Abstract
Leishmaniasis is considered as a zoonotic infection and neglected tropical disease. Leishmania treatment is not totally successful and imposes high expenditures, especially in developing countries. Since the natural infection leads to the robust immunity in most of the human cases, many bodies of research have been focusing on Leishmania vaccines, being capable to control Leishmania infection. First generation vaccines (such as Leishmune® and CaniLeish®) have proved robust protective immunity in dogs. In human, recombinant vaccines, including Leish-F1 could confer some degrees of protective immunity against natural infection. Recently, ChAd63-KH DNA vaccine has been accomplished in providing prevention against Leishmania infection; however, this vaccine should be further evaluated in other clinical trials.
Keywords: Leishmnia amazonensis, Leishmania donovani, Leishmania major, Leishmania mexicana, Leishmania vaccines
Introduction
Leishmaniasis, which is considered as a zoonotic and vector-borne protozoan infectious disease, transmits through >70 species of female sand flies assigned to Phlebotomus or Lutzemia genera.[1,2] This infection is second to malaria in its prevalence while 0.7–1.5 and 0.2–0.4 million new cases of cutaneous and visceral leishmaniasis (CL and VL) are annually reported.[3,4,5] Golden standard of Leishmania treatment is based on antimonial drugs; nevertheless, this approach is toxic and sometimes fails to achieve patient recovery due to antimicrobial resistance.[6,7] Furthermore, antimonial treatment imposes high expenditures, especially in the developing countries and the patients may poorly comply with the treatment regimen.[8,9,10,11]
On the other hand, natural infections of CL and VL dominantly cause robust immunity; hence, different studies have aimed to develop appropriate Leishmania vaccines. In the current study, we try to make a presentation of Leishmania vaccines, which are more likely to impact epidemiological aspect of this parasitological disease in the next coming years. Therefore, we aimed to focus on vaccines assessed in human clinical trials or animal field studies.
First generation vaccines
First generation antileishmanial vaccines comprises of three main subgroups: whole-killed parasites (i), fractionated Leishmania antigen (ii), Live-attenuated pathogens.
Whole-killed parasites
Killed Leishmania vaccines in new world
Whole-killed Leishmania vaccines have low cost and achieved the first senior success in animal modeling; nevertheless, none of the human vaccines in this subgroup has accomplished the World Health Organization (WHO) validity.[12] For instance, Leishvaccine, which comprised whole-killed promastigotes of Leishmania amazonensis (L. amazonensis) strain (IFLA/BR/1967/PH8) and Bacillus Calmette–Guérin (BCG), could play a prominent role in the protection of canine Leishmaniasis. In fact, this vaccine induced a significant increase in a mixed cytokine pattern. The vaccine stimulated innate immunity (especially neutrophils and eosinophils) and activated CD4+T, CD8+T, and B cells [Figure 1].[13] Leishvaccine in human was successfully applied in Phase I and II of clinical trials, which well documented its safety and immunogenicity; however, this vaccine failed to achieve satisfactory results in Phase III of the randomized clinical trial (RCT) [Figure 1 and Table 1].[14].
Figure 1.
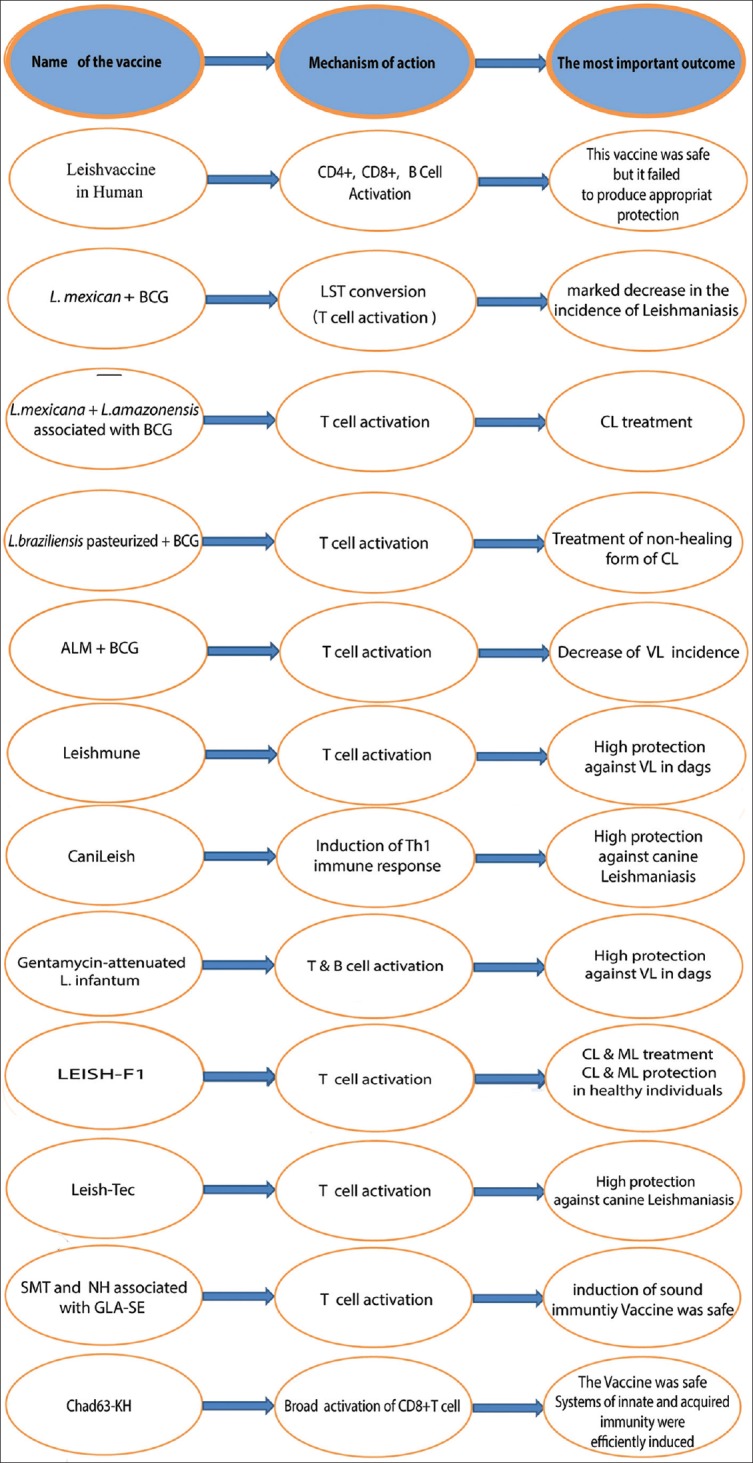
Diagram showing mechanism of action and the most important outcome of the vaccines
Table 1.
Status of Leishmania vaccines entered in clinical trials
| Vaccine name | Classification | Phase I | Phase II | Phase III | Reference |
|---|---|---|---|---|---|
| Leishvaccine | First generation | x | [14] | ||
| ALMϼ | First generation | x | [19] | ||
| Leishmune | First generation | x | [22] | ||
| CaniLeish | First generation | x | [23] | ||
| GALMα | First generation | x | [28] | ||
| LEISH-F1 | Second generation | x | [30] | ||
| LEISH-F2 | Second generation | x | [32] | ||
| LEISH-F3 | Second generation | x | [33] | ||
| Leish-Tec | Second generation | x | [36] | ||
| SMTγ + NHµ | Second generation | x | [38] | ||
| ChAd63-KH | Third generation | x | [40] |
ϼ=Autoclaved-killed, L. major α=Gentamycin-attenuated L. major, γ=Enzyme sterol 24-c-methyltranferase, µ=Nucleoside hydrolase
In different studies, the efficacy of autoclaved-killed Leishmania mexicana (L. mexicana) associated with BCG was assessed for both prophylaxis and immunotherapy aims.[15] The vaccine application resulted in low levels of leishmanin skin test (LST) conversion; however, it was noticeable that the incidence of Leishmaniasis significantly decreased in LST-converted participants.[15]
As a promising approach, a composite of two endemic species (L. mexican and L. amazonensis) associated with BCG protected 73% of healthy individuals in the Republic of Ecuador.[16] To assess the immunotherapy effects of the above vaccine (L. mexicana + L. amazonensis associated with BCG), 11,532 CL patients were recruited in a multicenter RCT implemented over 10 years. All of the recruited patients were afflicted with localized CL (LCL) and preliminary diagnosis was based on the LST conversion. In that study, the majority of the patients with CL were treated with almost no side effects and the treatment protocol was cost effective [Figure 1].[17]
The immunotherapy strategy achieved further success in the patients afflicted by mucocutaneous and diffuse forms of CL. They were treated with promastigotes of Leishmania braziliensis (L. braziliensis) killed by pasteurization and associated with viable BCG. This kind of immunotherapy offered a safe option in severe forms of CL, which did not respond to conventional chemotherapy. In comparison with autoclaved-killed Leishmania vaccines, pasteurization method achieved further efficacy since protein components of pasteurized and fresh promastigotes did not significantly differ. In Venezuela, pasteurized L. braziliensis + BCG is currently applied for the treatment of the non-healing form of CL, which does not respond to three courses (2 months) of antimonial treatment [Figure 1].[15]
In general, vaccination with killed Leishmania promastigotes could be considered as a safe and economical treatment; nevertheless, further trials aiming at evaluation of different adjuvants potentially pave the way for more efficient vaccines.[18]
Killed Leishmania vaccines in old world
In the old world, Leishmania major (L. major), as an immunogenic component, has been used in different clinical trials aiming at Leishmania treatment and prevention.[15] For instance, autoclaved-killed L. major (ALM) associated with BCG was evaluated in Phase I and II clinical trials implemented among healthy participants living in non-endemic areas of CL. Though the safety of the vaccine formula was approved, LST conversion occurred in just about 38% of the healthy participants and low levels of interferon-gamma was produced in response to soluble Leishmania antigen (SLA) [Figure 1].[12,19]
For further investigations, this vaccine was also assessed in healthy volunteers living in endemic areas of CL such as Bam (Kerman Province, Iran). The vaccine application led to LST conversion occurring in a small proportion of healthy participants (16.5%). In another clinical trial, a booster dose of the ALM vaccine associated with BCG was used in Sudan and the results of the study indicated a significant decrease (43%) of VL incidence in LST-converted individuals [Figure 1].[12,19,20]
In addition to preventive aims, ALM has also been used in clinical trials to assess what effects it might have. For example, in Sudan, a composition of sodium stibogluconate (Stb) and alum-precipitated ALM (alum/ALM) + BCG was used for the treatment of post-kala-azar dermal leishmaniasis (PKDL). The results of that study showed that the combination of the Leishmania vaccine and Stb was more efficient, compared with Stb alone (53% vs 87%) [Figure 1].[15,21]
Fractionated Leishmania antigens
Two fractionated vaccines, which are called Leishmune® and CaniLeish®, have achieved impressive success in the prevention of canine Leishmaniasis. These veterinary licensed vaccines protect dogs and block Leishmania transmission from dogs to human arising from sand fly biting [Figure 1].[22]
Leishmune® is based on fucose-mannose ligand (FML) and saponin as an adjuvant. FML, which is expressed in all cycles of Leishmania species, can be used as a suitable antigen in dog and human serodiagnosis. FML of Leishmune® has been purified from Leishmania donovani (L. donovani) promastigotes and saponin part of the vaccine includes QS21 and two deacylated saponins. The efficacy of Leishmune® was approved in endemic areas of Brazil, where 92–97% of the vaccinated dogs were protected against canine VL [Figure 1].[12,22]
LiESP/QA-21 vaccine or CaniLeish® (CaniLeish, Virbac, France) is the only Leishmania-licensed vaccine in Europe. This vaccine was produced through extracted secreted proteins of Leishmania infantum (LiESP). These purified proteins were derived in cell and serum-free culture patented by the Institut de Recherche pour le Développement (IRD). Furthermore, this protein was associated with a highly purified part of a fraction of saponin, which was called QA-21. The dogs vaccinated with CaniLeish® could develop Th1 immune response within 3 weeks [Figure 1].[23,24,25]
It seems that the fractionated Leishmania vaccines could be efficiently used in areas where there is a crucial need for the control of the Leishmania infection [Figure 1].[26]
Live-attenuated pathogens
Some research has shown that live attenuated form of Leishmania infantum (L. infantum) could be considered as an appropriate tool for the prevention of the canine Leishmaniasis.[27] In this regard, a field study was conducted among 103 dogs, grouped vaccinated (n = 55) and control (n = 44) trials. The process of Leishmania culture was done under the pressure of gentamicin (20 μg/ml). All of the dogs were not exposed to Leishmania infection, living in non-endemic areas of Iran. After the vaccination, all of the dogs were moved to Baft (Kerman, Iran), recognized as an endemic area of L. infantum. They were followed up for 24 months, experiencing four sand fly seasons (June and September).[27] At the end of the experience, the specific antibody for Leishmania-antigen wild type was found in 32% of the non-vaccinated dogs, whereas there was not any positive sample in the vaccinated group.[27] Clinical signs of Leishmaniasis were found in 29% and 2.2% of the control and vaccinated dogs, respectively [Figure 1].[27]
Documenting sound immunity of gentamycin-attenuated L. infantum, there is a real prospect that live-attenuated vaccines are capable to curb canine Leishmania infection in the near future.[27] In this regards, there is an ongoing clinical trial, aiming to employ gentamycin-attenuated L. major, has been implemented. This randomized and double-blind clinical trial was designed to assess the safety and protective effects of the L. major vaccine [Table 1].[28]
Second generation vaccines
Recombinant proteins, which are produced through genetically engineered-cells, are termed as “second generation vaccines.” LEISH-F1, formerly called Leish-111f, which has reached the Phase II of clinical trials. This artificial protein is encoded by three genes: L. major homologue of eukaryotic thiol-specific antioxidant (TSA), L. major stress-inducible protein-1 (LmSTI1), and L. braziliensis elongation and initiation factor (LeIF). This protein was produced by the Infectious Disease Research Institute (IDRI, Seattle, WA, USA) and emulsified with an adjuvant called “monophosphoryl lipid A in structure stimulating Toll-like receptor (TLR)” (MPL-SE). Not only could LEISH-F1+ MPL-SE efficiently treat patients afflicted by CL or ML, but also this vaccine efficiently induced protective immunity in healthy volunteers [Figure 1].[4,29,30,31]
In a different study, IDRI has launched another artificial protein, called LEISH-F2.[29] This protein excludes N-terminal histidine tag, resulting in more resemblance to natural proteins of wild species.[29] In addition, due to the substitution of glutamine for Lys274, the manufacturing process of LEISH-F2 has been improved, compared with LEISH-F1.[29] After safety and immunogenicity approval, the vaccine entered Phase II of a clinical trial, where its therapeutic effects on CL patients were assessed and compared with chemotherapy.[29] For this aim, LEISH-F2 (10 μg) was associated with MPL-SE adjuvant (25 μg) and the period of the clinical cure was determined for every patient.[29,32]
LEISH-F3 is another multicomponent vaccine comprised of two proteins: nucleoside hydrolase (NH) and sterol 24-c-methyltransferase (SMT), derived from L. donovani and L. infantum, respectively.[33] The vaccine was formulated with a TLR-4 ligand, namely glucopyranosyl lipid A-stable oil-in-water nanoemulsion (GLA-SE).[33] The application of the vaccine in healthy and adult individuals, living in Washington (US), showed promising results as a robust immune response against VL was induced.[29,33,34,35]
Leish-Tec®, licensed as a second generation vaccine in Brazil, contains A2 antigen of L. infantum. In a field trial, which was implemented among 847 seronegative dogs in southeastern part of Brazil, the dogs were assigned to either control (n = 418) or interventional (n = 429) group. The interventional group received three doses of the vaccine with 21-day intervals. Every single dose of the vaccine included 100 μg/mL of recombinant A2 protein and 500 μg/mL of saponin, which was applied as an adjuvant. The control group received a placebo. All of the dogs were followed up for 18 months through serological and parasitological methods. The results of that study showed that Leish-Tec® could efficiently prevent the incidence of canine Leishmaniasis among the dogs, which were naturally exposed to Leishmania parasite [Figure 1].[36]
Two recombinant proteins called “enzyme sterol 24-c-methyltranferase” (SMT) and “nucleoside hydrolase” (NH) can also be assumed as appropriate candidates for vaccine development.[34,37] SMT and NH sequences not only are conserved among Leishmania species but also do not exist in homospecies. The combination of SMT and NH proteins called NS was formulated with “glucopyranosyl lipid A-stable oil-in-water nanoemulsion” (GLA-SE), which was considered as a potent TLR-4 ligand. This structure was applied in a Phase I clinical trial study performed among healthy and uninfected individuals living in the USA. The results of the study showed that the combination of NS protein and GLA-SE adjuvant could induce safe and robust immunity against Leishmania infection [Figure 1].[34,37]
Third generation vaccines
Documenting the beneficial role of CD8+ T cells in the treatment and prevention of VL and PKDL, many bodies of research have been focusing on DNA vaccines.[38] In a very recent study, it was shown that a third generation vaccine, employing semian adenovirus (ChAd63) could effectively elicit a wide range of CD8+ T cells, specified for Leishmania antigens.[38] This vaccine encoded KH gene, constituted of two genes of L. donovani antigens: KMP-11 and HASPB.[38] The results of the study showed that not only intramuscular doses (1 × 1010 and 7.5 × 1010 ChAd63-KH) of ChAd63-KH were safe but also it efficiently induced interferon-gamma production and dendritic cell activation.[38] As a result, the application of ChAd63-KH vaccine as a promising approach for the prevention and treatment of L. donovani infection [Figure 1].[38]
In this regard, researchers have been evaluating the therapeutic effects of ChAd63-KH in Phase II of a non-randomized trial [Table 1].[39] This clinical trial has aimed to assess vaccine safety, as well as its cellular immune response and clinical changes in PKDL patients.[39]
Conclusions
Many bodies of research aimed to fulfill the hopes for an appropriate Leishmania vaccine; nevertheless, a small fracture of them has been found as a promising approach for Leishmania treatment and prevention. Dogs are considered as the primary reservoir of Leishmania infection and the animal vaccination can clearly impact the burden of the disease in the human population. Hence, animal vaccines such as Leishmune®, CaniLeish®, and Leish-Tec could be recommended as appropriate choices for the control and prevention of Leishmaniasis. Furthermore, second-generation vaccines such as LEISH-F2 could be adopted as a promising approach for the prevention of human Leishmaniasis. Recently, live attenuated and DNA vaccines have induced appropriate immune response against L. infantum and L. donovani infections, respectively. As a result, these vaccines could be considered as promising approaches to the prevention of Leishmania infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are greatly thankful of the wise counsel of Professor Khamesipour.
References
- 1.Moafi M, Rezvan H, Sherkat R, Taleban R, Asilian A, Esfahani SH, et al. Evaluation of IL-12RB1, IL-12B, CXCR-3 and IL-17a expression in cases affected by a non-healing form of cutaneous leishmaniasis: An observational study design. BMJ Open. 2017;7:e013006. doi: 10.1136/bmjopen-2016-013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali S, Rezvan H, McArdle S, Khodadadi A, Asteal F, Rees R. CTL responses to Leishmania mexicana gp63-cDNA vaccine in a murine model. Parasite Immunol. 2009;31:373–83. doi: 10.1111/j.1365-3024.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- 3.Gannavaram S, Bhattacharya P, Ismail N, Kaul A, Singh R, Nakhasi H. Modulation of innate immune mechanisms to enhance vaccine induced immunity: Role of co-inhibitory molecules. Front Immunol. 2016;7:187. doi: 10.3389/fimmu.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezvan H, Moafi M, editors. Veterinary Research Forum. Urmia, Iran: Faculty of Veterinary Medicine, Urmia University; 2015. An overview on Leishmania vaccines: A narrative review article. [PMC free article] [PubMed] [Google Scholar]
- 5.Moafi M, Rezvan H, Sherkat R, Taleban R, Asilian A, Hamid Zarkesh-Esfahani S, et al. Comparison of pro-inflammatory cytokines of non-healing and healing cutaneous leishmaniasis. Scand J Immunol. 2017;85:291–9. doi: 10.1111/sji.12534. [DOI] [PubMed] [Google Scholar]
- 6.Rezvan H, Khodadadi A, Ali S. CTL responses to DCs stimulated with leishmania antigens detected by DCs expressing Leishmania gp63. Iran J Immunol. 2014;11:65–73. [PubMed] [Google Scholar]
- 7.Imbert S, Palous M, Meyer I, Dannaoui E, Mazier D, Datry A, et al. In vitro combination of voriconazole and miltefosine against clinically relevant molds. Antimicrob Agents Chemother. 2014;58:6996–8. doi: 10.1128/AAC.03212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Griensven J, Gadisa E, Aseffa A, Hailu A, Beshah AM, Diro E. Treatment of cutaneous leishmaniasis caused by leishmania aethiopica: A systematic review. PLoS Negl Trop Dis. 2016;10:e0004495. doi: 10.1371/journal.pntd.0004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baptista C, Miranda Ld, Madeira Md, Leon LL, Conceição-Silva F, Schubach AdO. In vitro sensitivity of paired leishmania (viannia) braziliensis samples isolated before meglumine antimoniate treatment and after treatment failure or reactivation of cutaneous leishmaniasis. Dis Markers. 2015;2015:943236. doi: 10.1155/2015/943236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obonaga R, Fernández OL, Valderrama L, Rubiano LC, del Mar Castro M, Barrera MC, et al. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob Agents Chemother. 2014;58:144–52. doi: 10.1128/AAC.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanaerschot M, Dumetz F, Roy S, Ponte-Sucre A, Arevalo J, Dujardin JC. Treatment failure in leishmaniasis: Drug-resistance or another (epi-) phenotype? Expert Rev Anti-Infect Ther. 2014;12:937–46. doi: 10.1586/14787210.2014.916614. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S, Shankar P, Mishra J, Singh S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors. 2016;9:1. doi: 10.1186/s13071-016-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araújo MS, de Andrade RA, Vianna LR, Mayrink W, Reis AB, Sathler-Avelar R, et al. Despite leishvaccine and leishmune ® trigger distinct immune profiles, their ability to activate phagocytes and CD8+T-cells support their high-quality immunogenic potential against canine visceral leishmaniasis. Vaccine. 2008;26:2211–24. doi: 10.1016/j.vaccine.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira MCA, Oliveira GG, Santos PO, Bahiense TC, Silva VM, Rodrigues MS, et al. An experimental protocol for the establishment of dogs with long-term cellular immune reactions to Leishmania antigens. Mem Inst Oswaldo Cruz. 2011;106:182–9. doi: 10.1590/s0074-02762011000200011. [DOI] [PubMed] [Google Scholar]
- 15.Khamesipour A. Therapeutic vaccines for leishmaniasis. Expert Opin Biol Therapy. 2014;14:1641–9. doi: 10.1517/14712598.2014.945415. [DOI] [PubMed] [Google Scholar]
- 16.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30:134–41. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Convit J, Ulrich M, Zerpa O, Borges R, Aranzazu N, Valera M, et al. Immunotherapy of American cutaneous leishmaniasis in Venezuela during the period 1990-1999. Trans R Soc Trop Med Hyg. 2003;97:469–72. doi: 10.1016/s0035-9203(03)90093-9. [DOI] [PubMed] [Google Scholar]
- 18.Mutiso JM, Macharia JC, Taracha E, Gicheru MM. Leishmania donovani whole cell antigen delivered with adjuvants protects against visceral leishmaniasis in vervet monkeys (Chlorocebus aethiops) J Biomed Res. 2012;26:8–16. doi: 10.1016/S1674-8301(12)60002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca PM, Macedo ABB. Cutaneous leishmaniasis vaccination: A matter of quality. Front Immunol. 2016;7:151. doi: 10.3389/fimmu.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifi I, Aflatoonian MR, Fekri AR, Parizi MH, Afshar AA, Khosravi A, et al. A comprehensive review of cutaneous leishmaniasis in kerman province, southeastern iran-narrative review article. Iran J Public Health. 2015;44:299. [PMC free article] [PubMed] [Google Scholar]
- 21.Jain K, Jain N. Vaccines for visceral leishmaniasis: A review. J Immunol Methods. 2015;422:1–12. doi: 10.1016/j.jim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Wylie C, Carbonell-Antoñanzas M, Aiassa E, Dhollander S, Zagmutt F, Brodbelt D, et al. A systematic review of the efficacy of prophylactic control measures for naturally-occurring canine leishmaniosis, part I: Vaccinations. Prev Vet Med. 2014;117:7–18. doi: 10.1016/j.prevetmed.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Starita C, Gavazza A, Lubas G. Hematological, biochemical, and serological findings in healthy canine blood donors after the administration of CaniLeish ® vaccine. Vet Med Int. 2016;2016:4601893. doi: 10.1155/2016/4601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viana KF, Fiuza JA, Gannavaram S, Dey R, Selvapandiyan A, Bartholomeu DC, et al. Application of rapid in vitro co-culture system of macrophages and T-cell subsets to assess the immunogenicity of dogs vaccinated with live attenuated Leishmania donovani centrin deleted parasites (LdCen−/−) Parasites Vectors. 2016;9:1. doi: 10.1186/s13071-016-1528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gradoni L. Canine Leishmania vaccines: Still a long way to go. Vet Parasitol. 2015;208:94–100. doi: 10.1016/j.vetpar.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Shargh VH, Jaafari MR, Khamesipour A, Jaafari I, Jalali SA, Abbasi A, et al. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine. 2012;30:3957–64. doi: 10.1016/j.vaccine.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 27.Daneshvar H, Namazi MJ, Kamiabi H, Burchmore R, Cleaveland S, Phillips S. Gentamicin-attenuated Leishmania infantum vaccine: Protection of dogs against canine visceral leishmaniosis in endemic area of southeast of Iran. PLoS Negl Trop Dise. 2014;8:e2757. doi: 10.1371/journal.pntd.0002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iranian Registry of Clinical Trials. 2015. [Last accessed on 2019 Feb 06]. Available from: https://www.irct.ir/trial/20729 .
- 29.Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34:2992–5. doi: 10.1016/j.vaccine.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 30.Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29:3531–7. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 31.Llanos-Cuentas A, Calderón W, Cruz M, Ashman JA, Alves FP, Coler RN, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine. 2010;28:7427–35. doi: 10.1016/j.vaccine.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 32.U.S. National Library of Medicine. 2009. [Last accessed on 2019 Feb 06]. Available from: https://clinicaltrials.gov/ct2/show/NCT01011309?term=Leishmania+vaccines .
- 33.Christiaansen AF, Dixit UG, Coler RN, Beckmann AM, Reed SG, Winokur PL, et al. CD11a and CD49d enhance the detection of antigen-specific T cells following human vaccination. Vaccine. 2017;35:4255–61. doi: 10.1016/j.vaccine.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: Safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+ GLA-SE. Clin Transl Immunology. 2015;4:e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. National Library of Medicine. 2012. [Last accessed on 2019 Feb 06]. https://clinicaltrials.gov/ct2/show/NCT01751048 term=Leishmania+vaccines .
- 36.Regina-Silva S, Feres AM, França-Silva JC, Dias ES, Michalsky ÉM, de Andrade HM, et al. Field randomized trial to evaluate the efficacy of the Leish-Tec ® vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine. 2016;34:2233–9. doi: 10.1016/j.vaccine.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Mutiso JM, Macharia JC, Kiio MN, Ichagichu JM, Rikoi H, Gicheru MM. Development of Leishmania vaccines: Predicting the future from past and present experience. J Biomed Res. 2013;27:85. doi: 10.7555/JBR.27.20120064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis:First-in-human trial of ChAd63-KH. PLoS Negl Trop Dis. 2017;11:e0005527. doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. National Library of Medicine. [Last accessed on 2019 Jan 06]. Available from: https://clinicaltrials.gov/ct2/show/NCT01751048 term=Leishmania+vaccines&rank#x003D;6 .


