Abstract
CT-based radiotherapy workflow is limited by poor soft tissue definition in the pelvis and reliance on rigid registration methods. Current image-guided radiotherapy and adaptive radiotherapy models therefore have limited ability to improve clinical outcomes. The advent of MRI-guided radiotherapy solutions provides the opportunity to overcome these limitations with the potential to deliver online real-time MRI-based plan adaptation on a daily basis, a true “plan of the day.” This review describes the application of MRI guided radiotherapy in two pelvic tumour sites likely to benefit from this approach.
Introduction
Multiple challenges exist in radiotherapy (RT) delivery for gynaecological and rectal targets. The target consists of volumes encompassing the primary tumour and elective nodal regions, which are difficult to visualize on CT and move independently of each other. Tumour targets are highly mobile deformable structures and are influenced by adjacent rectal and bladder filling, which is difficult to standardize throughout treatment. Substantial tumour regression can occur, which results in normal tissue falling into high dose regions, and extended field treatments are susceptible to rotational setup error.1 Intensity modulated radiotherapy (IMRT) reduces dose to normal tissue in gynaecological and rectal RT,2,3 but tight conformity and sharp dose gradients mean that adequate planning target volume (PTV) safety margins to account for geometric uncertainty are essential to avoid a geographical miss.
The current PTV margins applied to targets are based on margin recipes that aim to ensure 95% of the prescribed dose is delivered to 99% of the target volume,4 or 95% of the prescribed dose is delivered to 100% of the target volume in 90% of patients.5 Significant interpatient variability in target motion results in population-based margins that are much larger than necessary in most patients and still miss the target in a small number of cases. The alternative to large margins and increased normal tissue dose is to individualize margins and implement adaptive treatment strategies.
RT is currently planned on a single CT data set obtained at treatment simulation. This may not reflect target and organ at risk (OAR) geometry at the time of treatment delivery. Adaptive radiotherapy (ART) uses information from imaging acquired before or during treatment delivery to modify the treatment plan based on changes in individual target and OAR geometry and biology. Adaptive strategies are classified based on their timescale relative to patient treatment.6 Offline strategies occur between treatment fractions and typically involve a single or multiple replans. Online adaptation is based on imaging acquired immediately prior to treatment and can be used daily or intermittently. In online adaptation, tumour target and OAR interfraction changes are accounted for, which means that PTV margins can be significantly reduced.7 Adaptive strategies can also use information from previous treatment imaging to track the actual dose delivered to the tumour target and OARs and correct for any discrepancy between the planned and delivered dose distributions.8 Implementation of online adaptive strategies is limited by technical challenges, which include image quality, image registration, target and OAR segmentation, and plan reoptimization. All of which, must be performed whilst the patient remains on the treatment couch in treatment position.
Currently, image-guided RT with cone beam CT (CBCT) is limited by its ability to visualize the target and OARs and by artefact from moving gas. MRI is the gold-standard imaging modality for diagnosis and staging in gynaecological and rectal cancer and transition from CT-based to MR-based workflow in these tumour sites offers immediate advantages. MRI-guided RT (MRIgRT) will provide superior image quality at treatment planning and treatment delivery for image registration and target and OAR localization and segmentation. This will facilitate implementation of online adaptive strategies to reduce normal tissue irradiation, whilst improving target coverage. The purpose of this article is to review the advantages and challenges in the clinical application of MRIgRT in RT treatment planning and treatment adaptation using rectal and gynaecological cancers as illustrative examples.
Search/ selection strategy
PubMed was searched using terms “Rectal Neoplasms/radiotherapy”[Mesh] or “Uterine Cervical Neoplasms/radiotherapy”[Mesh] or “Endometrial Neoplasms/radiotherapy”[Mesh] and “motion” or “adaptive” or “MR-guided” or “auto segmentation” or “auto contouring”. Search included meeting abstracts and was limited to English language. Further references were identified by cross-reference of articles. Identified studies were first screened by title and/or abstract, with further full paper screening to generate the final list of studies relevant to the scope of the present review. The last PubMed search was performed on 5 April 2018.
Rationale for MRI-guided adaptive radiotherapy (MRIgART) in gynaecological and rectal cancer
MRI is the imaging modality of choice for diagnosis and staging in gynaecological and rectal cancer where it characterizes tumour and local macroscopic extent to inform treatment decisions, assess treatment response and detect recurrent disease.9–11 It is essential in identifying patients for radiation treatment, determining the radiation treatment field extent and accurate definition of the tumour target from bladder, sigmoid and small bowel.
1. MRI improves target localization
Target volume delineation on the planning CT in both gynaecological and rectal tumours is difficult because it is not possible to discriminate between tumour and normal tissue. Figures 1 and 2 illustrate improved soft tissue contrast seen on MRI compared to CT for RT treatment planning in rectal and cervix cancer. Compared to CT, target volume delineation on MRI results in significantly smaller rectal and cervix volumes12,13 and low interobserver variability14,15 .Studies evaluating inter- and intraobserver variability in contour delineation on MRI in gynaecological and rectal RT are illustrated in Tables 1 and 2.12–23 In rectal RT, MRI delineation results in significantly reduced tumour length, width and distance of the proximal tumour edge to the anal verge p < 0.05.12 When gross tumour volume (GTV) is subdivided into tumour located in the sigmoid, rectal and anal sub regions, coverage of the CT contoured GTV was inadequate for tumours with MRI evidence of sigmoid or anal invasion.21
Figure 1.
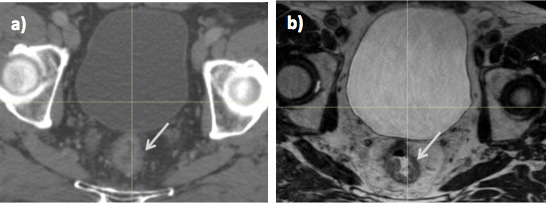
Radiotherapy planning imaging in a male patient with T3N1 rectal cancer; (a) CT and (b) MRI. On MRI, the tumour (arrow) is easily differentiated from normal rectum, which is not possible on CT.
Figure 2.
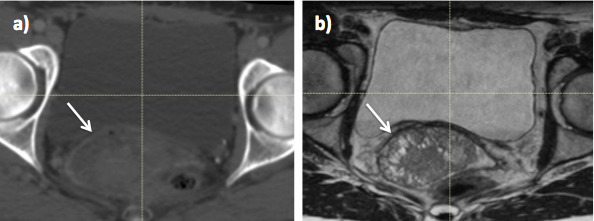
Radiotherapy planning imaging in Stage 2B cervix cancer (a) CT and (b) MRI. On MRI, the cervix tumour (arrow) is easily differentiated from normal bladder and rectum, which is not possible on CT.
Table 1.
Contour delineation on MRI for cervix cancer
| Ref | No of patients | Structures contoured/ contour guidelines used |
Method | MR sequence | Results |
| 13 | 10 | HRCTV and IRCTV GEC/ESTRO guidelines |
MRI vs CT 1 radiation oncologist |
T
2W Axial |
HRCTV height, thickness and total volume were similar Significant difference in width of HRCTV and IRCTV on CT compared to MRI Significant difference in volume of HRCTV treated to prescription dose or more (MRI 96%, CT 86% p ≤ 0.01) |
| 15 | 3 | GTV, nodal CTV, uterus and parametrium RTOG guidelines |
Interobserver variability 12 radiation oncologists |
T
2W Axial |
High GTV agreement (sensitivity 0.54–0.92, specificity 0.97–0.98) Moderate agreement for nodal CTV, uterus and parametrium (κ statistic 0.45–0.77 p < 0.0001) Contouring variability largest at cervix and vagina |
| 16 | 1 | GTV Cervix, uterus, vagina and parametrium RTOG guidelines |
Interobserver variability 19 radiation oncologists |
T 2WAxial | Good sensitivity and specificity for GVT (0.84 and 0.96 respectively) Moderate agreement for cervix, uterus and vagina (κ 0.42–0.57 P < 0.001) Parametrium good specificity 0.99 but low sensitivity 0.48 |
| 17 | 19 | GTV, HRCTV and IRCTV GEC/ESTRO guidelines |
Interobserver variability 2 radiation oncologists |
T
2W Axial |
No significant difference in mean volume of GTV and HRCTV p > 0.05 Significant difference in mean volume IRCTV p < 0.05 Conformity indices (range); GTV 0.6 (0.1–0.9), HRCTV 0.7 (0.4–0.8) and IRCTV 0.7 (0.5–0.8) |
| 18 | 6 | GTV HRCTV GEC/ESTRO guidelines |
Interobserver variability 10 radiation oncologists |
T
2W Axial |
Mean relative SD of 8–10% for GTV and HRCTV D90 Mean relative SD for D2cc was 5–8% for rectum and bladder, 11% for sigmoid |
| 19 | 13 | HRCTV GEC/ESTRO guidelines |
Interobserver variability two experienced observers |
T
2W Transverse vs para-transverse plane |
Interplane conformity index did not differ significantly between observers (0.72 v
s 0.71) Interobserver conformity index between planes was not significantly different (0.79 v s 0.78) Contouring on para-transverse plane was quicker No significant difference in DVH of plans using contours from transverse or para-transverse planes |
| 20 | 20 | Elective pelvic LN volume | MRI with iron oxide particles to delineate LNs and establish pelvic LN contouring guidelines | T 2W with administration of iron oxide particles | Blood vessels with a 7 mm margin, edited off muscle and bone, are a good surrogate target for the elective pelvic LN volume |
GEC/ESTRO, group european de curietherapie and european society for radiotherapy and oncology; GTV, gross tumour volume; HRCTV, high risk clinical target volume; IRCTV, intermediate risk clinical target volume; LN, lymph node; RTOG, radiation therapy oncology group.
Table 2.
Contour delineation on MRI for rectal cancer
| Reference | No of patients | Structures contoured | Method | MR sequence | Results |
| 12 | 10 | GTV (entire rectal wall at level of tumour) | MRI v CT (MR <2–3/52 from CT) one radiologist |
T 2W sagittal | CT overestimated all tumour radiological parameters Mean MRI GTV volume 18 cm3 smaller than on CT p < 0.05 Mean MRI GTV length, max width and distance of proximal tumour to anal verge significantly less than on CT (mean reduction 3.2 cm, 0.5 cm, 2.9 cm respectively) p < 0.05 |
| 21 | 15 | GTV | MRI V CT 1x radiologist in consultation with 1x radiation oncologist |
T 2 axial | Mean CT-GTV/ MRI- GTV volume ratio was 1.2cc (range 0.5–2.9) CT-GTV coverage inadequate for tumours with sigmoid or anal invasion and in the two cases this occurred there was significant underestimation of GTV on CT. |
| 14 | 24 | GTV | MRI T
2
v
s DWI Interobserver variation three radiation oncologists |
T
2W, DWI and a combination of both axial |
T
2 GTV volumes significantly larger than on DWI (approx. 2–3 x larger) No significant difference between observers per modality (mean conformity index 0.7 for T 2W and 0.71 for DWI) Mean distance between contours T 2 = 1.8 mm and DWI = 1.5 mm |
| 22 | 27 | GTV | MRI T
2W v
s DWI Interobserver variation two radiologists |
T 2W v s DWI axial |
T
2W MRI GTVs were slightly larger but not statistically different from DWI volumes Inter observer mean difference in volume was not improved with DWI Mean difference and 95% limits of agreement for T2W MRI and DWI GTVs were -9.8 (-55 to 35) cm3 and -14.8 (-54 to 24.4) cm3 respectively. |
| 23 | 50 | GTV | MRI pre- and post-CRT Interobserver variation two radiologists Histology reference standard for post-CRT radiology |
Pre- and Post-CRT DWI and T 2W MRI axial | Pre CRT MRI; Interobserver agreement for T
2W and DWI was excellent (ICC 0.97) ICC all modalities; pre-CRT 0.91–0.96 and post-CRT 0.61–0.79 ROC for post-CRT volume T 2W = 0.7, DWI = 0.93 and ADC = 0.54 |
CRT, chemoradiotherapy; DWI, diffusion weighted imaging; GTV, gross tumour volume; ICC, intraclass correlation coefficient; ROC, receiver operating characteristic.
In cervix cancer, geometric studies show that agreement between target volumes delineated on transverse and para-transverse planes of MRI is good with conformity index 0.71–0.72.19 In dosimetric studies, overestimation of tumour width on CT results in significant differences in the volume treated to the prescription dose or higher.13,24 Compared to the CT-based imaging RT workflow, MRIgRT will provide superior visualization of the target and normal tissue immediately before and during treatment delivery. Table 1 and 2 summarizes the published data for contour delineation on MRI in cervix and rectal cancer.
2. MRI for motion assessment
Extensive target motion occurs in gynaecological and rectal RT and has been reviewed previously.25,26 With RT for cervix cancer, the primary clinical target volume (CTV) includes any visible tumour, cervix, uterus, upper vagina and parametrium. The elective nodal CTV includes the pelvic and common iliac lymph nodes (LN) and the para-aortic LN in high-risk disease. Motion is largest at the uterine fundus and studies report maximum interfraction motion of over 30 mm.27 In one study, margins of 15 mm to the primary and nodal CTV failed in 32% of patients and margins of up to 30 mm were required to ensure coverage in 95% of fractions.27
With RT for rectal cancer the primary target volume includes the tumour and mesorectum, and the elective nodal volume includes the pelvic LN. The entire circumference of the rectum at the level of the tumour is included, because it is not possible to distinguish tumour from normal rectal tissue on CT. The anterior and lateral rectal wall move more than the posterior wall and motion is larger in the middle and upper rectum compared with the lower rectum.28 Maximum motion occurs anteriorly, particularly in the upper mesorectum, and anterior PTV margins of 24 mm in the upper mesorectum and 15 mm in the lower mesorectum have been recommended.29,30 Tables 3 and 4 summarize the published data for cervix and rectal interfraction target motion.27,28,31–43
Table 3.
Interfraction motion in cervix cancer radiotherapy
| Ref | Target measured | No of Pts | Imaging modality and Frequency | Method of measurement/ registration | Statistic used | Motion (mm) | Suggested Margins (mm) | Volume change | Bladder/ rectum correlation | ||||
| AP | LR | SI | AP | LR | SI | ||||||||
| 31 | Cervix | 16 | Weekly CT | Cervix COM Cervix contour |
Mean max Range Mean max |
16 5.1–25 A = 17 p = 18 |
8.2 4.4–14 L = 9.4 R = 7.6 |
21 12–33 S = 23 I = 13 |
Cervix volume reduced by mean 62.3% after 45 Gy | Bladder volume affects AP and SI but not lateral margins | |||
| 32 | Cervix Uterus |
20 | MR at baseline and weekly x5 | Cervical os Uterine canal Uterine fundus Cervical os Uterine canal Uterine fund us |
Grand mean Mean range |
2.4 4.8 4.6 11.2 13.1 14.5 |
1.5 5.7 7.8 11.3 15.7 24.4 |
Isotropic internal margin to encompass 90% of motion was 40 mm at the fundus and 15 mm at the cervix | Significant reduction in bladder volume during RT. No systematic change in rectosigmoid volume. |
Bladder volume associated with SI motion of fundus and AP motion of cervical os. Rectal volume associated with SI motion of uterine canal and cervical os. | |||
| 33 | Cervix Uterus Upper vagina |
33 | MR on 2 days 24 h apart | Post cervix Uterine body Upper vagina |
Mean (SD) CTV–PTV margins |
2.7 (2.8) 7 (9) 2.6 (3) |
0.3 (0.8) 0.8 (1.3) 0.3 (1) |
4.1 (4.4) 7.1 (6.8) |
15 30 11 |
7 8 |
13 25 7 |
SI uterine motion correlated to bladder filling. AP cervix and vaginal motion related to rectal filling |
|
| 34 | GTV CTV |
20 | MR at baseline and weekly | GTV CTV |
Margin to encompass 95% cases (internal motion) |
A = 12 p = 14 A = 24 p = 17 |
R = 12 L = 11 R = 12 L = 16 |
S = 4 I = 8 S = 11 I = 8 |
Significant regression GTV p ≤ 0.001 Mean GTV 57cc week 0, 43.3cc, 32cc and 23cc at weeks 2, 3 and 4 |
AP shift in GTV and CTV weakly correlated with rectal vol. Significant difference in margins required if pre-treat rectum volume >70 cc. |
|||
| 27 | CTV | 10 | Daily CBCT | COM | Mean SD Range |
3 5 −9.4–18.9 |
−0.28 1.3 +3.3–3.5 |
−4.6 3.9 −15.3–3.8 |
Mean margin to encompass CTV motion = 15 mm, but fails in 32% Margins up to 30 mm could be required to ensure coverage in ≥95% fractions. |
Mean reduction in CTV of 20% (586.4 to 469cc) Mean bladder volume relative to the planning CT −48.5 cc |
Increased rectal and bladder volume associated with significant superior shifts (p < 0.001) |
||
| 35 | Cervix | 10 | Daily EPID | Cervix fiducials | Mean of mean Random error Internal motion |
3.5 3.9 |
3.7 2.2 |
4.1 3.7 |
9.7 | 10.8 | 8.9 | ||
| 36 | Cervix | 15 | Portal films weekly | Radiopaque ring | Median Max |
16 23 |
10 24 |
8 36 |
50% reduction in tumour size at 30 Gy (21 days) | ||||
| 37 | Cervix | 10 | Daily 2D KVI | Cervix fiducials | Mean SD Max |
4.2 3.5 18 |
1.9 1.9 14 |
4.1 3.2 18 |
|||||
| 38 | Cervix | 10 | MVCT daily | Cervix contour Uterus contour 95% margin for internal motion and setup |
Mean SD Mean SD |
A = 0.4 P=-3 A = 10.1 p = 6.9 A = 3.3 p = 0.3 A = 11.9 p = 11.7 |
L = −3.5 R = 0.2 L = 4.9 R = 4.5 L = 0.7 R = −0.6 L = 8.1 R = 7.5 |
S = 2.2 I = 0.5 S = 8 I = 5 S = 6.1 I = 5 S = 11.6 I = 11.2 |
A = 17 p = 12 A = 19 p = 19 |
R = 8 L = 9 R = 13 L = 13 |
S = 15 I = 9 S = 20 I = 19 |
Significant reduction in mean cervix volume (106 cc pre-treatment to 74 cc last week of treatment) |
Average bladder volume reduced from 156 cc in wk1 to 88 cc in the last week (p < 0.01). |
| 39 | Cervix | 20 | MRI baseline and weekly x5 | GTV Cervix Uterus Upper vagina |
Euclidean vector displacement | 1.2 ± 0.4 (0.5–3) 1.1 ± 0.3 (0.5–2.8) 1.7 ± 0.2 (0.5–4.5) 0.7 ± 0.3 (0.3–1.3) |
15 mm GTV to PTV margin covered the GTV to >98% of prescription dose | The relative reduction in the GTV from baseline to the end treatment was 48–96%. | Individually, the planned dose was not the same as the simulated delivered dose | ||||
COM, centre of mass; CTV, clinical target volume; 2D, two-dimensional; EPID, electronic portal imaging device; GTV, gross tumour volume; KVI, kilovoltage imaging; MVCT, megavoltage CT; PTV, planning target volume; RTP, radiotherapy planning; SD, standard deviation.
Table 4.
Inter- and Intrafraction motion in rectal cancer
| Ref | Target measured | No of Pts | Imaging modality and frequency | Method of measurement/ registration | Statistic used | Motion (mm) | Suggested margins (mm) | Volume change | Other | ||||
| AP | LR | SI | AP | LR | SI | ||||||||
| 40 | GTV Rectum Mesorectum |
17 | RTP CT Wk1, 3 and 5 | Displacement of points on GTV, rectum and mesorectum surface | Mean (SD) GTV Rectum Mesorectum |
0.7 (3.1) 1.1 (5.1) 1.1 (2.7) |
1.2 (2.8) 0.2 (4.5) 0.3 (2.2) |
4.2 (3.6) |
A = 14 p = 7 A = 8 p = 9 A = 7 p = 6 |
L = 7 R = 8 L = 8 R = 8 L = 5 R = 4 |
S = 16 I = 12 | Greatest motion of rectum in upper 1/3 No correlation of motion direction and bladder filling |
|
| 41 | Mesorectum | 10 | Helical MVCT before and after treatment x2/week | Contour displacement by bony landmarks | Mean (SD) Margins for intrafraction motion and setup |
A=-2(6.8) p = −0.4 (3.8) |
L = −1.6 (4.2) R = 0.1 (4) |
S = −3.2 (5.6) I = −3.2 (6.8) |
A = 11 p = 7 |
8 |
S = 10 I = 12 |
If new margins applied instead of standard 1 cm margins, there would be an average decrease in PTV by 21.5% (SD, 1.45%). | |
| 28 | Rectum | 16 | CBCT D1-3, then weekly GTV to PTV margin |
Upper rectum Mid rectum Low rectum |
Mean of mean Mean of SD Mean of mean Mean of SD Mean of mean Mean of SD |
A= −4 p = −0.1 A = 7.4 p = 4.2 A= −1 p = −0.1 A = 7 p = 3.6 A = 1.8 p = 1.2 A = 4.2 p = 4.7 |
L = 1.3 R = −2.8 L = 6.9 R = 5.2 L = −0.4 R = 0 L = 5.1 R = 4.1 L = 0.1 R = 0.0 L = 3 R = 3 |
A = 17 p = 14.4 A = 16.7 p = 14.9 A = 14.2 p = 16 |
L = 4.2 R = 4.2 L = 11 R = 10.3 L = 9 R = 10.1 |
No significant change in rectal volume on CBCT compared to baseline CT | No relationship between rectal and bladder volume and time Significant day to day bladder volume variation |
||
| 42 | CTV Rectum |
10 | Weekly RTP CT CTV Rectum |
At AV 5.5 cm from anus 9 cm from anus At anus 4.5 cm from anus 9 cm from anus |
CTV SD of motion Rectum SD of motion |
A = 3–4 A = 6 A = 10 p = No motion p = 4 p = 7 p = 2 A= ‘very similar to CTV’ |
No motion observed Motion similar to CTV, i.e. no motion |
Motion dependent on location in pelvis Increased motion of CTV at ≥5.5 cm from anus caused by bladder filling Biggest motion at 10 cm from anus The biggest difference in CTV volume between a full and empty bladder was 51 cm3 |
|||||
| 43 | Mesorectum | 63 | Repeat RTP CT LCRT daily CT for first week and then weekly. SCRT cohort daily CT |
LCRT Upper Mesorectum Lower mesorectum SCRT Upper Mesorectum Lower Mesorectum |
PTV margins for 95% prescribed dose to 90% patients |
A = 24 p = 7 A = 15 p = 7 A = 32 p = 7 A = 18 p = 7 |
L = 7 R = 7 L = 7 R = 7 L = 7 R = 7 L = 10 R = 10 |
S = 10 I = 10 S = 10 I = 10 S = 10 I = 10 S = 10 I = 10 |
Significant reduction in rectal volume in LCRT by 35% Reduced bladder volume during RT |
Significant reduction in rectal volume resulted in 5 mm post shift of upper ant CTV | |||
AV, anal verge; CTV, clinical target volume; GTV, gross tumour volume; LCRT, long-course chemoradiotherapy; MVCT, megavoltage CT; PTV, planning target volume; RTP, radiotherapy planning; SCRT, stereotactic conformal radiotherapy; SD, standard deviation.
Bladder and rectal filling influence target motion in gynaecological and rectal RT. With cervix treatment, bladder volume is correlated with superior/inferior uterine motion and rectal volume is correlated with cervix and vaginal anterior/posterior motion.33 With rectal RT, deformation of the mesorectum is largely driven by changes in rectal volume.29 In both cervix and rectal RT, there is significant interpatient variation in bladder volume despite bladder filling protocols, and both bladder and rectal volumes reduce during treatment.27,28,34,44 Laxatives may not significantly reduce target anterior/posterior motion from rectal volume variation, because passage of gas can still cause significant target displacement.37 Figure 3 illustrates CTV positional changes related to bladder volume as seen on CBCT during cervix RT. MRIgART will facilitate implementation of margin reduction through adaptive strategies that account for these geometric changes.
Figure 3.
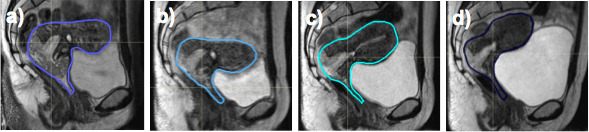
Changes in clinical target volume position during cervix radiotherapy as seen on MRI at (a) week 0, (b) week 2, (c) week 3 and (d) week 4.
3. MRI for anatomical response assessment and dose escalation
Significant tumour regression is observed during cervix and rectal RT.31,34,45,46 In 20 cervix patients having weekly MRI during chemoradiotherapy (CRT), average tumour volume reductions of 59.6% at week 4 were observed, which resulted in increased uterine motion, substantial changes in tumour position and movement of normal tissue, particularly small bowel, into the high dose region.47 Repeat MRI and planning after delivery of 30 Gy found that a second IMRT plan significantly reduced the volume of bowel irradiated if the primary GTV decreased >30 cc.47
In a study of 15 rectal cancer patients, mean tumour regression of 46.3% was seen on MRI by week 5 of CRT and regression was fastest in the first 3 weeks of treatment.45 A further study in 13 patients found that the majority of patients who had a good response to treatment had volume reduction and fibrotic changes during weeks 1–3.46 There is a move towards organ preservation in rectal patients with a complete radiological response to spare morbidity from surgery.48 Patients who respond to CRT are more likely to benefit from dose escalation to increase the rate of pathological complete response (pCR)46 and early assessment to identify these patients is therefore important. Response to neo-adjuvant CRT is dose-dependent with dose escalation of >60 Gy resulting in increased rates of pCR and acceptable toxicity.49 Tumour boost volume delineation on the initial RT planning CT does not take account of tumour regression during treatment. Repeat imaging during treatment could help select patients who would benefit from radiation dose escalation and would produce more accurate and smaller boost volumes, facilitating increased tumour dose without increased OAR dose and toxicity.50
Figures 4 and 5 illustrate changes in cervix and rectal tumour volume as seen on weekly MRI during RT.
Figure 4.
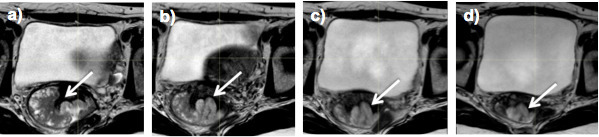
Changes in cervix tumour volume (arrow), as seen on weekly MRI during treatment at (a) week 0, (b) week 2, (c) week 3 and (d) week 4.
Figure 5.
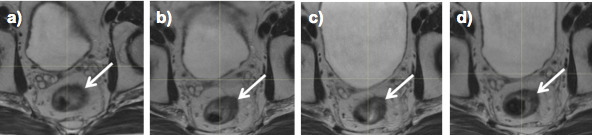
Changes in rectal tumour volume (arrow), as seen on weekly MRI during treatment at (a) week 0, (b) week 2, (c) week 3 and (d) week 4.
4. MRI for biological response prediction and dose delivery assessment
Functional MRI with diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) may predict biological response in rectal and cervix RT and identify patients for dose escalation.14,51
MRI has potential to act as a biomarker, identifying good and poorly responding tumours to select patients for dose adaptation in order to improve treatment outcomes.52–55 Studies suggest that DWI can predict pathological complete response early in rectal RT,53,54,56,57 but there are limitations to the current evidence preventing its routine implementation in patient selection for dose escalation. Most studies were small and did not prospectively determine MRI criteria to differentiate between complete and non-complete response to treatment. Retrospective identification of these parameters introduces selection bias. There was variability in the time points at which imaging was acquired and surgery was performed. For example, patients classified as achieving a non-pCR at 6 weeks following CRT may have been classified as a pCR if surgery was performed at a later date and meta-analysis reports 6% increase rate of pCR with an interval of greater than 6 weeks from the end of preoperative CRT.58
In cervix RT, DCE and DWI MRI may predict response to CRT and identify patients for dose escalation.51 Increasing apparent diffusion coefficient (ADC) values from DWI acquired during treatment can detect early signs of treatment response.55 DCE MRI during treatment detects tumour perfusion.59 Persistently low perfusion during CRT is correlated with treatment failure and patients with increases in perfusion during CRT have better outcomes.59 This could identify patients for dose escalation to hypoxic regions, which should increase tumour shrinkage prior to brachytherapy, which we know improves local control.60 There was however, no technical standardization in these studies, which limits assessment of reproducibility and generalizability. The optimal time to assess biological response and adapt treatment based on these finding has yet to be determined.
MRIgRT will also provide quantitative knowledge of the actual delivered dose and the impact of radiation dose on tumour and normal tissue. This would enable dose compensation strategies and tumour and normal tissue radiobiological modelling.
Adaptive radiotherapy (ART) strategies
1. Target volume modification based on individual internal motion
PTV modification based on data from setup and internal target motion acquired from planning or previous treatment, allows safe reduction of generic population based margins. This is also referred to as a composite volume technique. The range of target motion is modelled during the planning stage or first treatments to generate an internal target volume (ITV). The treatment plan is optimized offline and applied to subsequent treatments. Individualized ITVs in cervix RT account for the range of cervix and uterine motion with variable bladder volume and may be based on variable bladder filling CT scans acquired at simulation, or using bladder geometry as a predictive tool.61,62 Compared to population-based margins, individualized margins reduce CTV–PTV margins by 48% (±6%), and bladder and rectal volume within the PTV is reduced by 5–45% and 26–74% respectively.62
For rectal cancer, an average CTV can be acquired from the radiotherapy planning (RTP) CT and repeat CTs during the first week of treatment.30 Adaptation after day 4 resulted in a 7 mm reduction in the maximum required PTV margin from 24 to 17 mm and a significant reduction in PTV and dose to the small bowel.30
2. Online plan selection strategy
Online plan selection uses imaging acquired at treatment to select a plan from a library of treatment plans generated from multiple PTVs. In cervix RT, evaluated strategies include a plan library using individualized PTVs based on CTV position at different bladder volumes, or PTVs created by the application of incremental margins to the CTV as seen on RTP CT acquired with a full bladder.62,63 Compared to a standard population margin approach, plan selection results in significantly better target coverage and OAR sparing.62–64 Adaptation based on variable bladder filling CTVs enables reductions in PTV margins from 38 to 7 mm and better CTV D98% > 95% in comparison to the non-ART approach where 17% of treatment fractions have inadequate target coverage.62,64 When using an incremental margin approach, a 5 mm margin of the day plan could be used in 25% of fractions.63 Libraries based on variable bladder filling do not account for rectal filling variation or the passage of gas, which are difficult to predict and can significantly influence cervix motion.65
In rectal cancer, target motion is influenced more by rectal than bladder filling, so a library of plans strategy based on variable bladder volumes is not appropriate. Instead plan selection has been based on plans with variable PTV margins between −25 and +25 mm applied to the anterior CTV, which is where largest variation is seen.66 This reduced dose to the bladder and small bowel OARs, although the absolute reductions were small.67 Plan selection in rectal RT is feasible with good plan selection consistency between observers of 75%.66 Plan selection in both cervix and rectal radiotherapy is being implemented clinically, but is limited by the image quality of CBCT. MRIgRT would facilitate target and OAR localization for online plan selection.
3. Plan reoptimization
The optimal strategy to account for target and OAR motion and deformation, anatomical and biological response, is to generate a new plan with full reoptimization. This determines the dose distribution based on target and OAR geometry and/or physiology at the time of treatment delivery.6
A number of planning studies in cervix RT have simulated the benefit of online replanning.7,68,69 One study of 33 patients compared a 3 mm PTV margin plan without replanning, with an automated weekly replan on real-time patient geometry as seen on MRI.68,69 Pre-treatment optimization criteria were automatically reapplied to replans without any physics planner intervention. Without replanning, there was a significant reduction in accumulated dose to the primary CTV, with nine patients failing D98% > 95%.68 In patients who were replanned, there was a reduction in CTV between 8 and 68% (median 39%) and the D98 CTV constraint was met in all patients.68 There was no difference in dose to OARs, which might move with the target and remain in the high dose region. This may lead to increased OAR dose in patients where OAR movement is related to the target compared to patients where the OARs move independently.68,69
A study in 14 cervix patients used 15 mm PTV margins and replanning based on target and OAR geometry on MRI after 30 Gy.47 There was a reduction in OAR dose with replanning, but in this study the replans were interactively optimized to reflect new anatomy.47 A planning study to simulate the benefit of online MRIgRT replanned using weekly MRI in 11 patients receiving IMRT for cervix cancer with 4 mm PTV margins.7 This was compared to plans based on the pre-treatment MRI with primary and nodal PTV margins of 15 and 10 mm.7 There was a significant reduction in the dose to the bladder, rectum, sigmoid, and small bowel with online replanning.7
4. Dose compensation
Adaptation using dose tracking allows reduction in PTV margins because variations in the dose delivered to the CTV compared to the planned dose, can be compensated for in subsequent fractions. The pre-treatment imaging, together with any setup correction applied, is used to determine target and OAR position and the dose delivered at each treatment fraction. This is non-rigidly registered to the planning CT to model anatomical motion and deformation and allows calculation of the accumulated delivered dose. The treatment plan can then be reoptimized to compensate for any problems with dose coverage or to account for adaptation of treatment goals.
Lim et al looked at pre-treatment and weekly MRI in 30 cervix IMRT patients using a 3 mm PTV margin and dose accumulation.70 They modelled an anatomical driven approach with a single offline replan mid-treatment to account for tumour regression, and a dosimetrically triggered approach if the estimated accumulated D98 to the GTV or primary CTV was low. Without replanning, there was insufficient target coverage in 27% of patients. The anatomical approach improved target coverage and reduced OAR dose, but there were still three patients with insufficient target coverage. Dosimetrically triggered replanning resulted in target coverage in all patients, but no difference in the accumulated OAR dose.70 Deformable registration is not consistently accurate and validation is difficult. In deformable registration for dose accumulation, particular caution must be taken when tumours have undergone mass change and in areas with sharp dose gradients.
INTEGRATION OF MRI INTO RADIOTHERAPY AND ITS CHALLENGES
MRI can be integrated into RT workflow in a variety of ways. In a CT-MRI simulation workflow, the MRI is used for contour delineation at radiotherapy treatment planning (RTP) and the CT provides a robust geometric representation of the patient, an electron density map required for dose calculation and a reference image for patient set up during standard treatment. Any error in image registration will however lead to a systematic geometric error throughout patient treatment.71 MRI-only simulation reduces potential for image registration error at RTP, but the challenges of geometric distortion and lack of electron density information and material properties inherent to MRI need to be addressed. MRI for RT treatment localization, planning and verification have different demands to those acquired for diagnosis and staging. Specific solutions are required. The main differences relate to patient positioning, image acquisition and sequence parameters and the need for geometric accuracy (Table 5).
Table 5.
Different demands of MRI acquired for diagnostic and radiotherapy purposes in cervix and rectal cancer
| MRI for diagnosis | MRI for radiotherapy | |
| Couch | Soft, often concave Maximized for patient comfort |
Needs to be flat, the same as in RT delivery |
| Patient positioning | Comfortable Supine |
As for RT delivery Supine |
| Immobilization devices | None | Combifix knee support to stabilize pelvis |
| Bowel artefact management | IM Buscopan Anterior abdominal wall compression Saturation bands |
IM Buscopan may be used in MRI simulation but may not be acceptable during daily treatment within MRI treatment workflow |
| Bladder status | Empty | Full |
| Coil placement | Pelvic coil centred on tumour | Anterior coil supports prevent distortion of external body contour Customized MR simulators may incorporate posterior coils into a flat couch |
| Field strength | Increasing strength improves signal to noise, but is more expensive and requires more room | Increasing field strength increases geometric distortion |
| Coverage | High resolution FOV limited to tumour | High resolution FOV must encompass entire tumour target Sequences including external body contour required for dose calculation |
|
Preferred
sequence |
2d T
2W high resolution at tumour with ≤3 mm slice thickness, and voxel size <1 mm Imaging plane perpendicular to the rectum or cervical canal |
T
2W 3d < 1 mm isotropic voxel size for target delineation Imaging plane true axial acquired perpendicular to the system |
| Geometric accuracy | Less important | Essential to localize the target |
| Electron density/ material composition information | Not required | Not required in a CT/ MRI combined workflow, but essential in MR-only simulation and MR treatment workflow |
FOV, field of view;RT, radiotherapy.
A number of MRIgRT technologies are in active development, integrating MRI with external beam RT delivery, providing MRI data immediately before and after treatment, and simultaneously with treatment delivery.72–75 They differ in their imaging and treatment adaptation capabilities and their approach to tackling the technical challenges of magnetic and radiofrequency interference and treatment beam transmission through the magnet. Table 6 summarizes the different systems, each presenting advantages and disadvantages.72–77 The MRIdian system (ViewRay Inc, Oakwood Village, OH) has treated over 300 patients since 2014 and integrates a 0.35 Tesla (T) magnet with either three multileaf collimator (MLC)-equipped Cobolt-60 heads, or a 6 MV linac.73,78 The Elekta Unity MR-linac solution (Elekta AB, Stockholm, Sweden) started treating patients in 2017 under pre-CE mark clinical trial protocol. It integrates a 7 MV linac with a high field 1.5 T MRI system from Philips, which uses technology similar to the Philips Ingenia diagnostic systems.72 Lower magnetic field solutions benefit from a reduction in image artefacts and patient related geometric distortion, and lower energy deposition by the radiofrequency pulses. Higher field solutions benefit from enhanced signal to noise, which improves spatial and temporal resolution and functional imaging capabilities.
Table 6.
MRI guidance radiotherapy systems
|
Elekta Unity MR Linac
72,76 |
ViewRay MRIdian Cobalt 60
73,77 |
ViewRay MRIdian Linac |
Australian MRI-Linac
74 |
Canadian Aurora Magnet X MR Linac 75 | |
| Magnet | 1.5 T closed | 0.35 T split bore | 0.35 T split bore | 1.0 T split bore | 0.5 T biplanar rotating geometry |
| Radiotherapy source | 7 MV | 3 Cobalt-60 heads | 6 MV | 6 MV | 6 MV |
| MLC effective leaf width at isocentre | 0.72 cm | 1.05 cm | 0.83 cm | ||
| MLC maximum leaf speed | 6 cm/sec | 2.0 ± 0.1 cm/sec | >2 cm/ sec | ||
| Magnetic field orientation to delivery | Perpendicular | Perpendicular | Perpendicular | Perpendicular and parallel | Perpendicular and parallel |
| Bore Size | 70 cm | 70 cm | 70 cm | 50 cm | 60 cm |
| Magnetic field homogeneity | ≤2.0 ppm over 50 × 50×45 cm3 | <25 ppm over 45 cm DSV | <25 ppm over 45 cm DSV | ||
| Maximum imaging field of view | 50 cm DSV | 50 cm DSV | 50 cm DSV | ||
| Maximum treatment field size | 57.4 × 22 cm2 | 27.3 × 27.3 cm2 | 27.4 × 24.1 cm2 | ||
| 4D capabilities | Yes | Yes | Yes | No | No |
| Functional imaging | Yes | Yes | Yes | No | No |
| Treating patients | Yes | Yes | Yes | No | No |
| CE Marked/ FDA approved | Yes | Yes | Yes | No | No |
DSV, diameter of spherical volume; MLC, multileaf collimator; Ppm, parts per million.
TECHNICAL CHALLENGES IN THE REALIZATION OF REAL-TIME MRIGART
Generation of a new treatment plan based on target and OAR geometry or biology at the time of treatment delivery is the ultimate goal of MRIgART. The main challenge is achieving this in a short amount of time with the patient on the treatment couch. Its clinical implementation is limited by;
Requirement for robust automated real-time registration of the newly acquired MRI with the images used for treatment planning.
Requirement for electron density data necessary for dose calculation.
Target and OAR segmentation on the new MRI.
Plan reoptimization and dose calculation.
Quality assurance of the newly generated plan.
In the first clinical applications of MRIgART using the Elekta Unity MR-Linac (Elekta AB, Stockholm, Sweden) and the MRIdian system (ViewRay, Oakwood Village, OH), MRI are acquired immediately before treatment and registered to the reference planning MRI and planning CT using deformable registration.79,80 Electron density information from the reference planning CT is then transferred to the MRI of the day using the deformation map.79,80
The standard treatment-planning process requires segmented contours and generates the desired dose distribution from scratch. This is achieved through iterative optimization, driven by defined objective functions set by the planner, which specify the dose–volume constraints for tumour targets and OARs. The planner then fine-tunes the objective functions and repeats the optimization process to further improve the treatment plan by trial and error. This takes too long to be feasibly implemented in real-time MRIgART and faster automated replanning strategies are required.
Segmentation of target and OARs on the daily image is a major challenge in online replanning. Manual segmentation is time consuming and susceptible to inter- and intraobserver variability. Mean time required to manually delineate the pelvic nodal CTV alone is over 30 min, and automated strategies are necessary to reduce segmentation time and improve structure definition.81 Autosegmentation without prior knowledge uses imaging properties such as voxel intensities and gradients.82 Alternative strategies incorporate prior knowledge into the segmentation process to improve accuracy and reproducibility and include atlas-based segmentation, statistical shape models, machine learning and hybrid strategies.82
In atlas-based autosegmentation, an atlas of manually contoured structures is used to propagate structures onto a new data set using deformable registration voxels transformations.83–85 Use of multiple atlases further improves accuracy.86 Cervix target segmentation on MRI using machine learning results in mean sensitivity and specificity of 85–93%87 and is faster than atlas based strategies.88 Accuracy of autosegmentation is not perfect and visual verification is still required. In MRIgART using both the Elekta Unity MR-Linac and the ViewRay MRIdian systems, target and OAR contours are transferred to the online MRI from the reference image using deformable registration and are then checked and manually edited if necessary by a clinician.78,80,89
Daily plan reoptimization does not need to start from scratch and many components of the new plan can be extrapolated from the original fully optimized plan. Plan modification with aperture morphing reduces the number of steps in reoptimization.90 Segment aperture morphing adjusts the beam segment shape of the MLC, based on the new target position and shape, as seen in the projection from the beam’s eye view of each treatment beam. Segment weight optimization can then be applied to improve dosimetry.90 More complex aperture morphing methods rely on deformable registration.91,92
Plan adaptation based on previous knowledge from the original plan can also speed up the process. Gradient maintenance strategies maintain the same dose gradient around the target, towards the OARs, as in the original treatment plan.93 This requires segmentation of the new target but not segmentation of OARS. It may not be suitable for the larger target volumes seen in gynaecological and rectal RT. Interactive dose shaping is based on contoured structures and enables direct manipulation of the initial plan isodose surface shape or the dose to individual voxels.94,95 Advances in computer power, both graphical processing units and modified central core processing units, can now reduce the time of plan optimization and dose calculation from minutes to seconds.96,97 Commercial treatment planning systems incorporating advances in adaptive planning are now becoming available.
Plan approval and quality assurance (QA) in real-time MRIgART is challenging. Automation of image acquisition and registration, target and OAR segmentation, treatment dose calculation and adaptive planning optimization is essential in implementing online MRIgART, but creates additional problems. The detailed plan reviews and QA process that occur at pre-treatment during standard RT are not appropriate. Limiting physician plan approval to when plan quality is less than the original treatment plan would improve efficiency. Conventional patient specific QA approaches insert physical phantoms in the treatment beam, which cannot be used with the patient on the treatment couch. An alternative solution is to send the treatment plan to an independent dose calculation engine to verify that the dose distributions agree.98
Delivery of MRIgRT with the ViewRay MRIdian Cobalt 60 was feasible in 11 rectal patients receiving neoadjuvant chemoradiation with IMRT and simultaneous integrated boost.99 Daily MRI were acquired for patient setup and verification, and all patients completed treatment. The ViewRay MRIdian has also been used for imaging and RT planning in brachytherapy for cervical cancer.100 No studies have yet been published for MRIgRT delivery in cervix external beam RT.
Conclusions
MRIgRT in rectal and gynaecological RT will improve all aspects of the treatment workflow. Its most exciting application in gynaecological and rectal RT will be to refine GTV to CTV definition, increased accuracy and precision of target localization for treatment verification and implementation of adaptive strategies to personalize the therapeutic approach. This will facilitate reduced PTV margins and normal tissue irradiation whilst maintaining target coverage. Together with dose adaptation, this will translate into improved tumour control and reduced toxicity for patients. Optimal adaptive strategies need to be determined and challenges remain for the implementation of MRIgART clinical workflow. But technology is exponentially increasing and the ability to personalize and intensify treatment with MRIgART at these tumour sites is no longer an improbable blue-sky ideology but is now within reach.
Footnotes
Acknowledgment: This work was undertaken in The Royal Marsden NHS Foundation Trust who received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was supported by The Institute of Cancer Research and Cancer Research UK [CRUK] grant number C33589/A19727. We acknowledge NHS funding to the NIHR Biomedical Research Centre. The Institute of Cancer Research and Royal Marsden National Health Service Foundation Trust are part of the Elekta MR-Linac Research Consortium, which aims to coordinate international research into the magnetic resonance linear accelerator.
Conflicts of interest declaration: Ingrid White, Erica Scurr, Andreas Wetscherek, Gina Brown, Simeon Nill, Uwe Oelfke and Shree Bhide have nothing to disclose. David Dearnaley reports personal fees from ICR, grants from Cancer Research UK and National Institute for Health Research, outside the submitted work; In addition, David Dearnaley has a patent EP1933709B1 issued and receives personal fees for advisory board/consultancy from Takeda, Amgen, Astellas, Sandoz and Janssen. Susan Lalondrelle reports personal fees from ELEKTA, grants from MSD and personal fees and non-financial support from Roche, outside the submitted work.
The authors Susan Lalondrelle and Shreerang Bhide contributed equally to the work.
Contributor Information
Ingrid M White, Email: ingrid.white@icr.ac.uk.
Erica Scurr, Email: erica.scurr@rmh.nhs.uk.
Andreas Wetscherek, Email: andreas.wetscherek@icr.ac.uk.
Gina Brown, Email: gina.brown@icr.ac.uk.
Aslam Sohaib, Email: aslam.sohaib@rmh.nhs.uk.
Simeon Nill, Email: simeon.nill@icr.ac.uk.
Uwe Oelfke, Email: uwe.oelfke@icr.ac.uk.
David Dearnaley, Email: david.dearnaley@icr.ac.uk.
Susan Lalondrelle, Email: susan.lalondrelle@rmh.nhs.uk.
Shreerang Bhide, Email: sabhide@yahoo.com; shreerang.bhide@icr.ac.uk.
REFERENCES
- 1. Laursen LV , Elstrøm UV , Vestergaard A , Muren LP , Petersen JB , Lindegaard JC , et al. . Residual rotational set-up errors after daily cone-beam CT image guided radiotherapy of locally advanced cervical cancer . Radiother Oncol 2012. ; 105 : 220 – 5 . doi: 10.1016/j.radonc.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 2. Roeske JC , Lujan A , Rotmensch J , Waggoner SE , Yamada D , Mundt AJ . Intensity-modulated whole pelvic radiation therapy in patients with gynecologic Malignancies . International Journal of Radiation Oncology*Biology*Physics 2000. ; 48 : 1613 – 21 . doi: 10.1016/S0360-3016(00)00771-9 [DOI] [PubMed] [Google Scholar]
- 3. Duthoy W , De Gersem W , Vergote K , Boterberg T , Derie C , Smeets P , et al. . Clinical implementation of intensity-modulated Arc therapy (IMAT) for rectal cancer . International Journal of Radiation Oncology*Biology*Physics 2004. ; 60 : 794 – 806 . doi: 10.1016/j.ijrobp.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 4. Stroom JC , de Boer HCJ , Huizenga H , Visser AG . Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability . International Journal of Radiation Oncology*Biology*Physics 1999. ; 43 : 905 – 19 . doi: 10.1016/S0360-3016(98)00468-4 [DOI] [PubMed] [Google Scholar]
- 5. van Herk M , Remeijer P , Rasch C , Lebesque JV . The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy . International Journal of Radiation Oncology*Biology*Physics 2000. ; 47 : 1121 – 35 . doi: 10.1016/S0360-3016(00)00518-6 [DOI] [PubMed] [Google Scholar]
- 6. Yan D . Adaptive radiotherapy: merging principle into clinical practice . Semin Radiat Oncol 2010. ; 20 : 79 – 83 . doi: 10.1016/j.semradonc.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 7. Kerkhof EM , Raaymakers BW , van der Heide UA , van de Bunt L , Jürgenliemk-Schulz IM , Lagendijk JJW . Online MRI guidance for healthy tissue sparing in patients with cervical cancer: an IMRT planning study . Radiother Oncol 2008. ; 88 : 241 – 9 . doi: 10.1016/j.radonc.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 8. Orlandini LC , Coppola M , Fulcheri C , Cernusco L , Wang P , Cionini L . Dose tracking assessment for image-guided radiotherapy of the prostate bed and the impact on clinical workflow . Radiat Oncol 2017. ; 12 : 78 . doi: 10.1186/s13014-017-0815-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hricak H , Gatsonis C , Coakley FV , Snyder B , Reinhold C , Schwartz LH , et al. . Early invasive cervical cancer: CT and MR imaging in preoperative evaluation - ACRIN/GOG comparative study of diagnostic performance and interobserver variability . Radiology 2007. ; 245 : 491 – 8 . doi: 10.1148/radiol.2452061983 [DOI] [PubMed] [Google Scholar]
- 10. Haie-Meder C , Pötter R , Van Limbergen E , Briot E , De Brabandere M , Dimopoulos J , et al. . Recommendations from gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV . Radiother Oncol 2005. ; 74 : 235 – 45 . doi: 10.1016/j.radonc.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Brown G , Daniels IR , Richardson C , Revell P , Peppercorn D , Bourne M . Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer . Br J Radiol 2005. ; 78 : 245 – 51 . doi: 10.1259/bjr/33540239 [DOI] [PubMed] [Google Scholar]
- 12. O'Neill BDP , Salerno G , Thomas K , Tait DM , Brown G . MR vs CT imaging: low rectal cancer tumour delineation for three-dimensional conformal radiotherapy . Br J Radiol 2009. ; 82 : 509 – 13 . doi: 10.1259/bjr/60198873 [DOI] [PubMed] [Google Scholar]
- 13. Viswanathan AN , Dimopoulos J , Kirisits C , Berger D , Pötter R . Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours . International Journal of Radiation Oncology*Biology*Physics 2007. ; 68 : 491 – 8 . doi: 10.1016/j.ijrobp.2006.12.021 [DOI] [PubMed] [Google Scholar]
- 14. Burbach JPM , Kleijnen J-PJ , Reerink O , Seravalli E , Philippens MEP , Schakel T , et al. . Inter-observer agreement of MRI-based tumor delineation for preoperative radiotherapy boost in locally advanced rectal cancer . Radiother Oncol 2016. ; 118 : 399 – 407 . doi: 10.1016/j.radonc.2015.10.030 [DOI] [PubMed] [Google Scholar]
- 15. Lim K , Erickson B , Jürgenliemk-Schulz IM , Gaffney D , Creutzberg CL , Viswanathan A , et al. . Variability in clinical target volume delineation for intensity modulated radiation therapy in 3 challenging cervix cancer scenarios . Pract Radiat Oncol 2015. ; 5 : e557 – 65 . doi: 10.1016/j.prro.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim K , Small W , Portelance L , Creutzberg C , Jürgenliemk-Schulz IM , Mundt A , et al. . Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer . International Journal of Radiation Oncology*Biology*Physics 2011. ; 79 : 348 – 55 . doi: 10.1016/j.ijrobp.2009.10.075 [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos JCA , De Vos V , Berger D , Petric P , Dumas I , Kirisits C , et al. . Inter-observer comparison of target delineation for MRI-assisted cervical cancer brachytherapy: application of the GYN GEC-ESTRO recommendations . Radiother Oncol 2009. ; 91 : 166 – 72 . doi: 10.1016/j.radonc.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 18. Hellebust TP , Tanderup K , Lervåg C , Fidarova E , Berger D , Malinen E , et al. . Dosimetric impact of interobserver variability in MRI-based delineation for cervical cancer brachytherapy . Radiother Oncol 2013. ; 107 : 13 – 19 . doi: 10.1016/j.radonc.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 19. Petric P , Dimopoulos J , Kirisits C , Berger D , Hudej R , Pötter R . Inter- and intraobserver variation in HR-CTV contouring: intercomparison of transverse and paratransverse image orientation in 3D-MRI assisted cervix cancer brachytherapy . Radiother Oncol 2008. ; 89 : 164 – 71 . doi: 10.1016/j.radonc.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 20. Taylor A , Rockall AG , Reznek RH , Powell MEB . Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy . International Journal of Radiation Oncology*Biology*Physics 2005. ; 63 : 1604 – 12 . doi: 10.1016/j.ijrobp.2005.05.062 [DOI] [PubMed] [Google Scholar]
- 21. Tan J , Lim Joon D , Fitt G , Wada M , Lim Joon M , Mercuri A , et al. . The utility of multimodality imaging with CT and MRI in defining rectal tumour volumes for radiotherapy treatment planning: a pilot study . J Med Imaging Radiat Oncol 2010. ; 54 : 562 – 8 . doi: 10.1111/j.1754-9485.2010.02212.x [DOI] [PubMed] [Google Scholar]
- 22. Regini F , Gourtsoyianni S , Cardoso De Melo R , Charles-Edwards GD , Griffin N , Parikh J , et al. . Rectal tumour volume (GTV) Delineation using T2-weighted and diffusion-weighted MRI: implications for radiotherapy planning . Eur J Radiol 2014. ; 83 : 768 – 72 . doi: 10.1016/j.ejrad.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 23. Curvo-Semedo L , Lambregts DMJ , Maas M , Thywissen T , Mehsen RT , Lammering G , et al. . Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging . Radiology 2011. ; 260 : 734 – 43 . doi: 10.1148/radiol.11102467 [DOI] [PubMed] [Google Scholar]
- 24. Wachter-Gerstner N , Wachter S , Reinstadler E , Fellner C , Knocke TH , Pötter R . The impact of sectional imaging on dose escalation in endocavitary HDR-brachytherapy of cervical cancer: results of a prospective comparative trial . Radiother Oncol 2003. ; 68 : 51 – 9 . doi: 10.1016/S0167-8140(03)00083-5 [DOI] [PubMed] [Google Scholar]
- 25. Jadon R , Pembroke CA , Hanna CL , Palaniappan N , Evans M , Cleves AE , et al. . A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer . Clin Oncol 2014. ; 26 : 185 – 96 . doi: 10.1016/j.clon.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 26. Gwynne S , Webster R , Adams R , Mukherjee S , Coles B , Staffurth J . Image-guided radiotherapy for rectal cancer: a systematic review . Clin Oncol 2012. ; 24 : 250 – 60 . doi: 10.1016/j.clon.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 27. Tyagi N , Lewis JH , Yashar CM , Vo D , Jiang SB , Mundt AJ , et al. . Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer . International Journal of Radiation Oncology*Biology*Physics 2011. ; 80 : 273 – 80 . doi: 10.1016/j.ijrobp.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 28. Chong I , Hawkins M , Hansen V , Thomas K , McNair H , O’Neill B , et al. . Quantification of organ motion during chemoradiotherapy of rectal cancer using cone-beam computed tomography . International Journal of Radiation Oncology*Biology*Physics 2011. ; 81 : e431 – 8 . doi: 10.1016/j.ijrobp.2011.04.060 [DOI] [PubMed] [Google Scholar]
- 29. Nijkamp J , de Jong R , Sonke J-J , van Vliet C , Marijnen C . Target volume shape variation during irradiation of rectal cancer patients in supine position: comparison with prone position . Radiother Oncol 2009. ; 93 : 285 – 92 . doi: 10.1016/j.radonc.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 30. Nijkamp J , Marijnen C , van Herk M , van Triest B , Sonke J-J . Adaptive radiotherapy for long course neo-adjuvant treatment of rectal cancer . Radiother Oncol 2012. ; 103 : 353 – 9 . doi: 10.1016/j.radonc.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 31. Beadle BM , Jhingran A , Salehpour M , Sam M , Iyer RB , Eifel PJ . Cervix regression and motion during the course of external beam chemoradiation for cervical cancer . International Journal of Radiation Oncology*Biology*Physics 2009. ; 73 : 235 – 41 . doi: 10.1016/j.ijrobp.2008.03.064 [DOI] [PubMed] [Google Scholar]
- 32. Chan P , Dinniwell R , Haider MA , Cho Y-B , Jaffray D , Lockwood G , et al. . Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study . International Journal of Radiation Oncology*Biology*Physics 2008. ; 70 : 1507 – 15 . doi: 10.1016/j.ijrobp.2007.08.055 [DOI] [PubMed] [Google Scholar]
- 33. Taylor A , Powell MEB . An assessment of interfractional uterine and cervical motion: implications for radiotherapy target volume definition in gynaecological cancer . Radiother Oncol 2008. ; 88 : 250 – 7 . doi: 10.1016/j.radonc.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 34. van de Bunt L , Jürgenliemk-Schulz IM , de Kort GAP , Roesink JM , Tersteeg RJHA , van der Heide UA . Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need? Radiother Oncol 2008. ; 88 : 233 – 40 . doi: 10.1016/j.radonc.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 35. Kaatee RSJP , Olofsen MJJ , Verstraate MBJ , Quint S , Heijmen BJM . Detection of organ movement in cervix cancer patients using a fluoroscopic electronic portal imaging device and radiopaque markers . International Journal of Radiation Oncology*Biology*Physics 2002. ; 54 : 576 – 83 . doi: 10.1016/S0360-3016(02)02953-X [DOI] [PubMed] [Google Scholar]
- 36. Lee CM , Shrieve DC , Gaffney DK . Rapid involution and mobility of carcinoma of the cervix . International Journal of Radiation Oncology*Biology*Physics 2004. ; 58 : 625 – 30 . doi: 10.1016/j.ijrobp.2003.09.060 [DOI] [PubMed] [Google Scholar]
- 37. Haripotepornkul NH , Nath SK , Scanderbeg D , Saenz C , Yashar CM . Evaluation of intra- and inter-fraction movement of the cervix during intensity modulated radiation therapy . Radiother Oncol 2011. ; 98 : 347 – 51 . doi: 10.1016/j.radonc.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 38. Collen C , Engels B , Duchateau M , Tournel K , De Ridder M , Bral S , et al. . Volumetric imaging by megavoltage computed tomography for assessment of internal organ motion during radiotherapy for cervical cancer . International Journal of Radiation Oncology*Biology*Physics 2010. ; 77 : 1590 – 5 . doi: 10.1016/j.ijrobp.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 39. Lim K , Kelly V , Stewart J , Xie J , Cho Y-B , Moseley J , et al. . Pelvic radiotherapy for cancer of the cervix: is what you plan actually what you deliver? International Journal of Radiation Oncology*Biology*Physics 2009. ; 74 : 304 – 12 . doi: 10.1016/j.ijrobp.2008.12.043 [DOI] [PubMed] [Google Scholar]
- 40. Brierley JD , Dawson LA , Sampson E , Bayley A , Scott S , Moseley JL , et al. . Rectal motion in patients receiving preoperative radiotherapy for carcinoma of the rectum . International Journal of Radiation Oncology*Biology*Physics 2011. ; 80 : 97 – 102 . doi: 10.1016/j.ijrobp.2010.01.042 [DOI] [PubMed] [Google Scholar]
- 41. Tournel K , De Ridder M , Engels B , Bijdekerke P , Fierens Y , Duchateau M , et al. . Assessment of intrafractional movement and internal motion in radiotherapy of rectal cancer using megavoltage computed tomography . International Journal of Radiation Oncology*Biology*Physics 2008. ; 71 : 934 – 9 . doi: 10.1016/j.ijrobp.2008.02.032 [DOI] [PubMed] [Google Scholar]
- 42. Nuyttens JJ , Robertson JM , Yan D , Martinez A . The variability of the clinical target volume for rectal cancer due to internal organ motion during adjuvant treatment . International Journal of Radiation Oncology*Biology*Physics 2002. ; 53 : 497 – 503 . doi: 10.1016/S0360-3016(02)02753-0 [DOI] [PubMed] [Google Scholar]
- 43. Nijkamp J , Swellengrebel M , Hollmann B , de Jong R , Marijnen C , van Vliet-Vroegindeweij C , et al. . Repeat CT assessed CTV variation and PTV margins for short- and long-course pre-operative RT of rectal cancer . Radiother Oncol 2012. ; 102 : 399 – 405 . doi: 10.1016/j.radonc.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 44. Ahmad R , Hoogeman MS , Bondar M , Dhawtal V , Quint S , De Pree I , et al. . Increasing treatment accuracy for cervical cancer patients using correlations between bladder-filling change and cervix-uterus displacements: proof of principle . Radiother Oncol 2011. ; 98 : 340 – 6 . doi: 10.1016/j.radonc.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 45. Van den Begin R , Kleijnen JP , Engels B , Philippens M , van Asselen B , Raaymakers B , et al. . Tumor volume Regression during preoperative chemoradiotherapy for rectal cancer: a prospective observational study with weekly MRI . Acta Oncol 2017. ; 57 : 723 – 7 . [DOI] [PubMed] [Google Scholar]
- 46. Lambregts DMJ , Yassien AB , Lahaye MJ , Betgen A , Maas M , Beets GL , et al. . Monitoring early changes in rectal tumor morphology and volume during 5 weeks of preoperative chemoradiotherapy - An evaluation with sequential MRIs . Radiother Oncol 2018. ; 126 : 431 – 6 . doi: 10.1016/j.radonc.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 47. van de Bunt L , van der Heide UA , Ketelaars M , de Kort GAP , Jürgenliemk-Schulz IM , Conventional J-SIM . Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression . International Journal of Radiation Oncology*Biology*Physics 2006. ; 64 : 189 – 96 . doi: 10.1016/j.ijrobp.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 48. Appelt AL , Pløen J , Harling H , Jensen FS , Jensen LH , Jørgensen JCR , et al. . High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study . Lancet Oncol 2015. ; 16 : 919 – 27 . doi: 10.1016/S1470-2045(15)00120-5 [DOI] [PubMed] [Google Scholar]
- 49. Burbach JPM , den Harder AM , Intven M , van Vulpen M , Verkooijen HM , Reerink O . Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis . Radiother Oncol 2014. ; 113 : 1 – 9 . doi: 10.1016/j.radonc.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 50. Seierstad T , Hole KH , Saelen E , Ree AH , Flatmark K , Malinen E . MR-guided simultaneous integrated boost in preoperative radiotherapy of locally advanced rectal cancer following neoadjuvant chemotherapy . Radiother Oncol 2009. ; 93 : 279 – 84 . doi: 10.1016/j.radonc.2009.08.046 [DOI] [PubMed] [Google Scholar]
- 51. Bowen SR , Yuh WTC , Hippe DS , Wu W , Partridge SC , Elias S , et al. . Tumor radiomic heterogeneity: Multiparametric functional imaging to characterize variability and predict response following cervical cancer radiation therapy . J Magn Reson Imaging 2018. ; 47 : 1388 – 96 . doi: 10.1002/jmri.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lambrecht M , Deroose C , Roels S , Vandecaveye V , Penninckx F , Sagaert X , et al. . The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer . Acta Oncol 2010. ; 49 : 956 – 63 . doi: 10.3109/0284186X.2010.498439 [DOI] [PubMed] [Google Scholar]
- 53. Sun Y-S , Zhang X-P , Tang L , Ji J-F , Gu J , Cai Y , et al. . Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging . Radiology 2010. ; 254 : 170 – 8 . doi: 10.1148/radiol.2541082230 [DOI] [PubMed] [Google Scholar]
- 54. Barbaro B , Vitale R , Valentini V , Illuminati S , Vecchio FM , Rizzo G , et al. . Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy . International Journal of Radiation Oncology*Biology*Physics 2012. ; 83 : 594 – 9 . doi: 10.1016/j.ijrobp.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 55. Harry VN , Semple SI , Gilbert FJ , Parkin DE . Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer . Gynecol Oncol 2008. ; 111 : 213 – 20 . doi: 10.1016/j.ygyno.2008.07.048 [DOI] [PubMed] [Google Scholar]
- 56. Lambrecht M , Vandecaveye V , De Keyzer F , Roels S , Penninckx F , Van Cutsem E , et al. . Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results . International Journal of Radiation Oncology*Biology*Physics 2012. ; 82 : 863 – 70 . doi: 10.1016/j.ijrobp.2010.12.063 [DOI] [PubMed] [Google Scholar]
- 57. Curvo-Semedo L , Lambregts DMJ , Maas M , Beets GL , Caseiro-Alves F , Beets-Tan RGH . Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness . J Magn Reson Imaging 2012. ; 35 : 1365 – 71 . doi: 10.1002/jmri.23589 [DOI] [PubMed] [Google Scholar]
- 58. Petrelli F , Sgroi G , Sarti E , Barni S . Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies . Ann Surg 2016. ; 263 : 458 – 64 . doi: 10.1097/SLA.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 59. Mayr NA , Wang JZ , Zhang D , Grecula JC , Lo SS , Jaroura D , et al. . Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer . International Journal of Radiation Oncology*Biology*Physics 2010. ; 77 : 502 – 8 . doi: 10.1016/j.ijrobp.2009.04.084 [DOI] [PubMed] [Google Scholar]
- 60. Tanderup K , Fokdal LU , Sturdza A , Haie-Meder C , Mazeron R , van Limbergen E , et al. . Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer . Radiother Oncol 2016. ; 120 : 441 – 6 . doi: 10.1016/j.radonc.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 61. Bondar L , Hoogeman M , Mens JW , Dhawtal G , de Pree I , Ahmad R , et al. . Toward an individualized target motion management for IMRT of cervical cancer based on model-predicted cervix-uterus shape and position . Radiother Oncol 2011. ; 99 : 240 – 5 . doi: 10.1016/j.radonc.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 62. Bondar ML , Hoogeman MS , Mens JW , Quint S , Ahmad R , Dhawtal G , et al. . Individualized nonadaptive and online-adaptive intensity-modulated radiotherapy treatment strategies for cervical cancer patients based on pretreatment acquired variable bladder filling computed tomography scans . International Journal of Radiation Oncology*Biology*Physics 2012. ; 83 : 1617 – 23 . doi: 10.1016/j.ijrobp.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 63. Ahmad R , Bondar L , Voet P , Mens J-W , Quint S , Dhawtal G , et al. . A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: a dosimetric evaluation . Acta Oncologica 2013. ; 52 : 1430 – 6 . doi: 10.3109/0284186X.2013.813640 [DOI] [PubMed] [Google Scholar]
- 64. van de Schoot AJAJ , de Boer P , Visser J , Stalpers LJA , Rasch CRN , Bel A . Dosimetric advantages of a clinical daily adaptive plan selection strategy compared with a non-adaptive strategy in cervical cancer radiation therapy . Acta Oncol 2017. ; 56 : 667 – 74 . doi: 10.1080/0284186X.2017.1287949 [DOI] [PubMed] [Google Scholar]
- 65. Heijkoop ST , Langerak TR , Quint S , Bondar L , Mens JWM , Heijmen BJM , et al. . Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT . International Journal of Radiation Oncology*Biology*Physics 2014. ; 90 : 673 – 9 . doi: 10.1016/j.ijrobp.2014.06.046 [DOI] [PubMed] [Google Scholar]
- 66. de Jong R , Lutkenhaus L , van Wieringen N , Visser J , Wiersma J , Crama K , et al. . Plan selection strategy for rectum cancer patients: an interobserver study to assess clinical feasibility . Radiother Oncol 2016. ; 120 : 207 – 11 . doi: 10.1016/j.radonc.2016.07.027 [DOI] [PubMed] [Google Scholar]
- 67. Lutkenhaus LJ , de Jong R , Geijsen ED , Visser J , van Wieringen N , Bel A , et al. . Potential dosimetric benefit of an adaptive plan selection strategy for short-course radiotherapy in rectal cancer patients . Radiother Oncol 2016. ; 119 : 525 – 30 . doi: 10.1016/j.radonc.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 68. Stewart J , Lim K , Kelly V , Xie J , Brock KK , Moseley J , et al. . Automated Weekly replanning for intensity-modulated radiotherapy of cervix cancer . Int J Radiat Oncol Biol Phys 2010. ; 78 : 350 – 8 . doi: 10.1016/j.ijrobp.2009.07.1699 [DOI] [PubMed] [Google Scholar]
- 69. Oh S , Stewart J , Moseley J , Kelly V , Lim K , Xie J , et al. . Hybrid adaptive radiotherapy with on-line MRI in cervix cancer IMRT . Radiother Oncol 2014. ; 110 : 323 – 8 . doi: 10.1016/j.radonc.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 70. Lim K , Stewart J , Kelly V , Xie J , Brock KK , Moseley J , et al. . Dosimetrically triggered adaptive intensity modulated radiation therapy for cervical cancer . Int J Radiat Oncol Biol Phys 2014. ; 90 : 147 – 54 . doi: 10.1016/j.ijrobp.2014.05.039 [DOI] [PubMed] [Google Scholar]
- 71. Nyholm T , Jonsson J . Counterpoint: opportunities and challenges of a magnetic resonance imaging-only radiotherapy work flow . Semin Radiat Oncol 2014. ; 24 : 175 – 80 . doi: 10.1016/j.semradonc.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 72. Raaymakers BW , Lagendijk JJW , Overweg J , Kok JGM , Raaijmakers AJE , Kerkhof EM , et al. . Integrating a 1.5 T MRI scanner with a 6 mV accelerator: proof of concept . Phys Med Biol 2009. ; 54 : N229 – N237 . doi: 10.1088/0031-9155/54/12/N01 [DOI] [PubMed] [Google Scholar]
- 73. Mutic S , Dempsey JF . The ViewRay system: magnetic resonance-guided and controlled radiotherapy . Semin Radiat Oncol 2014. ; 24 : 196 – 9 . doi: 10.1016/j.semradonc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 74. Keall PJ , Barton M , Crozier S . Australian Mri-Linac program icfIIICCCLHSUUoNQSWS, Wollongong. The Australian magnetic resonance imaging-linac program . Semin Radiat Oncol 2014. ; 24 : 203 – 6 . [DOI] [PubMed] [Google Scholar]
- 75. Fallone BG . The rotating biplanar linac-magnetic resonance imaging system . Semin Radiat Oncol 2014. ; 24 : 200 – 2 . doi: 10.1016/j.semradonc.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 76. Woodings SJ , Bluemink JJ , de Vries JHW , Niatsetski Y , van Veelen B , Schillings J , et al. . Beam characterisation of the 1.5 T MRI-linac . Phys Med Biol 2018. ; 63 : 085015 . doi: 10.1088/1361-6560/aab566 [DOI] [PubMed] [Google Scholar]
- 77. Hu Y , Rankine L , Green OL , Kashani R , Li HH , Li H , et al. . Characterization of the onboard imaging unit for the first clinical magnetic resonance image guided radiation therapy system . Med Phys 2015. ; 42 : 5828 – 37 . doi: 10.1118/1.4930249 [DOI] [PubMed] [Google Scholar]
- 78. Fischer-Valuck BW , Henke L , Green O , Kashani R , Acharya S , Bradley JD , et al. . Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system . Adv Radiat Oncol 2017. ; 2 : 485 – 93 . doi: 10.1016/j.adro.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Acharya S , Fischer-Valuck BW , Kashani R , Parikh P , Yang D , Zhao T , et al. . Online magnetic resonance image guided adaptive radiation therapy: first clinical applications . Int J Radiat Oncol Biol Phys 2016. ; 94 : 394 – 403 . doi: 10.1016/j.ijrobp.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 80. Raaymakers BW , Jürgenliemk-Schulz IM , Bol GH , Glitzner M , Kotte ANTJ , van Asselen B , et al. . First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment . Phys Med Biol 2017. ; 62 : L41 – L50 . doi: 10.1088/1361-6560/aa9517 [DOI] [PubMed] [Google Scholar]
- 81. Young AV , Wortham A , Wernick I , Evans A , Ennis RD . Atlas-based segmentation improves consistency and decreases time required for contouring postoperative endometrial cancer nodal volumes . Int J Radiat Oncol Biol Phys 2011. ; 79 : 943 – 7 . doi: 10.1016/j.ijrobp.2010.04.063 [DOI] [PubMed] [Google Scholar]
- 82. Sharp G , Fritscher KD , Pekar V , Peroni M , Shusharina N , Veeraraghavan H , et al. . Vision 20/20: perspectives on automated image segmentation for radiotherapy . Med Phys 2014. ; 41 : 050902 . doi: 10.1118/1.4871620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Klein S , Staring M , Murphy K , Viergever MA , Pluim JPW . elastix: a toolbox for intensity-based medical image registration . IEEE Trans Med Imaging 2010. ; 29 : 196 – 205 . doi: 10.1109/TMI.2009.2035616 [DOI] [PubMed] [Google Scholar]
- 84. van der Put RW , Kerkhof EM , Raaymakers BW , Jürgenliemk-Schulz IM , Lagendijk JJW . Contour propagation in MRI-guided radiotherapy treatment of cervical cancer: the accuracy of rigid, non-rigid and semi-automatic registrations . Phys Med Biol 2009. ; 54 : 7135 – 50 . doi: 10.1088/0031-9155/54/23/007 [DOI] [PubMed] [Google Scholar]
- 85. Staring M , van der Heide UA , Klein S , Viergever MA , Pluim JPW . Registration of cervical MRI using multifeature mutual information . IEEE Trans Med Imaging 2009. ; 28 : 1412 – 21 . doi: 10.1109/TMI.2009.2016560 [DOI] [PubMed] [Google Scholar]
- 86. Sjöberg C , Lundmark M , Granberg C , Johansson S , Ahnesjö A , Montelius A . Clinical evaluation of multi-atlas based segmentation of lymph node regions in head and neck and prostate cancer patients . Radiat Oncol 2013. ; 8 : 229 . doi: 10.1186/1748-717X-8-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Torheim T , Malinen E , Hole KH , Lund KV , Indahl UG , Lyng H , et al. . Autodelineation of cervical cancers using multiparametric magnetic resonance imaging and machine learning . Acta Oncol 2017. ; 56 : 806 – 12 . doi: 10.1080/0284186X.2017.1285499 [DOI] [PubMed] [Google Scholar]
- 88. Lustberg T , van Soest J , Gooding M , Peressutti D , Aljabar P , van der Stoep J , et al. . Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer . Radiother Oncol 2018. ; 126 : 312 – 7 . doi: 10.1016/j.radonc.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 89. Bohoudi O , Bruynzeel AME , Senan S , Cuijpers JP , Slotman BJ , Lagerwaard FJ , et al. . Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (smart) for pancreatic cancer . Radiother Oncol 2017. ; 125 : 439 – 44 . doi: 10.1016/j.radonc.2017.07.028 [DOI] [PubMed] [Google Scholar]
- 90. Ahunbay EE , Peng C , Chen G-P , Narayanan S , Yu C , Lawton C , et al. . An on-line replanning scheme for interfractional variations . Med Phys 2008. ; 35 : 3607 – 15 . doi: 10.1118/1.2952443 [DOI] [PubMed] [Google Scholar]
- 91. Mohan R , Zhang X , Wang H , Kang Y , Wang X , Liu H , et al. . Use of Deformed intensity distributions for on-line modification of image-guided IMRT to account for interfractional anatomic changes . Int J Radiat Oncol Biol Phys 2005. ; 61 : 1258 – 66 . doi: 10.1016/j.ijrobp.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 92. Feng Y , Castro-Pareja C , Shekhar R , Yu C . Direct aperture deformation: an interfraction image guidance strategy . Med Phys 2006. ; 33 : 4490 – 8 . doi: 10.1118/1.2374675 [DOI] [PubMed] [Google Scholar]
- 93. Ahunbay EE , Li XA . Gradient maintenance: a new algorithm for fast online replanning . Med Phys 2015. ; 42 : 2863 – 76 . doi: 10.1118/1.4919847 [DOI] [PubMed] [Google Scholar]
- 94. Otto K . Real-time interactive treatment planning . Phys Med Biol 2014. ; 59 : 4845 – 59 . doi: 10.1088/0031-9155/59/17/4845 [DOI] [PubMed] [Google Scholar]
- 95. Ziegenhein P , Ph Kamerling C , Oelfke U . Interactive dose shaping Part 1: a new paradigm for IMRT treatment planning . Phys Med Biol 2016. ; 61 : 2457 – 70 . doi: 10.1088/0031-9155/61/6/2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ziegenhein P , Kamerling CP , Bangert M , Kunkel J , Oelfke U . Performance-optimized clinical IMRT planning on modern CPUs . Phys Med Biol 2013. ; 58 : 3705 – 15 . doi: 10.1088/0031-9155/58/11/3705 [DOI] [PubMed] [Google Scholar]
- 97. Men C , Gu X , Choi D , Majumdar A , Zheng Z , Mueller K , et al. . GPU-based ultrafast IMRT plan optimization . Phys Med Biol 2009. ; 54 : 6565 – 73 . doi: 10.1088/0031-9155/54/21/008 [DOI] [PubMed] [Google Scholar]
- 98. Noel CE , Parikh PJ , Spencer CR , Green OL , Hu Y , Mutic S , et al. . Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy . Acta Oncol 2015. ; 54 : 1474 – 82 . doi: 10.3109/0284186X.2015.1062541 [DOI] [PubMed] [Google Scholar]
- 99. Cellini F , Chiloiro G , Boldrini L , Massaccesi M , Mattiucci G , Antonelli MV , et al. . EP-1501: feasibility of MRgRT neoadjuvant treatment for locally advanced rectal cancer . Radiotherapy and Oncology 2018. ; 127 : S814 . doi: 10.1016/S0167-8140(18)31810-3 [DOI] [Google Scholar]
- 100. Ko HC , Huang JY , Miller JR , Das RK , Wallace CR , De Costa A-MA , et al. . Novel use of ViewRay MRI guidance for high-dose-rate brachytherapy in the treatment of cervical cancer . Brachytherapy 2018. ; 17 : 680 – 8 . doi: 10.1016/j.brachy.2018.04.005 [DOI] [PubMed] [Google Scholar]


