Abstract
Background:
This study was performed to evaluate the effects of carnitine administration on carotid intima-media thickness (CIMT) and inflammatory markers in women with polycystic ovary syndrome (PCOS).
Methods:
This randomized, double-blind, placebo-controlled trial was conducted among 60 women diagnosed with PCOS according to the Rotterdam criteria, aged 18–40 years. Participants were randomly allocated into two groups to intake either 250 mg/day carnitine (n = 30) or placebo (n = 30) for 12 weeks. High-resolution carotid ultrasonography was conducted at baseline and after the 12-week intervention.
Results:
After the 12-week intervention, compared with the placebo, carnitine supplementation resulted in a significant decrease in maximum levels of the left CIMT (−0.01 ± 0.02 vs. +0.002 mm ± 0.006 mm, P = 0.001), mean levels of the left CIMT (−0.01 ± 0.02 vs. +0.001 mm ± 0.01 mm, P = 0.001), maximum levels of the right CIMT (−0.01 ± 0.02 vs. +0.006 mm ± 0.01 mm, P < 0.001), and mean levels of the right CIMT (−0.01 ± 0.02 vs. +0.002 mm ± 0.01 mm, P = 0.001). Change in plasma nitric oxide (NO) (+2.4 ± 3.6 vs. +0.2 ± 2.3 μmol/L, P = 0.007) was significantly different between the supplemented patients and placebo group. We did not see any significant effect in serum high sensitivity C-reactive protein (hs-CRP) following the supplementation of carnitine compared with the placebo.
Conclusions:
Overall, carnitine administration for 12 weeks to participants with PCOS had beneficial effects on CIMT and plasma NO, but did not affect serum hs-CRP levels.
Keywords: Carnitine, carotid intima-media thickness, inflammation, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the cause of hyperandrogenism and menstrual disorders with chronic anovulation and infertility which affects 5%–10% of women in reproductive age.[1] PCOS patients with metabolic syndrome, overweight, and/or type 2 diabetes mellitus due to androgen excess and insulin resistance are at increased risk of cardiovascular diseases (CVD) that are evaluated by carotid intima-media thickness (CIMT).[2,3] PCOS subjects present with increased CIMT levels compared with nonhyper androgenic women have shown the presence of subclinical atherosclerosis associated with androgen excess and not with obesity or insulin resistance.[4] In addition, increased circulating levels of inflammatory markers, such as C-reactive protein (CRP), interleukins, endothelin-1, fibrinogen can progress atherosclerotic process, and CVD.[5]
The beneficial effects of carnitine administration on metabolic profiles in patients with PCOS[6] and without PCOS[7] have previously reported. The effects of carnitine administration on human atherosclerosis progression were evaluated in a few small studies in individuals without PCOS, which were inconclusive. Stasi et al.[8] and McMackin et al.[9] indicated that propionyl-L-carnitine accelerated blood flow recovery and the restoration of vascular function. Furthermore, Loffredo et al.[10] demonstrated that propionyl-L-carnitine infusion was related to increased flow-mediated dilation (FMD) in individuals with peripheral vascular disease. Findings of a meta-analysis also indicated the clinically relevant benefit of L-carnitine administration in reducing levels of CRP.[11] In addition, L-carnitine administration at a dosage of 3 g/day to young soccer players provided strong antioxidant function through increasing glutathione and nitric oxide (NO) concentrations.[12] However, after 1 month of L-carnitine therapy at dosage of 1500 mg/day in hemodialysis patients; there was no significant change in FMD and CIMT levels.[13]
Existing evidence shows that carnitine intake in women with PCOS may have beneficial effects on CIMT and inflammatory factors. To the best of our knowledge, data on carnitine administration on CIMT and inflammatory factors in patients with PCOS are limited. The current study was, therefore, conducted to evaluate the effects of carnitine administration on CIMT and inflammatory factors to patients with PCOS.
Methods
Trial design and participants
This randomized, double-blind, placebo-controlled clinical trial was performed among sixty women with PCOS diagnosed according to the Rotterdam criteria,[14] aged 18–40 years who were referred to the Naghavi Clinic in Kashan, Iran, between October 2016 and February 2017. Exclusion criteria were as follows: Pregnant women, elevated levels of prolactin, and endocrine diseases. This study was approved by the research Ethics Committee of Kashan University of Medical Sciences and informed consent form was taken from all individuals. Subjects were randomized into two groups to intake either 250 mg/day of carnitine (Avecina, Tehran, Iran) or placebo (Barij Essence, Kashan, Iran) (n = 30 each group) for 12 weeks. We used the above-mentioned dose based on the beneficial effects of carnitine administration on metabolic profiles in women with PCOS.[15] All participants were consuming metformin tablet at the initial dose of 500 mg, which was increased in a stepwise manner during the first 3 weeks to a total of 1500 mg/day.[16] Shape, size, and color of the placebo capsules were identical with the carnitine capsules. Randomization assignment was done using computer-generated random numbers. Randomization and allocation concealment were carried out by the researchers and participants and were carried out by trained staff at the gynecology clinic. Participants were requested not to change their routine physical activity or usual dietary intakes throughout the study and not to take any supplements other than the one provided to them by the investigators during the 12-week intervention. Daily dietary macro- and micronutrient intakes were analyzed by nutritionist 4 software (First Databank, San Bruno, CA). Physical activity was assessed through asking subjects to record their routine physical activities 1 day in each month and expressed as metabolic equivalents (METs) in hours/day.[17]
Treatment adherence
To assess the compliance, we counted the remaining supplements. In addition, to increase compliance, all individuals received short messages every day to remind them about taking the capsules.
Assessment of anthropometric measures
Weight and height of participants were determined using a standard scale (Seca, Hamburg, Germany) at pre-and post-intervention. BMI was calculated as weight in kg divided by height in meters squared.
Assessment of outcomes
We considered CIMT as primary outcome measurement and inflammatory markers as secondary outcomes measurements.
Clinical assessment
Measurements of the CIMT (maximum and mean of left and right CIMT) were carried out in the patients at the 2-cm distance of the common carotid bifurcation[18,19] at baseline and after the 12-week treatment using a Doppler ultrasonography device (Samsung Medison V20, Korea) with linear multifrequencies of 7.5-to 10-MHz probe. All CIMT (mean and maximum thickness) measurements were evaluated blindly by a single experienced ultrasonographer. CIMT evaluation was performed in the far wall (posterior wall of the common carotid artery). Moreover, auto IMT software version 2.05.02.1122 was used for border detection. Reproducibility information was obtained from the duplicate ultrasound examinations at baseline and at follow-up. The mean (standard deviation [±SD]) difference in common CIMT between the two baseline measurements (screening visit and randomization visit) was 0.0004 mm ± 0.056 mm. The mean (±SD) absolute mean difference was 0.036 mm ± 0.040 mm. The intra- and interobserver coefficient variances (CVs) for the repeated measurements of mean CIMT were 6.6% and 9.8%, respectively. In addition, the intra- and inter-observer CVs for the repeated measurements of maximum CIMT were 7.2% and 10.9%, respectively.
Biochemical assessment
Ten milliliters fasting blood samples were collected at baseline and after the 12-week treatment at Kashan reference laboratory. Serum high sensitivity CRP (hs-CRP) values were quantified by an ELISA kit (LDN, Nordhorn, Germany) with intra- and inter-assay CVs of 3.1 and 5.3%, respectively. The plasma NO levels were evaluated using Griess method[20] with inter-and intra-assay CVs <5%.
Statistical methods
The Kolmogorov–Smirnov test was applied to control the normal distribution of variables. Independent sample t-test was used to establish changes in anthropometric measures and dietary intakes between the two groups. To determine the effects of carnitine administration on CIMT, biomarkers of inflammation and oxidative stress, we used one-way repeated measures analysis of variance. To evaluate confounding variables, including baseline values of biochemical variables, age and baseline BMI, we used analysis of covariance (ANCOVA). P < 0.05 were considered statistically significant. All statistical analyses conducted using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
Thirty participants in each group completed the trial out of 70 subjects which were recruited in our study (10 subjects were excluded from the study because of not living in Kashan and not meeting inclusion criteria) [Figure 1]. On average, higher than 90% of capsules were taken in both groups. No side effects were reported following the administration of carnitine in women with PCOS throughout the study.
Figure 1.
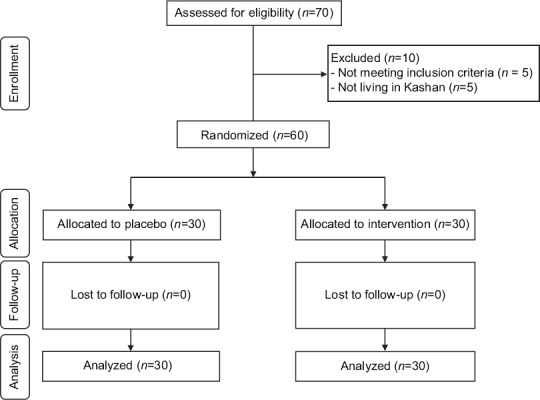
Summary of patient flow diagram
Mean age, height, weight, and METs at baseline and end-of-trial of study subjects were not statistically different between two groups [Table 1]. After the 12-week intervention, consuming carnitine resulted in a significant reduction in weight (−2.3 ± 1.1 vs. −0.2 ± 1.0 kg, P < 0.001), BMI (−0.9 ± 0.4 vs. −0.1 ± 0.4 kg/m2, P < 0.001) compared with the placebo.
Table 1.
General characteristics of study participants
| Placebo group (n=30) | Carnitine group (n=30) | P* | |
|---|---|---|---|
| Age (year) | 250.0±5.4 | 23.6±4.6 | 0.28 |
| Height (cm) | 162.1±6.8 | 160.8±5.3 | 0.41 |
| Weight at study baseline (kg) | 74.3±17.3 | 75.1±9.4 | 0.82 |
| Weight at end-of-trial (kg) | 74.1±17.5 | 72.8±9.3 | 0.71 |
| Weight change (kg) | −0.2±1.0 | −2.3±1.1 | <0.001 |
| BMI at study baseline (kg/m2) | 28.2±6.5 | 29.4±4.1 | 0.53 |
| BMI at end-of-trial (kg/m2) | 28.2±6.6 | 28.2±4.0 | 0.97 |
| BMI change (kg/m2) | −0.1±0.4 | −0.9±0.4 | <0.001 |
| MET-h/day at study baseline | 27.8±1.5 | 28.2±1.3 | 0.26 |
| MET-h/day at end-of-trial | 28.0±1.7 | 28.3±1.3 | 0.38 |
| MET-h/day change | 0.1±0.5 | 0.1±0.3 | 0.56 |
*Obtained from independent t-test, data are means±SDs. BMI=Body mass index, METs=Metabolic equivalents, SD=Standard deviation, BMI=Body mass index
The mean dietary macro-and micro-nutrient intakes at both baseline and after the 12-week treatment as well as throughout the intervention were not significantly different between the two groups (Data not shown).
After the 12-week intervention, compared with the placebo, carnitine supplementation resulted in a significant decrease in maximum levels of the left CIMT (−0.01 ± 0.02 vs. +0.002 mm ± 0.006 mm, P = 0.001), mean levels of the left CIMT (−0.01 ± 0.02 vs. +0.001 mm ± 0.01 mm, P = 0.001), maximum levels of the right CIMT (−0.01 ± 0.02 vs. +0.006 mm ± 0.01 mm, P < 0.001), and mean levels of the right CIMT (−0.01 ± 0.02 vs. +0.002 mm ± 0.01 mm, P = 0.001) [Table 2]. Change in plasma NO (+2.4 ± 3.6 vs. +0.2 ± 2.3 μmol/L, P = 0.007) was significantly different between the supplemented patients and placebo group. We did not see any significant effect in serum hs-CRP following the supplementation of carnitine compared with the placebo.
Table 2.
Carotid intimamedia thickness and inflammatory markers at baseline and 12 weeks after the intervention in patients with polycystic ovary syndrome
| Placebo group (n=30) | Carnitine group (n=30) | P# | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End-of-trial | Change | P* | Baseline | End-of-trial | Change | P* | ||
| Mean left CIMT (mm) | 0.48±0.04 | 0.48±0.05 | 0.001±0.01 | 0.47 | 0.49±0.05 | 0.47±0.05 | −0.01±0.02 | 0.001 | 0.001 |
| Maximum left CIMT (mm) | 0.58±0.05 | 0.58±0.05 | 0.002±0.006 | 0.07 | 0.58±0.06 | 0.57±0.04 | −0.01±0.02 | 0.005 | 0.001 |
| Mean right CIMT (mm) | 0.47±0.04 | 0.47±0.04 | 0.002±0.01 | 0.33 | 0.49±0.06 | 0.48±0.05 | −0.01±0.02 | 0.001 | 0.001 |
| Maximum right CIMT (mm) | 0.57±0.05 | 0.57±0.04 | 0.006±0.01 | 0.04 | 0.59±0.06 | 0.57±0.04 | −0.01±0.02 | 0.002 | <0.001 |
| hs-CRP (ng/mL) | 3030.0±1380.2 | 3300.3±1283.3 | 270.3±752.7 | 0.05 | 3116.7±2549.5 | 3121.9±3188.0 | 5.1±2016.5 | 0.89 | 0.50 |
| NO (μmol/L) | 42.2±5.4 | 42.4±5.0 | 0.2±2.3 | 0.64 | 43.2±5.4 | 45.7±4.4 | 2.4±3.6 | 0.001 | 0.007 |
*P values represent paired-samples t-test, #P values represent the time×group interaction (computed by analysis of the one-way repeated measures ANOVA), all values are means±SDs. CIMT=Carotid intima-media thickness, hs-CRP=High-sensitivity C-reactive protein, NO=Nitric oxide, SD=Standard deviation, ANOVA=Analysis of variance
We adjusted the analyses for baseline values of biochemical variables, age, and baseline BMI. When we adjusted the analysis for baseline values of biochemical parameters, age, and baseline BMI, our findings did not alter [Table 3].
Table 3.
Adjusted changes in carotid intima-media thickness and inflammatory markers in patients with polycystic ovary syndrome
| Placebo group (n=30) | Carnitine group (n=30) | P* | |
|---|---|---|---|
| Mean left CIMT (mm) | 0.001±0.003 | −0.01±0.003 | 0.001 |
| Maximum left CIMT (mm) | 0.003±0.003 | −0.01±0.003 | <0.001 |
| Mean right CIMT (mm) | 0.0001±0.003 | −0.01±0.003 | 0.003 |
| Maximum right CIMT (mm) | 0.003±0.003 | −0.01±0.003 | <0.001 |
| hs-CRP (ng/mL) | 309.6±278.4 | −34.2±278.4 | 0.38 |
| NO (μmol/L) | 0.1±0.5 | 2.5±0.5 | 0.001 |
*Obtained from ANCOVA, all values are means±SEs, values are adjusted for baseline values, age, and BMI at baseline. CIMT=Carotid intima-media thickness, hs-CRP=High-sensitivity C-reactive protein, NO=Nitric oxide, SE=Standard error, BMI=Body mass index, ANCOVA=Analysis of covariance
Discussion
To the best of our knowledge, this study is the first report the effects of carnitine administration on CIMT and inflammatory factors to patients with PCOS. We found that carnitine administration for 12 weeks to patients with PCOS had beneficial effects on CIMT and plasma NO, but did not affect serum hs-CRP concentrations.
Women with PCOS are susceptible to multiple metabolic disturbances.[21,22,23] The current study indicated that carnitine administration for 12 weeks to patients with PCOS led to a significant reduction in maximum and mean levels of the left and right CIMT compared with the placebo. Some animal and human studies have reported the effects of carnitine supplementation on risk factors of CVD. For instance, Stasi et al.[8] and McMackin et al.[9] demonstrated that propionyl-L-Carnitine accelerated blood flow recovery and also resorted vascular function. In addition, Loffredo et al.[10] indicated that propionyl-L-carnitine infusion was associated with a significant increase in FMD among patients with peripheral vascular disease. In another study, Volek et al.[24] reported that 2 g/day of L-carnitine supplementation for 3 weeks improved FMD in healthy individuals after high-fat meals. Silvestro et al.[25] also concluded that in participants with intermittent claudication, supplementation with propionyl-L-carnitine provided a protective effect against deterioration of FMD. In addition, in subjects with arterial disease, intravenous administration of propionylcarnitine significantly decreased biomarkers of oxidative stress and also significantly improved FMD,[10,25] and a combination of acetyl-l-carnitine and alpha lipoic acid significantly elevated brachial artery diameter.[9] However, data on CVD events in women with PCOS are scarce; however, a recent meta-analysis indicated that participants with PCOS had twice the relative risk of CVD or stroke than controls.[26] Moreover, in a meta-analysis study conducted by Meyer et al.,[27] it was seen that IMT artery in participants with PCOS was significantly higher than healthy women. CIMT has been widely used as a surrogate index of atherosclerosis and CVD events.[28,29] Carnitine intake may improve CIMT through to decrease measures of oxidative stress[30,31] and protect against lipid peroxidation.[32] In addition, carnitine supplementation due to a positive impact on NO metabolism[24] may decrease CIMT in women with PCOS.
Our study demonstrated that carnitine supplementation to patients with PCOS for 12 weeks was associated with a significant elevation in plasma NO levels, but did not influence serum hs-CRP concentrations. The previous studies in animal models have reported that L-carnitine and its propionate improved endothelial responses by reducing O2 production and increasing NO availability.[33,34] Furthermore, they suggested that L-carnitine prevents the progression of atherosclerotic lesions and endogenous carnitine depletion and/or deficiency.[33,34] Furthermore, taking L-carnitine at a dosage of 3 g/day in young soccer players provided strong antioxidant function through increasing the glutathione and NO levels.[12] Endothelial dysfunction and tissue decrease of NO production also improved by propionyl-L-carnitine treatment for 9 weeks in diet-induced obese mice.[35] In relation to the effect of carnitine supplementation on CRP concentrations, few studies have documented decreasing effects of canrnitine on CRP,[36,37] but such effect was reported by others.[38] Inflammatory cytokines stimulate mononuclear cells to release tissue factors which are important to initiation of coagulation reactions, complement activation, and neutralization of platelet-activating factor, which in turn these factors promote thrombotic response.[39] Maintenance of NO levels after carnitine treatment may be associated with decreased NADPH oxidase activation,[40] an enzyme which subsequently results in superoxide radical generation.[41] In addition, carnitine intake may increase endothelial NO synthase,[42] the major enzyme responsible for NO production.
This study had few limitations. Due to funding limitation, we did not evaluate the effects of carnitine administration on plasma carnitine levels. In addition, our study was relatively of short duration of intervention. Long-term intervention might result in better change in anatomical sign of subclinical atherosclerosis and serum hs-CRP levels.
Conclusions
Overall, carnitine administration for 12 weeks among patients with PCOS had beneficial effects on CIMT and plasma NO levels; however, did not affect serum hs-CRP concentrations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The current study was funded by a grant from the Vice-chancellor for Research, KUMS, and Iran.
References
- 1.Marciniak A, Nawrocka Rutkowska J, Brodowska A, Wiśniewska B, Starczewski A. Cardiovascular system diseases in patients with polycystic ovary syndrome – The role of inflammation process in this pathology and possibility of early diagnosis and prevention. Ann Agric Environ Med. 2016;23:537–41. doi: 10.5604/12321966.1226842. [DOI] [PubMed] [Google Scholar]
- 2.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 3.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–9. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 4.Luque-Ramírez M, Mendieta-Azcona C, Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reprod. 2007;22:3197–203. doi: 10.1093/humrep/dem324. [DOI] [PubMed] [Google Scholar]
- 5.Venugopal SK, Devaraj S, Jialal I. Effect of C-reactive protein on vascular cells: Evidence for a proinflammatory, proatherogenic role. Curr Opin Nephrol Hypertens. 2005;14:33–7. doi: 10.1097/00041552-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi Z, et al. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2016;84:851–7. doi: 10.1111/cen.13003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JJ, Wu ZB, Cai YJ, Ke B, Huang YJ, Qiu CP, et al. L-carnitine ameliorated fasting-induced fatigue, hunger, and metabolic abnormalities in patients with metabolic syndrome: A randomized controlled study. Nutr J. 2014;13:110. doi: 10.1186/1475-2891-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasi MA, Scioli MG, Arcuri G, Mattera GG, Lombardo K, Marcellini M, et al. Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol. 2010;30:426–35. doi: 10.1161/ATVBAHA.109.201533. [DOI] [PubMed] [Google Scholar]
- 9.McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, et al. Effect of combined treatment with alpha-lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9:249–55. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loffredo L, Marcoccia A, Pignatelli P, Andreozzi P, Borgia MC, Cangemi R, et al. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J. 2007;28:608–12. doi: 10.1093/eurheartj/ehl533. [DOI] [PubMed] [Google Scholar]
- 11.Sahebkar A. Effect of L-carnitine supplementation on circulating C-reactive protein levels: A Systematic review and meta-analysis. J Med Biochem. 2015;34:151–9. doi: 10.2478/jomb-2014-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atalay Guzel N, Erikoglu Orer G, Sezen Bircan F, Coskun Cevher S. Effects of acute L-carnitine supplementation on nitric oxide production and oxidative stress after exhaustive exercise in young soccer players. J Sports Med Phys Fitness. 2015;55:9–15. [PubMed] [Google Scholar]
- 13.Sabri MR, Fahimi F, Hajialiasgar S, Etminan A, Nazemi S, Salehi F, et al. Does L-carnitine improve endothelial function in hemodialysis patients? J Res Med Sci. 2012;17:417–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153:778–89. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulghesu AM, Romualdi D, Di Florio C, Sanna S, Tagliaferri V, Gambineri A, et al. Is there a dose-response relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012;27:3057–66. doi: 10.1093/humrep/des262. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Plasencia Martínez JM, García Santos JM. Is manual ultrasonographic measurement of carotid intima-media thickness a reproducible cardiovascular biomarker? Radiologia. 2017;59:478–86. doi: 10.1016/j.rx.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–38. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–50. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Asemi Z, Samimi M, Tabassi Z, Sabihi SS, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. 2013;29:619–24. doi: 10.1016/j.nut.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, et al. Calcium plus Vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and Vitamin D-deficient women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Clin Endocrinol (Oxf) 2015;83:888–94. doi: 10.1111/cen.12840. [DOI] [PubMed] [Google Scholar]
- 23.Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A, et al. Calcium plus Vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese Vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015;34:586–92. doi: 10.1016/j.clnu.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Volek JS, Judelson DA, Silvestre R, Yamamoto LM, Spiering BA, Hatfield DL, et al. Effects of carnitine supplementation on flow-mediated dilation and vascular inflammatory responses to a high-fat meal in healthy young adults. Am J Cardiol. 2008;102:1413–7. doi: 10.1016/j.amjcard.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Silvestro A, Schiano V, Bucur R, Brevetti G, Scopacasa F, Chiariello M, et al. Effect of propionylcarnitine on changes in endothelial function and plasma levels of adhesion molecules induced by acute exercise in patients with intermittent claudication. Angiology. 2006;57:145–54. doi: 10.1177/000331970605700203. [DOI] [PubMed] [Google Scholar]
- 26.de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 27.Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:112–26. doi: 10.1093/humupd/dmr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Meer IM, Iglesias del Sol A, Hak AE, Bots ML, Hofman A, Witteman JC, et al. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: The rotterdam study. Stroke. 2003;34:2374–9. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- 29.Hurst RT, Ng DW, Kendall C, Khandheria B. Clinical use of carotid intima-media thickness: Review of the literature. J Am Soc Echocardiogr. 2007;20:907–14. doi: 10.1016/j.echo.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Volek JS, Kraemer WJ, Rubin MR, Gómez AL, Ratamess NA, Gaynor P, et al. L-carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am J Physiol Endocrinol Metab. 2002;282:E474–82. doi: 10.1152/ajpendo.00277.2001. [DOI] [PubMed] [Google Scholar]
- 31.Sachan DS, Hongu N, Johnsen M. Decreasing oxidative stress with choline and carnitine in women. J Am Coll Nutr. 2005;24:172–6. doi: 10.1080/07315724.2005.10719462. [DOI] [PubMed] [Google Scholar]
- 32.Bertelli A, Conte A, Palmieri L, Ronca G, Segnini D, Yu G, et al. Effect of propionyl carnitine on energy charge and adenine nucleotide content of cardiac endothelial cells during hypoxia. Int J Tissue React. 1991;13:37–40. [PubMed] [Google Scholar]
- 33.Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res. 2001;44:235–42. doi: 10.1006/phrs.2001.0852. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez de Sotomayor M, Bueno R, Pérez-Guerrero C, Herrera MD. Effect of L-carnitine and propionyl-L-carnitine on endothelial function of small mesenteric arteries from SHR. J Vasc Res. 2007;44:354–64. doi: 10.1159/000102303. [DOI] [PubMed] [Google Scholar]
- 35.Mingorance C, Duluc L, Chalopin M, Simard G, Ducluzeau PH, Herrera MD, et al. Propionyl-L-carnitine corrects metabolic and cardiovascular alterations in diet-induced obese mice and improves liver respiratory chain activity. PLoS One. 2012;7:e34268. doi: 10.1371/journal.pone.0034268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BJ, Lin JS, Lin YC, Lin PT. Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition. 2015;31:475–9. doi: 10.1016/j.nut.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Rafraf M, Karimi M, Jafari A. Effect of L-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese women. J Sports Med Phys Fitness. 2015;55:1363–70. [PubMed] [Google Scholar]
- 38.Malek Mahdavi A, Mahdavi R, Kolahi S. Effects of l-carnitine supplementation on serum inflammatory factors and matrix metalloproteinase enzymes in females with knee osteoarthritis: A Randomized, double-blind, placebo-controlled pilot study. J Am Coll Nutr. 2016;35:597–603. doi: 10.1080/07315724.2015.1068139. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 40.Pignatelli P, Lenti L, Sanguigni V, Frati G, Simeoni I, Gazzaniga PP, et al. Carnitine inhibits arachidonic acid turnover, platelet function, and oxidative stress. Am J Physiol Heart Circ Physiol. 2003;284:H41–8. doi: 10.1152/ajpheart.00249.2002. [DOI] [PubMed] [Google Scholar]
- 41.Zalba G, San José G, Moreno MU, Fortuño MA, Fortuño A, Beaumont FJ, et al. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension. 2001;38:1395–9. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 42.de Sotomayor MA, Mingorance C, Rodriguez-Rodriguez R, Marhuenda E, Herrera MD. L-carnitine and its propionate: Improvement of endothelial function in SHR through superoxide dismutase-dependent mechanisms. Free Radic Res. 2007;41:884–91. doi: 10.1080/10715760701416467. [DOI] [PubMed] [Google Scholar]


