Abstract
Background:
There has been no report of the vitamin D status of the professional athletes from Iran to date. This study was performed to evaluate the efficacy of weekly vitamin D supplementation on athletic performance in Iranian athletes expedited to Asian competition in Taipei, China, 2015.
Methods:
This study was a randomized controlled clinical trial. Seventy subjects were enrolled in the study. The athletes were randomly divided into two groups: vitamin D supplement (D; received 50,000 IU of vitamin D supplement weekly) and control (P, received a placebo weekly). Duration of the study was 8 weeks. Anthropometric, dietary, athletic performance, and biochemical evaluations were performed for all subjects in the beginning and in the end of the intervention period.
Results:
A significant rise in circulating 25(OH)D concentration was observed in D group (17.3 ± 16.9 ng/mL, P < 0.001), whereas in P group, there was a statistically significant decrement (−3.1 ± 8.4 ng/mL, P = 0.040). There were no either within- or between-group significant differences in Ergo jump, vertical jump, and agility tests. In strength leg press tests, both groups showed a significant improvement. However, comparisons of changes revealed that the improvement in D group was significantly higher than in P group (P = 0.034). Moreover, in sprint test (one repetition-Max, 1RM), only D group had a significant within-group improvement (P = 0.030).
Conclusions:
Weekly supplementation with 50,000 IU vitamin D resulted in nearly 17 ng/mL increment in circulating calcidiol. This increase was associated with significant improvement of power leg press and sprint tests in D-supplemented group.
Keywords: Athletes, exercise, Vitamin D
Introduction
Vitamin D is largely known for its effects on calcium metabolism and bones health. However, a growing body of evidence has suggested a wide variety of other functions for vitamin D in the body, including immune regulation, protein synthesis, inflammatory response, cell growth, and skeletal muscle strength and function.[1]
The possible relationship between vitamin D and physical function was known many years ago when seasonality of athletic performance was observed, that is, the performance was commonly better in late summer, whereas it had a decreasing trend in winter.[2] Because of this observation, ultraviolet (UV) irradiation was used to improve physical performance in a group of medical students.[3]
Vitamin D has two main isoforms, D2 or ergocalciferol, which is found in plant dietary sources, and D3 or cholecalciferol, which is synthesized in skin upon direct exposure to solar beam. Though sun generously donates its warmth and UV rays to many parts of the world, poor vitamin D status is a global problem, surprisingly even in sunny areas.[4] Sun exposure behaviors, type of clothing, use of sunscreens, air pollution, and indoor living are among the determinant factors.[5] It is expected that athletes, as a part of general population, are affected by some degrees of vitamin D deficiency (VDD). Several observational studies endorse this notion. These observations raise very important questions as “is physical performance affected by vitamin D deficiency?” and if yes, “can athletic physical performance be improved by vitamin D supplementation?” The results coming from different studies are controversial.[6,7,8]
One of the clinical features of severe VDD is muscle weakness and myopathy.[9] Discovery of vitamin D receptor (VDR) in skeletal muscle indicated its function in the myocytes and has attracted scientists' attention to the possible role of vitamin D athletic performance and sport injuries.[10,11]
Despite especial attention made to the diet of athletes, some micronutrient inadequacies may be neglected. It is generally believed that by having a balanced diet, there will be no need for supplementation.[12,13] However, this notion may be over-simplistic. First, determination of dietary adequacy in athletes may be challenging. Micronutrient requirements of athletes may vary depending on duration, intensity, and the type of workouts.[14] Second, for some micronutrients, notably vitamin D, there may not be abundant dietary sources.[1] The importance of this issue lies in the fact that micronutrient status of an athlete can affect his/her physical performance.[15] As for vitamin D, undesirable vitamin D status has been associated with many chronic diseases including periodontitis, cardiovascular disease, cancers, type 2 diabetes, autoimmune disorders, infectious diseases, and neurological disorders.[1] Obviously, all these conditions could adversely affect athletic performance and could remarkably shorten duration of professional athletic life.
Though some investigators have reported the improving effect of vitamin D supplementation on physical performance, the subject is still controversial.[6,16] Several studies have reported high prevalence of poor vitamin D status among different age and sex groups of Iranians.[17,18,19] However, there has been no report of the vitamin D status of the professional athletes from Iran to date. This study was performed to reach the following aims in Iranian athletes expedited to Asian competition in Taipei, China, 2015:
-
(1)
to determine vitamin D status;
-
(2)
to evaluate the efficacy of weekly vitamin D supplementation in athletic performance.
Methods
Calculation of sample size
This randomized controlled trial (RCT) was done on national football and futsal athletes of both sexes. Sample size was calculated based on past studies and considering type I error α = 0.05 and type II error β = 0.20.[20]
Study protocol
This was a double-blind RCT. Based on the calculated sample size, 70 national football and futsal athletes (18- to 23-year old) were enrolled in the study. They were healthy people without any physical injuries and immune insufficiency. They did not use sport or nutritional supplement, wanted to enroll in the study, and were accessible. The objectives and protocol of the study was explained for the subjects before they signed the informed consent form. Health examinations were performed. General and demographic questionnaires were completed. Then, the athletes comprising 34 women (47.8%) and 36 men (52.2%) were randomly divided into two groups; that is, intervention, n1 = 35, vitamin D supplement (D) and control, n2 = 35, placebo (P). Duration of the interventional period was 8 weeks. Both vitamin D supplements (50,000 IU) and placebos (containing corn oil) were obtained from Zahravi Pharmaceutical Company. The supplement and placebo had similar shapes, colors, and size; they were given weekly to the intervention and control groups, respectively.[9] Though different doses of vitamin D has been recently examined in athletes (35,000 vs. 70,000 IU, weekly), findings indicated that there might potentially be adverse effects by using 70,000 IU vitamin D a week.[21] The weekly dose of 50,000 IU vitamin D seemed, therefore, appropriate to have both maximum beneficial effects and minimum potential adverse effects of the supplementation.
Anthropometric, dietary, athletic performance, and biochemical evaluations were performed for all subjects in the beginning and in the end of the intervention period. The study was approved by the Ethical Committee of the National Nutrition and Food Technology Research Institute. Figure 1 shows the study protocol.
Figure 1.
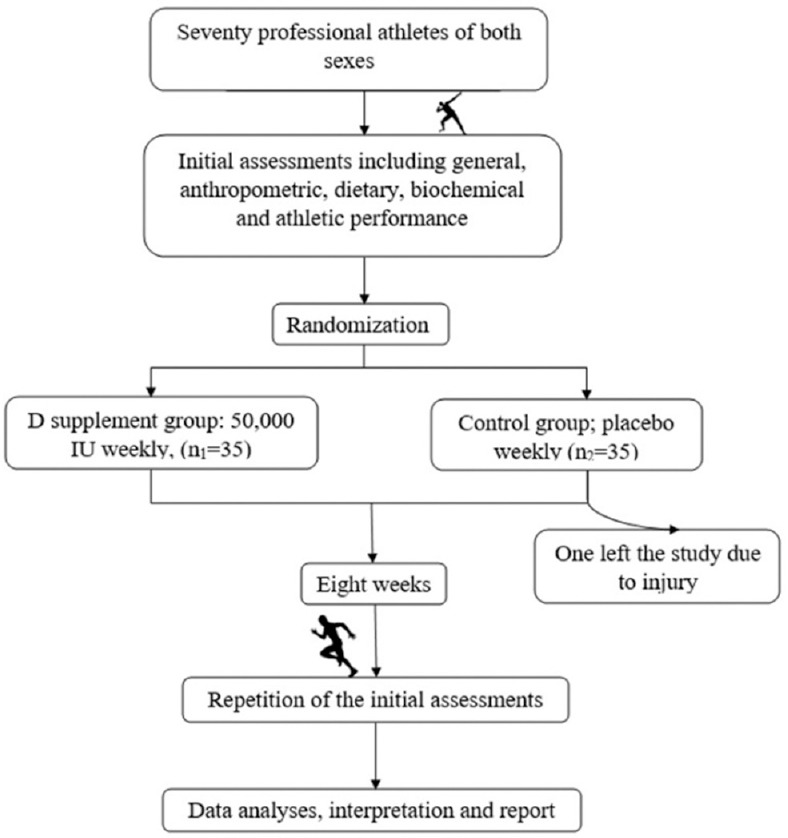
Study protocol at a glance
Evaluation of general health
Health status was evaluated using clinical examination by different medical specialists (orthopedist, cardiologist, internist, and physical medicine specialist). Those athletes who had serious acute or chronic injuries were excluded from the study.
Dietary assessment
Dietary intake was assessed using 24-hour dietary recall, which were performed four times (including a holiday) biweekly during 8 weeks intervention period. Dietary data were translated to energy and nutrients using Nutritionist 4 software (N-Squared Computing, USA).
Anthropometric measurement and evaluation of truncal fat
Weight was measured using a digital scale (Seca 755, Germany) to the nearest 0.1 kg. Height was measured using a stadiometer (Seca 213, Germany) to the nearest 0.1 cm. Body mass index was calculated using the formula weight (kg)/height2 (m). Waist circumference was measured using a tape measure lies parallel to the floor and snug but without compressing the skin in iliac crest area with less clothing as well as possible. Truncal and visceral fat was evaluated using bioimpedance system (Viscan, AB-140, Tanita, Japan).
Athletic performance
To evaluate physical performance, the following tests were applied: strength leg press test, Ergo jump test, vertical jump test, Illinois agility, test, and 40 yard speed test. The mean score of all tests was considered as the athlete's performance score.
Laboratory investigations
Blood collection and handling
Briefly, 5 mL of fasting venous blood was taken from all subjects. Blood samples were kept at room temperature (RT) for 30–45 min followed by centrifugation at 800g atRT. Sera, thus, separated were aliquoted in fresh and clean microtubes, which were then stored at −80°C till the day of analysis.
Biochemical tests
Circulating concentrations of 25-hydroxycalciferol (25(OH)D), as an indicator of vitamin D status, and parathyroid hormone (PTH) were determined using Immune Electrochemiluminescence (Liaison® Immunoassay Analyzer, Diasorin, Italy). In this study, serum 25(OH) concentrations <30 ng/mL (75 nmol/L) was considered as undesirable vitamin D status.
Statistical analyses
Kolmogrov–Smirnov test was used for evaluating normality of data distribution. Quantitative and qualitative data were expressed by mean ± standard deviation and relative or absolute frequency, respectively. Within- between-group comparisons were done using paired t-test and Student's t-test, respectively. Comparisons of vitamin D status between two groups at baseline and the end of study were evaluated by Chi-square test. McNemar test was used for within-group comparison of vitamin D status. P < 0.05 was considered significant.
Results
Sixty-nine subjects completed the study; one from the control group could not finish the study due to injury. The study population comprised of 33 women (47.8%) and 36 men (52.2%). There was no significant difference in general as well as demographic [Table 1], dietary intake (data not shown), or anthropometric variables [Table 2] between two groups.
Table 1.
Comparison of some general and demographic variables between D-supplement and control groups
| Variable | Group | P | |
|---|---|---|---|
| D-supplement (n=35) | Control (n=34) | ||
| Sex, n (%) | |||
| Female | 16 (45.7) | 17 (50) | 0.811a |
| Male | 19 (54.3) | 17 (50) | |
| Age (year) | 24.09±5.06 | 22.71±4.07 | 0.246b |
| Marital status, n (%) | |||
| Single | 8 (22.9) | 7 (20.6) | 0.585a |
| Married | 26 (74.3) | 27 (79.4) | |
| Other | 1 (2.8) | 0 | |
aSignificance of differences in the distribution of data between the two groups (Chi-square test), bSignificance of differences in mean between the two groups (t-test)
Table 2.
Between and within group comparisons of anthropometric and body fat (mean±standard deviation)
| Variable | Group | Pb | |||||
|---|---|---|---|---|---|---|---|
| D-supplement | Control | ||||||
| Initial | Final | P* | Initial | Final | Pa | ||
| Weight (kg) | 66.7±10.4 | 66.1±10.4 | 0.665 | 63.6±9.1 | 64.3±8.4 | 0.204 | 0.063 |
| BMI (kg/m2) | 22.6±2.3 | 22.6±2.2 | 0.572 | 22.2±2.6 | 22.5±2.5 | 0.194 | 0.065 |
| Waist (cm) | 83.7±6.6 | 83.7±5.4 | 0.968 | 81.8±5.4 | 82.5±4.7 | 0.233 | 0.481 |
| Truncal fat (%) | 21.6±8.4 | 21.2±8.2 | 0.297 | 22.1±7.4 | 22.0±8.3 | 0.810 | 0.577 |
| Visceral fat (%) | 4.9±1.9 | 4.7±1.4 | 0.316 | 4.4±1.7 | 4.3±1.2 | 0.626 | 0.687 |
BMI=Body mass index. aWithin-group comparison. bBetween-group comparison of changes of the variables
Table 3 shows intra- and intergroup comparisons of 25(OH)D and PTH serum concentrations. Initial concentrations of both calcidiol and PTH did not differ significantly between groups. However, a significant rise in circulating 25(OH)D concentration was expectedly observed in D group (17.3 ± 16.9 ng/mL, P < 0.001), whereas in P group, there was a small but statistically significant decrement (−3.1 ± 8.4 ng/mL, P = 0.040). Serum PTH concentration significantly increased in P group, whereas in D group, no significant change occurred. Accordingly, between-group comparison of changes of serum PTH showed a significant difference (P = 0.046).
Table 3.
Within- and between-group comparisons of serum 25-hydroxycalciferol and parathyroid hormone concentrations (mean±standard deviation)
| Variable | Group | Pb | |||||
|---|---|---|---|---|---|---|---|
| D-supplement (n1=35) | Control (n2=34) | ||||||
| Initial | Final | P | Initial | Final | Pa | ||
| 25(OH)D (ng/mL) | 27.5±17.9 | 44.9±19.8 | <0.001* | 24.4±12.7 | 21.3±8.9 | 0.04* | <0.001* |
| PTH (pg/mL) | 12.1±4.0 | 13.3±3.3 | 0.082 | 12.8±6.6 | 16.5±7.7 | <0.001* | 0.046* |
25(OH)D=25-hydroxycalciferol, PTH=Parathyroid hormone. aWithin-group comparison. bBetween-group comparison of changes of the variables. *Significant difference
Initially, 66.7% in D group and 70.6% in P group had lower serum concentrations of 25(OH)D than 30 ng/mL. Finally, however, the percent of the subjects with undesirable vitamin D status showed a significant decrease only in D group (20%, P < 0.001) and an increase in P group (91.2%, P = 0.039).
Table 4 demonstrates within- and between-group comparisons of physical functional tests. There were no either within- or between-group significant differences in Ergo jump, vertical jump, and agility tests. In strength leg press tests, both groups showed a significant improvement. However, comparisons of changes revealed that the improvement in D group was significantly higher than P group (P = 0.034). Moreover, in sprint test (one repetition-Max, 1RM), only D group had a significant within-group improvement (P = 0.030). However, comparison of between-group changes showed no significant difference (P = 0.345).
Table 4.
Within- and between-group comparisons of physical function tests (mean±standard deviation)
| Test | Group | Pb | |||||
|---|---|---|---|---|---|---|---|
| D-supplement | Control | ||||||
| Initial | Final | P | Initial | Final | Pa | ||
| Strength leg press (%) | 124.9±26.6 | 149.6±32.2 | <0.001* | 129.4±28.3 | 144.8±32 | <0.001* | 0.034* |
| Ergo jump (%) | 68.9±14.4 | 69.4±15.9 | 0.787 | 73±16 | 70.2±21.4 | 0.368 | 0.367 |
| Vertical jump (%) | 78.8±17 | 77.7±12.6 | 0.883 | 85.1±15.3 | 79.5±13.2 | 0.158 | 0.199 |
| Agility (%) | 47.3±35.3 | 44.4±29.4 | 0.838 | 57.3±31.7 | 40.5±32.1 | 0.256 | 0.586 |
| Speed (%) | 55.1±26.2 | 62.5±23.6 | 0.030* | 59.6±19.5 | 63.1±20.6 | 0.163 | 0.345 |
aWithin-group comparison. bBetween-group comparison of changes of the variables. *Significant difference
Discussion
A significant rise in circulating 25(OH)D concentration was observed in D group, whereas in P group, there was a statistically significant decrement. There were no either within- or between-group significant differences in Ergo jump, vertical jump, and agility tests. In strength leg press tests, both groups showed a significant improvement, comparisons of changes revealed that the improvement in D group was significantly higher than in P group. Moreover, in sprint test (one repetition-Max, 1RM), only D group had a significant within-group improvement.
We found weekly supplementation with 50,000 IU (1,250 μg/week or ~178.6 μg/day) vitamin D resulted in nearly 17 ng/mL increment in circulating calcidiol corresponding to 0.1 ng/mL rise in 25(OH)D per 1 μg/day vitamin D daily intake. This is much less than earlier estimation that daily intake of 1 μg vitamin D would result in 0.48 ng/mL increment in serum calcidiol.[22] In our previous experiments, we found that daily intake of 1,000 IU (25 μg) vitamin D through fortified food or drink would result in about 12–15.6 ng/mL increase in circulating 25(OH)D corresponding to 0.48-0.60 ng/mL rise in calcidiol for 1 μg/d vitamin D intake.[23,24,25] The reason for this discrepancy could be the baseline concentrations of serum 25(OH)D in the current study, which were much higher than those in our previous experiments. Initial concentrations of serum calcidiol has a very significant contribution in the response to supplementation, that is, the higher in initial concentration the lower serum rise of calcidiol following supplementation.[26]
The improvement in vitamin D status in D group did not result in any significant change in anthropometric or body fat measures. In contrast with this finding, we have already reported a small but statistically significant reduction in visceral fat due to improvement of vitamin D status in healthy adults.[25] Having an athletic body with minimum truncal and visceral fat, which can be less affected by supplementation in the current study, could be a possible explanation for this finding. In support of this notion, a significant decrease in waist to hip ratio was reported in overweight/obese young adults supplemented with 4000 IU/d vitamin D combined with resistance exercise, as compared with the placebo group.[27]
Our finding of significant improvement of power leg press and sprint tests in D-supplemented group is noticeable. The pivotal role of vitamin D in musculoskeletal health and its trophic function especially in elderly and athletes has attracted huge attention in recent years.[28] Studies on vitamin D effect on leg press are limited. Some studies showed that vitamin D supplementation has significant positive small effect on muscle strength. Supplementation of deficient people resulted in a significant improvement compared to those had 25[OH]D level ≥30 nmol/L. People who had an increase of at least 25 nmol/L and in other studies 50 nmol/L 25[OH]D concentration within the duration of the study showed higher muscle strength. Supplementation 65 years or older people resulted in a significant improvement of muscle strength, but it was not shown for younger people.[29] Despite the evidence from many observational as well as some interventional studies for the association of vitamin D status and physical performance, this subject is still controversial.[7,8]
The identification of the VDR, its various polymorphisms, and variable expression with aging has provided some insight into the complex mechanisms by which vitamin D and its metabolic pathways may affect muscle function. The recognition of both genomic and nongenomic effects of vitamin D in skeletal muscle, with the resultant impact on both calcium metabolism and protein transcription, further illustrates the significance of vitamin D in muscle function.[30] Some studies have shown that daily doses of 800–1,000 IU vitamin D improve muscular strength and balance in older adults.[31,32,33] This effect is potentiated if D-supplementation is accompanied by resistance exercise.[34] A systematic review by considering several interventional studies with various durations (from 6 weeks to 6 months) and different vitamin D doses (from 4,000 IU/day to 60,000 IU/week) concluded that vitamin D supplementation increases muscular strength in upper and lower limbs of healthy adults.[35] Another study reported that vitamin D supplement has a small beneficial effect on muscular strength and more studies on dosage, mode of administration and duration are needed to maximize the effect.[36] On the other hand, a meta-analytic study reported that vitamin D supplementation may not be efficient in terms of muscular strength in adults with circulating concentrations of 25(OH)D >10 ng/mL (25 nmol/L).[37]
The number of vitamin D supplementation studies in athletes is much less than those on other subpopulations. Recently, a study reported that even a single dose of 150,000 IU oral vitamin D resulted in improved muscular strength in elite Judo athletes who had vitamin D insufficiency before supplementation.[38] In support of our finding of improved sprint test following vitamin D supplementation, in another RCT, 5,000 IU/day vitamin D supplementation for 8 weeks resulted in significant improvement of sprint and vertical jump of supplemented athletes as compared with the control group.[39] In contrast, biweekly supplementation with 50,000 IU vitamin D of professional rugby athletes for 11–12 weeks did not result in improvement of their performance tests, including sprint. It is noteworthy that the baseline 25(OH)D concentrations of all subjects were >20 ng/mL (50 nmol/L).[40] Along the same line of evidence, using higher doses of vitamin D (5,000 IU/day) in professional football players did not have any beneficial effect on 30 m sprint running time after 8 weeks of supplementation.[41]
The reasons of these controversial results could be the type of sport (indoor vs. outdoor) and initial calcidiol concentrations, dosages used, route of administration, duration of supplementation, and performance tests used to evaluate athletes.
To date, data coming from experimental animal models propose various tenable mechanisms for the role of vitamin D in neuromuscular adaptation and remodeling following exercise and injury.[42] Some studies have revealed the association of circulating 25(OH)D and androgens with a similar variations[43] which is independent of vitamin D-related gene polymorphisms.[44] However, post hoc analyses of three small RCTs in men with normal initial serum concentrations of testosterone, did not confirm any significant rise in serum testosterone due to vitamin D supplementation.[45] Vitamin D-mediated enhanced delivery of insulin-like growth factor to the muscles is another proposed mechanism for the effect of vitamin D on muscular performance.[46] Notwithstanding, the beneficial effect of vitamin D on athletic performance may be related to the vitamin D isoform as a research group found that 6 weeks vitamin D2 (ergocalciferol) supplementation in National Association for Stock Car Auto Racing pit crew athletes not only decreased serum concentrations of 25(OH) D3 by 21% but resulted in augmented muscular damage biomarkers, as well.[47]
Conclusions
We found high occurrence of undesirable vitamin D status among the studied Iranian professional athletes. Apart from possible adverse effects of VDD on general health, this condition could impair athletic performance. Though we found weekly intake of 50,000 IU vitamin D improved certain athletic performance tests in our subjects, the optimum dosage for athletes needs further studies. But until then, regular checking of serum calcidiol especially in indoor athletes and (if needed) appropriate intervention is warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This paper represents part of Ph.D. thesis of KA. The whole project was supervised By TRN. Ali AR and BN had great intellectual input in several stages of the project including study design and data analysis. MZ, NS, and AK were involved in data collection and manuscript preparation. All vitamin D and placebo pearls were purchased from Zahravi Pharmaceuticals, Tabriz, Iran. All biochemical tests were performed at the Medical Lab of Resalat Hospital, Tehran, Iran. Body fat analyses were done at the Laboratory of Nutrition Research, NNFTRI. Authors do not have any conflict of interest.
References
- 1.Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–65. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 2.Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41:1102–10. doi: 10.1249/MSS.0b013e3181930c2b. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann G, Mueller E. [Ultraviolet irradiation and altitude fitness] luftfahrtmedizin. 1944;9:37–43. [Google Scholar]
- 4.Nikooyeh B, Hajifaraji M, Yarparvar A-H, Abdollahi Z, Sahebdel M, Dehkordi AM, et al. Hypovitaminosis D in adults living in a Sunny City: Relation to some cardiometabolic risk factors, national food and nutrition surveillance. Nutrition and Food Sci Res. 2018;5:11–6. [Google Scholar]
- 5.Tønnesen R, Hovind PH, Jensen LT, Schwarz P. Determinants of vitamin D status in young adults: Influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health. 2016;16:385. doi: 10.1186/s12889-016-3042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girgis CM, Clifton-Bligh RJ, Turner N, Lau SL, Gunton JE. Effects of vitamin D in skeletal muscle: Falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf) 2014;80:169–81. doi: 10.1111/cen.12368. [DOI] [PubMed] [Google Scholar]
- 7.Dahlquist DT, Dieter BP, Koehle MS. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J Int Soc Sports Nutr. 2015;12:33. doi: 10.1186/s12970-015-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stachowicz M, Lebiedzińska A. The role of vitamin D in health preservation and exertional capacity of athletes. Postepy Hig Med Dosw (Online) 2016;70:637–43. doi: 10.5604/17322693.1205363. [DOI] [PubMed] [Google Scholar]
- 9.Rasheed K, Sethi P, Bixby E. Severe vitamin d deficiency induced myopathy associated with rhabydomyolysis. N Am J Med Sci. 2013;5:334–6. doi: 10.4103/1947-2714.112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker T, Henriksen VT, Martins TB, Hill HR, Kjeldsberg CR, Schneider ED, et al. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients. 2013;5:1253–75. doi: 10.3390/nu5041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyon MA, Koutedakis Y, Wolman R, Nevill AM, Allen N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J Sci Med Sport. 2014;17:8–12. doi: 10.1016/j.jsams.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2000;100:1543–56. doi: 10.1016/S0002-8223(00)00428-4. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez NR, DiMarco NM, Langley S. American Dietetic Association, Dietitians of Canada, American College of Sports Medicine: Nutrition and Athletic Performance, et al. Position of the American dietetic association, dietitians of Canada, and the American college of sports medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109:509–27. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Volpe SL. Micronutrient requirements for athletes. Clin Sports Med. 2007;26:119–30. doi: 10.1016/j.csm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Lukaski HC. Vitamin and mineral status: Effects on physical performance. Nutrition. 2004;20:632–44. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald JS, Peterson BJ, Warpeha JM, Johnson SC, Ingraham SJ. Association between vitamin D status and maximal-intensity exercise performance in junior and collegiate hockey players. J Strength Cond Res. 2015;29:2513–21. doi: 10.1519/JSC.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 17.Neyestani TR, Hajifaraji M, Omidvar N, Eshraghian MR, Shariatzadeh N, Kalayi A, et al. High prevalence of vitamin D deficiency in school-age children in tehran, 2008: A red alert. Public Health Nutr. 2012;15:324–30. doi: 10.1017/S1368980011000188. [DOI] [PubMed] [Google Scholar]
- 18.Nikooyeh B, Abdollahi Z, Hajifaraji M, Alavi-Majd H, Salehi F, Yarparvar AH, et al. Vitamin D status and cardiometabolic risk factors across latitudinal gradient in Iranian adults: National food and nutrition surveillance. Nutr Health. 2017;23:87–94. doi: 10.1177/0260106017702918. [DOI] [PubMed] [Google Scholar]
- 19.Nikooyeh B, Abdollahi Z, Hajifaraji M, Alavi-Majd H, Salehi F, Yarparvar AH, et al. Vitamin D Status, Latitude and their Associations with Some Health Parameters in Children: National Food and Nutrition Surveillance. J Trop Pediatr. 2017;63:57–64. doi: 10.1093/tropej/fmw057. [DOI] [PubMed] [Google Scholar]
- 20.Koundourakis NE, Androulakis NE, Malliaraki N, Margioris AN. Vitamin D and exercise performance in professional soccer players. PLoS One. 2014;9:e101659. doi: 10.1371/journal.pone.0101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens D, Tang J, Bradley W, Sparks A, WD F, Morton J, et al. Efficacy of high-dose Vitamin D supplements for elite athletes. Med Sci Sports Exerc. 2017;49:349–56. doi: 10.1249/MSS.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 22.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012;142:1102–8. doi: 10.3945/jn.112.158014. [DOI] [PubMed] [Google Scholar]
- 23.Neyestani TR, Djazayery A, Shab-Bidar S, Eshraghian MR, Kalayi A, Shariátzadeh N, et al. Vitamin D receptor fok-I polymorphism modulates diabetic host response to vitamin D intake: Need for a nutrigenetic approach. Diabetes Care. 2013;36:550–6. doi: 10.2337/dc12-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, et al. Daily consumption of vitamin D- or vitamin D+ calcium-fortified yogurt drink improved glycemic control in patients with Type 2 diabetes: A randomized clinical trial. Am J Clin Nutr. 2011;93:764–71. doi: 10.3945/ajcn.110.007336. [DOI] [PubMed] [Google Scholar]
- 25.Nikooyeh B, Neyestani TR, Zahedirad M, Mohammadi M, Hosseini SH, Abdollahi Z, et al. Vitamin D-fortified bread is as effective as supplement in improving vitamin D status: A randomized clinical trial. J Clin Endocrinol Metab. 2016;101:2511–9. doi: 10.1210/jc.2016-1631. [DOI] [PubMed] [Google Scholar]
- 26.Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7:5111–42. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, et al. Impact of Vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr. 2013;32:375–81. doi: 10.1016/j.clnu.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingues-Faria C, Boirie Y, Walrand S. Vitamin D and muscle trophicity. Curr Opin Clin Nutr Metab Care. 2017;20:169–74. doi: 10.1097/MCO.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 29.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. The Journal of Clinical Endocrinology and Metabolism. 2014;99:4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182–90. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 32.Walrand S. Effect of vitamin D on skeletal muscle. Geriatr Psychol Neuropsychiatr Vieil. 2016;14:127–34. doi: 10.1684/pnv.2016.0599. [DOI] [PubMed] [Google Scholar]
- 33.Dawson-Hughes B. Serum 25-hydroxyvitamin D and muscle atrophy in the elderly. Proc Nutr Soc. 2012;71:46–9. doi: 10.1017/S0029665111003260. [DOI] [PubMed] [Google Scholar]
- 34.Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open. 2017;7:e014619. doi: 10.1136/bmjopen-2016-014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson PB, Joseph C, Angioi M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport. 2015;18:575–80. doi: 10.1016/j.jsams.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 37.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos Int. 2011;22:859–71. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 38.Wyon MA, Wolman R, Nevill AM, Cloak R, Metsios GS, Gould D, et al. Acute effects of vitamin D3 supplementation on muscle strength in judoka athletes: A Randomized placebo-controlled, double-blind trial. Clin J Sport Med. 2016;26:279–84. doi: 10.1097/JSM.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 39.Close GL, Russell J, Cobley JN, Owens DJ, Wilson G, Gregson W, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J Sports Sci. 2013;31:344–53. doi: 10.1080/02640414.2012.733822. [DOI] [PubMed] [Google Scholar]
- 40.Fairbairn KA, Ceelen IJ, Skeaff CM, Cameron CM, Perry TL. Vitamin D3 supplementation does not improve sprint performance in professional rugby players: A randomised, placebo-controlled double blind intervention study. Int J Sport Nutr Exerc Metab. 2017:1–24. doi: 10.1123/ijsnem.2017-0157. [DOI] [PubMed] [Google Scholar]
- 41.Jastrzębska M, Kaczmarczyk M, Jastrzębski Z. Effect of Vitamin D supplementation on training adaptation in well-trained soccer players. J Strength Cond Res. 2016;30:2648–55. doi: 10.1519/JSC.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 42.Minshull C, Biant LC, Ralston SH, Gleeson N. A systematic review of the role of vitamin D on neuromuscular remodelling following exercise and injury. Calcif Tissue Int. 2016;98:426–37. doi: 10.1007/s00223-015-0099-x. [DOI] [PubMed] [Google Scholar]
- 43.Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–8. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 44.Rafiq R, van Schoor NM, Sohl E, Zillikens MC, Oosterwerff MM, Schaap L, et al. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol. 2016;164:11–7. doi: 10.1016/j.jsbmb.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Heijboer AC, Oosterwerff M, Schroten NF, Eekhoff EM, Chel VG, de Boer RA, et al. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol (Oxf) 2015;83:105–10. doi: 10.1111/cen.12711. [DOI] [PubMed] [Google Scholar]
- 46.Darr RL, Savage KJ, Baker M, Wilding GE, Raswalsky A, Rideout T, et al. Vitamin D supplementation affects the IGF system in men after acute exercise. Growth Horm IGF Res. 2016;30-31:45–51. doi: 10.1016/j.ghir.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Nieman DC, Gillitt ND, Shanely RA, Dew D, Meaney MP, Luo B, et al. Vitamin D2 supplementation amplifies eccentric exercise-induced muscle damage in NASCAR pit crew athletes. Nutrients. 2013;6:63–75. doi: 10.3390/nu6010063. [DOI] [PMC free article] [PubMed] [Google Scholar]


