Abstract
Objective:
Anxiety and depression predict poor physical health longitudinally, but are neglected in primary care settings compared to other risk factors such as obesity and smoking. Further, anxiety has been less commonly studied than depression, and whether anxiety has unique predictive effects for physical health is unknown. We compared anxiety and depression to obesity and smoking as predictors of physical health indices and examined unique predictive effects of anxiety and depression.
Method:
Using data from the Health and Retirement study, a US population-based cohort study of older adults, we tested longitudinal associations of anxiety and depression symptoms with onset of self-reported physical health indices (N= 15,418; M age = 68). Medical illnesses (heart disease, stroke, arthritis, high blood pressure, diabetes, and cancer) and somatic symptoms (stomach problems, shortness of breath, dizziness, back pain, headache, pain, and eyesight difficulties) were assessed on two occasions over four years. Anxiety and depression were measured at the initial time point and tested as predictors of medical illness and somatic symptom onset.
Results:
Anxiety and depression symptoms predicted greater incidence of nearly all medical illnesses and somatic symptoms. Effects were as strong as or stronger than those of obesity and smoking, and anxiety and depression independently increased risk for most physical health indices assessed.
Conclusions:
Findings suggest that anxiety and depression are as strongly predictive of poor future physical health as obesity and smoking and that anxiety is independently linked to poor physical health. Greater attention should be paid towards these conditions in primary care.
Keywords: anxiety, depression, chronic disease, somatic symptoms
Anxiety and depression symptoms are strongly linked to poor physical health in both cross-sectional and longitudinal studies. Yet these conditions continue to receive limited attention in primary care settings compared with other risk factors such as obesity and smoking. Further, although anxiety has also been linked with a multitude of medical conditions and somatic symptoms, more research has focused on depression as a predictor of poor physical health than anxiety, and our understanding of the unique predictive effects of anxiety and depression symptoms is limited. Finally, the majority of prior studies have focused on one medical condition at a time, limiting our ability to understand the broad physical health effects of anxiety and depression and underestimating the overall physical health burden. Clarifying the strength of the association between anxiety and depression symptoms, both in terms of combined and unique predictive effects, with multiple physical health indices in a longitudinal study could ultimately help guide clinical decision making in primary care and highlight potential novel targets for preventative intervention. The goals of the current study were to examine the predictive effects of anxiety and depression symptoms on multiple self-reported major medical illnesses and somatic symptoms, to compare the strength of these predictive effects with those of obesity and smoking, and to examine independent contributing effects of anxiety and depression symptoms.
Longitudinal studies have linked anxiety and depression with increased risk for disease onset, including diabetes (Farvid et al., 2014; Knol et al., 2006; Vimalananda et al., 2014), heart disease (Eaker, Pinsky, & Castelli, 1992; Eaker, Sullivan, Kelly-Hayes, D’Agostino, & Benjamin, 2005), arthritis and other autoimmune disorders (Aguilar-Gaxiola et al., 2016; Andersson et al., 2015; Euesden, Danese, Lewis, & Maughan, 2017; Lu et al., 2016), blood clots (Sumner et al., 2016), stroke (Jonas & Mussolino, 2000), cancer (Archer, Pikhart, & Head, 2015), and high blood pressure (Jonas, Franks, & Ingram, 1997; Meng, Chen, Yang, Zheng, & Hui, 2012). These studies provide strong evidence that anxiety and depression symptoms contribute to disease onset. Because anxiety and depression impact multiple biological systems, including the hypothalamic pituitary adrenal axis and the autonomic nervous, immune, and telomere maintenance systems (Bruce & Musselman, 2005; Maes et al., 1998; O’Donovan et al., 2017; Thayer, 2006), as well as a range of health behaviors (Allgöwer, Wardle, & Steptoe, 2001; Strine, Chapman, Kobau, & Balluz, 2005), many disease outcomes are likely affected. Prior studies, with a few exceptions (El-Gabalawy, Mackenzie, Pietrzak, & Sareen, 2014; Kang et al., 2017), have tended to focus on one medical condition at a time, making it difficult to determine the relative strength of the association between anxiety and depression symptoms and the various conditions to which these symptoms are linked. Further, although many prior studies aim to communicate the strength of the association by reporting incident rate ratios or percent of variance explained, another way to contextualize these findings is to directly compare the strength of the predictive effect of high anxiety and depression symptoms with that of well-known risk factors that receive significant attention in primary care settings, such as obesity and smoking. Researchers have previously taken this approach to quantify the risks associated with social isolation (Holt-Lunstad, Robles, & Sbarra, 2017; Pantell et al., 2013). To our knowledge, however, no prior study has directly compared anxiety and depression symptoms with obesity and smoking as prospective risk factors for disease onset.
Prior studies have also examined the link of anxiety and depression symptoms with somatic symptoms (e.g., fatigue, headache, low back pain, and gastrointestinal distress). Subjective somatic complaints are common (Eriksen, Svendsrod, Ursin, & Ursin, 1998), and medical providers often have difficulty identifying a medical diagnosis that explains the symptoms (Kroenke et al., 1994). Nonetheless, these symptoms contribute significantly to long-term sickness compensation (i.e., benefits provided as a result of work incapacitation) and disability (Tellnes, Svendsen, Bruus-gaard, & Bjerkedal, 1989). Given that the etiology of these symptoms is sometimes unclear, a better understanding of the link of anxiety and depression symptoms with somatic complaints may guide future research on possible psychological interventions. Cross-sectional studies have shown links between elevated depression and anxiety and physical symptoms such as pain, stomach distress, heart palpitations, shortness of breath, and impaired hearing and vision (Haug, Mykletun, & Dahl, 2004; Kroenke, Jackson, & Chamberlin, 1997; Simon & VonKorff, 1991; Simon, VonKorff, Piccinelli, Fullerton, & Ormel, 1999). To our knowledge, no prior studies have examined longitudinal predictive effects of anxiety and depression symptoms with somatic complaints, making it difficult to determine whether somatic complaints precede anxiety and depression or vice versa. Further, little is known about how anxiety and depression symptoms may be more strongly linked with certain somatic complaints, as prior studies have examined a somatic symptom composite rather than each symptom separately.
Finally, unique contributions of anxiety and depression symptoms to physical health outcomes are poorly understood. Anxiety and depression tend to be highly comorbid and have a number of overlapping symptoms. Yet these conditions remain distinct in the Diagnostic And Statistical Manual of Mental Disorders (5th ed.; American Psychiatric Association, 2013) and have unique biological and cognitive characteristics. (Martin & Nemeroff, 2010; Mathews & MacLeod, 2005; Toker, Shirom, Shapira, Berliner, & Melamed, 2005). Identifying independent contributions of anxiety and depression symptoms to multiple physical health outcomes may inform the biological pathways by which these conditions confer greater disease risk. Few prior studies have tested unique contributions of anxiety and depression to physical health. In one study, anxiety was a unique predictor of cardiac rehospitalization and prognosis over and above depression, but not vice versa (Strik, Denollet, Lousberg, & Honig, 2003), and in another, depression and anxiety each independently predicted cardiac events (Janszky, Ahnve, Lundberg, & Hemmingsson, 2010). In one cross-sectional study testing multiple medical conditions, anxiety was associated with greater odds of ulcer over and above depression, and depression with greater odds of heart disease, migraine, and eyesight difficulties over and above anxiety (Niles et al., 2015). These studies, however, were either cross-sectional, limiting conclusions regarding predictive effects of anxiety and depression for disease onset, or focused only on heart disease. Thus, longitudinal studies that examine unique effects of anxiety and depression symptoms for multiple medical conditions are needed.
The present study tested the longitudinal effects of anxiety and depression symptoms on the onset of medical illnesses and somatic symptoms over a 4-year period in the Health and Retirement Study (HRS). HRS is a population-based longitudinal cohort study focused on older adults in the United States. This sample is particularly valuable for testing predictors of physical health outcomes given the higher prevalence of physical health conditions and somatic complains in older adults. First, we examined whether a composite of anxiety and depression symptoms predicted medical illness and somatic symptom onset, predicting that this symptom composite would predict greater onset of all physical health outcomes. Second, we compared the strength of the effect for anxiety and depression symptoms predicting medical illness and somatic symptoms with that of obesity and smoking, hypothesizing that effect sizes would be larger for obesity and smoking than for anxiety and depression. Finally, we examined unique predictive effects of anxiety and depression symptoms for all physical health indices, hypothesizing that anxiety and depression symptoms would be independently associated with greater onset of all physical health outcomes.
Method
Participants
HRS is a longitudinal study of a U.S. population-based sample of more than 20,000 adults, launched in 1992. HRS is sponsored by the National Institute on Aging (Grant Number NIA U01AG009740) and is conducted by the University of Michigan. The study was approved by the University of Michigan Institutional Review Board (Protocol HUM00061128). HRS used amul-tistage area probability sample design. Data were collected in four stages. In the first stage, participants were sampled using probability proportionate to size selection of U.S. Metropolitan Statistical Areas (MSAs) and non-MSA counties. The second stage included sampling of area segments (SSUs) within sampled primary stage units. In the third stage, a complete listing (enumeration) of all the housing units (HUs) physically located in the bounds of the selected SSU was collected, and HUs were systematically selected from the SSUs. Finally, the household financial unit was selected from within the sample HU. Additional details regarding sampling procedures can be found in Heeringa and Conner (1995). Depression symptoms were assessed throughout the study. Anxiety was assessed in one cohort of participants in 2006 who were followed up in 2010, and in another cohort of participants in 2008, followed up in 2012. We combined data from the two cohorts and treated data from 2006 and 2008 as Time 1 (T1), and data from 2010 and 2012 as Time 2 (T2). Cohort was included as a covariate. All participants with at least one available anxiety and depression measure were included in analyses when maximum likelihood (ML) was possible (see Statistical Analyses section; N= 15,300). When ML was not possible, participants were included only if they had all available data (N = 11,502). Further, some somatic symptoms were not collected for Cohort 2, reducing the sample sizes for analyses of these variables. Participant characteristics at T1 are shown in Table 1.
Table 1.
Time 1 Patient Characteristics
| Characteristic | Descriptive statistic |
|---|---|
| Age (years), mean (SD) | 68.0 (10.49) |
| Male, n (%) | 6301 (40.9) |
| Race, n (%) | |
| White | 12,695 (82.3) |
| Black | 1,994 (12.9) |
| Hispanic ethnicity, n (%) | 1,329 (8.6) |
| Body mass index, M (SD) | 28.2 (5.9) |
| Obese, n (%) | 4,737 (31.4) |
| Vigorous physical activity, n (%) | 3,597 (23.4) |
| Currently smoke, n (%) | 2,125 (13.9) |
| Heavy alcohol use, n (%) | 928 (6.0) |
| Married, n (%) | 9,785 (63.5) |
| High school educated, n (%) | 11,637 (75.5) |
| Medical illnesses, n (%) | |
| 0 | 2,159 (14.1) |
| 1 | 3,877 (25.2) |
| 2 | 4,266 (27.8) |
| >3 | 5,069 (33.0) |
| Somatic symptoms, n (%) | |
| 0 | 1,499 (24.2) |
| 1 | 1,356 (21.9) |
| 2 | 1,157 (18.7) |
| >3 | 2,184(35.2) |
| Anxiety, M (SD) | 7.9 (3.0) |
| Depression, M (SD) | 1.9 (2.2) |
| High anxiety and depression, n (%) | 2,225 (15.8) |
We examined whether participants with all data for both time points (n = 4,755) differed from those with available data at the first time point but not the second (n = 1,433). A number of significant differences emerged between these two groups with participants with incomplete data reporting more medical conditions (p < .001), more somatic symptoms (p < .001), higher depression (p < .001) and anxiety (p < .001) scores, older age (p < .001), higher likelihood of smoking (p < .01) and sedentary lifestyle (p < .001), less education (p < .001), and less likelihood of being married (p < .001).
Measures
Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977).
Depression symptoms were assessed using a nine-item version of the CES-D. Participants answered “yes” or “no” to items assessing depression symptoms in the past week, and scale scores were a sum of responses across the nine items. In our sample, this abbreviated scale had good internal consistency (α = .80-.81 across the cohorts).
Beck Anxiety Inventory—Shortened Version (BAI; Beck, Epstein, Brown, & Steer, 1988).
Anxiety symptoms were assessed with a five-item version of the BAI. Respondents rated how often during the past week they experienced five anxiety symptoms on a 4-item Likert scale. Scale scores were a sum of responses across the five items. In our sample, this measure had good internal consistency (α = .81-.82 across the cohorts).
Anxiety and depression symptom composite.
We created a composite of anxiety and depression symptoms by standardizing CES-D and BAI scores and averaging them. The composite was created because of significant overlap between anxiety and depression symptoms (current sample, r = .46 at T1 and r = .47 at T2) and our interest in examining how both unique and shared variance of anxiety and depression symptoms is associated with physical health. To compare anxiety and depression symptoms with obesity and smoking, we created a dichotomous variable with high anxiety and depression defined as scores greater than one standard deviation from the mean (approximately 16% of the sample). This cutoff likely roughly captures those meeting full criteria for anxiety or depression given that estimated prevalence rates are 15% and 12% for these disorders, respectively, in this age group (Kessler, Chiu, Demler, Merikangas, & Walters, 2005).
Medical illness.
Medical illnesses were assessed at T1 and T2. If participants had not reported a condition at the previous time point, they were asked whether a doctor told them, since the prior interview, that they had “high blood pressure or hypertension,” “diabetes or high blood sugar,” “cancer of any kind, excluding skin,” “heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems,” “stroke,” and “arthritis or rheumatism.” If a participant reported one of these conditions at a previous time point, the interviewer would probe about whether the condition persisted or whether a change had occurred since the prior positive response. Total medical illnesses was the number of medical illnesses each participant reported.
Somatic symptoms.
Somatic symptoms were assessed at T1 and T2. Participants were asked, “Have you had any of the following persistent or troubling problems?” and then were asked about symptoms including “shortness of breath,” “dizziness or lightheadedness,” “back pain or problems,” “headaches,” “taking prescription medications for stomach problems,” and “trouble with pain.” Participants were also asked to rate their eyesight from 5 (excellent) to 1 (poor), with poor coded as 1 and all other responses coded as 0. Because of potential overlap between “back pain or problems” and “trouble with pain,” we examined the number of people reporting only one of these symptoms. We found that 51% of people endorsing pain and/or back pain reported only having one or the other, but not both symptoms, suggesting these are, in fact, unique constructs. Total somatic symptoms was the number of symptoms each participant reported.
Demographics and health behaviors.
Race included Caucasian, African American, or other, and, in a separate question, participants were asked whether they identified their ethnicity as Hispanic or Latino. Education was categorized as “≥high school” and “<high school.” Smoking was categorized as currently smoking cigarettes versus not. Because interviewers would skip this question if participants had previously reported that they never smoked, for missing data, this variable was imputed based on previous or future reports of having smoked. Body mass index (BMI) was calculated using the equation (Weight/Height2) × 703 based on self-reported weight and height. For a subset of participants (n = 12,573), weight was measured during an in-person visit, and analyses are also conducted with this subset to replicate the findings based on self-report. Obesity was defined as a BMI more than 30. Participants who drank more than seven drinks per week for women and 14 for men were categorized as heavy drinkers (Department of Health and Human Services & U.S. Department of Agriculture, 2015). Marital status included currently or not currently married. Physically active participants were those who participated in vigorous activity more than once per week. Covariates were assessed at T1.
Statistical Analyses
Multiple regression in Stata 14 was used to test the longitudinal relationship of anxiety and depression symptoms with physical health indices over the 4-year study period. We first examined the predictive effect of the anxiety and depression symptom composite (described in Measures section) on total number of medical conditions and somatic symptoms using linear regression, and then for incidence of each individual condition and symptom using logistic regression. We ran separate models for total number of medical conditions, total number of somatic symptoms, and for each individual physical health indicator. For models testing total number of conditions or symptoms, the outcome was the physical health indicator at T2, and the predictor was the composite of anxiety and depression symptoms. Covariates included the physical health indicator at T1, age, gender, race, ethnicity, marital status, education, physical activity, alcohol use, smoking, and BMI. For models testing incidence of individual medical conditions, the sample was restricted to those who did not report the condition or symptom at T1, and incidence was coded as 0 if they did not develop the condition at T2, and 1 if they did. Incidence was used as the outcome, and covariates included age, gender, race, ethnicity, marital status, education, physical activity, alcohol use, smoking, and BMI. Models did not control for other medical conditions at T1.
To compare anxiety and depression symptoms as predictors of medical illness and somatic symptoms with obesity and smoking, we first created a dichotomous variable to indicate high/low anxiety and depression symptoms as described in the Measures section. We also created a dichotomous variable to indicate presence or absence of obesity (smoking was already dichotomous). We then reran the multiple regression models using the dichotomous anxiety and depression symptoms, obesity, and smoking variables as predictors (as well as all covariates). Finally, we tested a linear combination of the parameter estimates for each model. Specifically, we calculated the difference in the betas for high anxiety and depression (bad) from the betas for obesity (bo) and smoking (bs), and tested whether the difference was significantly greater or less than zero (i.e., the null hypothesis was that the difference between the betas was equal to zero).
Finally, to test unique predictive effects of anxiety and depression symptoms, we ran the same statistical models described for our first research question, but instead of using the composite anxiety and depression measure as the predictor, we included both the CES-D (depression symptoms) and the BAI (anxiety symptoms) as separate predictors within the same model. Depression and anxiety symptom scales were standardized to allow for comparison of odds ratios. For models with continuous outcomes, we used ML, which does not exclude participants with incomplete data (Allison, 2012), and we accounted for clustering of individuals within households using the Huber-White sandwich estimator (R. L. Williams, 2000). Models with dichotomous outcomes (e.g., heart disease) did not allow for use of ML, and multiple imputation could not incorporate the alternative standard error calculation. Therefore, these models included only participants with complete data, and we accounted for clustering within households using random intercepts. To determine whether inclusion of missing data would affect the results, we conducted analyses using multiply-imputed data (10 imputations using chained equations) but ignoring clustering within households, as we could not incorporate both of these complexities in one analysis. Results remained largely unchanged with imputed analyses, and because these analyses did not account for clustering, we report analyses based on nonimputed data.
Results
We first examined incidence of physical health indices and the association between covariates and physical health indices using multivariable regression. Supplementary Table 1 of the online supplemental materials provides the 4-year incidence of each medical condition and somatic symptom. Supplementary Table 2 provides the associations between covariates and physical health indices. Exact sample sizes for models are shown in Table 2.
Table 2.
Prediction of Total Number of and Incident Physical Health Indices at T2 by Anxiety and Depression Symptoms at T1 Over and Above Covariates
| Anxiety and depression composite T1 |
Unique anxiety T1 (BAI) |
Unique depression T1 (CES-D) |
||
|---|---|---|---|---|
| Physical health indices | N | OR [95% CI] | OR [95% CI] | OR [95% CI] |
| Medical illness T2 | ||||
| No. of medical illnesses (B) | 15,300 | .04 [.03, .05] | .03 [.02, .05] | .02 [.00, .03] |
| Incidence of: | ||||
| Heart condition | 8,821 | 1.27 [1.17, 1.37] | 1.13 [1.04, 1.23] | 1.17 [1.07, 1.27] |
| Stroke | 10.843 | 1.30 [1.18, 1.43] | 1.22 [1.10, 1.35] | 1.12 [1.01, 1.24] |
| Arthritis | 4,441 | 1.33 [1.21, 1.47] | 1.10 [.99, 1.22] | 1.28 [1.15, 1.42] |
| High blood pressure | 4,919 | 1.21 [1.12, 1.32] | 1.14 [1.04, 1.24] | 1.10 [1.01, 1.20] |
| Diabetes | 9,274 | 1.10 [1.01, 1.20] | 1.02 [.93, 1.12] | 1.10 [1.00, 1.21] |
| Cancer | 9,833 | 1.09 [.99, 1.19] | 1.09 [.98, 1.20] | 1.01 [.91, 1.12] |
| Somatic symptoms T2 | ||||
| No. of somatic symptoms (B) | 7,199 | .14 [.09, .19] | .06 [.03, .09] | .10 [.06, .14] |
| Incidence of: | ||||
| Stomach problems | 7,241 | 1.39 [1.27, 1.52] | 1.30 [1.18, 1.43] | 1.14 [1.03, 1.25] |
| Shortness of breath | 4,579 | 1.58 [1.41, 1.77] | 1.22 [1.09, 1.37] | 1.40 [1.25, 1.57] |
| Dizziness | 4,781 | 1.83 [1.61,2.07] | 1.46 [1.39, 1.65] | 1.40 [1.24, 1.57] |
| Back pain | 3,412 | 1.46 [1.31, 1.61] | 1.23 [1.11, 1.36] | 1.27 [1.14, 1.42] |
| Headache | 4,977 | 1.62 [1.40, 1.87] | 1.31 [1.13, 1.53] | 1.34 [1.15, 1.57] |
| Pain | 7,595 | 1.45 [1.35, 1.55] | 1.16 [1.09, 1.25] | 1.34 [1.24, 1.44] |
| Eye sight difficulties | 11,008 | 1.75 [1.57, 1.96] | 1.37 [1.23, 1.53] | 1.41 [1.26, 1.58] |
Note. All regression models were adjusted for age, gender, race, ethnicity, marital status, education, alcohol use, physical activity, smoking, and body mass index. T1 = Time 1; T2 = Time 2; B= standardized beta; OR= odds ratio; CI = confidence interval; BAI = Beck Anxiety Inventory; CES-D = Center for Epidemiologic Studies Depression Scale.
Anxiety and Depression Composite Predicting Physical Health Indices
As shown in Table 2, the anxiety and depression symptom composite at T1 significantly predicted total medical illnesses (p < .001) and total somatic symptoms (p < .001) at T2 over and above medical illnesses or somatic symptoms, and covariates at T1. Further, in terms of specific medical conditions, the anxiety and depression symptom composite at T1 predicted incidence of self-reported heart conditions (p < .001), stroke (p < .001), arthritis (p < .001), high blood pressure (p < .001), and diabetes (p = .025), but not cancer (p = .084) at T2 over and above covariates at T1. In terms of somatic symptoms, the anxiety and depression symptom composite at T1 predicted incidence of all somatic symptoms (all ps < .001) at T2 over and above covariates at T1.
Comparison of High Anxiety and Depression Symptoms With Obesity and Smoking as Predictors of Physical Health Indices
Obesity.
For total number of medical illnesses at T2, the strength of the prediction for high anxiety and depression at T1 was less than that of obesity at T1 (bad - bo = –.06, 95% confidence interval [CI] [ – .11, –.01], p = .017). For individual medical illnesses at T2 (see Figure 1), the magnitude of the prediction for high anxiety and depression at T1 did not differ from that of obesity at T1 for heart conditions (bad - bo = .10, 95% CI [ – .16, .36], p = .454), stroke (bad- bo = .31, 95% CI [ – .03, .65], p = .071), arthritis (bad- bo = .22, 95% CI [ – .11, .55], p = .194), high blood pressure (bad - bo = .11, 95% CI [ – .17, .39], p = .431), or cancer (bad - bo = .11, 95% CI [ – .23, .45], p = .538), and was significantly smaller than that of obesity for diabetes (bad - bo = –.93, 95% CI [–1.23, –.63], p < .001). Results were mainly unchanged when we reran these analyses using BMI as measured by study personnel on the subset of 12,573 participants with this measure, with the exception that obesity was no longer a significantly stronger predictor of total medical illnesses compared with high anxiety and depression.
Figure 1.
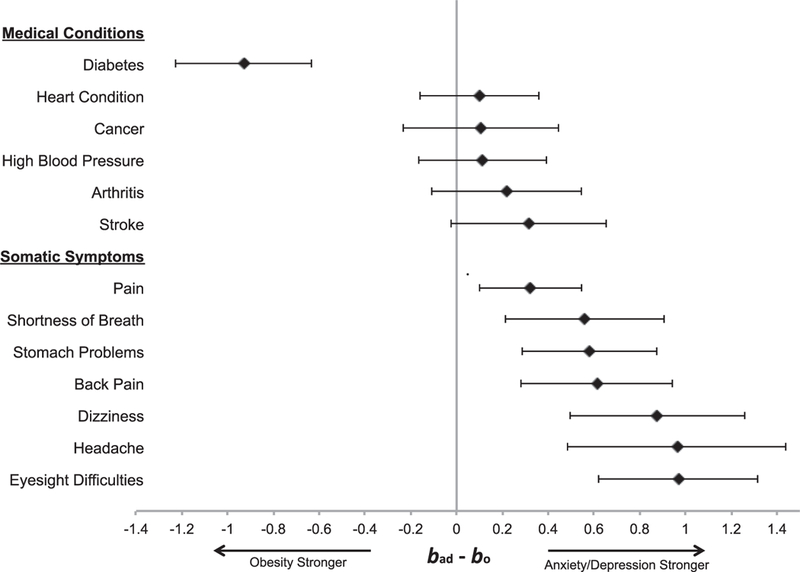
Forest plot comparing the beta for high anxiety and depression with incidence of physical health indices (bad) to the beta for obesity with incidence of physical health indices (bo). Values greater than zero indicate that high anxiety/depression is more strongly associated with the medical condition or somatic symptom than obesity, and values less than zero indicate that obesity is more strongly associated with the medical condition or somatic symptom than high anxiety/depression.
For total number of somatic symptoms at T2, the strength of the prediction for high anxiety and depression at T1 was significantly greater than that of obesity at T1 (bad - bo = .37, 95% CI [.22, .51], p < .001). For individual somatic symptoms at T2 (see Figure 1), the strength of the prediction for high anxiety and depression at T1 was significantly greater than that of obesity at T1 for stomach problems (bad - bo = .58, 95% CI [.29, .88], p < .001), shortness of breath (bad - bo = .56, 95% CI [.21, .91], p = .002), dizziness (bad - bo = .88, 95% CI [.49, 1.26], p < .001), back pain (bad - bo = .61, 95% CI [.28, .95], p < .001), headache (bad - bo = .96, 95% CI [.49, 1.44], p = .001), pain (bad - bo = .32, 95% CI [.10, .55], p = .005), and eyesight difficulties (bad - bo = .97, 95% CI [.62, 1.32], p < .001). Results were unchanged when we compared high anxiety and depression with obesity as measured by study personnel.
Smoking.
For total number of medical illnesses at T2, the strength of the prediction for high anxiety and depression at T1 was not significantly different than that of smoking at T1 (bad - bs = .01, 95% CI [ – .04, .07], p = .645). Moreover, for individual medical illnesses at T2 (see Figure 2), the strength of the prediction for high anxiety and depression at T1 did not differ from that of smoking at T1 for heart conditions (bad - bs = .19, 95% CI [ – .11, .49], p = .223), stroke (bad - bs = .11, 95% CI [ – .28, .50], p = .581), high blood pressure (bad - bs = .20, 95% CI [ – .10, .51], p = .196), cancer (bad - bs = –.15, 95% CI [ – .53, .23], p = .439), or diabetes (bad- bs = –.02, 95% CI [ – .37, .32],p = .902), and was significantly larger than that of smoking for arthritis (bad - bs = .55, 95% CI [.17, .93], p = .004).
Figure 2.
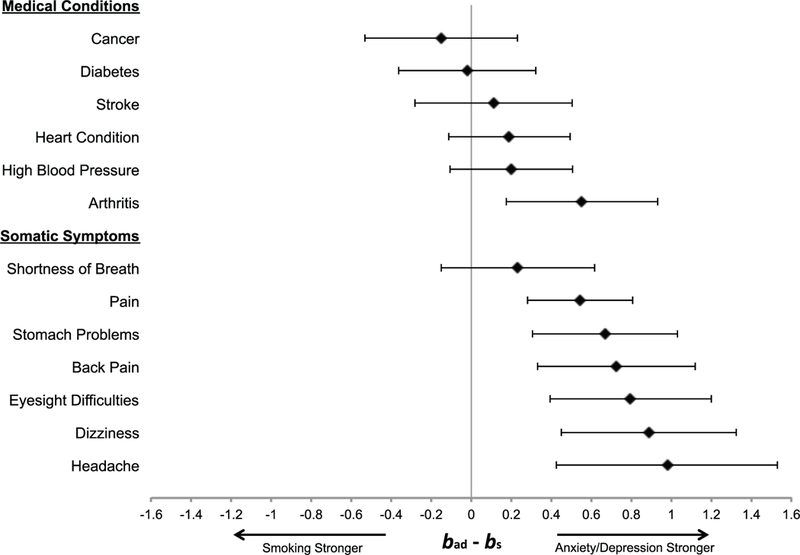
Forest plot comparing the beta for high anxiety and depression with incidence of physical health indices (bad) to the beta for smoking with incidence of physical health indices (bs). Values greater than zero indicate that high anxiety/depression is more strongly associated with the medical condition or somatic symptom than smoking, and values less than zero indicate that smoking is more strongly associated with the medical condition or somatic symptom than high anxiety/depression.
For total number of somatic symptoms at T2, the strength of the prediction for the anxiety and depression composite at T1 was significantly greater than that of smoking at T1 (bad - bs = .35, 95% CI [.15, .55], p = .001). Further, for individual somatic symptoms at T2 (see Figure 2), the strength of the association for high anxiety and depression at T1 was significantly larger than that of smoking at T1 for stomach problems (bad - bs = .67, 95% CI [.31, 1.03], p < .001), dizziness (bad - bs = .89, 95% CI [.45, 1.32], p < .001), back pain (bad-bs = .73, 95% CI [.33, 1.12], p < .001), headache (bad - bs = .98, 95% CI [.43, 1.53], p = .001), pain (bad - bs = .55, 95% CI [.28, .81], p < .001), and eyesight difficulties (bad - bs = .80, 95% CI [.39, 1.20], p < .001), and did not differ from that of smoking for shortness of breath (bad - bs = .23, 95% CI [ – .15, .62], p = .232).
Anxiety and Depression as Independent Predictors of Physical Health Indices
Anxiety symptoms.
As shown in Table 2, anxiety symptom severity at T1 significantly predicted total number of medical illnesses (p < .001) and somatic symptoms (p < .001) at T2 over and above depression symptom severity, medical conditions or somatic symptoms, and covariates at T1. Further, anxiety at T1 independently predicted incidence of heart condition (p = .003), stroke (p < .001), high blood pressure (p = .003), and all somatic symptoms (ps < .001) at T2. Anxiety symptom severity at T1 was not independently associated with incidence of arthritis, diabetes, or cancer (ps > .062) at T2.
Depression symptoms.
As shown in Table 2, depression symptom severity at T1 significantly predicted total number of total medical illnesses (p = .014) and somatic symptoms (p < .001) at T2 over and above anxiety symptom severity, medical conditions or somatic symptoms, and covariates at T1. Further, depression symptoms at T1 independently predicted incidence of heart condition (p < .001), stroke (p .038), arthritis (p < .001), high blood pressure (p .023), diabetes (p .044), and all somatic symptoms (ps < .007) at T2. Depression symptom severity at T1 was not independently associated with incidence of cancer at T2 (p .837).
Discussion
The present study examined whether anxiety and depression symptoms were predictive of worsening physical health in terms of major medical conditions and somatic symptoms over a 4-year period in a sample of older adults in the Health and Retirement Study. Largely consistent with hypotheses, we found that a composite of anxiety and depression symptoms predicted an increasing number of all self-reported medical illnesses and somatic symptoms assessed, with the exception of cancer. Further, inconsistent with hypotheses, we found that the strength of the predictive effect for high anxiety and depression was comparable with, or larger than that of, obesity and smoking for all individual medical conditions and somatic symptoms assessed, with the exception of diabetes, for which obesity was a stronger predictor. Finally, partially consistent with hypotheses, we showed unique predictive effects of anxiety and depression symptoms for all somatic symptoms assessed, and that anxiety symptoms uniquely predicted heart condition, stroke, and high blood pressure, and depression symptoms uniquely predicted heart condition, stroke, arthritis, high blood pressure, and diabetes. These findings highlight the relative importance of anxiety and depression compared with widely accepted risk factors for medical illnesses and somatic complaints such as obesity and smoking, and suggest that anxiety has been overlooked as an independent risk factor.
Consistent with prior longitudinal studies (Andersson et al., 2015; Eaker et al., 2005; Engum, 2007; Farvid et al., 2014; Jonas et al., 1997; Jonas & Mussolino, 2000; Knol et al., 2006; Lu et al., 2016; Meng et al., 2012; Vimalananda et al., 2014), self-reported anxiety and depression symptom severity was associated with new onset of individual medical conditions, including heart conditions, stroke, arthritis, high blood pressure, and diabetes. The current findings are also in line with prior analyses of HRS data showing that depression increased risk for heart problems, arthritis, and diabetes, but not cancer, at 12-year follow-up (Karakus & Patton, 2011); that higher baseline depression symptoms were associated with earlier development of heart disease over 12-and 20-year periods (Maryam & Shervin, 2016; Xiang & An, 2015); and that having a psychiatric condition increased the risk of developing heart disease and diabetes (Fluegge, 2016). In the current analysis, the strongest effects were found for heart conditions, stroke, and arthritis. Despite a large body of literature examining links between depression and diabetes, our findings suggest that other conditions may be more strongly linked with anxiety and depression symptoms, although depression symptoms in particular appear to be more strongly associated with diabetes than anxiety symptoms. In terms of high blood pressure, some studies have found a link (Jonas et al., 1997; Markovitz, Matthews, Kannel, Cobb, & D’Agostino, 1993), whereas others have not (e.g., Goldberg, Comstock, & Graves, 1980; Russek, King, Russek, & Russek, 1990). Although there was a significant association of anxiety and depression symptoms with high blood pressure in the current study, the strength of the association is smaller than that of other medical conditions, which may explain conflicting findings in the literature. Null findings for cancer are generally consistent with prior research showing either small or null predictive effects for anxiety and depression symptoms with cancer (Huang et al., 2015; Poole, Kubzansky, Sood, Okereke, & Tworoger, 2016; Reeves et al., 2018). Interestingly, both anxiety and depression symptoms uniquely predicted onset of heart conditions, stroke, and high blood pressure, indicating that depression does not explain the link between anxiety symptoms and medical illness onset. Thus, anxiety has additional predictive utility over and above depression, providing evidence that assessment of both anxiety and depression symptoms may more reliably indicate risk compared with depression alone.
Anxiety and depression symptoms were also strong predictors of worsening somatic symptoms, which extends prior findings from cross-sectional samples (Haug et al., 2004; Kroenke et al., 1997; Simon & VonKorff, 1991; Simon et al., 1999) and shows that effects are maintained in longitudinal analyses that control for baseline somatic symptom reporting. It is important to note that somatic symptoms in the present analysis were entirely subjective and were not meant to indicate a diagnosable condition or disease. Thus, somatic complaints may be manifestations of anxiety or depression symptoms, which could explain the relatively greater strength of the association between anxiety and depression and somatic symptoms compared with medical conditions. Regardless, for patients presenting in primary care with somatic complaints such as back pain, migraines, or stomach distress, it may be difficult for a provider to identify the etiology. Because of stigma regarding mental illness and treatment, and fear of communicating to patients that symptoms are not real, doctors may be reticent to suggest mental health treatment. However, evidence supports biological pathways that could explain causal relationships between depression and anxiety symptoms and physical pain and discomfort (e.g., Campbell, Clauw, & Keefe, 2003; Collins, Surette, & Bercik, 2012), and cognitive-behavioral therapy has been shown to reduce medically unexplained symptoms (for a meta-analysis, see Menon, Rajan, Kuppili, & Sarkar, 2017). Because somatic complaints comprise a large portion of presenting problems in primary care settings, and often prove difficult to treat using strictly medical interventions, greater normalization and integration of mental health treatment in primary care could expand potential treatment options.
One important limitation of our study is the reliance on self-report. We were therefore unable to explore relationships of clinician-diagnosed anxiety and depression with physical health. However, the prevalence of high anxiety and depression in our sample (greater than 1 SD from the mean) at 15.8% is consistent with estimates of the prevalence of these conditions in the population (Kessler et al., 2005). In terms of medical illnesses, although participants were asked about physician-diagnosed illnesses, findings are mixed on whether people are able to accurately report medical diagnoses (Okura, Urban, Mahoney, Jacobsen, & Rode-heffer, 2004; Smith et al., 2008; St Clair et al., 2017). Patients with elevated anxiety and depression symptoms may be more likely to report being diagnosed with medical conditions, which could partially explain the current findings. However, we examined new onset of medical conditions. Thus, reporting bias should be controlled for by accounting for conditions reported at the initial time point. Nonetheless, these findings should be replicated using more objective indicators of disease. Second, as a result of the response options available in HRS, our indicator of “physically active” was quite liberal, defined as engaging in vigorous physical activity more than once per week. A more conservative definition may have explained additional variance in the outcome, thereby reducing the strength of the relationship between anxiety and depression symptoms with physical health indices. Third, we selected obesity and smoking as comparators because these two are such widely accepted risk factors for ill health. Other potential comparators are physical inactivity and excessive drinking. Finally, participants with complete data generally were healthier than participants with data available only at the first time point, and it is also possible that those who remained in the study long enough to complete the assessments in 2006 and 2008 may have differed from participants who dropped from the study prior to these time points. If participants with more severe depression and anxiety symptoms and with more severe medical comorbidity were more likely to drop from the study, it is unlikely that the attrition of such participants would increase the strength of the association examined in the present study. In fact, because of the attrition of these individuals, the present results may underestimate the strength of this association by potentially reducing variability in the measures.
Conclusion
In the current study, we showed that high levels of anxiety and depression symptoms are comparable with obesity and smoking as predictors of disease and somatic symptom onset. These findings suggest that anxiety and depression symptoms deserve greater attention in primary care settings as important risk factors for medical illness and somatic symptoms. Second, we found that anxiety and depression each contributed uniquely to disease onset, indicating that anxiety has been neglected in the literature as an important risk factor for disease. Few patients presenting with these mental health conditions in primary care are adequately assessed, and even fewer receive evidence-based treatments (Stein et al., 2011; J. W. Williams et al., 1999). Although further research is needed on the effect of empirically supported treatments for anxiety and depression on physical health outcomes, it is possible that such treatments could prevent onset of health conditions and alleviate distress associated with somatic symptoms that frequently require far more costly and invasive treatment procedures.
Supplementary Material
Acknowledgments
Andrea N. Niles is supported by the U.S. Department of Veteran’s Affairs Women’s Health Fellowship.
References
- Aguilar-Gaxiola S, Loera G, Geraghty EM, Ton H, Lim CC, de Jonge P, … Scott KM (2016). Associations between DSM-IV mental disorders and subsequent onset of arthritis. Journal of Psychosomatic Research, 82, 11–16. http://dx.doi.org/10.10167j.jpsychores.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgöwer A, Wardle J, & Steptoe A (2001). Depressive symptoms, social support, and personal health behaviors in young men and women. Health Psychology, 20, 223–227. 10.1037/0278-6133.20.3.223 [DOI] [PubMed] [Google Scholar]
- Allison P (2012). Handling missing data by maximum likelihood. SAS Global Forum Retrieved from https://statisticalhorizons.com/wp-content/uploads/MissingDataByML.pdf [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jorgensen P, & Goodwin RD (2015). Depression and the risk of autoimmune disease: A nationally representative, prospective longitudinal study. Psychological Medicine, 45, 3559–3569. 10.1017/S0033291715001488 [DOI] [PubMed] [Google Scholar]
- Archer G, Pikhart H, & Head J (2015). Do depressive symptoms predict cancer incidence?: 17-year follow-up of the Whitehall II study. Journal of Psychosomatic Research, 79, 595–603. 10.1016/j.jpsychores.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56, 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Bruce EC, & Musselman DL (2005). Depression, alterations in platelet function, and ischemic heart disease. Psychosomatic Medicine, 67, S34–S36. 10.1097/01.psy.0000164227.63647.d9 [DOI] [PubMed] [Google Scholar]
- Campbell LC, Clauw DJ, & Keefe FJ (2003). Persistent pain and depression: A biopsychosocial perspective. Biological Psychiatry, 54, 399–409. 10.1016/S0006-3223(03)00545-6 [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, & Bercik P (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology, 10, 735–742. 10.1038/nrmicro2876 [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services & U.S. Department of Agriculture. (2015). 2015–2020 Dietary guidelines for Americans (8th ed). Washington, DC: Author. [Google Scholar]
- Eaker ED, Pinsky J, & Castelli WP (1992). Myocardial infarction and coronary death among women: Psychosocial predictors from a 20-year follow-up of women in the Framingham Study. American Journal of Epidemiology, 135, 854–864. 10.1093/oxfordjournals.aje.a116381 [DOI] [PubMed] [Google Scholar]
- Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB Sr., & Benjamin EJ (2005). Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: The Framingham Offspring Study. Psychosomatic Medicine, 67, 692–696. 10.1097/01.psy.0000174050.87193.96 [DOI] [PubMed] [Google Scholar]
- El-Gabalawy R, Mackenzie CS, Pietrzak RH, & Sareen J (2014). A longitudinal examination of anxiety disorders and physical health conditions in a nationally representative sample of U.S. older adults. Experimental Gerontology, 60, 46–56. http://dx.doi.org/10.1016Zj.exger.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Engum A (2007). The role of depression and anxiety in onset of diabetes in a large population-based study. Journal of Psychosomatic Research, 62, 31–38. 10.1016/j.jpsychores.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Eriksen HR, Svendsrod R, Ursin G, & Ursin H (1998). Prevalence of subjective health complaints in the Nordic European countries in 1993. European Journal of Public Health, 8, 294–298. 10.1093/eurpub/8.4.294 [DOI] [Google Scholar]
- Euesden J, Danese A, Lewis CM, & Maughan B (2017). A bidirectional relationship between depression and the autoimmune disorders - New perspectives from the National Child Development Study. PLoS ONE, 12, e0173015 10.1371/journal.pone.0173015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farvid MS, Qi L, Hu FB, Kawachi I, Okereke OI, Kubzansky LD, & Willett WC (2014). Phobic anxiety symptom scores and incidence of type 2 diabetes in U.S. men and women. Brain, Behavior, and Immunity, 36, 176–182. http://dx.doi.org/10.1016Zj.bbi.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluegge KR (2016). Comorbidities among persons with incident psychiatric condition. Gerontology & Geriatric Medicine, 2, 233372141663500 10.1177/2333721416635001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EL, Comstock GW, & Graves CG (1980). Psychosocial factors and blood pressure. Psychological Medicine, 10, 243–255. 10.1017/S0033291700044007 [DOI] [PubMed] [Google Scholar]
- Haug TT, Mykletun A, & Dahl AA (2004). The association between anxiety, depression, and somatic symptoms in a large population: The HUNT-II study. Psychosomatic Medicine, 66, 845–851. 10.1097/01.psy.0000145823.85658.0c [DOI] [PubMed] [Google Scholar]
- Heeringa SG, & Conner J (1995). Technical description of the Health and Retirement Study sample design. Ann Arbor, MI: Institute for Social Research, University of Michigan; 10.7826/ISR-UM.06.585031.001.05.0001.1995 [DOI] [Google Scholar]
- Holt-Lunstad J, Robles TF, & Sbarra DA (2017). Advancing social connection as a public health priority in the United States. American Psychologist, 72, 517–530. 10.1037/amp0000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK, … Tworoger SS (2015). Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecologic Oncology, 139, 481–486. 10.1016/j.ygyno.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky I, Ahnve S, Lundberg I, & Hemmingsson T (2010). Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. Journal of the American College of Cardiology, 56, 31–37. 10.1016/jjacc.2010.03.033 [DOI] [PubMed] [Google Scholar]
- Jonas BS, Franks P, & Ingram DD (1997). Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Archives of Family Medicine, 6, 43–49. 10.1001/archfami.6.1.43 [DOI] [PubMed] [Google Scholar]
- Jonas BS, & Mussolino ME (2000). Symptoms of depression as a prospective risk factor for stroke. Psychosomatic Medicine, 62, 463–471. 10.1097/00006842-200007000-00001 [DOI] [PubMed] [Google Scholar]
- Kang HJ, Bae KY, Kim SW, Shin HY, Shin IS, Yoon JS, & Kim JM (2017). Impact of anxiety and depression on physical health condition and disability in an elderly Korean population. Psychiatry Investigation, 14, 240–248. 10.4306/pi.2017.14.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakus MC, & Patton LC (2011). Depression and the onset of chronic illness in older adults: A 12-year prospective study. The Journal of Behavioral Health Services & Research, 38, 373–382. 10.1007/s11414-011-9234-2 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Twisk JWR, Beekman ATF, Heine RJ, Snoek FJ, & Pouwer F (2006). Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia, 49, 837–845. 10.1007/s00125-006-0159-x [DOI] [PubMed] [Google Scholar]
- Kroenke K, Jackson JL, & Chamberlin J (1997). Depressive and anxiety disorders in patients presenting with physical complaints: Clinical predictors and outcome. The American Journal of Medicine, 103, 339–347. 10.1016/S0002-9343(97)00241–6 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Linzer M, Hahn SR, deGruy FV III, & Brody D (1994). Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Archives of Family Medicine, 3, 774–779. 10.1001/archfami.3.9.774 [DOI] [PubMed] [Google Scholar]
- Lu MC, Guo HR, Lin MC, Livneh H, Lai NS, & Tsai TY (2016). Bidirectional associations between rheumatoid arthritis and depression: A nationwide longitudinal study. Scientific Reports, 6, 20647 10.1038/srep20647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, … Scharpé S (1998). Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiat-rica Scandinavica, 98, 328–335. http://dx.doi.org/10.1111Zj.1600-0447.1998.tb10092.x [DOI] [PubMed] [Google Scholar]
- Markovitz JH, Matthews KA, Kannel WB, Cobb JL, & D’Agostino RB (1993). Psychological predictors of hypertension in the Framingham Study: Is there tension in hypertension? Journal of the American Medical Association, 270, 2439–2443. 10.1001/jama.1993.03510200045030 [DOI] [PubMed] [Google Scholar]
- Martin EJ, & Nemeroff CB (2010). Diagnostic issues in depression and generalized anxiety disorder: Refining the research agenda for the DSM-V In Goldberg D, Kendler KS, & Sirovatka PJ (Eds.), Diagnostic issues in depression and generalized anxiety disorder: Refining the research agenda for DSM-V (pp. 45–70). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Maryam ML, & Shervin A (2016). Baseline depressive symptoms predict subsequent heart disease, a 20-year cohort study. International Cardiovascular Research Journal, 10, 29–34. 10.17795/icrj-10(1)29 [DOI] [Google Scholar]
- Mathews A, & MacLeod C (2005). Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology, 1, 167–195. 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- Meng L, Chen D, Yang Y, Zheng Y, & Hui R (2012). Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. Journal of Hypertension, 30, 842–851. 10.1097/HJH.0b013e32835080b7 [DOI] [PubMed] [Google Scholar]
- Menon V, Rajan TM, Kuppili PP, & Sarkar S (2017). Cognitive behavior therapy for medically unexplained symptoms: A systematic review and meta-analysis of published controlled trials. Indian Journal of Psychological Medicine, 39, 399–406. 10.4103/IJPSYM.IJPSYM_17_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles AN, Dour HJ, Stanton AL, Roy-Byrne PP, Stein MB, Sullivan G, … Craske MG (2015). Anxiety and depressive symptoms and medical illness among adults with anxiety disorders. Journal of Psychosomatic Research, 78, 109–115. 10.1016/j.jpsychores.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, & Cohen BE (2017). Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain, Behavior, and Immunity, 60, 198–205. 10.1016/j.bbi.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, & Rodeheffer RJ (2004). Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology, 57, 1096–1103. 10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, & Adler N (2013). Social isolation: A predictor of mortality comparable to traditional clinical risk factors. American Journal of Public Health, 103, 2056–2062. 10.2105/AJPH.2013.301261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole EM, Kubzansky LD, Sood AK, Okereke OI, & Tworoger SS (2016). A prospective study of phobic anxiety, risk of ovarian cancer, and survival among patients. Cancer Causes & Control, 27, 661–668. 10.1007/s10552-016-0739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Reeves KW, Okereke OI, Qian J, Tamimi RM, Eliassen AH, & Hankinson SE (2018). Depression, antidepressant use, and breast cancer risk in pre-and postmenopausal women: A prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention, 27, 306–314. 10.1158/1055-9965.EPI-17-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russek LG, King SH, Russek SJ, & Russek HI (1990). The Harvard Mastery of Stress Study 35-year follow-up: Prognostic significance of patterns of psychophysiological arousal and adaptation. Psychosomatic Medicine, 52, 271–285. 10.1097/00006842-199005000-00002 [DOI] [PubMed] [Google Scholar]
- Simon GE, & VonKorff M (1991). Somatization and psychiatric disorder in the NIMH Epidemiologic Catchment Area study. The American Journal of Psychiatry, 148, 1494–1500. 10.1176/ajp.148.11.1494 [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Piccinelli M, Fullerton C, & Ormel J (1999). An international study of the relation between somatic symptoms and depression. The New England Journal of Medicine, 341, 1329–1335. 10.1056/NEJM199910283411801 [DOI] [PubMed] [Google Scholar]
- Smith B, Chu LK, Smith TC, Amoroso PJ, Boyko EJ, Hooper TI, … Millennium Cohort Study Team. (2008). Challenges of self-reported medical conditions and electronic medical records among members of a large military cohort. BMC Medical Research Methodology, 8, 37 10.1186/1471-2288-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair P, Gaudette E, Zhao H, Tysinger B, Seyedin R, & Goldman DP (2017). Using self-reports or claims to assess disease prevalence: It’s complicated. Medical Care, 55, 782–788. 10.1097/MLR.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Roy-Byrne PP, Craske MG, Campbell-Sills L, Lang AJ, Golinelli D, … Sherbourne CD (2011). Quality of and patient satisfaction with primary health care for anxiety disorders. The Journal of Clinical Psychiatry, 72, 970–976. 10.4088/JCP.09m05626blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik JJMH, Denollet J, Lousberg R, & Honig A (2003). Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. Journal of the American College of Cardiology, 42, 1801–1807. 10.1016/j.jacc.2003.07.007 [DOI] [PubMed] [Google Scholar]
- Strine TW, Chapman DP, Kobau R, & Balluz L (2005). Associations of self-reported anxiety symptoms with health-related quality of life and health behaviors. Social Psychiatry and Psychiatric Epidemiology, 40, 432–438. 10.1007/s00127-005-0914-1 [DOI] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Kabrhel C, Roberts AL, Chen Q, Winning A, ... Koenen KC (2016). Associations of trauma exposure and posttraumatic stress symptoms with venous thromboembolism over 22 years in women. Journal of the American Heart Association, 5(5), e003197 10.1161/JAHA.116.003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellnes G, Svendsen K-OB, Bruusgaard D, & Bjerkedal T (1989). Incidence of sickness certification. Proposal for use as a health status indicator. Scandinavian Journal of Primary Health Care, 7, 111–117. 10.3109/02813438909088657 [DOI] [PubMed] [Google Scholar]
- Thayer JF (2006). On the importance of inhibition: Central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose-Response, 4, 2–21. 10.2203/dose-response.004.01.002.Thayer [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, & Melamed S (2005). The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. Journal of Occupational Health Psychology, 10, 344–362. 10.1037/1076-8998.10.4.344 [DOI] [PubMed] [Google Scholar]
- Vimalananda VG, Palmer JR, Gerlovin H, Wise LA, Rosenzweig JL, Rosenberg L, & Ruiz-Narvaez EA (2014). Depressive symptoms, antidepressant use, and the incidence of diabetes in the Black Women’s Health Study. Diabetes Care, 37, 2211–2217. 10.2337/dc13-2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW Jr., Rost K, Dietrich AJ, Ciotti MC, Zyzanski SJ, & Cornell J (1999). Primary care physicians’ approach to depressive disorders. Effects of physician specialty and practice structure. Archives of Family Medicine, 8, 58–67. 10.1001/archfami.8.1.58 [DOI] [PubMed] [Google Scholar]
- Williams RL (2000). A note on robust variance estimation for cluster-correlated data. Biometrics, 56, 645–646. 10.1111/].0006-341X.2000.00645.x [DOI] [PubMed] [Google Scholar]
- Xiang X, & An R (2015). Depression and onset of cardiovascular disease in the U.S. middle-aged and older adults. Aging & Mental Health, 19, 1084–1092. 10.1080/13607863.2014.1003281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


