Platelet-white blood cell (WBC) interaction, WBC apoptosis, and procoagulant activity in stored red blood cells. Transfusion 2011;51:1086–95. [DOI] [PubMed] [Google Scholar]
- 123.Fu X, Felcyn JR, Odem-Davis K, et al. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion 2016;56: 2560–70. [DOI] [PubMed] [Google Scholar]
- 124.Vlaar AP, Kulik W, Nieuwland R, et al. Accumulation of bio-active lipids during storage of blood products is not cell but plasma derived and temperature dependent. Transfusion 2011;51:2358–66. [DOI] [PubMed] [Google Scholar]
- 125.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of pre-storage leukoreduced red blood cells and cause acute lung injury. Transfusion 2011;51:2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 127.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Trans-fusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood 2003;101:454–62. [DOI] [PubMed] [Google Scholar]
- 128.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication.J Proteom 2010;73:1907–20. [DOI] [PubMed] [Google Scholar]
- 129.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007; 21:157–71. [DOI] [PubMed] [Google Scholar]
- 130.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol 2008;84:1316–25. [DOI] [PubMed] [Google Scholar]
- 131.Leroyer AS, Isobe H, Lesèche G, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol 2007;49: 772–7. [DOI] [PubMed] [Google Scholar]
- 132.Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol 2005;17:879–87. [DOI] [PubMed] [Google Scholar]
- 133.Dey-Hazra E, Hertel B, Kirsch T, et al. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag 2010;6:1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rubin O, Crettaz D, Tissot JD, et al. Pre-analytical and methodological challenges in red blood cell microparticle proteomics. Talanta 2010;82:1–8. [DOI] [PubMed] [Google Scholar]
- 135.Jy W, Mao WW, Horstman L, et al. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol Dis 1995;21:217–31; discussion 231a. [DOI] [PubMed] [Google Scholar]
- 136.Rubin O, Crettaz D, Canellini G, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang 2008;95:288–97. [DOI] [PubMed] [Google Scholar]
- 137.Baj-Krzyworzeka M, Majka M, Pratico D, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol 2002;30:450–9. [DOI] [PubMed] [Google Scholar]
- 138.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183: 1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014;123:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bakkour S, Acker JP, Chafets DM, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang 2016;111:22–32. [DOI] [PubMed] [Google Scholar]
- 141.Bicalho B, Pereira AS, Acker JP. Buffy coat (top/bottom)-and whole-blood filtration (top/top)-produced red cell concentrates differ in size of extracellular vesicles. Vox Sang 2015;109:214–20. [DOI] [PubMed] [Google Scholar]
- 142.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol 2009;19:43–51. [DOI] [PubMed] [Google Scholar]
- 143.Sadallah S, Eken C, Martin PJ, et al. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol 2011;186:6543–52. [DOI] [PubMed] [Google Scholar]
- 144.Vasina EM, Cauwenberghs S, Feijge MA, et al. Micro-particles from apoptotic plateletspromote resident macrophage differentiation. Cell Death Dis 2011;2: e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004;104:2543–8. [DOI] [PubMed] [Google Scholar]
- 146.Ren Y, Yang J, Xie R, et al. Exosomal-like vesicles with immune-modulatory features are present in human plasma and can induce CD41 T-cell apoptosis in vitro. Transfusion 2011;51:1002–11. [DOI] [PubMed] [Google Scholar]
- 147.Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014;8:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Théry C, Duban L, Segura E, et al. Indirect activation of naive CD41 T cells by dendritic cell-derived exosomes. Nat Immunol 2002;3:1156–62. [DOI] [PubMed] [Google Scholar]
- 149.Qazi KR, Gehrmann U, Domange Jordö E, et al. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood 2009;113:2673–83. [DOI] [PubMed] [Google Scholar]
- 150.Kim OY, Hong BS, Park KS, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol 2013;190:4092–102. [DOI] [PubMed] [Google Scholar]
- 151.Lee WH, Choi HI, Hong SW, et al. Vaccination with Klebsi-ella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med 2015;47:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Assinger A Platelets and infection—an emerging role of platelets in viral infection. Front Immunol 2014;5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lacroix J, Hébert PC, Fergusson DA, et al. Age of trans-fused blood in critically ill adults. N Engl J Med 2015; 372:1410–8. [DOI] [PubMed] [Google Scholar]
- 154.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 2012;308:1443–51. [DOI] [PubMed] [Google Scholar]
- 155.Ramirez-Arcos S, Marks DC, Acker JP, et al. Quality and safety of blood products. J Blood Transfus 2016;2016: 2482157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chassé M, Tinmouth A, English SW, et al. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med 2016;176: 1307–14. [DOI] [PubMed] [Google Scholar]
- 157.Almizraq RJ, Yi QL, Acker JP, et al. Impact of technical and assay variation on reporting of hemolysis in stored red blood cell products. Clin Chim Acta 2017; 468:90–7. [DOI] [PubMed] [Google Scholar]
- 158.Acker JP, Marks DC, Sheffield WP. Quality assessment of established and emerging blood components for transfusion. J Blood Transfus 2016;2016:4860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hall MW, Geyer SM, Guo CY, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 2013;41:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muszynski JA, Nofziger R, Greathouse K, et al. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock 2014;42:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 2011; 37:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med 2008;178: 276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011;306:2594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muszynski JA, Nofziger R, Greathouse K, et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care 2014;18:R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Engele LJ, Straat M, van Rooijen IH, et al. Transfusion of platelets, but not of red blood cells, is independently associated with nosocomial infections in the critically ill. Ann Intensive Care 2016;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]