Abstract
Background:
Today, the importance of physical activity as a preventative way for cardiovascular disease has attracted much attention.The aim of this study is to investigate the effect of 6 weeks of interval training with or without extract of Ziziphus jujuba on lipocalcin-2 (LCN2) and adiponectin levels in heart tissue in male Wistar rats with myocardial infarction.
Methods:
Thirty male Wistar rats (mean weight, 180–220 g and age, 2–3 months) were divided into five groups, including (1) Healthy control; (2) Isoprenaline-treated group (ISO); (3) ISO + jujube extracts (JE); (4) Trained ISO rats; and (5) Trained ISO rats + JE. Exercise was performed (5 days/week, for 6 week including 54-min cycles with speed of 23 m/min and 54-min cycles with speed of 15 m/min). After 48 h of the last training session, the rats were sacrificed, and their heart tissue was excised. The significant level of statistical data was analyzed by one-way ANOVA test.
Results:
LCN2 levels significantly decreased in trained ISO rats + JE group after 6 weeks of interval training with JE consumption, compared to ISO group. However, the consumption of jujuba extracts with and without interval training did not show any significant changes in adiponectin levels of rat's heart tissue, compared to ISO (P < 0.05).
Conclusions:
Because the LCN2 inflammatory factor decreased after 6 weeks of exercise and consumption of the extract, it seems that performing interval training with JE consumption can be an effective method in the cardiac rehabilitation phase after a heart attack.
Keywords: Adiponectin, exercise training, lipocalin-2, myocardial infarction, Ziziphus
Introduction
Acute myocardial infarction (MI) is one of the most common causes of mortality in the world.[1] Exercise training and medical antioxidants plants using (Jujube) is a strategy for heart rehabilitation.[2,3] The search for new adipokines gained added fervor with the correlation of the reconciliation between cardiovascular diseases (CVDs) and metabolic syndrome.[4] Lipocalin-2 (LCN2) expression is significantly augmented in patients with CVDs and MI.[5] Adiponectin protects the heart against the development of systolic malfunction after MI, and it protects the heart against loss of heart cells and capillaries by suppressing heart hypertrophy and interstitial fibrosis.[6] Exercise training is accepted as a fundamental non-pharmacological intervention strategy in cardiac rehabilitation. In particular, maximal oxygen uptake (VO2 max), which has been noted as the best single predictor of death among cardiac patients.[2] In addition, the use of medicinal plants has long been considered in the treatment of many diseases. Natural antioxidants increase plasma antioxidants and reduce the incidence of certain diseases such as cancer, heart disease, and brain stroke.[3] Several therapeutic properties have been reported for jujuba, including anti-inflammatory, anti-cancer, lipid lowering, anti-epileptic, anti-diabetic, and antioxidant properties.[7] As far as we know, no study has ever been done investigating the effect of interactive exercise and jujuba extracts (JEs) in patients with myocardial infarction. Studies show that doing different exercises in rehabilitation protocols may have beneficial effects, sometimes harmful or no effects on heart function and the deformation structure of heart tissue, but research on the interactive effect of exercise and the Z. JE is limited. Therefore, the purpose of current study is to examine the effect of 6 weeks aerobic interval training with or without the consumption of JEs on LCN2 and adiponectin levels in the heart tissue of the MI in rats.
Methods
Animals
Thirty healthy male Wistar albino rats, weighting 180–220 g, were obtained from Birjand University of Medical Sciences Laboratory Animal Breeding and Duplication Center. The animals were kept in standard polypropylene cages (six rats/cage), at room temperature 23–22 ± 2°C with a 12-h light/dark cycle. The animals had free access to tap water and food. The rats were randomly assigned into five groups: (1) Healthy control (2) Isoprenaline-treated group (ISO) (3) ISO + JEs (4) Trained ISO rats, and 5. Trained ISO rats + JE. Groups 4 and 5 were run on treadmill for 52 min/day and 5 days/week for 6 weeks under interval aerobic exercise program: 52 min/day with an 8-min warm-up and 4-min cool down at 10 m/min and exercise at 15 m/min 4 × 4 min interspersed with 4 × 4 min at 23 m/min [Table 1]. Groups 2 and 4 received orally jujube supplementation (400 mg/kg body weight) according to the schedule.
Table 1.
Mean and standard deviations of research variables
| Groups | Healthy control | ISO | ISO+JE | Trained ISO | Trained ISO+JE | F | Sig | |
|---|---|---|---|---|---|---|---|---|
| Variables | M±SEM | |||||||
| LCN.TISSU | M±SEM | 1.37±0.13 | 2.25±0.31 | 1.46±0.14 | 1.66±0.08 | 1.43±0.20 | 3.408 | 0.023 |
| ADP.TISSU | M±SEM | 1.70±0.22 | 0.96±0.04 | 1.13±0.10 | 1.26±0.06 | 1.00±0.07 | 6.126 | 0.001 |
Myocardial infarction induction
Experimental MI was induced by subcutaneous injection of ISO (85 mg/kg body weight) in two consecutive days.[8]
The selection of injectable dose was from a pilot study by injection of 50, 85, and 150 mg/kg of body weight. The histochemical evaluation of cardiac tissue showed that the doses of 85 and 150 mg/kg could induce necrotic area in the heart tissue. Therefore, the dose of 85 mg/kg was chosen for the induction of MI in rats. The confirmation of MI was investigated using histochemical techniques for hematoxylin-eosin staining and presence of white areas indicating an infarction-induced necrosis damage in the heart tissue was confirmed by microscope. A total of 30 rats received ISO; among these, four rats died after the second injection. Twenty-four rats were randomly assigned to four groups (n = 6) as mentioned before.
Preparation of jujuba extract
Z. jujuba 50 g (Herbarium No. 2470) was coarsely powdered and dissolved in 1000 cc of 80% ethanol and stayed on an electrical grinder for 24 h at room temperature. Then, the mixture was filtered by using a filter paper. To remove the solvent, the samples are poured into glass plates and placed in a temperature of 40°C for 1 to 2 days; after the evaporation of the solvent, the samples are placed in the freezer at −20°C.[9] The JE was given to each rat in 6 weeks (400 mg/kg) after the training session.[10,11]
Aerobic interval training protocol
After 2 weeks of adaptation, rats were familiarized with running on a treadmill for 5 days (10 min/day at a speed of 10 m/min).[12] Then, aerobic interval training was performed 5 days/week, for 6 weeks on the treadmill, and the overload principle was applied through a gradual increase in speed. The aerobic interval training programs were performed for 52 min/day, including 8 min of warm-up and 4 min of cool down at speed of 10 m/min and 54-min exercise at 23 m/min interspersed with 54-min at 15 m/min.[13] However, the control group was without activity under the standard environmental conditions of the laboratory.
Sample collection
At the end of the experiment, after 48 h of the last session of exercise, rats were anesthetized by intraperitoneal injection of ketamin/xylasin 70/10 mg/kg.[14] Blood samples were taken directly from the heart, and serum was stored at −80°C. Then, the heart was isolated and washed in a normal saline and was immediately frozen in liquid nitrogen and then transferred to −80°C for subsequent measurements. The heart tissue was homogenized by liquid nitrogen and then buffer containing a protease inhibitor tablet (Sigma, Saint Louis, USA) added to each sample and centrifuged for 10 min at 1400 rpm and at 4°C, and the supernatant liquid to measure the protein was collected by the Bradford method. Heart tissue levels of adiponectin and LCN2 were evaluated by using the German kits Rat LCN2 manufactured by ZellBio GmbH, coefficient of variation (CV) = 10% and sensitivity of 0.2 ng/ml and rat adiponectin kit with CV = 10% and sensitivity of 28 pg/ml were evaluated by ELISA method.
Statistical analysis
The statistical analyses were performed in SPSS 22 software environment. The Shapiro-Wilk test was used to determine the normal distribution of data. The one-way ANOVA test was used for testing the hypotheses, and Turkey's post hoc test was used for determining the different groups at a significant level of P < 0.05.
Results
A total of 30 male Wistar rats (mean weight, 180–220 g and age, 2–3 months) in five groups, participated in this study. It should be noted that four rats died after the second ISO injection and dropped out study. Finally, 30 rats successfully completed all training sessions and consumpted JE and were included in the subsequent analyses. As shown in Table 1, the one-way ANOVA's test results show that after 6 weeks performing interval training and consumption of JE following the induction of MI by ISO, the levels of LCN2 significantly changed (P = 0.023). To find meaningful, the Tukey's post hoc test showed that the levels of LCN2 significantly declined in trained ISO rats + JE, compared to ISO group (P = 0.047). In addition, the data showed a significant increase in LCN2 levels of heart tissue in ISO group, compared to the healthy control group (P = 0.029). There was no significant difference in LCN2 levels between ISO + JE, trained ISO, and trained ISO JE groups (P ≥ 0.05) [Figure 1]. Moreover, One-way ANOVA's test results in levels of adiponectin show that applying interval training and/or JE have significantly changes between groups (P = 0.001). To find meaningful, Tukey's post hoc test showed that the levels of adiponectin significantly decreased in ISO group compare to healthy control group (P = 0.002). Although the levels of adiponectin in ISO + JE and trained ISO + JE groups were significantly lower than healthy control group (respectively P = 0.019, P = 0.003), it seems that performing 6 weeks interval training can increase the levels of adiponectin in trained ISO group in comparison with ISO group (0.433) and cause a slight and insignificant improvement [Figure 2]. According to the data, it can be induced that adiponectin levels in trained ISO group were increased insignificantly more than those in ISO + JE group (0.940). In addition, no significant variations were observed in adiponectin levels in ISO + JE, trained ISO, and trained ISO rats + JE groups, compared to ISO group (P ≥ 0.05). The observed quantity in the table is related to the significant reduction of adiponectin in ISO group, compared to the healthy control group (P = 0.001).
Figure 1.
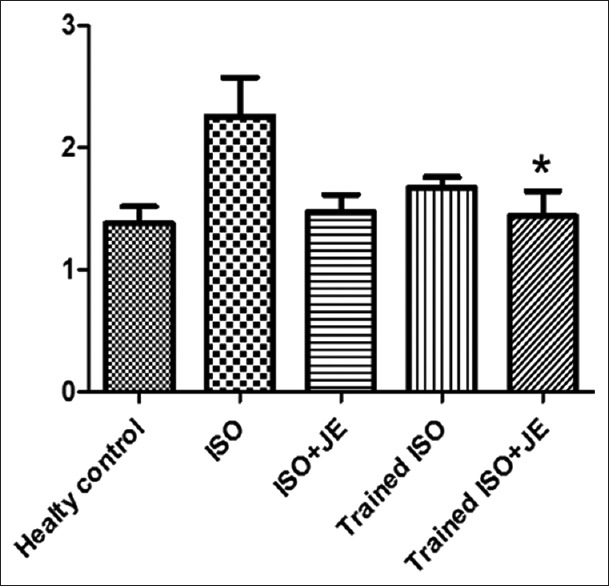
Effect of interval training and consumption Ziziphus jujuba extract on lipocalin-2 levels in heart tissue (ng/mg). *(P<0.05)
Figure 2.
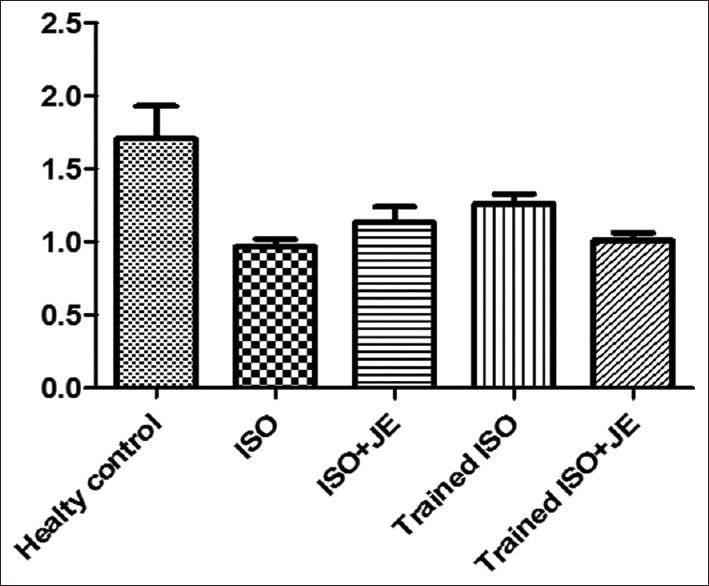
Effect of interval training and consumption Ziziphus jujuba extract on adiponectin levels in heart tissue (ng/mg)
Discussion
In general, the findings showed that performing 6 weeks of interval training with Z. jujuba supplementation decreased the LCN2 levels in heart tissue in rats with MI. LCN2 has many functions such as transferring retinol, pheromone, and prostaglandin synthesis and contributing to the transfer of iron and fatty acids, inhibiting the bacterial growth, and modulating the inflammatory responses.[15] In the current study, it was shown that the levels of LCN2 as an inflammatory marker have been reduced by performing interval training and consuming JEs. It seems that LCN2 has been implicated in the regulation of cell death in various cell types, although whether LCN2 directly regulates cardiomyocyte apoptosis remains unknown.[5] It has been shown that the changes in intracellular iron content can mediate apoptosis in a variety of cell types. Furthermore, iron overload is associated with cardiomyopathy, involving apoptosis and fibrosis, leading to heart failure. Because LCN2 has the capability to bind iron, there is a hypothesis that the alteration of intracellular iron levels is an important mechanism in LCN2-induced apoptosis.[5] Hence, the decrease in LCN2 levels following the interval training and supplementation with JE in heart tissue may be because of the modulation of inflammatory responses. Over recent years, some authors have studied regular exercise as a natural anti-atherogenic activity and its effects on oxidative stress and inflammatory pathways.[16] In this regard, Ranjbar et al. (2018) suggested that 10 weeks resistance training and Crataegus oxyacantha can synergistically decrease ischemia-reperfusion injury, and this mechanism may be related to a reduction in oxidative stress, which is normally associated with ischemia-reperfusion.[17] In this study, interval training combined with JE supplementation was more effective in improving the cardiac condition, compared to the levels of LCN2 between trained ISO rats or ISO + JE group alone. However, the results showed that performing interval training with Z. jujube after MI had no significant increase in the levels of adiponectin in heart tissue; however, an insignificant increase was observed. There is strong evidence regarding the cardiac protection role of adiponectin in various pathological conditions, including MI, ischemic/reperfusion, heart failure, and coronary artery disease, and multiple beneficial effect of adiponectin such as insulin sensitivity, anti-inflammation, and energy balance adjustment.[18] Although it seems that the use of JE with and without interval training has not been effective in sufficiently increasing the levels of protective adipokine in heart tissue, a slight increase in adiponectin levels (as an anti-inflammatory index) was observed in these groups. Physical activity as an important part of post-cardiac rehabilitation have different effects on cardiac rehabilitation, depending on severity, duration, type of protocol, and rest duration to start post-MI exercise program. Because of inducing favorite effects with less exercise volume or optimizing the time, aerobic interval training has been considered for patients suffering from chronic heart failure, MI, hypertension, and coronary heart diseases (CHDs).[19] Hence, some studies are consistent with our research; Choi et al. (2008) investigated the implications of LCN2 and visfatin levels in patients with CHD and reported that LCN2 levels were significantly higher in patients with CHD, compared to the control subjects; they concluded that serum LCN2 levels were significantly elevated in patients with CHD and were independently associated with CHD.[20] Mohebbati et al. (2018) investigated the effects of sub-chronic administration of Z. jujuba fruits hydroalcoholic extract to NG-nitro-L-arginine methyl ester (L-NAME) hypertensive rats, and they suggested that Z. jujuba has potential beneficial effects in prevention of hypertension induced by nitric oxide deficiency.[21] Buturak et al. (2016) reported a reduction in adiponectin levels after percutaneous coronary intervention (PCI) and says this decline in adiponectin levels may be the consequence of plaque rupture that impairs endothelial integrity leading to adiponectin accumulation in subendothelial space in response to PCI.[22] Roca et al. (2012) reported the levels of adiponectin and leptin remained stably after a 2-month cardiac rehabilitation program that was on the basis of nutritional and exercise.[16] However, other factors such as the severity of MI is important in the efficacy of the exercise following heart infarction. Doing exercise a short period after MI could exacerbate the development of the infarction region and the transformation of the heart tissue.[23] A number of studies are countered with this study; Damirchi et al. (2011) studied the levels of LCN2 in obese and normal weight men after a short term treadmill protocol, and they found that levels of LCN2, hs-CRP, and the number of white blood cells increased significantly in both groups.[23] Choi et al. (2009) evaluated the effect of exercise training on A-FABP, LCN2, and RBP4, and they reported no significant change in the LCN2 and RBP4 levels after the exercise program. These results suggest that A-FABP and LCN2 might not be simple markers of inflammation. It is likely that there is a complex interconnection between LCN family proteins, obesity-induced metabolic disorders, and inflammation.[24] Zhu et al. (2015) observed an increase in the expressions of four adiponectin-related proteins: AdipoR1, PPARα, AMPK, and P-AMPK, in the myocardial tissue of the ApoE mice.[25] During the past decade, the reactive oxygen species generation and oxidative stress have been implicated in the development of many diverse diseases, including hypertension, cardiac dysrhythmia, and myocardial damage.[26] The jujubosides, flavonoids, and terpenes are important compounds of Z. jujuba that have anti-inflammatory activities, which may produce beneficial cardiovascular effect of Z. jujuba.[21] Gao et al. (2013) stated that Z. jujuba fruit with anticancer, anti-inflammatory, antiobesity, immunostimulating, antioxidant, and hepatoprotective properties is useful for predicting other medicinal uses and potential drug.[27] In the current study, findings from LCN2 indicated that exercise and extract consumption could reduce the inflammatory effects of MI in the heart tissue, whereas the findings revealed a little increasing trend in adiponectin levels but adiponectin levels did not change significantly after 6 weeks of intervention.
Conclusions
It can be concluded that 6 weeks of aerobic interval training with consumption of Z. jujuba extract has beneficial effect on LCN2 levels in the heart tissue of MI rats. In addition, interval training alone had a positive but negligible effect on the levels of adiponectin in the heart tissue. In conclusion, some potentiation effects have been observed between aerobic training and JE on LCN2 levels, and probably a longer period of training with consumption of JE could reveal potentiation effect on the levels of adiponectin as well.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Leistner DM, Zeiher AM. Novel avenues for cell therapy in acute myocardial infarction. Circ Res. 2012;110:195–7. doi: 10.1161/CIRCRESAHA.111.260281. [DOI] [PubMed] [Google Scholar]
- 2.Tschakert G, Kroepfl JM, Mueller A, Harpf H, Harpf L, Traninger H, et al. Acute physiological responses to short-and long-stage high-intensity interval exercise in cardiac rehabilitation. A pilot study. J Sports Sci Med. 2016;15:80–91. [PMC free article] [PubMed] [Google Scholar]
- 3.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovas Res. 2011;89:499–506. doi: 10.1093/cvr/cvq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 5.Buonafine M, Martinez E, Jaisser F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin Sci. 2018;132:909–23. doi: 10.1042/CS20171592. [DOI] [PubMed] [Google Scholar]
- 6.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nooriahmadabadi A, Hojjati M, Sedighihafashjani M. The effect of hydroalcoholiccziziphus jujube extract on peripheral blood cells of Balb/c. Phy Pha. 2013;71:224–30. [Google Scholar]
- 8.Kumaran KS, Prince PS. Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chap. 2010;15:791–806. doi: 10.1007/s12192-010-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dashtban M, Sarir H, Omidi A. The effect of Prosopisfarcta beans extract on blood biochemical parameters in streptozotocin-induced diabetic male rats. Adv Biomed Res. 2016;5:116–24. doi: 10.4103/2277-9175.185575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahuja M, Mehla J, Reeta K, Joshi S, Gupta YK. Hydroalcoholic extract of Zizyphusjujuba ameliorates seizures, oxidative stress, and cognitive impairment in experimental models of epilepsy in rats. Epilepsy Behav. 2011;21:356–63. doi: 10.1016/j.yebeh.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Ebrahimi S, Sadeghi H, Pourmahmoudi A, Askariyan SH, Askari S. Protective effect of zizphus vulgaris extract, on liver toxicity in laboratory rats. Armaghan Danesh. 2012;16:172–80. [Google Scholar]
- 12.Xu X, Zhao W, Lao S, Wilson BS, Erikson JM, Zhang JQ. Effects of exercise and L-arginine on ventricular remodeling and oxidative stress. Med Sci Sports Exerc. 2010;42:346–54. doi: 10.1249/MSS.0b013e3181b2e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunes RB, Alves JP, Kessler LP, Dornelles AZ, Stefani GP, Lago PD. Interval and continuous exercise enhances aerobic capacity and hemodynamic function in CHF rats. Braz J Phys Ther. 2015;19:257–63. doi: 10.1590/bjpt-rbf.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YL, Chen CL, Chen CM, Ko WC. Hesperetin-5, 7, 3'-O-triacetate suppresses airway hyperresponsiveness in ovalbumin-sensitized and challenged mice without reversing xylazine/ketamine-induced anesthesia in normal mice. BMC Pharmacol Toxicol. 2017;18:39. doi: 10.1186/s40360-017-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowland JB, Sørensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol. 2003;171:6630–9. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 16.Roca-Rodríguez MD, Garrido-Sánchez L, García-Almeida JM. Effects of exercise on inflammation in cardiac rehabilitation. Nutr Hosp. 2015;31:2633–40. doi: 10.3305/nh.2015.31.6.8868. [DOI] [PubMed] [Google Scholar]
- 17.Ranjbar K, Zarrinkalam E, Salehi I, Komaki A, Fayazi B. Cardioprotective effect of resistance training and Crataegusoxyacantha extract on ischemia reperfusion–induced oxidative stress in diabetic rats. Biomed Pharmacother. 2018;100:455–60. doi: 10.1016/j.biopha.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Caselli C, D'amico A, Cabiati M, Prescimone T, Del Ry S, Giannessi D. Back to the heart: The protective role of adiponectin. Pharmacol Res. 2014;82:9–20. doi: 10.1016/j.phrs.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang HK, Wang YH, Sun L, He X, Zhao M, Feng ZH, et al. Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction: Roles of mitochondrial network dynamics. Int J Mol Sci. 2014;15:5304–22. doi: 10.3390/ijms15045304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. 2008;158:203–7. doi: 10.1530/EJE-07-0633. [DOI] [PubMed] [Google Scholar]
- 21.Mohebbati R, Bavarsad K, Rahimi M, Rakhshandeh H, Rad AK, Shafei MN. Protective effects of long-term administration of Ziziphusjujuba fruit extract on cardiovascular responses in L-NAME hypertensive rats. Avicenna J Phytomed. 2018;8:143–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Buturak A, Degirmencioglu A, Bayrak F, Kırış T, Karakurt H, Demir AR, et al. Elective percutaneous coronary intervention leads to significant changes in serum resistin, leptin, and adiponectin levels regardless of periprocedural myocardial injury: An observational study. Anatol J Cardiol. 2016;16:940–6. doi: 10.14744/AnatolJCardiol.2016.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damirchi A, Rahmani-Nia F, Mehrabani J. Lipocalin-2: Response to a short-term treadmill protocol in obese and normal-weight men. J Human Sports and Exer (JHSE) 2011;6:59–67. doi: 10.5812/asjsm.34821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KM, Kim TN, Yoo HJ, Lee KW, Cho GJ, Hwang TG, et al. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin Endocrinol. 2009;70:569–74. doi: 10.1111/j.1365-2265.2008.03374.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XJ, Chen LH, Li JH. The effects of aerobic exercise on plasma adiponectin level and adiponectin-related protein expression in myocardial tissue of ApoE-/-mice. J Sports Sci Med. 2015;14:877–82. [PMC free article] [PubMed] [Google Scholar]
- 26.Santilli F, Guagnano MT, Azania N, La Barba S, Davi G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: From biomarkers to therapeutic approach. Curr Med Chem. 2015;22:582–95. doi: 10.2174/0929867322666141128163739. [DOI] [PubMed] [Google Scholar]
- 27.Gao QH, Wu CS, Wang M. The jujube (Ziziphusjujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J Agri Food Chem. 2013;61:3351–63. doi: 10.1021/jf4007032. [DOI] [PubMed] [Google Scholar]


