Abstract
Objective:
To evaluate the clinical performance of contrast-enhanced spectral mammography (CESM) on asymmetries detected on a mammogram (MG).
Methods:
This study was approved by the Scientific Research Review Board of the Radiology Department, and waiver of informed consent was applied for the uses of data of the included cases. The study included 125 female patients,33 (26.4%) who presented for screening and 92 (73.6%) who presented for a diagnostic MG. All had breast asymmetries on MG. Ultrasound examination and CESM using dual-energy acquisitions were performed for all patients.
Results:
In all, 88/125 (70.4%) females had focal asymmetry (seen in two views and occupying less than a quadrant), 26/125 (20.8%) had global asymmetry (occupying more than one quadrant), 10/125 (8%) had asymmetry (seen in a single view and occupying less than a quadrant), and 1/125 had developing asymmetry (0.8%) (not present in the previous MG). Malignant lesions represented 91 cases, benign lesions represented 30 cases, and 4 cases were high-risk lesions. CESM sensitivity was 100% (v s 97.8 % for sono-mammography), specificity was 55.88% (v s 81.8% for sono-mammography), and the positive- and negative-predictive values were 85.85 and 100% (v s 93.7 and 93% for sono-mammography respectively) .
Conclusion:
In our study, we conclude that focal and global asymmetries with other suspicious mammographic findings were statistically significant for malignancy and CESM played an important role in delineating tumor size and extension. Any non-enhancing asymmetrical density correlated with a benign pathology, if not associated with other suspicious imaging findings.
Advances in knowledge:
Our study is the first to explore the added value of CESM to asymmetries detected in screening and diagnostic mammography.
Introduction
Screening and diagnostic mammography assessments encounter many presentations as masses, calcifications, distortion and asymmetries. Asymmetry commonly seen in healthy females, however in some cases it may be a presentation of underlying malignant disease. Thus, thoroughly reviewing mammograms is crucial when breast asymmetry is present.1
The Breast Imaging Reporting and Data System has set definitions related to breast asymmetry; focal asymmetry is when the same features are observable on standard mammographic views, occupying less than a single quadrant, but lacking convex margins and containing interspersed fat. Asymmetry shares similarities with focal asymmetry, yet it is only visible on one of the standard mammography views. Conversely, developing asymmetry is focal asymmetry not present on previous mammograms and is more conspicuous or displays increases in size.2
The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) lexicon has updated its guidelines regarding asymmetric breast findings. The nomenclature in the fourth edition replaced “asymmetric breast tissue” with “global asymmetry,” “density seen in only a single projection” with “asymmetry” and “focal asymmetric density” with “focal asymmetry.” The evaluation of a perceived asymmetry, whether it is a definite lesion or not, remains a diagnostic challenge.1
The incidence of asymmetric findings on screening mammograms varies, where focal asymmetry was reported in 0.87%,2 asymmetry was found in 3.3% and developing asymmetry was observed in 0.16% of screens, with the latter comprising 0.11% of diagnostic mammogram findings.3,4 Still, these lesions comprise a minor percentage of screening-detected breast malignancy.
Digital mammography is considered the most consistent imaging modality utilized in the early detection of breast cancer. Dense breasts still represent a challenge, as they are associated with limited mammographic sensitivity and specificity when detecting and characterizing breast lesions.3 Contrast-enhanced spectral mammography (CESM) is an evolving technology budding from digital mammography; it includes information from tumor angiogenesis to improve the sensitivity of breast lesion characterization.4
Contrast-enhanced MRI (MRI) is currently considered a sensitive imaging tool for breast cancer detection, but it carries the burdens of limited availability and high costs. Conversely, CESM is a rapid scanning technique and is available in the mammography suite, thus saving time and there is no need for appointment reservation.5 In our study, we evaluated the clinical performance of CESM on asymmetry detected during mammography.
Methods and materials
Patients
This study is a retrospective analysis that included 125 females, 33 (26.4%) of whom presented for screening and 92 (73.6%) of whom were symptomatic and referred from the clinic for a diagnostic mammogram in the period spanning from March 2015 to March 2016. The patients’ ages ranged from 25 to 81 years (mean: 48.87 years). None of the patients were treated with hormone replacement therapy. The study was approved by “Baheya Centre for Early Detection and Treatment of Breast Cancer” ethics committee and all enrolled patients provided their informed consent. During the mammogram, contrast injection was used to further evaluate any detected asymmetries. Patients with renal impairment, pregnant patients, and those with a history of allergy to contrast media were excluded from the study. The remaining patients were eligible to undergo CESM, the requirement to obtain informed consent was waived by our ethics committee. Compression magnification views were applied and revealed fibroglandular tissue in five cases presenting with focal asymmetries and these patients were scheduled for annual follow-up. Complementary ultrasound examination was performed for all cases. In our study, the reference standard was histopathology after ultrasound-guided true-cut biopsy and follow up for five cases.
Contrast-enhanced spectral mammography system
Dual-energy (CESM)was performed using Senographe Essential, (Seno DS; GE, Buc, France) which obtained low-energy images that were comparable to the standard mammography image, and high-energy images were also acquired to show the contrast-enhanced areas for each mammography view.
Examination technique
The examination was performed with a digital mammography device developed by GE Healthcare; it allowed for dual-energy CESM image acquisition by means of an intravenous injection of an iodinated contrast agent (iohexol, 300 mg I/mL) at a dose of 1.5 mL/kg before the application of breast compression. This was followed by a 2 min wait prior to a mammography exam and was performed using the two standard positions (craniocaudal and mediolateral oblique views). Low- and high-energy images were consecutively acquired in each view. Low-energy images were acquired at peak kilovoltage values ranging from 26 to 31 kVp, which is below the K-edge of iodine. High-energy images were acquired at 45–49 kVp, which is above the K-edge of iodine. Enhanced images were calculated by weighted logarithmic subtraction of the two images through appropriate image processing, thus reducing the visibility of the parenchyma and generating contrast-enhanced images.
Iimage analysis
The analysis of sono-mammography and CESM images was performed using two different dedicated breast radiologists. The radiologists provided a BI-RADS classification for conventional mammography and ultrasound examination using the BI-RADS lexicon designed by the American College of Radiology.5 Subsequently, CESM images were viewed and the radiologists were allowed to up- or downgrade their BI-RADS classification. Images were analyzed with respect to:
Localization and type of asymmetry.
Associated distortion, microcalcifications, and skin and nipple changes.
Assessment of ultrasound-detected masses regarding their number, shape, margins, and echogenicity. Any parenchymal heterogenicity and enlarged axillary lymph nodes were assessed. Any asymmetrical breast density associated with other suspicious mammographic or ultrasonographic findings was considered suspicious.
Regarding CESM images, the presence or absence of contrast enhancement of asymmetrical density was assessed. In case of enhancement, its morphology——whether as a mass (margins, enhancement pattern) or non-mass (ductal, segmental, regional, or diffuse enhancement) was analyzed. The assessment also included the presence of other enhancing lesions in the same breast or on the other side. An ill-defined mass with heterogeneous enhancement or non-mass enhancement (ductal, segmental, regional, or unilateral diffuse) were considered suspicious and were categorized as BI-RADS four lesions. Well-defined homogenously enhancing masses or non-enhancing asymmetries that were not associated with other suspicious mammographic findings were scored on a scale of 1–3 and were considered benign.
Statistical analysis
Data were coded and entered using the Statistical Package for the Social Sciences (SPSS) v. 24 (IBM Corporation, Armonk, NY). Data were summarized using the mean, standard deviation, median, minimum, and maximum for quantitative data, while frequency (count) and relative frequency (percentage) were used for categorical data. Standard diagnostic indices including sensitivity, specificity, positive-predictive value (PPV), and negative-predictive value (NPV) were calculated as described by Galen. To compare categorical data, a χ2 test was performed. This test was used when the expected frequency was less than 5 (Chan, 2003). A p-value < 0.05 was considered statistically significant.
results
Classification of asymmetries
Our study included 125 females; 88/125 (70.4%) females had focal asymmetry (seen in two views and occupying less than one quadrant), 26/125 (20.8%) had global asymmetry (occupying more than one quadrant), 10/125 (8%) had asymmetry (seen in a single view and occupying less than one quadrant), and 1/125 had developing asymmetry (0.8%); not present in the previous mammogram.
Malignant lesions represented 91 cases, including 53 cases of invasive duct carcinoma, 16 cases of invasive lobular carcinomas (ILC), 3 cases of invasive mixed carcinomas, 2 cases of ductal carcinomas in situ,15 cases of invasive ductal carcinomas with ductal carcinomas in situ, 1 case of mucinous carcinoma and 1 case of tubular carcinoma. Four high-risk lesions were recorded, including three atypical ductal hyperplasias and one papilloma. Benign lesions represented 30 cases, including 5 granulomatous mastitis, 5 abscess cavities, 3 mastopathy, 3 fibroadenoma, 3 ductal hyperplasia, 2 fibrocystic changes 2 fat necrosis, 1 periductal mastitis and 1 fibroadenomatoid changes. Five cases of asymmetries were considered as condensed benign glandular tissue and were scheduled for annual follow-up (Table 1).
Table 1.
Histopathology of 125 cases of asymmetries
| Histopathologic diagnosis | Number of lesions (n = 125) |
| Malignant lesions | 91 |
| Invasive duct carcinoma | 53 |
| Invasive lobular carcinoma | 16 |
| Invasive ductal with DCIS | 15 |
| Invasive mixed carcinoma | 3 |
| DCIS | 2 |
| Mucinous carcinoma | 1 |
| Tubular carcinoma | 1 |
| High-risk lesions | 4 |
| Atypical ductal hyperplasia | 3 |
| Papilloma | 1 |
| Benign lesions | 30 |
| Granulomatous mastitis | 5 |
| Abscess | 5 |
| Mastopathy | 3 |
| Fibroadenoma | 3 |
| Ductal hyperplasia | 3 |
| Fibrocystic changes | 2 |
| Fat necrosis | 2 |
| Fibroadenomatoid changes | 1 |
| Periductal mastitis | 1 |
| Benign tissue | 5 |
DCIS, ductal carcinoma in situ.
Further, 23 (26.1%) cases of focal asymmetry were benign and 65 cases (73.9%) were malignant. 7 (26.9%) out of 26 cases of global asymmetry were benign and 19 (73.1%) were malignant. Four cases of asymmetry (40%) were benign and six (60%) were malignant. The only case of developing asymmetry was malignant (100%) (Table 2).
Table 2.
Asymmetrical densities and their correlation to their final diagnosis
| Asymmetry | Benign | Malignant | Total |
| Focal | 23 (26.1%) | 65 (73.9%) | 88 (70.4%) |
| Global | 7 (26.9%) | 19 (73.1%) | 26% (20.8%) |
| Asymmetry | 4 (40%) | 6 (60%) | 10 (8%) |
| Developing | 0 (0%) | 1 (100%) | 1 (0.8%) |
| Total | 34 (27.2%) | 91 (72.8%) | 125 (100%) |
Other mammographic findings
Other mammographic findings associated with asymmetrical densities and their correlation with the final diagnosis are listed in Table 3. Among the associated mammographic findings, there was a significant correlation between focal asymmetry associated with distortion, suspicious calcification, skin/nipple changes and malignancy. Focal asymmetries with no other associated mammographic findings were significantly correlated with a benign pathology (p ≤ 0.001).
Table 3.
Mammographic findings of asymmetries and their correlation to the final diagnosis
| Other associated mammographic findings | Asymmetries | |||||||
| Asymmetry | Focal | Global | Developing | |||||
| Benign | Malignant | Benign | Malignant | Benign | Malignant | Benign | Malignant | |
| Distortion | 0 | 2 | 5 | *34 | 1 | 6 | 0 | 0 |
| 0% | −2.20% | −14.70% | −37.40% | −2.90% | −6.60% | 0% | 0% | |
| Suspicious calcifications | 0 | 1 | 0 | *11 | 0 | 5 | 0 | 0 |
| 0% | −1.10% | 0% | −12.10% | 0% | −5.50% | 0% | 0% | |
| Skin/nipple changes | 2 | 1 | 5 | *31 | 3 | 16 | 0 | 0 |
| −5.90% | −1.10% | −14.70% | −34.10% | −8.80% | −17.60% | 0% | 0% | |
| Axillary LN | 1 | 1 | 0 | 13 | 1 | 4 | 0 | 0 |
| −2.90% | −1.10% | 0% | −14.30% | −2.90% | −4.40% | 0% | 0% | |
| No other findings | 1 | 2 | *14 | 7 | 4 | 0 | 0 | 1 |
| −2.90% | −2.20% | −41.20% | −7.70% | −11.80% | 0% | 0% | −1.10% | |
LN, lymph node.
34.4%of patients who had focal asymmetry and distortion had a malignancy.
12.1%of patients who had focal asymmetry and suspicious calcifications had a malignancy.
34.1%of patients who had focal asymmetry and skin/nipple changes had a malignancy.
41.2%of patients who had focal asymmetry with no other mammographic findings were considered benign cases.
p≤0.001
Asymmetry enhancement pattern
On CESM, enhancing asymmetrical densities represented 114 (91.2%) cases. Further, 59 out of 114 cases (52%) showed mass enhancement, 10 of which (17%) were benign and 49 (83%) of which were malignant. In addition, 55 cases (48.2%) showed non-mass enhancement,13 (24%) of which were benign and 42 (76.4%) of which were malignant. Any enhancing asymmetry showing a mass or non-mass enhancement was significantly correlated with malignant pathology (p ≤ 0.001), with 15 false positive cases, as listed in Table 4.
Table 4.
Histopathology of 15 CESM false-positive cases
| Histopathologic diagnosis | Number of lesions (n = 15) |
| High -risk lesions | 4 |
| Atypical ductal hyperplasia | 3 |
| Papilloma | 1 |
| Inflammatory lesions | 8 |
| Granulomatous mastitis | 4 |
| Abscess | 3 |
| Periductal mastitis | 1 |
| Benign lesions | 3 |
| Fibrocystic mastopathy | 1 |
| Fatnecrosis | 1 |
There was a significant correlation between non-enhancing asymmetrical findings and benign pathology with no other associated suspicious mammographic findings (p ≤ 0.001); this was observed in 11 cases (8.8%). The enhancement pattern of breast asymmetries and their correlation to the final diagnosis are listed in Table 5. Focal asymmetry showing mass enhancement was significantly correlated with malignancy, while non-enhancing focal asymmetry was correlated with benign pathology (p ≤ 0.001).
Table 5.
The enhancement pattern of breast asymmetries and their correlation to a final diagnosis
| Asymmetries | ||||||||
| CESM | Asymmetry | Focal | Global | Developing | ||||
| Benign | Malignant | Benign | Malignant | Benign | Malignant | Benign | Malignant | |
| Mass | 2 | 5 | 7 | a 43 | 1 | 0 | 0 | 1 |
| −5.90% | −5.50% | −20.60% | −47.30% | −2.90% | 0% | 0% | −1.10% | |
| Non-mass | 0 | 1 | 7 | 23 | 6 | 18 | 0 | 0 |
| 0% | −1.10% | −20.60% | −25.50% | −17.60% | −19.80% | 0% | 0% | |
| No enhancement | 2 | 0 | a8 | 0 | 1 | 0 | 0 | 0 |
| −5.90% | 0% | −23.50% | 0% | −2.90% | 0% | 0% | 0% | |
CESM, contrast-enhanced spectral mammography.
47.3% of patients with malignant focal asymmetry had mass enhancement on CESM.
23.5% of patients with benign focal asymmetry had no enhancement on CESM.
p≤0.001
Other detected breast lesions on CESM
26 cases of asymmetrical density had other enhancing malignant lesions. 22 cases had enhancing multifocal/multicentric carcinoma on the same side-of the breast asymmetry and 4 cases had bilateral enhancing lesions (3 were malignant lesions and 1 was a high-risk lesion). Sono-mammography diagnosed 17 out of 26 cases of multifocal/multicentric or bilateral malignant lesions.
Diagnostic performance of sono-mammography and CESM
Sono-mammography sensitivity, specificity, PPV and NPV were 97.8%, 81.8%, 93.7%, and 93%, respectively. CESM sensitivity, specificity, PPV and NPV were 100%, 55.88%, 85.85% and 100% respectively, with 15 false-positive and no false-negative findings.
Discussion
Breast asymmetries encountered during screening and diagnostic mammographic evaluation pose a challenge for radiologists in terms of proper assessment and management.
Our study is the first to evaluate the added value of using CESM in detected breast asymmetries in 125 females over the course of 1 year.
The increased sensitivity offered by CESM was demonstrated by Jochelson et al.6 In their study, the sensitivity of mammography increased from 81 to 96%,owing to CESM. Similar observations were published by Fallenberg et al, who showed that the sensitivity of mammography (82.5%) increased to 100% owing to CESM.7 The study of Lobbes et al also showed an increase in the sensitivity of CESM to 100%, specificity to 87.7%, PPV to 76.2% and NPV to 100%. The NPV of 100% found in this study population suggests that a negative CESM can rule out breast cancer.8 The study of Tardivel et al, also stated that the high CESM NPV was of great help when resolving cases with indeterminate lesions (i.e. BI-RADS 3 or 4a) by avoiding the additional need for biopsy;9 this matched the high sensitivity and high NPV of CESM. All non- enhancing asymmetries in our study were of benign histopathology (p ≤ 0.001). Also, in our study, non-enhancing focal and global asymmetries on CESM with no associated suspicious mammographic findings were of benign pathology (p ≤ 0.001). From this, we emphasize that any non-enhancing asymmetry on CESM that do not feature any other associated suspicious mammographic findings can be scheduled for follow up rather than biopsy.
Dromain et al were able to demonstrate the depiction of tumor angiogenesis of breast cancer independent of histologic type.10 Initial clinical trials,10–13 and the findings that emerged from the study of Tradivel et al, have described same false-positives9 that were encountered in the present study, including fibrocystic changes, atypical ductal hyperplasia, adenosis, and steatonecrosis. Bhimani et al, reported that CESM has false-positive results that are similar to MRI.14 15 false-positive findings were present in our study. 10 of which were categorized as benign findings by ultrasound examination (Figure 1). Thus, target ultrasound examination can categorize asymmetrical mammographic density as benign findings, thus reducing the need for further imaging studies and reducing biopsy rates.
Figure 1.
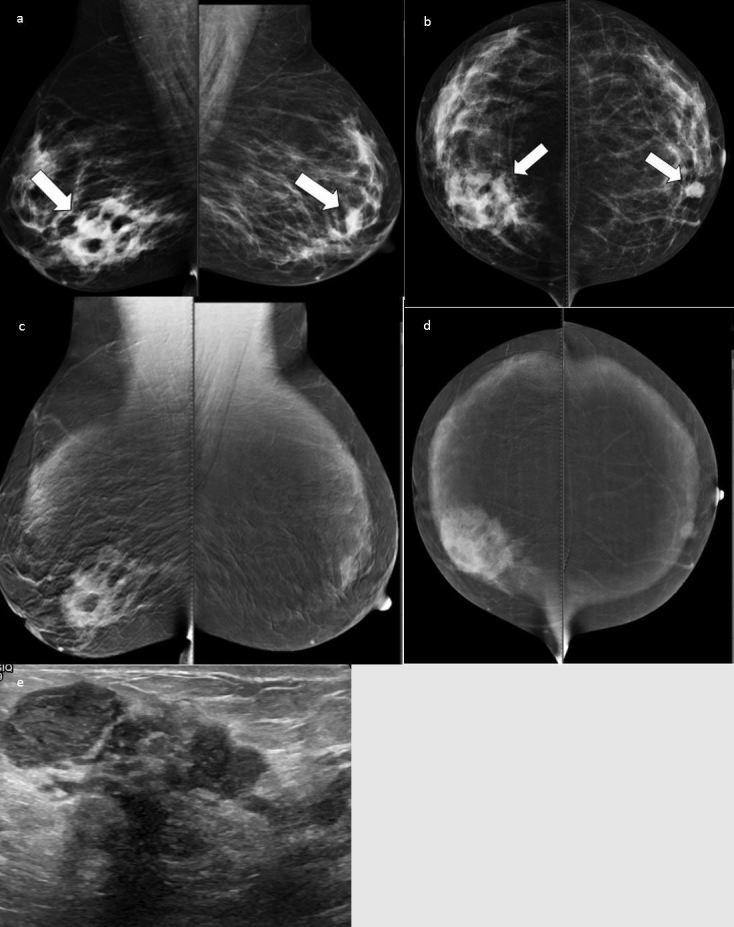
Low-energy images showing focal asymmetry at the right lower inner quadrant, a small well-circumscribed mass at the left para-areolar region is also noted (a, b). High-energy level images showing an ill-defined, heterogeneous enhancing lesion corresponding to asymmetry and homogenous enhancement of the left mass (c, d). Ultrasound showed multiple localized collections (e). The pathology was right granulomatous mastitis and left fibroadenoma.
Focal asymmetry was the most frequently encountered asymmetrical density in the present study. Harvey et al considered focal asymmetry as being more suspicious than global asymmetry, especially if companion parenchymal distortion is present.15 In fact, most of our focal asymmetry cases were malignant, especially in instances when the focal asymmetry was associated with suspicious mammographic findings (p ≤ 0.001). Conversely, focal asymmetry cases were more likely to be associated with a benign pathology if they did not present with other suspicious findings (p ≤ 0.001). All non- enhancing focal asymmetries were benign (p ≤ 0.001).
Most of the patients in our study with malignant focal asymmetry showed mass enhancement in our study (43 cases); these individuals were diagnosed using sono-mammography. 23 malignant cases of focal asymmetry showed non-mass enhancement, the extension and size of those depicted nonmass enhancement were better delineated by CESM when compared to sono-mammography.
Global asymmetry was the second most frequently represented asymmetrical density in our study; it was statistically correlated with benign findings if it was not associated with other suspicious findings on mammogram (Figure 2). On CESM, non -enhancing global asymmetry was associated with benign findings (p ≤ 0.001). Further, 18 out of 26 cases of global asymmetry in our study were malignant. CESM was of value in the assessment of size and extension of enhancing global asymmetry due to malignant infiltration compared to sono-mammography examination (Figure 3). The studies6–8 confirmed the potential of CESM in being a reliable alternative to breast MRI in the assessment of the extent of the disease. In our study, 26 cases had other enhancing lesions detected by CESM compared to 17 out of 26 cases detected by sono-mammography. Asymmetric density seen only in one plane, as well as developing asymmetry, were the least represented asymmetries in our study, yet CESM was able to better delineate disease extension in two out of six cases of malignant asymmetry (Figure 4). Lee et al reported a series of 86 indeterminate breast lesions in the form of asymmetric breast densities or architectural distortion, of which 39 cases (45%) were seen in one mammographic plane.17 The authors acknowledged the valuable additional role of breast MRI in solving some problematic cases identified by initial mammographic assessment. These cases warranted further histopathological diagnosis on positive MR results and follow up of mammographic surveillance on negative MR results.17
Figure 2.
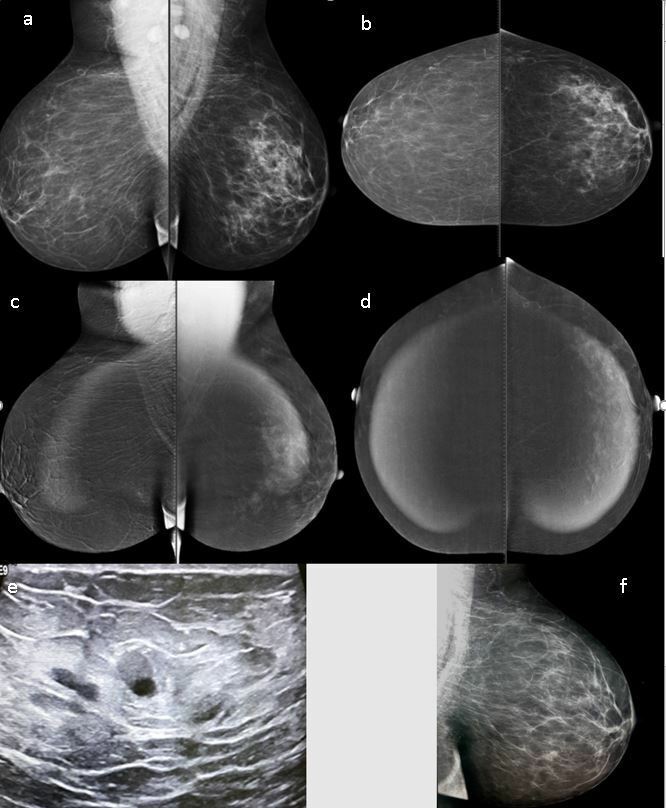
Low-energy level images (a, b) showing left global asymmetry. High-energy images (c, d) showing diffuse homogenous nonmass enhancement. On ultrasound examination no parenchymal changes are seen, and only a few small scattered cysts with partial turbid fluid contents are evident (e). At annual follow up, the left MLQ view showed almost complete resolution of the breast asymmetry (f). The pathology was fat necrosis. MLQ, mediolateral oblique.
Figure 3.
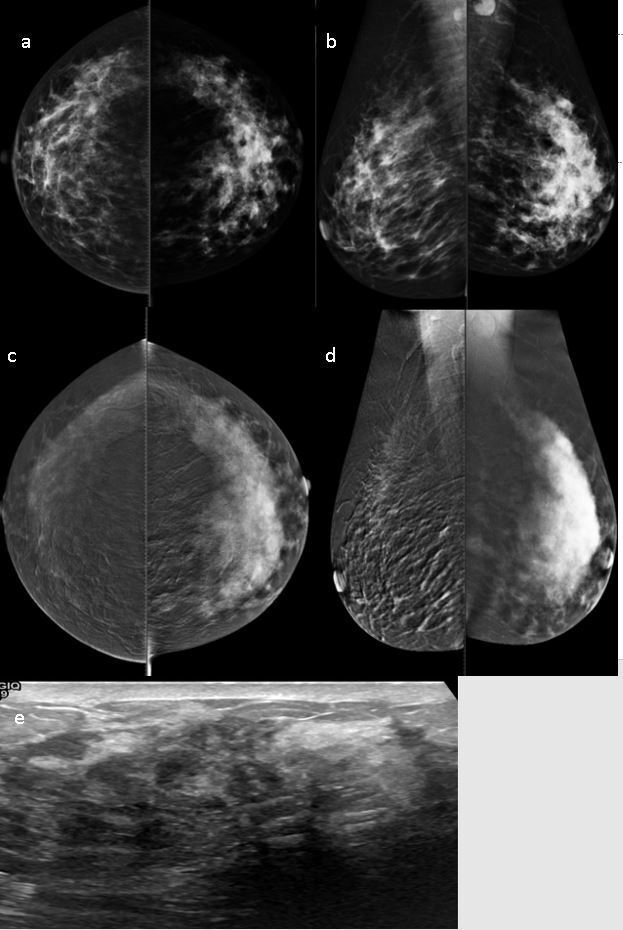
CESM showing left global asymmetry in low-energy images with minimal focal skin thickening and enlarged axillary LN (a, b). High-energy level images (c, d) showing diffuse heterogeneous nonmass enhancement with better delineations of tumor size and extension.Heterogeneous hypoechoic parenchyma with shadowing is noted on ultrasound examination (e). The pathology was IDC. CESM,contrast-enhanced spectral mammography; IDC, invasive duct carcinoma; LN,lymph node.
Figure 4.
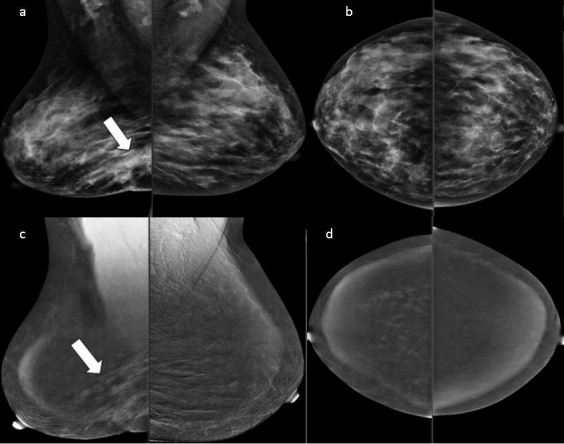
Asymmetry is seen in the lower region of the right breast with overlying skin retraction (MLQ view) (a, b). CESM showing regional clumped nonmass enhancement involving a larger area than the one seen on sono-mammography (c, d). The pathology was DCIS with microinvasion. CESM,contrast-enhanced spectral mammography; DCIS, ductal carcinomas in situ; MLQ,mediolateral oblique
In our study, we conclude that focal and global asymmetries that present with other suspicious mammographic findings were statistically significant with malignancy and CESM played important roles in the delineation of tumor size and extension. Any non-enhancing asymmetrical densities that correlated with a benign pathology did not present with other suspicious imaging findings.
Footnotes
Acknowledgment: This research was carried out at Baheya Charity Females's Cancer Hospital which is fully equipped by modern machines for breast cancer diagnosis.We want to thanks our colleagues who helped us to do such research work.
Contributor Information
Rasha Wessam, Email: rashakao@yahoo.com.
Mohammed Mohammed Mohammed Gomaa, Email: mohammedgomaa555@yahoo.com.
Mona Ahmed Fouad, Email: mona.fouad@kasralainy.edu.eg.
Sherif Mohamed Mokhtar, Email: Dr_sherifmokhtar1@yahoo.com.
Yasmin Mounir Tohamey, Email: yasminmounir@gmail.com.
REFERENCES
- 1. Youk JH , Kim E-K , Ko KH , Kim MJ . Asymmetric mammographic findings based on the fourth edition of BI-RADS: types, evaluation, and management . Radiographics 2009. ; 29 : e33 – 47 . doi: 10.1148/rg.e33 [DOI] [PubMed] [Google Scholar]
- 2. Sickles EA , D’Orsi CJ , Bassett LW . et al. . ACR BI-RADS Mammography . : Res ton , ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th . VA: : The British Institute of Radiology. ; 2013. . . 1 – 175 . [Google Scholar]
- 3. Jong RA , Yaffe MJ , Skarpathiotakis M , Shumak RS , Danjoux NM , Gunesekara A , et al. . Contrast-enhanced digital mammography: initial clinical experience . Radiology 2003. ; 228 : 842 – 50 . doi: 10.1148/radiol.2283020961 [DOI] [PubMed] [Google Scholar]
- 4. Hill ML , Mainprize JG , Carton A-K , Muller S , Ebrahimi M , Jong RA , et al. . Anatomical noise in contrast-enhanced digital mammography. Part I. Single-energy imaging . Med Phys 2013. ; 40 : 051910 . doi: 10.1118/1.4801905 [DOI] [PubMed] [Google Scholar]
- 5. Dromain C , Thibault F , Muller S , Rimareix F , Delaloge S , Tardivon A , et al. . Dual-energy contrast-enhanced digital mammography: initial clinical results . Eur Radiol 2011. ; 21 : 565 – 74 . doi: 10.1007/s00330-010-1944-y [DOI] [PubMed] [Google Scholar]
- 6. Jochelson MS , Dershaw DD , Sung JS , Heerdt AS , Thornton C , Moskowitz CS , et al. . Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma . Radiology 2013. ; 266 : 743 – 51 . doi: 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fallenberg EM , Dromain C , Diekmann F , Engelken F , Krohn M , Singh JM , et al. . Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size . Eur Radiol 2014. ; 24 : 256 – 64 . doi: 10.1007/s00330-013-3007-7 [DOI] [PubMed] [Google Scholar]
- 8. Lobbes MBI , Lalji U , Houwers J , Nijssen EC , Nelemans PJ , van Roozendaal L , et al. . Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme . Eur Radiol 2014. ; 24 : 1668 – 76 . doi: 10.1007/s00330-014-3154-5 [DOI] [PubMed] [Google Scholar]
- 9. Tardivel A-M , Balleyguier C , Dunant A , Delaloge S , Mazouni C , Mathieu M-C , et al. . Added value of contrast-enhanced spectral mammography in Postscreening assessment . Breast J 2016. ; 22 : 520 – 8 . doi: 10.1111/tbj.12627 [DOI] [PubMed] [Google Scholar]
- 10. Dromain C , Balleyguier C , Muller S , Mathieu M-C , Rochard F , Opolon P , et al. . Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography . AJR Am J Roentgenol 2006. ; 187 : W528 – W537 . doi: 10.2214/AJR.05.1944 [DOI] [PubMed] [Google Scholar]
- 11. Dromain C , Thibault F , Muller S , Rimareix F , Delaloge S , Tardivon A , et al. . Dual-energy contrast-enhanced digital mammography: initial clinical results . Eur Radiol 2011. ; 21 : 565 – 74 . doi: 10.1007/s00330-010-1944-y [DOI] [PubMed] [Google Scholar]
- 12. Lewin JM , Isaacs PK , Vance V , Larke FJ . Dual-energy contrast-enhanced digital subtraction mammography: feasibility . Radiology 2003. ; 229 : 261 – 8 . doi: 10.1148/radiol.2291021276 [DOI] [PubMed] [Google Scholar]
- 13. Jong RA , Yaffe MJ , Skarpathiotakis M , Shumak RS , Danjoux NM , Gunesekara A , et al. . Contrast-enhanced digital mammography: initial clinical experience . Radiology 2003. ; 228 : 842 – 50 . doi: 10.1148/radiol.2283020961 [DOI] [PubMed] [Google Scholar]
- 14. Bhimani C , Matta D , Roth RG , Liao L , Tinney E , Brill K , et al. . Contrast-enhanced spectral mammography: technique, indications, and clinical applications . Acad Radiol 2017. ; 24 : 84 – 8 . doi: 10.1016/j.acra.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 15. Harvey JA , Nicholson BT , Cohen MA . Finding early invasive breast cancers . Radiology 2008. ; 248 : 61 – 76 . [DOI] [PubMed] [Google Scholar]
- 16. Chesebro AL , Winkler NS , Birdwell RL , Giess CS . Developing asymmetries at mammography: a multimodality approach to assessment and management . Radiographics 2016. ; 36 : 322 – 34 . doi: 10.1148/rg.2016150123 [DOI] [PubMed] [Google Scholar]
- 17. Lee CH , Smith RC , Levine JA , Troiano RN , Tocino I . Clinical usefulness of MR imaging of the breast in the evaluation of the problematic mammogram . AJR Am J Roentgenol 1999. ; 173 : 1323 – 9 . doi: 10.2214/ajr.173.5.10541112 [DOI] [PubMed] [Google Scholar]


