Abstract
Background
The aim of this study was to assess the effects of a new treatment strategy for envenomation that consists of multiple small incisions and negative-pressure wound therapy (NPWT) on injured limb swelling and systemic inflammatory reaction.
Material/Methods
This was a prospective randomized controlled trial on snakebite envenomation. The enrolled patients were randomly divided into 2 groups: an observation group and a control group. The traditional comprehensive treatment was administered in both groups, but the observation group also received combined treatment with multiple small incisions and NPWT. Reduction in limb swelling, mean admission duration, complication rate, and changes in the levels of relevant cytokines were recorded and compared between the 2 groups.
Results
The mean duration of hospital stay was significantly lower in the observation group than in the control group (5.44±0.89 days vs. 7.71±1.70 days). The complication rate and IL-6 concentration were significantly lower in the observation group than in the control group.
Conclusions
Multiple small incisions combined with NPWT proved effective for controlling the release of inflammatory cytokines and accelerating the relief of systemic inflammatory reaction. As a consequence, the complication rate decreased. Therefore, our new treatment strategy is safe and effective.
MeSH Keywords: Bite Envenomation, Negative-Pressure Wound Therapy, Systemic Inflammatory Response Syndrome
Background
Snakebite envenomation is a common cause of lethal wounds and has become an important global public health problem [1]. Approximately 4.2 to 18.4 million cases of snakebite envenomation occur each year, resulting in 2 to 9.2 million deaths and 4 million amputation cases. The highest burden from snakebite envenomation and death was recorded in South and Southeast Asia and in sub-Saharan Africa [2]. In 2009, snakebite envenomation was ranked as a neglected public health problem by the World Health Organization, as it has not received adequate attention [3]. Patients bitten by venomous snakes are more prone to liver, heart, and kidney injuries; systemic inflammatory response syndrome; and multiple-organ failure [4]. Approximately 98.1% of patients develop swelling of the limb and injured tissue, and body fluid exudation, which can cause osteofascial compartment syndrome if the wound is not properly treated.
Administration of antivenoms is the most effective method currently available for the treatment of snakebite envenomation [5]; it can combine and neutralize the free venom involved in the pathophysiological reaction that forms the antigen-antibody complex, thus resulting in toxin loss [6]. Although antivenoms can neutralize the venom and relieve the whole body from the toxic reaction, they cannot reduce the tissue swelling and exudation, necrosis, and other pathological reactions. Winger and Chan [7] showed that patients who underwent incision along bite marks were not relieved, which could easily result in tendon, nerve, and blood vessel injuries, and increased incision inflammation rate. In China, the main therapeutic regimen for snakebite envenomation is comprehensive symptomatic treatment, but this technique has some defects.
Fleischmann, a trauma surgeon specialist at Ulm University, was the first to use negative-pressure wound therapy (NPWT) [8]. This strategy is capable of effectively draining local exudates and cell components that accumulate as a result of local inflammatory reaction and necrosis. Consequently, NPWT can eliminate the inflammatory response triggered by these substances and accelerate reduction in local inflammation [9]. Using vacuum suction, multiple small incisions were made proximal to the limb envenomation site, and NPWT was applied. The present study assessed the effects of this new treatment method on injured limb swelling and systemic inflammatory reaction.
Our study was approved by the China Ethics Committee of Clinical Trial Registration, with Ethic Committee Approved No. ChiECRCT-20130308 (http://www.chictr.org.cn/showproj.aspx?proj=5379).
Material and Methods
Inclusion and exclusion criteria of subjects
Fifty patients with envenomation who were admitted to the Emergency Department of Southwest Hospital between April 2015 and November 2016 were enrolled. The inclusion criteria were: (1) confirmed diagnosis of envenomation from Protobothrops mucrosquamatus bite in the first 48 h or patient admission to the Emergency Department within 48 h, with progressive worsening of extremity swelling; (2) patients with class 2 or 3 injury according to the Downey grading system [10]; and (3) patients who signed the clinical trial and surgery consent. The exclusion criteria were: (1) not meeting the inclusion criteria; (2) patients with mental disease who were unable to coordinate; (3) patients with a confirmed diagnosis of envenomation from a Deinagkistrodon acutus (long-nosed pit viper) snakebite; and (4) patients who were breastfeeding or pregnant. The included patients were then randomly divided into 2 groups: an observation group (n=25) and a control group (n=25). This study was conducted in accordance with the Declaration of Helsinki and was conducted with approval from the Ethics Committee of the China Ethics Committee, Ethic Committee Approved No. ChiECRCT-20130308. Written informed consent was obtained from all participants.
Intervention method
The control group was provided traditional comprehensive treatments, including wound debridement, antivenom injection, tetanus antitoxin injection, methylprednisolone anaphylaxis treatment, dehydration and dieresis treatment, oral or topical Jidesheng Sheyao tablets (traditional Chinese medicine), ceftizoxime sodium, and fluid replacement therapy.
The observation group underwent the same traditional treatments as the control group, but with small incisions combined with NPWT. The procedure was as follows: After preoperative preparation and local anesthesia infiltration, the patients were placed in a horizontal position and incisions were made into the limb skin 15–25 cm proximal to the injured area with high tension, avoiding large vessels and nerves. Normally, 6 small incisions were made in the lower extremities, divided into 2 lines parallel to the vertical axis of the body, with 3 incisions in each line. The incisions were 1.5 cm in length, with a 1-cm interval between each incision and 2-cm in-line spacing (Figure 1), and were made deep into subcutaneous tissue. Four incisions were made in the upper limbs, divided into 2 lines parallel to the vertical axis of the body, with 2 incisions in each line and 2-cm line spacing. After the incisions were made, the NPWT device was installed with a negative pressure of 125 mmHg. The NPWT duration was normally 2 to 3 days, depending on the skin tension, degree of swelling, and ambulatory state of the injured and contralateral limbs.
Figure 1.
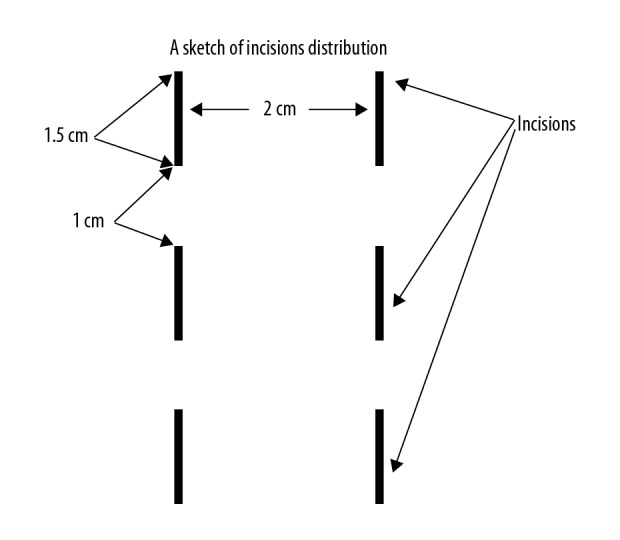
Six incisions were divided into 2 lines, 3 incisions in each line, 1.5 cm in length, 1 cm interval between each incision, 2 cm in-line spacing.
Observation index
1. General Data
Upon admission to the Emergency Department, the patient’s sex, age, length of time from injury to admission, injured area, injury severity, occupation, and regional distributions, and other indexes were recorded as general observation indexes.
2. Circumference Difference
Before treatment (0 h) and at 12, 24, 48, and 72 h after treatment, the circumference values of the injured limb were measured in 3 planes: at 10 and 20 cm above the rascela and at 10 cm above the olecranon. These planes were defined as the distal, middle, and proximal limbs, respectively, and circumference values of the contralateral limb were also measured in the same planes.
The circumference difference between the injured and contralateral healthy limbs was calculated after each measurement. The reduction in local swelling was objectively reflected through a comparison of the circumference difference.
3. Complications
The incidence rates of local and general complications were calculated at the time of admission. Complications included osteofascial compartment syndrome, amputation, skin necrosis, acute kidney failure, rhabdomyolysis, and inflammation, among others.
4. Length of hospital stay
The total length of hospital stay, including the minimum and maximum times, was recorded.
5. Inflammatory markers
The serum concentrations of IL-6, TNF-α, IL-10, and endocan were detected and recorded at each time point (0, 12, 24, 48, and 72 h after treatment).
Sample collection and detection
Peripheral venous blood samples were collected prior to treatment (0 h) and at 12, 24, 48, and 72 h after treatment. The blood samples were centrifuged, and the supernatant was stored at −70°C until analysis. The samples were analyzed using the enzyme-linked immunosorbent assay (ELISA) detection method with ab46042, ab10065, and ab46034 reagents (Abcam) and the ESM-1 kit (America Avisceral Bioscience Company).
Statistical analyses
All statistical analyses were performed using PASW Statistics Version 18. Categorical variables were tested using the χ2 test, while continuous data were expressed as mean ± standard deviation (χ̄±s) and tested using an independent-samples t test and repeated-measures analysis of variance (ANOVA) for 2 groups. A linear correlation analysis was also performed. A p value of <0.05 was considered statistically significant.
Results
General data
Most patients were admitted within the first 24 h after injury, with the earliest admission being 2 h after injury and the latest admission being 48 h after injury. Eleven patients were admitted 24 h after injury. No statistically significant differences in age, sex ratio, injured area, admission time after injury, and grade of injury were found between the groups (Table 1).
Table 1.
Comparison of two groups’ age, gender ratio, injured area, admission time after injury and Downey grading.
| Group | Cases | Age (y.o) | Gender ratio (Male/Female) | Injured area Upper extremity/lower extremity | Admission time after injury(h) | Downey grading (2/3) |
|---|---|---|---|---|---|---|
| Observation | 25 | 49.76±15.18 | 17/8 | 5/20 | 17.32±10.22 | 12/13 |
| Control | 25 | 51.62±15.93 | 16/9 | 8/17 | 16.79±13.61 | 14/11 |
| χ2 or t | t=0.403 | χ2=0.089 | χ2=0.936 | t=−0.153 | χ2=0.321 | |
| P | 0.689 | 0.765 | 0.333 | 0.879 | 0.571 |
Correlation between the degree of limb swelling and admission time
The results showed no significant correlation between the degree of distal injured limb swelling and admission time after injury (r=0.103, p=0.483). However, the degree of swelling in the middle and proximal limbs demonstrated a significant positive relationship with the time of admission after injury (r=0.313, p=0.028 and r=0.331, p=0.020, respectively). Therefore, the later the time of admission after injury, the more severe the degree of swelling in the middle or proximal limb.
Comparison of the degree of injured limb swelling
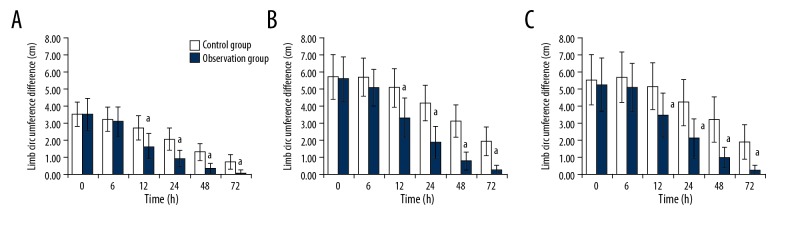
After treatment, repeated-measures ANOVA according to time showed a significant difference in distal limb circumferences between the 2 groups (p<0.001; Figure 2A). Moreover, a significant difference in middle limb circumference over time was observed between the observation and control groups (p<0.001; Figure 2B). Lastly, the circumference differences in the proximal limb over time were significantly different between the 2 groups (p<0.001; Figure 2C).
Figure 2.
Variation tendency of the circumference difference between the injured limb and the healthy limb. (A) Changes of distal limb circumference difference. (B) Changes of middle limb circumference difference. (C) Changes of proximal limb circumference difference. a P<0.05, compared with the control group.
Comparison of admission complications
During admission, the complication rates in the 2 groups were observed and recorded. Four patients in the control group had complications, including 2 cases of skin necrosis, 1 case of rhabdomyolysis, and 1 case of acute kidney failure, with an incidence rate of 19%. No complications were observed in the observation group. Consequently, a significant difference in complication rates was found between the 2 groups (χ2=4.348, p=0.037).
Comparison of admission duration
The mean length of hospital stay was significantly lower in the observation group than in the control group (t=5.70, p<0.001; Table 2).
Table 2.
Admission days (χ̄±s, d).
| Group | Case | The shortest admission days (d) | The longest admission days (d) | The mean admission days (d) |
|---|---|---|---|---|
| Observation | 25 | 4 | 7 | 5.44±0.89 |
| Control | 25 | 5 | 12 | 7.71±1.70 |
| t=5.70 | ||||
| P<0.001 |
Serum levels of cytokines
Before treatment, the serum IL-6 and TNF-α levels were increased in both groups. The baseline concentrations of IL-6 and TNF-α showed no significant differences between the groups (t=0.036, p=0.972 and t=−0.086, p=0.932, respectively). However, after treatment, the IL-6 concentration decreased in the 2 groups. The repeated-measures ANOVA according to time showed a significant difference in IL-6 concentration between the observation and control groups (p=0.032; Table 3).
Table 3.
Comparison of IL-6, TNF-α, IL-10, Endocan concentration changes in each time point between the two groups (pg/mL, χ̄±s).
| Group | 0 h | 6 h | 12 h | 24 h | 48 h | 72 h | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | Observation | 21.61±12.42 | 14.82±7.69 | 7.16±2.79 | 4.22±1.48 | 2.36±1.14 | 0.83±0.53 | <0.001 | <0.001 | 0.032 |
| Control | 21.76±15.97 | 16.99±10.34 | 12.46±6.27 | 9.10±4.26 | 5.93±3.02 | 3.42±2.00 | ||||
| TNF-α | Observation | 477.11±300.9 | 289.20±168.9 | 168.91±96.9 | 106.44±62.0 | 50.16±32.6 | 26.38±20.7 | <0.001 | <0.001 | 0.147 |
| Control | 466.58±309.9 | 389.14±288.8 | 289.45±219.5 | 214.85±154.5 | 118.62±62.7 | 54.55±27.6 | ||||
| IL-10 | Observation | 202.44±103.8 | 124.75±74.5 | 80.58±58.0 | 38.40±25.4 | 23.99±15.8 | 13.48±7.6 | <0.001 | <0.001 | 0.158 |
| Control | 238.68±165.9 | 161.11±108.8 | 112.27±80.0 | 61.61±45.1 | 34.85±30.1 | 17.81±12.8 | ||||
| Endocan* | Observation | 2.63±1.79 | 3.35±1.76 | 4.06±1.43 | 4.21±1.24 | 3.89±1.20 | 3.30±1.15 | <0.001 | <0.001 | 0.468 |
| Control | 2.72±1.07 | 3.63±1.42 | 4.02±1.19 | 3.86±0.96 | 3.33±0.99 | 2.41±0.92 |
P1 – P value for Mauchly’s Test of sphericity; P2 – P value for tests of Within-Subjects Effects; P3 – P value for tests of Between-Subjects Effects.
The unit of measurement of Endocan is ng/mL.
After treatment, the TNF-α concentration in the 2 groups began to decrease, but the repeated-measures ANOVA of the 2 groups indicated no significant difference in TNF-α concentration between the observation and control groups (p=0.147; Table 3).
The IL-10 concentration before treatment in both groups showed a relatively high level of up to 598.30 pg/ml. The independent t test of the IL-10 concentration between the groups did not show a significant difference (t=0.912, p=0.367). After treatment, IL-10 levels decreased in both groups. The repeated-measures ANOVA of the 2 groups showed a significant difference in IL-10 concentration (p=0.158; Table 3).
Comparison of endocan levels between the groups
Before treatment, the mean serum endocan levels of the 2 groups were relatively high, with an increasing trend. Serum endocan concentration began to decrease in the observation and control groups at 12 and 24 h after treatment, respectively. The serum endocan levels showed no significant difference between the observation and control groups (t=−0.209, p=0.835). Moreover, repeated-measures ANOVA did not reveal a significant difference between the 2 groups after treatment (p=0.468).
Correlation analysis of cytokine levels and admission time
The linear correlation analysis revealed no statistical significance in the correlation between serum IL-6 concentration and admission time after injury (r=−0.072, p=0.620). By contrast, a negative correlation was found between serum TNF-α and IL-10 levels and admission time after injury (r=−0.419, p=0.003 and r=−0.828, p=0.002, respectively). These results mean that earlier admission time was associated with higher TNF-α and IL-10 concentrations in serum.
Lastly, a statistically significant difference was found between the serum endocan levels and admission time after injury (r=0.649, p=0.000). Therefore, a positive correlation was found, which means that the endocan levels were expected to be higher during later admission after injury.
Discussion
Limb swelling and treatment effect
In this study, we observed that all the patients presented limb swelling on admission. Moreover, the highest swelling plane in the lower and upper limbs was at the inguinal and shoulder joint levels, respectively. These results were consistent with those reported by Lavonas et al. [11]. Correlation analysis between the degree of limb swelling and admission time after injury revealed that the degree of middle and proximal limb swelling had an obvious correlation with the admission time after injury. Therefore, when the time between injury and hospitalization was longer, more proximal swelling was observed, and, consequently, the degree of swelling was also higher. Snake venom contains many kinds of enzymes. The proteolytic enzyme can injure blood vessel endometrial cells, increase vascular wall permeability, and result in plasma exosmosis. Through its action, hyaluronidase can dissolve cells and intercellular substances and destroy connective tissue integrity. Moreover, the venom can stimulate the human body to release plasmakinin, histamine, and 5-hyforxytryptamine, which can ultimately cause limb swelling. Some studies found that tissue swelling showed a temporal correlation dependent mostly on the amount of injected venom. Low-dose venom caused tissue swelling that peaked within 1 h and then declined, but in the case of high-dose venom, the peak tissue swelling persisted for a relatively longer time [12]. Our study found that limb swelling regression was not significant within 6 h of treatment, especially in the proximal limb. In addition, an increasing trend in limb swelling was observed in the early stages, which indicated that the previous toxic effect persisted after the venom absorption. The rationale was that the local injury might still have residual venom and might not yet reach the swelling peak at the time of admission; thus, the swelling would continue developing proximally. Although both groups of patients received an antivenom treatment after admission, the regression in limb swelling was faster in the observation group (12 h after treatment) than in the control group, indicating that the limb swelling regression was obviously accelerated after using the combined treatment with multiple small incisions and NPWT. Hence, we consider it necessary to use NPWT after antivenom treatment.
Advantages of the combination of multiple small incisions and NPWT
NPWT promotes the biology of wound healing, which includes angiogenesis, neurogenesis, granulation tissue formation, cellular proliferation, differentiation, and migration [13]. The small incision should be approximately 1.5 to 2 cm deep into the subcutaneous tissue. The multiple small incisions made in the proximal skin will firstly reduce skin tension, relieve the patient’s agony caused by high-degree swelling, lessen swelling compression on local nerves and vessels, improve blood circulation in the injured limb, prevent consistent swelling that may cause further local damage, and provide a quick channel for edema fluid drainage and venom expulsion. Combined treatment with multiple small incisions and NPWT consists of applying NPWT on small incisions. This will not only accelerate the expulsion of edema fluid but also block the physical absorption of snake venom, reducing the systemic reaction after venom absorption, and play an important role in relieving swelling and venom drainage. At the same time, NPWT can quickly expel local tissue exudates, necrotic substances, and cell components, keep the wound microenvironment stable, reduce the consistent influence of the inflammatory medium, and accelerate local tissue healing. We found that the limb swelling in the observation group regressed almost completely by 72 h after treatment, which shows the benefit of this treatment over the traditional treatment methods used in the control group.
IL-6 and TNF-α levels
Venom- and toxin-induced limb swelling are the pathophysiological processes where multiple factors act, causing direct damage to microvessels such as capillaries and venules. Toxins such as hemorrhagic toxins can cause the release of many inflammatory mediators, increasing the permeability of microvessels [14]. Most studies have proved that snakebite envenomation can cause massive systemic cytokine responses, that metalloproteinase can cause local generation of IL-6 and IL-1β [15], and that TNF-α is involved in the pathophysiological process of skin necrosis and systemic hemodynamic changes [16]. IL-6 and TNF-α can be used as biomarkers for evaluating the intensity of systemic inflammatory reaction and the severity of clinical symptoms. In our study, the IL-6 level had no significant correlation with admission time after injury and Downey grading, while TNF-α level had a negative correlation with hospital admission after injury. These data are consistent with those from other studies. TNF-α can reach a relatively high level; in the first 2–5 h, its peak can reach 1465 pg/ml [17]. Some researchers reported that venom metalloproteinases cleave the glutathione S-transferase tumor necrosis factor-alpha fusion protein (GST-TNF-α) to generate a biologically active TNF-α, while inhibition or neutralization of endogenous TNF-α appears to be a result of a significant reduction of venom-induced necrosis. All these observations indicate that increased TNF-α level after snakebite envenomation might play an important role in local necrosis [18]. Our study also showed that the reduced speed of TNF-α in the observation group was faster than that in the control group. The result was not statistically significant, which might be due to the limited sample size.
IL-10 level
In our study, the IL-10 levels in both groups before treatment were relatively high, which can be explained by the inflammatory and anti-inflammatory reactions provoked by snakebites. These data indicate that snakebites can cause an immunizing inflammatory reaction at an early stage. After treatment, serum IL-10 levels were reduced, with no significant difference between the groups, which led to the conclusion that body inflammation was controlled after administration of antivenom serum and other treatments. We found that earlier hospital admission time was associated with higher IL-10 serum level, which means that the IL-10 level can rapidly reach its peak in an early stage of envenomation. No statistically significant difference in IL-10 concentrations was found between the groups, which might be caused by a time-dependent change in the IL-10 concentration or the control of inflammatory reaction by traditional treatment. The comparison cannot be based on between-group differences.
Endocan level
Endocan, also called endothelial cell-specific molecule 1 (ESM-1), is a soluble proteoglycan of 50 kDa, secreted by the endometrium [19]. Endocan is a key player in the regulation of the proliferation, differentiation, migration, and adhesion of different cell types owing to its ability to bind a wide range of bioactive molecules associated with cellular signaling and adhesion. The mechanism by which endocan expression is regulated by a series of cytokines is relatively complex. Endocan expression is upregulated by VEGF-A, VEGF-C, IL-1, TNF-α, TGF-β1, and FGF-2, whereas phosphatidylinositide 3-kinases (PI3K) and interferon-γ cause its downregulation. TNF-α can promote time- and dose-dependent endometrial expressions of endocan, while IFN-γ inhibits the upregulation of endocan [20]. The concentration of the secretory-type endocan in a healthy body has been reported to be 1.081 ng/ml, which rises rapidly to 7.815 ng/ml in patients with septic shock. Endocan is thought to be upregulated in acute and severe inflammatory reactions [21].
In our study, serum endocan level continued to increase up to 2 days after treatment. This might be related to the continuous expression of some cytokines that induce endocan mRNA expression. Under a certain amount of TNF-α stimulation, endocan levels are expected to rapidly rise and persist in the system for a certain period of time. In our study, the mean TNF-α serum concentration was 0.4 ng/ml, which can stimulate endocan secretion in an earlier stage and increase over time. The fact that the downregulation of endocan was observed earlier in the observation group than in the control group suggests that the combination of multiple small incisions and NPWT is better at relieving systemic inflammation.
Other complications and control method
The observation group received a combination of multiple small incisions and NPWT. The complication rate in the control group was significantly higher than that in the observation group. Complications consisted mainly of skin necrosis and some relatively serious cases of existing rhabdomyolysis and acute kidney failure. From this point of view, multiple small incisions and NPWT had a significantly lower complication rate than the traditional therapy, and its safety and effectiveness were proved.
Conclusions
The patients with snakebite envenomation in this study presented different degrees of limb swelling and systemic inflammatory reactions. The degree of local injury worsens with prolonged delay of hospital admission. Multiple small incisions combined with NPWT can significantly accelerate limb-swelling resolution and decrease the length of hospital stay, which brings significant short-term effects. IL-6, TNF-α, IL-10, and endocan levels in patients with envenomation can reflect the severity of systemic inflammatory reaction before treatment. Combined treatment with multiple small incisions and NPWT can effectively control the release of inflammatory cytokines and accelerate the relief of systemic inflammatory reaction, thereby decreasing the incidence rates of other complications. Therefore, our new treatment strategy is safe and effective.
Footnotes
Source of support: This research was funded by the Chongqing Science and Technology Benefiting Project (CSTC2013jcsfC1001-4) and Scientific Research Innovation Fund of AMU (SWH2013LC11)
Conflicts of interest
None.
References
- 1.Gutiérrez JM, Williams D, Fan HW, Warrell DA. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon. 2010;56:1223–35. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams D, Gutiérrez JM, Harrison R, et al. The Global Snake Bite Initiative: An antidote for snake bite. Lancet. 2010;375:89–91. doi: 10.1016/S0140-6736(09)61159-4. [DOI] [PubMed] [Google Scholar]
- 4.Gay CC, Maruñak SL, Teibler P, et al. Systemic alterations induced by a Bothrops alternatus hemorrhagic metalloproteinase (baltergin) in mice. Toxicon. 2009;53:53–59. doi: 10.1016/j.toxicon.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 5.WHO/SEARO Guidelines for the clinical management of snake bites in the Southeast Asian region. Southeast Asian J Trop Med Public Health. 1999;30(Suppl 1):1–85. [PubMed] [Google Scholar]
- 6.Gutierrez JM, Leon G, Burnouf T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals. 2011;39:129–42. doi: 10.1016/j.biologicals.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;148:37–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmanian-Schwarz A, Willkomm LM, Gonser P, et al. A novel option in negative pressure wound therapy (NPWT) for chronic and acute wound care. Burns. 2012;38:573–77. doi: 10.1016/j.burns.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Daeschlein G, Napp M, Lutze S, et al. Comparison of the effect of negative pressure wound therapy with and without installation of polyhexanide on the bacterial kinetic in chronic wounds. Wound Medicine. 2016;13:5–11. [Google Scholar]
- 10.Downey DJ, Omer GE, Moneim MS. New Mexico rattlesnake bites: Demographic review and guidelines for treatment. J Trauma. 1991;31:1380–86. [PubMed] [Google Scholar]
- 11.Lavonas EJ, Kokko J, Schaeffer TH, et al. Short-term outcomes after Fab antivenom therapy for severe crotaline snakebite. Ann Emerg Med. 2011;57:128–37.e3. doi: 10.1016/j.annemergmed.2010.06.550. [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez JM, Rucavado A, Chaves F, et al. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon. 2009;54:958–75. doi: 10.1016/j.toxicon.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301–31. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Moreira V, Teixeira C, Borges da Silva H, et al. The role of TLR2 in the acute inflammatory response induced by Bothrops atrox snake venom. Toxicon. 2016;118:121–28. doi: 10.1016/j.toxicon.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Otero R, Gutiérrez J, Beatriz Mesa M, et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon. 2002;40:1107–14. doi: 10.1016/s0041-0101(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira CF, Zamunér SR, Zuliani JP, et al. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve. 2003;28:449–59. doi: 10.1002/mus.10453. [DOI] [PubMed] [Google Scholar]
- 17.Açıkalın A, Gökel Y. Serum IL-6, TNFα levels in snakebite cases occurring in Southern Turkey. Emerg Med J. 2011;28:208–11. doi: 10.1136/emj.2009.078428. [DOI] [PubMed] [Google Scholar]
- 18.Moura-da-Silva AM, Laing GD, Paine MJ, et al. Processing of pro-tumor necrosis factor-α by venom metalloproteinases: A hypothesis explaining local tissue damage following snake bite. Eur J Immunol. 1996;26:2000–5. doi: 10.1002/eji.1830260905. [DOI] [PubMed] [Google Scholar]
- 19.Mihajlovic DM, Lendak DF, Brkic SV, et al. Endocan is useful biomarker of survival and severity in sepsis. Microvasc Res. 2014;93:92–97. doi: 10.1016/j.mvr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Voiosu T, Bălănescu P, Benguş A, et al. Serum endocan levels are increased in patients with inflammatory bowel disease. Clin Lab. 2014;60:505–10. doi: 10.7754/clin.lab.2013.130333. [DOI] [PubMed] [Google Scholar]
- 21.Bechard D, Meignin V, Scherpereel A, et al. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37:417–25. doi: 10.1159/000025758. [DOI] [PubMed] [Google Scholar]