Abstract
Background
Chemokines are important in inflammation, immunity, tumor progression, and metastasis. The purpose of this research was to find an integrated-RNA signature of chemokine family genes to predict the survival prognosis in head and neck squamous carcinoma (HNSC) patients.
Material/Methods
Relevant data of 504 HNSC patients were extracted from The Cancer Genome Atlas (TCGA) database. Through analyzing RNA sequencing data, the univariate Cox model was used to identify chemokine family genes associated with survival and then to develop a multiple-RNA signature in the training set. The prediction value of this multiple-RNA signature was further verified in the validation and entire sets. The receiver operating characteristic curves were used to assess the predictive value of this multiple-RNA signature.
Results
Eleven chemokines were included in this prognostic signature. Based on this 11-chemokine signature, we further categorized patients as high or low risk. Compared with low-risk patients, high-risk patients had shorter overall survival (OS) time in the training set [hazard ratio (HR)=3.497, 95% confidence interval (CI)=2.142–5.711, p<0.001], validation set (HR=3.575, 95% CI=1.988–6.390, p<0.001), and entire set (HR=3.416, 95% CI=2.363–4.939, p<0.001). This 11-chemokine signature was an independent prognostic factor for OS in these datasets (p<0.05). The AUC values for predicting overall survival within 48 months in the training, validation, and entire sets were 0.71, 0.69, and 0.69, respectively.
Conclusions
This 11-chemokine signature could serve as a reliable prognostic tool for HNSC patients and might be useful to guide individualized treatment or even gene target therapy for high-risk patients.
MeSH Keywords: Chemokines, CC; Head and Neck Neoplasms; Survival Analysis; Transcriptome
Background
Head and neck squamous cell carcinoma (HNSC) is the sixth most common and frequently lethal cancer worldwide, with about 350 000 cancer-related deaths per year [1]. The current staging system has limitation in identifying high-risk HNSC because large variability in clinical outcomes was found in same-stage patients [2–6]. To identify high-risk HNSC patients, new prognostic biomarkers are urgently needed for guiding the personalized treatment.
Chemokines are a particular group of cytokines that were originally described as being chemotactic to leukocytes [7]. They can bind to 7-transmembrane domain G-protein-coupled receptors that are predominantly expressed by leukocytes [8]. Chemokines are classified into 4 different subgroups (CXC, CC, CX3C, or C) depending on the position of the conserved cysteine residue [9]. Chemokines are closely associated with inflammation, immunity, tumor development, and prognosis [10–12]. Chemokines play vital roles in all phases of oncogenesis, tumor growth, angiogenesis, malignant transformation, and metastatic dissemination in HNSC patients [9–13]. Many studies have shown that these single biomarkers cannot be widely used for the prediction of tumor prognosis because of their controversial conclusions and the heterogeneity between tumors [14–17]. Risk stratification may require combined multiple-molecular biomarkers. The gene expression profiles were produced simultaneously by high-throughput sequencing during the past 2 decades. Therefore, we can use a bioinformatic discovery approach to identify a multiple-RNA classifier that can improve the prediction of overall survival in HNSC patients.
Material and Methods
Data collection
The clinical data for age, gender, primary sites, and clinical stage were downloaded from the TCGA database using the cBioPortal platform (2018.12.01). The inclusion criteria were: (i) histological diagnosis of HNSC; and (ii) adequate clinical characteristics (gender, age, primary sites, clinical stage, overall survival status, and time). Altogether, 504 HNSC patients were included and randomly divided into the training set (n=252) and validation set (n=252, detail shown in Table 1). The numbers of stage I, II, III, and IV patients were 20, 97, 104, and 283, respectively. In addition, 10 HNSC patients had received neoadjuvant chemoradiotherapy; the other 494 patients had not. There were 334 HNSC patients <65 years, and the other 170 patients were >65 years. A total of 371 patients were male, and the other 133 patients were female. The RNA expression data of level 3 were downloaded from the cBioPortal platform and normalized. Chemokines were selected for which the expression data of >80% of HNSC patients were more than 0.
Table 1.
The characteristics of 11 chemokines associated with overall survival in the training set of 252 HNSC patients (n=252, TCGA).
| Gene symbol | Type | HR (95% CI) | Coefficients | p Value | Putative function |
|---|---|---|---|---|---|
| CCL2 | Ligand | 1.173 | 0.16 | 0.026 | Risky |
| CCL7 | Ligand | 1.154 | 0.143 | 0.016 | Risky |
| CCL22 | Ligand | 0.857 | −0.154 | 0.03 | Protective |
| XCL2 | Ligand | 0.856 | −0.155 | 0.037 | Protective |
| CXCL5 | Ligand | 1.113 | 0.107 | 0.01 | Risky |
| CXCL8 | Ligand | 1.14 | 0.131 | 0.011 | Risky |
| CCR4 | Receptor | 0.888 | −0.119 | 0.025 | Protective |
| CCR6 | Receptor | 0.772 | −0.258 | 0.003 | Protective |
| CCR7 | Receptor | 0.846 | −0.167 | 0.005 | Protective |
| XCR1 | Receptor | 0.831 | −0.185 | 0.005 | Protective |
| CX3CR1 | Receptor | 0.859 | −0.152 | 0.025 | Protective |
CI – confidence index; HR – hazard ratio.
Statistical analysis
In the training set (n=252), we estimated the expression of the selected chemokines with overall survival (OS) by using the univariate Cox model. The candidate chemokines with a p-value of less than 0.05 were used to construct a predictive model by a multivariate Cox model. We calculated the prognostic risk score by the selected chemokines and their regression coefficients in the multivariate Cox model [18–20], as follow: Risk Score=expCCL2 * βCCL2+expCCL7 * βCCL7+expCCL22 * βCCL22+expXCL2 * βXCL2+expCXCL5 * βCXCL5+expCXCL8 * βCXCL8+expCCR4 * βCCR4+expCCR6 * βCCR6+expCCR7 * βCCR7+expXCR1 * βXCR1+expCX3CR1 * βCX3CR1 (exp=expression level; β=the regression coefficient derived from the multivariate Cox model). To plot the Kaplan-Meier curves in these sets, we classified the patients into low or high risk by the same cutoff point, based on the Youden index in the training set [21]. Survival differences between different risk groups were assessed and compared by the Kaplan-Meier estimate and log-rank test in the training, validation, and entire set. The time-dependent receiver operating characteristic (ROC) curve analysis for this 11-chemokine signature was performed for the prediction ability of OS within 48 months by using the “survival ROC” package. If the p values were less than 0.05, the log-rank test, Cox regression analysis, and ROC curve analysis were considered to be significant.
Gene functional analysis
To better understand the underlying function of these selected chemokines, the gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were analyzed using the Database for Annotation Visualization and Integrated Discovery (DAVID) [22].
Results
Identification and selection of potential chemokines in the training set
The research flow for the development of this prognostic signature is shown in Figure 1. Gene expression data of 46 chemokines were extracted from TCGA sequencing data, and we further fitted these 46 chemokines in the univariate Cox model for the training set (n=252, shown in Supplementary Table 1). Therefore, we identified 11 chemokines whose expressions were significantly correlated with OS (p<0.05, shown in Table 1). Among the 11 chemokines, the coefficients in univariate Cox regression of CCL2, CCL7, CXCL5, and CXCL7 in univariate Cox model were positive, indicating that their higher levels of gene expression were associated with worse prognosis. In contrast, the coefficients of CCL22, XCL2, CCR4, CCR6, CCR7, XCR1, and CX3CR1 were negative, indicating that their higher levels of gene expression were associated with better prognosis.
Figure 1.

Study flow for the analysis of these survival-related chemokine genes.
Comparisons of survival between low-risk and high-risk groups
The entire study cohort of 504 patients was randomly grouped into training (n=252) and validation (n=252) sets. Based on these chemokines and their regression coefficients in the multivariate Cox model, we calculated the risk scores for each patient in the training (n=252), validation (n=252), and entire (n=504) sets (Figure 2). Using the cutoff value of risk scores (0.83074), HNSC patients were divided into a low-risk group and a high-risk group for training (low-risk/high-risk: 94/158) and validation (low-risk/high-risk: 58/194) sets. After integrating analysis of these 2 sets, there were 152 low-risk patients and 352 high-risk patients in the entire set (n=504). As shown in Figure 2, high-risk HNSC patients tended to have higher risk of death in the training, validation, and entire sets.
Figure 2.
The distribution of risk score and overall survival status in the 3 datasets.
Table 2 lists the comprehensive clinical features of HNSC patients in the low-risk and high-risk groups. As shown in Figure 3A and Table 3, further validation of this 11-chemokine signature using Kaplan-Meier and log-rank analysis significantly predicted OS in the training [hazard ratio (HR)=3.497, 95% confidence interval (CI)=2.142–5.711, p<0.001], validation (HR=3.575, 95% CI=1.988–6.390, p<0.001), and entire (HR=3.324, 95% CI=2.363–4.939, p<0.001) sets. These results indicated that high-risk HNSC patients had significantly shorter OS than low-risk patients.
Table 2.
Clinical characteristics of HNSC patients according to this 11-chemokine classifier in the training (n=252, TCGA), validation (n=252, TCGA), and entire (n=504, TCGA) sets.
| Characteristics | Training set (n=252) | Validation set (n=252) | Entire set (n=504) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| High-risk (n=158) | Low-risk (n=94) | p Value | High-risk (n=194) | Low-risk (n=58) | p Value | High-risk (n=352) | Low-risk (n=152) | p Value | |
| Age | |||||||||
| <65y | 96 | 65 | 0.180 | 132 | 41 | 0.703 | 228 | 106 | 0.279 |
| ≥65y | 62 | 29 | 62 | 17 | 124 | 46 | |||
| Gender | |||||||||
| Male | 114 | 71 | 0.557 | 144 | 42 | 0.518 | 258 | 113 | 0.807 |
| Female | 44 | 23 | 50 | 16 | 94 | 39 | |||
| Stage | |||||||||
| I–II | 32 | 23 | 0.433 | 43 | 19 | 0.100 | 75 | 42 | 0.050 |
| III–IV | 126 | 71 | 151 | 39 | 277 | 110 | |||
| Primary sites | |||||||||
| Oral cavity | 109 | 73 | 0.137 | 146 | 45 | 0.716 | 255 | 118 | 0.223 |
| Pharynx and larynx | 49 | 21 | 48 | 13 | 97 | 34 | |||
Figure 3.
Identification and performance evaluation of these 11 chemokines signature in training, validation, and entire sets. (A) Kaplan-Meier survival curve analysis for overall survival of HNSC patients using the 11-chemokines signature in these 3 datasets. (B) ROC curve analysis of the 11-chemokines signature in these 3 datasets.
Table 3.
Log-rank test of overall survival according to this 11-ckemokine classifier in the training (n=252), validation (n=252), and entire (n=504) sets.
| Datasets | Risk group (n) | Disease-free survival | ||||
|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | HR (95% CI) | p. Value | ||
| Training set (n=252) | High-risk (n=158) | 76.5% | 40.4% | 33.1% | 3.497 (2.142–5.711) | <0.001 |
| Low-risk (n=94) | 97.9% | 82.3% | 65.7% | |||
| Validation set (n=252) | High-risk (n=194) | 76.4% | 50.7% | 38.2% | 3.575 (1.988–6.390) | <0.001 |
| Low-risk (n=58) | 96.5% | 80.6% | 76.9% | |||
| Entire set (n=504) | High-risk (n=352) | 76.4% | 46.1% | 36.0% | 3.416 (2.363–4.939) | <0.001 |
| Low-risk (n=152) | 97.3% | 81.6% | 69.3% | |||
CI – confidence index; HR – hazard ratio.
Multivariate Cox model
We further assessed whether this risk score was independent of these clinical factors (age, sex, clinical stage, and primary sites) by a multivariate Cox model. As shown in Table 4, this integrated 11-chemokine signature was identified as an independent prognostic factor in the training, validation, and entire sets (all p<0.001) for HNSC patients. Hence, our findings suggest that this integrated 11-chemokine signature may become a reliable and independent biomarker for the prediction of overall survival in HNSC patients.
Table 4.
Multivariate Cox regression analysis of this eleven-chemokine classifier, gender, age, stage, and primary sites for overall survival in the training (n=252), validation (n=252), and entire (n=504) sets.
| Datasets | Variable | Disease-free Survival | |
|---|---|---|---|
| HR (95% CI) | p-Value | ||
| Training set (n=246) | The 11-chemokine classifier (high- vs. low-risk) | 3.557 (2.165–5.845) | <0.001 |
| Age (≥65 years vs. <65 years) | 1.562 (1.056–2.312) | 0.026 | |
| Gender (Female vs. Male) | 1.66 (1.081–2.548) | 0.021 | |
| Tumor stage (II–IV vs. I–II) | 0.999 (0.614–1.626) | 0.998 | |
| Primary sites (oral cavity vs. pharynx/larynx) | 0.964 (0.617–1.505) | 0.87 | |
| Validation set (n=246) | The eleven-chemokine classifier (high- vs. low-risk) | 3.442 (1.919–6.172) | <0.001 |
| Age (≥65 years vs. <65 years) | 1.009 (0.672–1.515) | 0.97 | |
| Gender (Female vs. Male) | 1.065 (0.688–1.647) | 0.78 | |
| Tumor stage (II–IV vs. I–II) | 1.226 (0.780–1.927) | 0.38 | |
| Primary sites (oral cavity vs. pharynx/larynx) | 0.741 (0.462–1.188) | 0.21 | |
| Entire set (n=504) | The eleven-chemokine classifier (high- vs. low-risk) | 3.360 (2.320–4.867) | <0.001 |
| Age (≥65 years vs. <65 years) | 1.250 (0.944–1.654) | 0.117 | |
| Gender (Female vs. Male) | 1.301 (0.962–1.759) | 0.088 | |
| Tumor stage (II–IV vs. I–II) | 1.097 (0.789–1.525) | 0.579 | |
| primary sites (oral cavity vs. pharynx/larynx) | 0.860 (0.622–1.188) | 0.361 | |
HR – hazard ratio; NR – not reported; CI – confidence index.
ROC curve analysis
As shown in Figure 3B, the AUC values for predicting overall survival within 48 months in the training, validation, and entire sets were 0.71, 0.69, and 0.69, respectively, highlighting the validity of this 11-chemokine signature.
Gene functional analysis
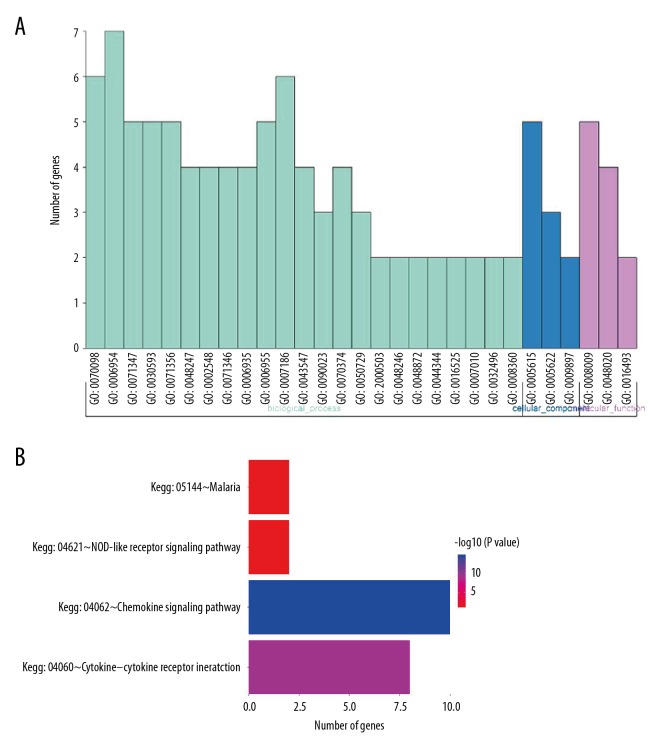
Gene functional analysis indicated 29 GO terms and 4 KEGG pathways which these 11 chemokines were enriched in (Figure 4A, 4B). The main 9 participating GO terms contained chemokine-mediated (GO: 0070098), inflammatory response (GO: 0006954), cellular response to interleukin-1 (GO: 00071347), neutrophil chemotaxis (GO: 0030593), cellular response to tumor necrosis factor (GO: 0071356), lymphocyte chemotaxis (GO: 0048247), monocyte chemotaxis (GO: 0002548), chemokine activity (GO: 0008009), and CCR chemokine receptor (GO: 0048020). The key involved KEGG pathways were chemokine signaling pathway (Kegg: 04062), cytokine-cytokine receptor (Kegg: 04060), NOD-like receptor signaling pathway (Kegg: 04621), and Malaria (Kegg: 05144).
Figure 4.
Gene function and pathway analysis. (A) GO enrichment analysis. (B) Significant pathway analysis.
Discussion
Although the current tumor staging system has been used to define the risk stratification of HNSC for many years, it is inadequate at identifying high-risk HNSC patients [4,6,23,24]. Inconsistent clinical outcomes always existed among the same-stage patients with HNSC [5,25]. Many studies demonstrated that chemokine family genes play a pivotal role in tumor inflammation, immunity, progression, and metastasis [26–29]. A multiple-gene prognostic biomarker of chemokine family genes is urgently needed and may contribute to the identification of potential HNSC patients with worse prognosis. To identify this multiple-gene prognostic biomarker, we profiled chemokine ligands and their receptors by mining the RNA sequencing data of TCGA. Based on the bioinformatic discovery and validation method, we constructed an 11-chemokine signature that can improve the prognostic prediction of overall survival in HNSC patients. The clinical utility of this signature can improve the predictive ability of the current staging system. In addition, the clinical application of this signature could classify patients with HNSC into low-risk and high-risk groups after radical surgery. High-risk patients with HNSC had worse prognosis than low-risk patients. This signature may be used as an additional biomarker for identifying potentially high-risk patients, which may contribute to personalized treatment for HNSC.
There are 7 protective genes (CCL22, XCL2, CCR4, CCR6, CCR7, XCR1, CX3CR1) and 4 risk genes (CCL2, CCL7, CXCL5, CXCL8) in this 11-chemokine signature. Previous research indicated that CCL2 and CCL7 are associated with tumor proliferation, invasion, migration, and tumor burden [30,31]. In HNSC cells, decreased CXCL5 expression inhibits cell proliferation and reduces cell migration and invasion in vitro and inhibits tumor formation in vivo [32]. CXCL8 is known to be a promoter of angiogenesis and a regulator of cell growth and motility in HNSC [33]. The serum level of CXCL8 was used to predict a lower survival rate in patients, because the chemokine promotes metastasis by neutrophil infiltration and stimulates vascular endothelial cell proliferation, survival, and migration [34–36].
Several studies found that CCR7 expression was significantly associated with nodal metastasis [37–40]. Tsujikawa et al. concluded that CCR4 expression in primary HNSCC cells may be an attractive diagnostic biomarker to predict lymph node metastasis and subsequent prognosis of HNSC patients [41]. However, levels of CCL22 in the peripheral blood have no correlation with tumor stage of HNSC [42]. A previous study showed that high expression of CCR7 was associated with disease-free survival and CCR4 was correlated with tumor site, showing higher immune-reactive scores in tumors of the oral cavity [24]. CCR6 and CCR7 mRNA levels were significantly decreased in lymph node (+) patients with laryngeal squamous cell carcinoma (LSCC) [43]. The prognostic effect of these remaining chemokines (XCL2, XCR1, and CX3CR1) in HNSC should be further explored.
We should acknowledge some potential limitations for this 11-chemokine signature. Firstly, gene enrichment analysis found that this 11-chemokine signature was mainly involved in 9 GO terms and 4 KEGG pathways., but the GO terms and KEGG pathways involved by these 11 chemokines were not confirmed by cell, animal, or clinical studies. Further studies may should be performed to provide potential therapeutic targets for HNSC. Secondly, only 46 chemokine family genes were selected for in this study. The connection between overall survival and mRNA levels of the remaining 14 chemokines should be studied by tumor specimens of HNSC furtherly. Thirdly, this 11-chemokine signature was constructed by a bioinformatic discovery and validation approach. This prognostic signature was not further verified by protein level data of Western blot or immunohistochemistry or RNA level data of quantitative reverse transcription polymerase chain reaction from clinical tumor specimens. Moreover, research in other databases is needed to verify the potential significance of this signature among different cohorts of HNSC patients.
Conclusions
We performed a comprehensive analysis of chemokines mRNA expression profiles and clinical data of HNSC patients in the TCGA database. Then, we identified an 11-chemokine signature that may improve the prediction of overall survival in HNSC patients. This is the first study to demonstrate the link between an 11-chemokine signature and overall survival in HNSC patients. Our results may support useful risk stratification of overall survival in HNSC patients, which would contribute to gene target treatment for HNSC. However, this 11-chemokine signature should be further tested by tumor specimens of HNSC before clinical application.
Supplementary Table
Supplementary Table 1.
Univariate Cox regression analysis of chemokine family genes associated with overall survival in the training set (n=252, TCGA).
| Gene Symbol | Type | Sub-family | HR (95% CI) | Coefficient | p Value | Gene Symbol | Type | Sub-family | HR (95% CI) | Coefficient | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 | Ligand | CC | 1.173 (1.019–1.351) | 0.16 | 0.026 | CXCL9 | Ligand | CXC | 0.966 (0.894–1.042) | −0.035 | 0.371 |
| CCL3 | Ligand | CC | 1.13 (0.978–1.305) | 0.122 | 0.097 | CXCL10 | Ligand | CXC | 1.006 (0.935–1.082) | 0.006 | 0.878 |
| CCL7 | Ligand | CC | 1.154 (1.027–1.296) | 0.143 | 0.016 | CXCL11 | Ligand | CXC | 1.028 (0.957–1.103) | 0.027 | 0.452 |
| CCL11 | Ligand | CC | 1.024 (0.916–1.146) | 0.024 | 0.671 | CXCL12 | Ligand | CXC | 1.054 (0.923–1.204) | 0.053 | 0.434 |
| CCL13 | Ligand | CC | 1.07 (0.952–1.203) | 0.068 | 0.256 | CXCL14 | Ligand | CXC | 0.956 (0.881–1.036) | −0.045 | 0.274 |
| CCL14 | Ligand | CC | 0.992 (0.897–1.097) | −0.008 | 0.876 | CXCL16 | Ligand | CXC | 1.079 (0.823–1.415) | 0.076 | 0.583 |
| CCL17 | Ligand | CC | 0.912 (0.796–1.044) | −0.092 | 0.183 | CXCL17 | Ligand | CXC | 0.933 (0.867–1.004) | −0.069 | 0.064 |
| CCL18 | Ligand | CC | 0.997 (0.896–1.109) | −0.003 | 0.954 | CXCR1 | Receptor | CXC | 0.997 (0.904–1.098) | −0.003 | 0.945 |
| CCL19 | Ligand | CC | 0.945 (0.875–1.021) | −0.056 | 0.153 | CXCR2 | Receptor | CXC | 0.917 (0.824–1.019) | −0.087 | 0.108 |
| CCL20 | Ligand | CC | 1.03 (0.948–1.12) | 0.03 | 0.48 | CXCR3 | Receptor | CXC | 0.909 (0.82–1.008) | −0.096 | 0.069 |
| CCL21 | Ligand | CC | 0.928 (0.858–1.004) | −0.075 | 0.063 | CXCR4 | Receptor | CXC | 0.871 (0.745–1.017) | −0.138 | 0.081 |
| CCL22 | Ligand | CC | 0.857 (0.746–0.985) | −0.154 | 0.03 | CXCR6 | Receptor | CXC | 0.886 (0.78–1.007) | −0.121 | 0.064 |
| CCL23 | Ligand | CC | 1.079 (0.912–1.275) | 0.076 | 0.375 | XCL1 | Ligand | XC | 0.959 (0.85–1.083) | −0.041 | 0.503 |
| CCL24 | Ligand | CC | 1 (0.876–1.143) | 0 | 0.997 | XCL2 | Ligand | XC | 0.856 (0.74–0.99) | −0.155 | 0.037 |
| CCL27 | Ligand | CC | 1.014 (0.891–1.155) | 0.014 | 0.83 | XCR1 | Receptor | XC | 0.831 (0.73–0.946) | −0.185 | 0.005 |
| CCL28 | Ligand | CC | 0.917 (0.801–1.049) | −0.087 | 0.207 | CX3CL1 | Ligand | CX3C | 1.008 (0.904–1.124) | 0.008 | 0.885 |
| CCR1 | Receptor | CC | 1.07 (0.928–1.233) | 0.067 | 0.352 | CX3CR1 | Receptor | CX3C | 0.859 (0.752–0.981) | −0.152 | 0.025 |
| CCR3 | Receptor | CC | 1.014 (0.816–1.261) | 0.014 | 0.899 | CXCL2 | Ligand | CXC | 1.062 (0.948–1.188) | 0.06 | 0.299 |
| CCR4 | Receptor | CC | 0.888 (0.8–0.985) | −0.119 | 0.025 | CXCL3 | Ligand | CXC | 1.074 (0.965–1.196) | 0.072 | 0.191 |
| CCR6 | Receptor | CC | 0.772 (0.652–0.915) | −0.258 | 0.003 | CXCL5 | Ligand | CXC | 1.113 (1.008–1.23) | 0.107 | 0.01 |
| CCR7 | Receptor | CC | 0.846 (0.752–0.951) | −0.167 | 0.005 | CXCL6 | Ligand | CXC | 1.053 (0.966–1.148) | 0.052 | 0.241 |
| CCR8 | Receptor | CC | 0.961 (0.849–1.088) | −0.039 | 0.533 | CXCL8 | Ligand | CXC | 1.14 (1.031–1.261) | 0.131 | 0.011 |
| CCR10 | Receptor | CC | 0.909 (0.785–1.052) | −0.096 | 0.201 |
CI – confidence index; HR – hazard ratio.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takes RP, Rinaldo A, Silver CE, et al. Future of the TNM classification and staging system in head and neck cancer. Head Neck. 2010;32:1693–711. doi: 10.1002/hed.21361. [DOI] [PubMed] [Google Scholar]
- 3.Huang SH, O’Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18:40. doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, David O, Cabay RJ, et al. Molecular classification of lymph node metastases subtypes predict for survival in head and neck cancer. Clin Cancer Res. :2018. doi: 10.1158/1078-0432.CCR-18-1884. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 6.Patel SG, Shah JP. TNM staging of cancers of the head and neck: Striving for uniformity among diversity. Cancer J Clin. 2005;55:242–58. doi: 10.3322/canjclin.55.4.242. quiz 261–62, 264. [DOI] [PubMed] [Google Scholar]
- 7.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 8.Lira SA, Furtado GC. The biology of chemokines and their receptors. Immunol Res. 2012;54:111–20. doi: 10.1007/s12026-012-8313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlotnik A, Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–27. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 10.Maggiolini M, Statti G, Vivacqua A, et al. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82:315–22. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 11.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011;317:685–90. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo) 2015;13:469–73. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Adjemian S, Galluzzi L, et al. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. Oncoimmunology. 2014;3:e27663. doi: 10.4161/onci.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw R, Maragos C, May JT. Bovine herpes mammillitis therapy. Vet Rec. 1986;119:436. doi: 10.1136/vr.119.17.436-a. [DOI] [PubMed] [Google Scholar]
- 15.Igawa S, Nishinarita N, Takakura A, et al. Real-world evaluation of carboplatin plus a weekly dose of nab-paclitaxel for patients with advanced non-small-cell lung cancer with interstitial lung disease. Cancer Manag Res. 2018;10:7013–19. doi: 10.2147/CMAR.S189556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–18. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke K, Chen G, Cai Z, et al. Evaluation and prediction of hepatocellular carcinoma prognosis based on molecular classification. Cancer Manag Res. 2018;10:5291–302. doi: 10.2147/CMAR.S178579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–37. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28:1721–26. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, D’Andrea AD, Kozono D. A DNA repair pathway-focused score for prediction of outcomes in ovarian cancer treated with platinum-based chemotherapy. J Natl Cancer Inst. 2012;104:670–81. doi: 10.1093/jnci/djs177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 22.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 23.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3305–13. doi: 10.1200/JCO.2015.62.0963. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Arriagada WA, Lozano-Burgos C, Zuniga-Moreta R, et al. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J Oral Pathol Med. 2018;47:755–63. doi: 10.1111/jop.12736. [DOI] [PubMed] [Google Scholar]
- 25.Zhai TT, van Dijk LV, Huang BT, et al. Improving the prediction of overall survival for head and neck cancer patients using image biomarkers in combination with clinical parameters. Radiother Oncol. 2017;124:256–62. doi: 10.1016/j.radonc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Subat S, Mogushi K, Yasen M, et al. Identification of genes and pathways, including the CXCL2 axis, altered by DNA methylation in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2019;45(3):675–84. doi: 10.1007/s00432-018-2824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalejska-Fiolka J, Hubkova B, Birkova A, et al. Prognostic value of the modified atherogenic index of plasma during body mass reduction in Polish obese/overweight people. Int J Environ Res Public Health. 2018;16 doi: 10.3390/ijerph16010068. pii: E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De la Fuente Lopez M, Landskron G, Parada D, et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 2018;40 doi: 10.1177/1010428318810059. 1010428318810059. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Xu Q, Wu Y, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35:1362–70. doi: 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- 31.Jung DW, Che ZM, Kim J, et al. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: A pivotal role of CCL7. Int J Cancer. 2010;127:332–44. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 32.Argiris A, Lee SC, Feinstein T, et al. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol. 2011;47:961–66. doi: 10.1016/j.oraloncology.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupisz K, Chibowski D, Klatka J, et al. Tumor angiogenesis in patients with laryngeal cancer. Eur Arch Otorhinolaryngol. 1999;256:303–5. doi: 10.1007/s004050050251. [DOI] [PubMed] [Google Scholar]
- 35.Lentsch EJ, Goudy S, Sosnowski J, et al. Microvessel density in head and neck squamous cell carcinoma primary tumors and its correlation with clinical staging parameters. Laryngoscope. 2006;116:397–400. doi: 10.1097/01.MLG.0000195286.29613.E1. [DOI] [PubMed] [Google Scholar]
- 36.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shareef H, Hiraoka SI, Tanaka N, et al. Use of NRP1, a novel biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol Rep. 2016;36:2444–54. doi: 10.3892/or.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katayama A, Ogino T, Bandoh N, et al. Expression of CXCR4 and its down-regulation by IFN-gamma in head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:2937–46. doi: 10.1158/1078-0432.CCR-04-1470. [DOI] [PubMed] [Google Scholar]
- 39.Ueda M, Shimada T, Goto Y, et al. Expression of CC-chemokine receptor 7 (CCR7) and CXC-chemokine receptor 4 (CXCR4) in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2010;37:488–95. doi: 10.1016/j.anl.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Xi L, Hunt JL, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–66. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 41.Tsujikawa T, Yaguchi T, Ohmura G, et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int J Cancer. 2013;132:2755–66. doi: 10.1002/ijc.27966. [DOI] [PubMed] [Google Scholar]
- 42.Schott AK, Pries R, Wollenberg B. Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. Int J Mol Med. 2010;26:67–75. doi: 10.3892/ijmm_00000436. [DOI] [PubMed] [Google Scholar]
- 43.Chen B, Zhang D, Zhou J, et al. High CCR6/CCR7 expression and Foxp3+ Treg cell number are positively related to the progression of laryngeal squamous cell carcinoma. Oncol Rep. 2013;30:1380–90. doi: 10.3892/or.2013.2603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Univariate Cox regression analysis of chemokine family genes associated with overall survival in the training set (n=252, TCGA).
| Gene Symbol | Type | Sub-family | HR (95% CI) | Coefficient | p Value | Gene Symbol | Type | Sub-family | HR (95% CI) | Coefficient | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 | Ligand | CC | 1.173 (1.019–1.351) | 0.16 | 0.026 | CXCL9 | Ligand | CXC | 0.966 (0.894–1.042) | −0.035 | 0.371 |
| CCL3 | Ligand | CC | 1.13 (0.978–1.305) | 0.122 | 0.097 | CXCL10 | Ligand | CXC | 1.006 (0.935–1.082) | 0.006 | 0.878 |
| CCL7 | Ligand | CC | 1.154 (1.027–1.296) | 0.143 | 0.016 | CXCL11 | Ligand | CXC | 1.028 (0.957–1.103) | 0.027 | 0.452 |
| CCL11 | Ligand | CC | 1.024 (0.916–1.146) | 0.024 | 0.671 | CXCL12 | Ligand | CXC | 1.054 (0.923–1.204) | 0.053 | 0.434 |
| CCL13 | Ligand | CC | 1.07 (0.952–1.203) | 0.068 | 0.256 | CXCL14 | Ligand | CXC | 0.956 (0.881–1.036) | −0.045 | 0.274 |
| CCL14 | Ligand | CC | 0.992 (0.897–1.097) | −0.008 | 0.876 | CXCL16 | Ligand | CXC | 1.079 (0.823–1.415) | 0.076 | 0.583 |
| CCL17 | Ligand | CC | 0.912 (0.796–1.044) | −0.092 | 0.183 | CXCL17 | Ligand | CXC | 0.933 (0.867–1.004) | −0.069 | 0.064 |
| CCL18 | Ligand | CC | 0.997 (0.896–1.109) | −0.003 | 0.954 | CXCR1 | Receptor | CXC | 0.997 (0.904–1.098) | −0.003 | 0.945 |
| CCL19 | Ligand | CC | 0.945 (0.875–1.021) | −0.056 | 0.153 | CXCR2 | Receptor | CXC | 0.917 (0.824–1.019) | −0.087 | 0.108 |
| CCL20 | Ligand | CC | 1.03 (0.948–1.12) | 0.03 | 0.48 | CXCR3 | Receptor | CXC | 0.909 (0.82–1.008) | −0.096 | 0.069 |
| CCL21 | Ligand | CC | 0.928 (0.858–1.004) | −0.075 | 0.063 | CXCR4 | Receptor | CXC | 0.871 (0.745–1.017) | −0.138 | 0.081 |
| CCL22 | Ligand | CC | 0.857 (0.746–0.985) | −0.154 | 0.03 | CXCR6 | Receptor | CXC | 0.886 (0.78–1.007) | −0.121 | 0.064 |
| CCL23 | Ligand | CC | 1.079 (0.912–1.275) | 0.076 | 0.375 | XCL1 | Ligand | XC | 0.959 (0.85–1.083) | −0.041 | 0.503 |
| CCL24 | Ligand | CC | 1 (0.876–1.143) | 0 | 0.997 | XCL2 | Ligand | XC | 0.856 (0.74–0.99) | −0.155 | 0.037 |
| CCL27 | Ligand | CC | 1.014 (0.891–1.155) | 0.014 | 0.83 | XCR1 | Receptor | XC | 0.831 (0.73–0.946) | −0.185 | 0.005 |
| CCL28 | Ligand | CC | 0.917 (0.801–1.049) | −0.087 | 0.207 | CX3CL1 | Ligand | CX3C | 1.008 (0.904–1.124) | 0.008 | 0.885 |
| CCR1 | Receptor | CC | 1.07 (0.928–1.233) | 0.067 | 0.352 | CX3CR1 | Receptor | CX3C | 0.859 (0.752–0.981) | −0.152 | 0.025 |
| CCR3 | Receptor | CC | 1.014 (0.816–1.261) | 0.014 | 0.899 | CXCL2 | Ligand | CXC | 1.062 (0.948–1.188) | 0.06 | 0.299 |
| CCR4 | Receptor | CC | 0.888 (0.8–0.985) | −0.119 | 0.025 | CXCL3 | Ligand | CXC | 1.074 (0.965–1.196) | 0.072 | 0.191 |
| CCR6 | Receptor | CC | 0.772 (0.652–0.915) | −0.258 | 0.003 | CXCL5 | Ligand | CXC | 1.113 (1.008–1.23) | 0.107 | 0.01 |
| CCR7 | Receptor | CC | 0.846 (0.752–0.951) | −0.167 | 0.005 | CXCL6 | Ligand | CXC | 1.053 (0.966–1.148) | 0.052 | 0.241 |
| CCR8 | Receptor | CC | 0.961 (0.849–1.088) | −0.039 | 0.533 | CXCL8 | Ligand | CXC | 1.14 (1.031–1.261) | 0.131 | 0.011 |
| CCR10 | Receptor | CC | 0.909 (0.785–1.052) | −0.096 | 0.201 |
CI – confidence index; HR – hazard ratio.