Abstract
Objective:
To report on the use of RFA for the treatment of symptomatic benign and autonomously functioning thyroid nodules (AFTNs) in the first reported UK cohort.
Methods:
Patients treated over a 19-month period were retrospectively reviewed. Nodules were assessed pre-treatment and at 1 and 6 months post-treatment. Nodule volume was calculated and cosmetic assessment and thyroid-related quality of life (QoL) scores were recorded at each time point.
Thyroid function tests (TFTs) were recorded at all three time points for patients with ATFNs.
Results:
46 patients with 50 nodules were treated with no complications. The mean volume reduction 1-month post-treatment was 53 +- 14.9 % ( p < 0.0001). Six month data was available for 31 nodules and showed a mean 67 +- 17.6% vol reduction ( p < 0.0001).
Five of the six patients with ATFNs were euthyroid at 1-month post-procedure. 6-month data was available on three of these patients, and all remained euthyroid.
The thyroid-related QoL and cosmetic scores also improved. Data from 23 patients was available pre-treatment and at 6 months post-treatment and there was a significant ( p < 0.0001) reduction in QoL score. Pre-treatment, 82 % of nodules were readily visible at rest, decreasing to 12.5 % 6 months after treatment ( p < 0.0001).
Conclusions:
Results align with published data suggesting that RFA is effective at reducing nodule volume and at treating ATFNs and leads to improvement in thyroid-related QoL and cosmetic scores.
Advances in knowledge:
This early UK experience demonstrates that day-case radiofrequency ablation can provide safe and effective treatment of benign symptomatic thyroid nodules.
Introduction
Whilst many benign thyroid nodules are asymptomatic and do not require treatment, a subset cause symptoms related to compression of adjacent structures, visible neck swelling, or result in a clinical or biochemical hyperthyroidism. Until recently, treatment options were limited to surgery for symptomatic benign thyroid nodules and radioiodine therapy, carbimazole or surgery for treatment of autonomously functioning thyroid nodules (AFTNs).
Radiofrequency ablation (RFA) is a minimally-invasive treatment for symptomatic and toxic thyroid nodules that was first described in 20061 and has since become an established technique, particularly in Europe and Asia. Large series have shown a 33–58% and 51–85% reduction in nodule volume at 1 and 6 months respectively, as well as significant improvement in symptom scores.1,2 Adoption in the UK has however awaited approval by the National Institute of Health and Care Excellence (NICE).3 The first service in the UK was established at our institution in February 2017.
The treatment involves insertion of an RFA electrode needle into the nodule under direct ultrasound guidance. The needle used has an active tip which heats due to alternating current passing through it. This heating of the adjacent 1 cm of thyroid tissue results in localised coagulative necrosis. The trans-isthmic moving-shot technique2,4 employs moving the needle during treatment resulting in multiple small overlapping ablation zones which collectively encompass the majority of the target nodule while avoiding critical medial structures including the recurrent laryngeal nerve and the cervical oesophagus that lie within a ‘danger triangle’.
Indications for thyroid nodule RFA include visible neck swelling, dysphagia, a feeling of lump in the throat and in the case of AFTNs, clinical or biochemical thyrotoxicosis. Furthermore, patients who are not suitable for surgical complete or partial thyroidectomy, including those with pre-existing vocal cord palsy, where thyroidectomy may put the other recurrent laryngeal nerve at risk, or those with co-morbidities which preclude general anaesthetic, can be treated. RFA is also a potential treatment for those patients who want to be treated but do not wish to undergo surgery.
Here we describe outcomes and experience of the first 19 months of treating benign thyroid nodules with RFA.
Methods
This retrospective review was undertaken at a single tertiary head and neck referral centre. The study was reviewed by the University College London (UCL) joint research and development office and considered to be service evaluation exempt from research ethics committee approval.
Patients
Selection criteria for RFA treatment included patients with symptomatic nodules causing compressive symptoms or a visible neck swelling or in the case of AFTNs, thyrotoxicosis. All nodules were ultrasonographically graded as U2 using the British Thyroid Association 2014 guidelines5 and all had to have had a fine needle aspiration cytology (FNAC) sample yielding a benign cytological result (Thy2 or Thy2C).
Exclusion criteria included any nodules that returned indeterminate, suspicious or malignant cytology (Thy 3a/Thy 3f and above) or patients who were asymptomatic from their thyroid nodular disease.
Patients were seen in an outpatient clinic where ultrasound measurements of the nodule in the transverse (T), anteroposterior (AP) and craniocaudal (CC) planes were made and nodule volume calculated using the formula [(3.14 x AP x T X CC)/6]. Nodules were also classified as predominantly cystic, mixed solid-cystic or solid.
Thyroid-related quality of life was assessed using relevant goitre appearance and quality of life Likert scales within the abbreviated ThyPro Patient-Reported Outcome – a validated assessment tool.6 Subjective assessment of whether a nodule was readily visible at rest was made by a consultant head and neck radiologist at baseline and assigned a score as follows; 4: visible mass, 3: mass visible on swallowing only 2: no visible mass but palpable lump; 1: no visible or palpable mass.
All patients provided fully informed written consent for treatment.
Technique
All the cases in our institution were performed by one of three dedicated head and neck radiologists.
A generator with a maximum output of 200W at a frequency of 400 +- 10% KHz was used (MYGEN M-3004, RF Medical Co., Ltd. Korea) with an internally cooled monopolar 18-gauge electrode with an active tip of 0.7 or 1.0 cm (MYGEN M-3004, RF Medical Co., Ltd. Korea). A mixture of long and shorter acting anaesthetic was infiltrated in a 1:1 ratio (0.5% bupivacaine: 1% lidocaine) at the electrode skin entry point in the mid-line of the neck to facilitate the trans-isthmic moving shot technique. Infiltration of the local anaesthetic continued to the deeper subcutaneous tissues, including into the plane between the thyroid capsule and the overlying strap muscles. If the nodule contained cystic material, this was aspirated to reduce the volume of nodule requiring treatment and to reduce heat-sink effect of the electrode.
Dispersive electrodes were applied to the skin and were connected to the RF generator and the generator connected to the RF electrode. A pump was used to perfuse cooled water through the electrode to prevent tissue charring and improve the dispersion of RF energy.
A trans-isthmic approach was utilised to introduce the electrode, whereby the electrode was inserted into the isthmus aiming towards the lateral part of the nodule. This facilitated visualisation of the entire length of the electrode as it treated the nodule, and enabled avoidance of the ‘danger-triangle’ structures including the recurrent laryngeal nerve and the cervical oesophagus. The electrode needle was directly inserted into the skin without the need for a scalpel incision, and treatment performed under direct ultrasound guidance without use of a needle guide.
The electrode was passed into the most inferior posterior portion of the nodule and a moving-shot technique was utilised whereby multiple small units were systematically ablated as the electrode was passed backwards to the most superficial and medial portion of the nodule. This also minimised disruption of the image that would be caused by gas bubbles forming from vaporised tissue.
The RF power varied between 30–120W depending on the size of the active tip and the nodule constituents. The larger the electrode tip, the greater starting energy that was usually required. Treatment ended when all the thyroid parenchyma safely amenable to treatment was replaced by echogenic gas artefact. Post-procedure, patients were monitored in a recovery area and a cool pack applied to the skin for approximately 30 min. Patients were assessed approximately 30 min after the procedure in a recovery area and discharged home once they felt well enough with advice and a written information sheet.
Assessment of safety and efficacy
Adverse events during or after treatment were recorded and scored using the Clavien-Dindo classification system for surgical complications. The target nodule triplanar dimension and volume were recorded pre-treatment at baseline and at 1 month and 6 months post-procedure in the outpatient clinic. Symptoms scores using the same standardised QoL as was utilised at baseline and also recorded at 1 month and 6 months post-procedure. Cosmetic scores were recorded by clinical assessment by one of the operating consultant head and neck radiologists at baseline, 1 month and 6 months post-procedure. Thyroid function tests (free T3 and T4 and TSH) were also recorded at each of the three time points for patients with AFTNs.
Statistical analysis
Patients with thyroid nodule volume measurements at each of the three time points (pre-treatment, 1- and 6-month post treatment) were compared using repeat measures analysis of variance (ANOVA). Change in thyroid-related QoL and cosmetic score at 6 months was evaluated using the Wilcoxon test. Analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, California). p < 0.05 was taken to indicate significance in all methods.
Results
53 patients were referred for thyroid RFA at our institution between February 2017 and August 2018. Three of these patients were considered unsuitable for treatment as despite having a multinodular thyroid, they were asymptomatic. Two further patients were excluded because the FNA yielded Thy3a in one patient and Thy3f in the second patient. After discussing RFA treatment at the initial clinic consultation, two patients decided against any treatment at all for their benign thyroid nodules, despite being symptomatic. Over the reviewed 19-month period, 46 patients (7 male and 39 female; mean/range age 50.9/30–83) and 50 thyroid nodules were treated. Of the 50 treated nodules, 43 were benign and symptomatic. Seven treated nodules were AFTNs, two of which were in a single patient, i.e. six thyrotoxic patients were treated in total over the study period.
22 of the treated nodules were solid (Figure 1), three were cystic and 25 were characterised as mixed solid-cystic.
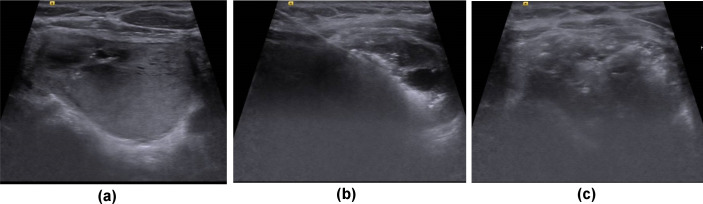
Figure 1.
Intra procedural ultrasound during radiofrequency ablation in a 58-year-old male. Pre-ablation scan (a) demonstrates the target nodule with maximum dimension of 28 mm in the medial left lobe. After local anaesthetic injection to the skin and subcutaneous tissues, extending to the thyroid capsule, the ablation probe is inserted using the trans-isthmic approach in a medial to lateral direction (b) avoiding critical structures in the danger triangle – bottom left of image. Heating at the tip of the probe leads to tissue vaporisation and echogenic artefact. At the end of treatment (c) heterogeneous echogenic artefact is seen throughout the nodule.
At baseline, mean (SD) nodule volume was 25.9 (27.7) ml, with a range from 0.7 to 130.8 ml. All but one patient (n = 45) attended their 1-month follow up assessment. Mean (SD) volume at 1 month was 13.0 (14.3) ml, with a range of 0.2–51.4 ml. This represented a mean (SD) volume reduction in the first month post-treatment of 53 (14.9) %. At 6 months, data was available for 31 of the 50 treated nodules (28 symptomatic benign nodules and 3 AFTNs) representing data in 27 patients, with a mean (SD) nodule volume of 10.1 (13.6) ml and mean (SD) volume reduction from baseline of 67 (17.6) %. Figure 2 shows an example case. Figure 3 summarises change in volume in all patients over the 6-month period. None of the patients treated re-presented with nodule recurrence over the time period studied.
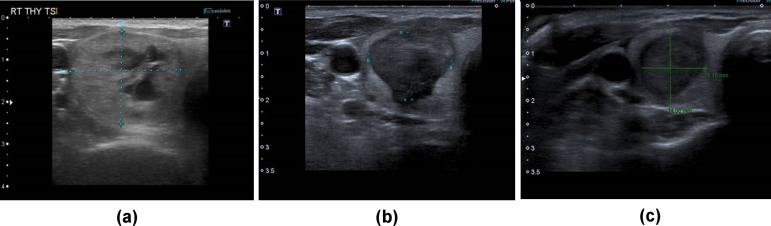
Figure 2.
Thyroid nodule before treatment (a) measuring 23 × 17×38 mm; and after treatment showing reduction in size of the thyroid nodule to 14 × 17×30 mm at 1 month (b) and 6 months (c) where it measured 14 × 14×25 mm. Note the small residuum at the inferomedial portion of the nodule in (b) and (c) lying adjacent to the 'danger-triangle'.
Figure 3.
Line plot illustrating thyroid nodule volume change with treatment. Asterisk denotes statistical significance.
Volume data at all three time points was available for 31 treated nodules and repeat measures ANOVA showed the reduction in nodule volume was significant (p < 0.0001) at both 1 and 6 months.
Five of the six thyrotoxic patients were euthyroid at 1-month post treatment. Three of these six patients were also seen at 6 months and remained euthyroid. A single thyrotoxic patient was still thyrotoxic at 1-month post procedure and at 6 month data was not yet available in this patient.
The thyroid-related quality of life and cosmetic score also improved following treatment. 23 patients returned completed questionnaires before and at 6 months after treatment. Wilcoxon test comparison showed a significant (p < 0.0001) reduction in score – Figure 4. Subjective cosmetic score showed 82% of nodules were visible at rest before treatment, which decreased to 12.5% at 6 months after treatment.
Figure 4.
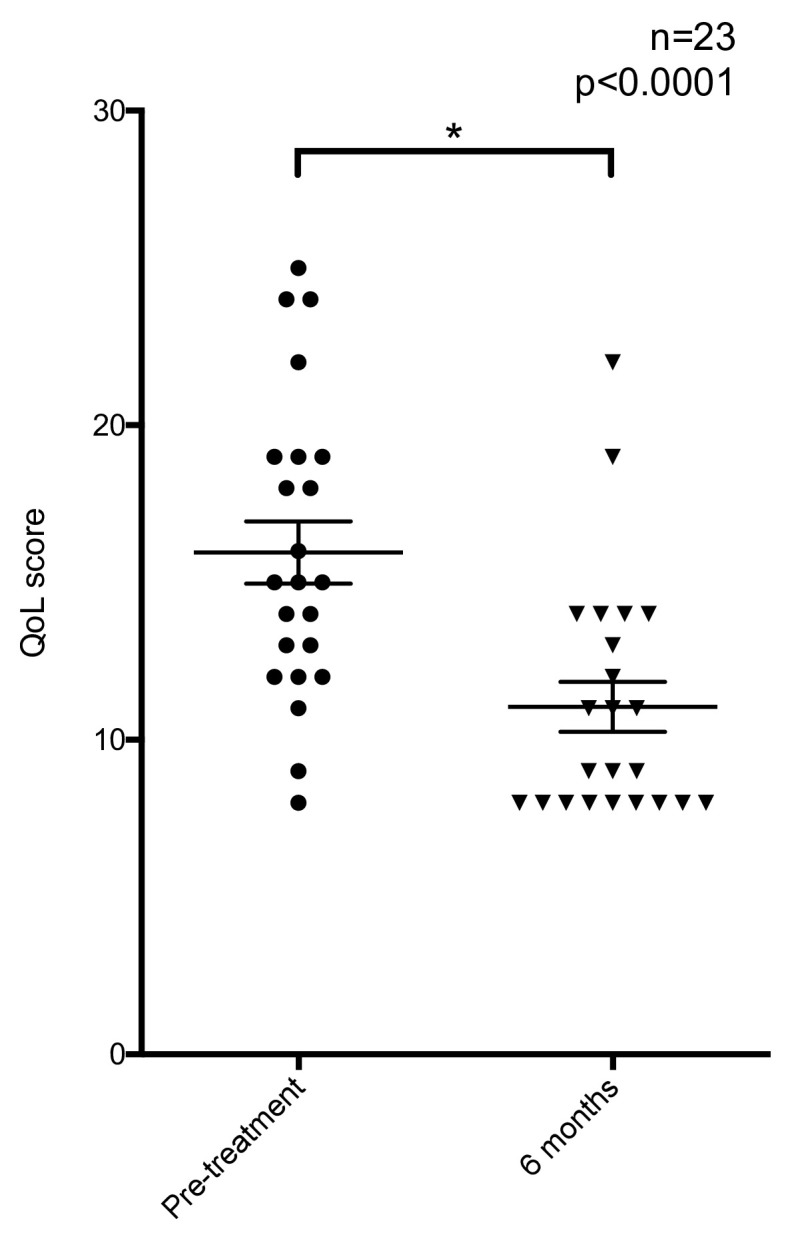
Scatter plot illustrating the distribution of thyroid-related symptom scores before and after treatment.
Complications
No significant complications (Clavian-Dindo Grade three or above) occurred in any of the patients treated and all patients were discharged home within an hour of the end of the procedure.
Discussion
In this service evaluation, we demonstrate that radiofrequency ablation can be used to safely treat benign symptomatic thyroid nodules and achieve effective reduction in nodule size and or function. We have successfully established a thyroid RFA service, offering treatment under local anaesthesia as a day case procedure at a UK tertiary head and neck centre.
The use of minimally invasive tissue ablation treatment has grown rapidly over the past two decades – with ablation offering effective local treatment with low complication risk and rapid recovery. Use of this technology has spread from cancer treatment to a number of benign diseases, with varying levels of supporting evidence.
Until recently, patients with symptomatic benign thyroid nodules were presented with limited treatment options, the mainstay being surgical resection, but the presence of a visible scar on the neck or anaesthetic limitations as well as patient choice excluded surgical treatment as an option for some. The use of RFA as a minimally invasive treatment option was developed in South Korea and has since gained international adoption. A systematic review that included two randomised controlled trials comparing RFA to placebo or another treatment, with 284 patients treated with RFA. The review concluded that RFA was safe and effective but encouraged further level one evidence comparing RFA to other treatments.7
Adoption of thyroid RFA within the UK has awaited approval by the National Institute of clinical Excellence (NICE). NICE published their guidance on RFA (IPG562) in June 2016, recommending that current evidence supported the use of this procedure, provided that standard arrangements were in place for clinical governance, consent and audit.3
Soon after publication of the updated NICE guidance, we sought permission for introduction of thyroid RFA from local clinical governance and new procedures committees, as well as support from the benign head and neck multidisciplinary team (MDT). Following a model developed by the interventional oncology service at our centre, we established a dedicated thyroid RFA pathway that included a single point of referral, MDT review, an outpatient clinic appointment and clear written patient information to ensure consent was fully informed. Pathway oversight was from one of three named dedicated head and neck radiology consultants who already regularly performed neck and thyroid interventional procedures and were well known to the wider head and neck service, with support from an interventional radiology clinical nurse specialist. Follow-up appointments at 1-month and 6 months post-procedure were also conducted as a formal clinic consultation, with outcome data recorded prospectively into a database and a letter sent back to the referring physician after each clinic appointment. Review timing was based on a study by Lim et al who followed up 111 patients over 4 years and showed that maximum volume reduction ratio was achieved at 6 months post treatment, with reduction remaining significant at 3 years. The recurrence rate over the study period was low and there were no reported delayed complications.8
This initial service review had several limitations. As the first described UK cohort, the number of patients treated was small and follow-up period relatively short. 6-month follow-up data was available in a limited number of case at the time of publication, but results were sufficient to demonstrate benefit. More robust future assessment of nodule volume reduction requires a complete 6-month dataset9 and these data will continue to be collected.
None of the patients treated suffered any major complication. All procedures were performed by dedicated head and neck radiologists using the trans-isthmic technique to avoid the ‘danger triangle’ structures. Instilling subcutaneous local anaesthesia facilitated passing the electrode through the skin and administering the RFA treatment. Despite this, some patients, especially those with deeper nodules or those nodules draped over the trachea experienced discomfort during the treatment due to the challenge of administering local anaesthetic deep to especially large nodules and those overlying the trachea. Some patients also experienced referred pain to the mandible, chest and ear during the procedure when the needle tip was close to the thyroid capsule. Reducing the power delivered to these nodules, as well as moving the tip of the electrode away from the thyroid capsule afforded relief to the patient in some of these cases.
Further minimally invasive techniques are emerging for the treatment of benign symptomatic thyroid nodules. Percutaneous microwave ablation (MWA) has been established as an alternative thermoablative technique for benign and malignant tumours of the lung, kidneys and liver – some technical advantages. In recent years MWA has been used to treat thyroid nodules with reported reduction in thyroid nodule volume of up to 65%.10 However a study comparing the volume and maximal diameter reduction of benign thyroid nodules treated with MWA versus RFA concluded that the volume and maximal diameter reduction were significantly greater in the RFA group versus the MWA group at 6 months; with no statistically significant difference in complications.11 High frequency ultrasound (HIFU) is another non-invasive emerging technique where coagulative necrosis is induced by a focused ultrasound beam from outside the skin. The lesion is divided into treatment voxel zones each requiring 4–8 sec of treatment with a 20–40 sec cooling interval.12 A recent systematic review of HIFU treatment of benign thyroid nodules reported a volume reduction of between 48.8 and 68.8%.13 However despite being the least invasive technique, HIFU takes significantly longer to ablate a nodule with HIFU compared to RFA and MWA13 and therefore most nodules treated with HIFU were small compared to other ablation techniques; and only nodules at a maximum depth of 2.8 cm can be effectively treated.13 Pharmaceutical ablation of predominantly (>90% constituent) cystic nodules with 100% ethanol can be performed. A prospective randomised study to compare single session treatment of benign cystic thyroid nodules (with greater than 90% cystic content) using ethanol ablation (EA) versus RFA showed that the primary end point of volume reduction was high in both the study groups; with EA superior to RFA. EA group mean volume reduction was 96.9% + −4.1% and RFA was 93.3% + −5.4%. All patients in both groups also achieved symptom and cosmetic improvement, with no significant difference between the two groups.14 This study concluded that for minimally invasive treatment cystic thyroid nodules, EA should be the first line modality treatment due to the comparable therapeutic efficacy but relatively lesser cost.14
To our knowledge ours was the first UK centre to perform RFA of benign symptomatic thyroid nodules and AFTNs since NICE approval in 2016. Our outcomes align with published data demonstrating RFA as an effective and safe treatment modality for patients with symptomatic thyroid nodules and AFTNs. RFA and other minimally invasive thermal and pharmaceutical ablation techniques are emerging as effective alternatives to traditional forms of treatment for benign thyroid nodular disease, especially in those patients not suitable for, or preferring not to undergo surgery.
Contributor Information
Simon Morley, Email: simon.morley3@nhs.net.
Sofia Otero, Email: sofia.otero@nhs.net.
Timothy Beale, Email: tim.beale@nhs.net.
Steven Bandula, Email: s.bandula@ucl.ac.uk.
REFERENCES
- 1. Kim Y-S , Rhim H , Tae K , Park DW , Kim ST . Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience . Thyroid 2006. ; 16 : 361 – 7 . doi: 10.1089/thy.2006.16.361 [DOI] [PubMed] [Google Scholar]
- 2. Jeong WK , Baek JH , Rhim H , Kim YS , Kwak MS , Jeong HJ , et al. . Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients . Eur Radiol 2008. ; 18 : 1244 – 50 . doi: 10.1007/s00330-008-0880-6 [DOI] [PubMed] [Google Scholar]
- 3.https://www.nice.org.uk/guidance/ipg562
- 4. Baek JH , Moon W-J , Kim YS , Lee JH , Lee D . Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules . World J Surg 2009. ; 33 : 1971 – 7 . doi: 10.1007/s00268-009-0130-3 [DOI] [PubMed] [Google Scholar]
- 5.https://onlinelibrary.wiley.com/doi/pdf/
- 6. Watt T , Bjorner JB , Groenvold M , Cramon P , Winther KH , Hegedüs L , et al. . Development of a short version of the thyroid-related patient-reported outcome ThyPRO . Thyroid 2015. ; 25 : 1069 – 79 . doi: 10.1089/thy.2015.0209 [DOI] [PubMed] [Google Scholar]
- 7. Fuller CW , Nguyen SA , Lohia S , Gillespie MB . Radiofrequency ablation for treatment of benign thyroid nodules: systematic review . Laryngoscope 2014. ; 124 : 346 – 53 . doi: 10.1002/lary.24406 [DOI] [PubMed] [Google Scholar]
- 8. Lim HK , Lee JH , Ha EJ , Sung JY , Kim JK , Baek JH , et al. . Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients . Eur Radiol 2013. ; 23 : 1044 – 9 . doi: 10.1007/s00330-012-2671-3 [DOI] [PubMed] [Google Scholar]
- 9. Deandrea M , Limone P , Basso E , Mormile A , Ragazzoni F , Gamarra E , et al. . US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules . Ultrasound Med Biol 2008. ; 34 : 784 – 91 . doi: 10.1016/j.ultrasmedbio.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 10. Yue W , Wang S , Wang B , Xu Q , Yu S , Yonglin Z , et al. . Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients . Eur J Radiol 2013. ; 82 : e11 – 16 . doi: 10.1016/j.ejrad.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 11. Cheng Z , Che Y , Yu S , Wang S , Teng D , Xu H , et al. . US-Guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study . Sci Rep 2017. ; 7 : 9554 . doi: 10.1038/s41598-017-09930-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barile A , Quarchioni S , Bruno F , Ierardi AM , Arrigoni F , Giordano AV , et al. . Interventional radiology of the thyroid gland: critical review and state of the art . Gland Surg 2018. ; 7 : 132 – 46 . doi: 10.21037/gs.2017.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang BH , Wu ALH . The efficacy and safety of high-intensity focused ultrasound ablation of benign thyroid nodules . Ultrasonography 2018. ; 37 : 89 – 97 . doi: 10.14366/usg.17057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sung JY , Baek JH , Kim KS , Lee D , Yoo H , Kim JK , et al. . Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study . Radiology 2013. ; 269 : 293 – 300 . doi: 10.1148/radiol.13122134 [DOI] [PubMed] [Google Scholar]