Abstract
There is a critical need to include female subjects in disease research; however, in Parkinson’s disease, where the male-to-female incidence is about 1.5-to-1, the majority of preclinical research is conducted in male animals. The mitochondrial complex I inhibitor, rotenone, is selectively toxic to dopaminergic neurons, and reproduces several neuropathological features of Parkinson’s disease, including α-synuclein pathology. Rotenone has been primarily utilized in male Lewis rats; however, pilot studies in age-matched female Lewis rats revealed that our usual dose (2.8 mg/kg/day intraperitoneal [i.p.]) did not cause dopaminergic neurodegeneration. Therefore, we compared rotenone-treated males (2.8 mg/kg/day, i.p.) to females at increasing doses (2.8 mg/kg/day, 3.2 mg/kg/day, 3.6 mg/kg/day, and 1.6 mg/kg bis in die, i.p.). Female rats receiving 3.2 mg/kg, and 3.6 mg/kg rotenone displayed significant loss of dopaminergic neurons in the substantia nigra as assessed by stereology, which was accompanied by a loss of striatal dopaminergic terminals. Even at these higher doses, however, females showed less inflammation, and less accumulation of α-synuclein and transferrin, possibly as a result of preserved autophagy. Thus, the bias toward increased male incidence of human Parkinson’s disease is reflected in the rotenone model. Whether such sex differences will translate into differences in responses to mechanism-driven therapeutic interventions remains to be determined.
Keywords: rotenone, Parkinson’s disease, sex differences, toxin models, neurodegeneration
The male-to-female odds ratio for incidence of Parkinson’s disease (PD) is 1.49, indicating that sex differences likely play a role in the pathogenesis of the disease (Wooten et al., 2004). In addition, clinical reports indicate that men and women differ in presentation of primary symptoms, nonmotor morbidities, and timing of onset (Picillo et al., 2017). Despite these observations, animal modeling of PD often lacks the integration of sex as a variable, possibly overlooking important etiological factors and misrepresenting females in preclinical therapeutic development.
Rotenone, an organic pesticide and prototypical mitochondrial complex I inhibitor, reliably reproduces parkinsonism in rats, including motor behavioral deficits of postural instability, rigidity, and bradykinesia (Cannon et al., 2009). Systemic exposure to rotenone causes the selective neurodegeneration of dopamine neurons in the substantia nigra (SN) and their terminal projections in the striatum (ST), endogenous α-synuclein accumulation, microglial activation, and changes in iron metabolism (Betarbet et al., 2000; Cannon et al., 2009; Mastroberardino et al., 2009). However, most in vivo rodent studies heavily favor the use of adult male rats (Capello et al., 2014). Currently, approximately 20% of rotenone studies reported in peer-reviewed literature involve the use of female animals, and there are no direct female-to-male pathological assessments following daily dosing of rotenone. In addition, the use of rotenone-induced neurodegeneration in adult rats is a common tool to assess the efficacy of preclinical therapeutics; therefore the underrepresentation of female animals could result in a sex-bias during translational therapeutics development (Aksoy et al., 2017; Anusha et al., 2017; Javed et al., 2016; Khadrawy et al., 2017; Zharikov et al., 2015). To this end, we sought to compare neurodegeneration and pathology of adult female age-matched male Lewis rats treated with a standardized rotenone dosing paradigm designed to produce maximal nigrostriatal loss without animal mortality (Cannon et al., 2009). Interestingly, our initial results indicated that female rats are not equivalently susceptible to rotenone toxicity as male rats, as they did not display significant loss of dopaminergic neurons from the SN or their terminal projections to the ST. In addition, female animals did not succumb to morbidity of daily rotenone treatment, despite containing the same concentration of rotenone in the brain, suggesting that female animals may not be as susceptible to rotenone-induced neurodegeneration.
The observed sex differences in PD may be hormonal (Brann et al., 2007; Gillies et al., 2004; Green and Simpkins, 2000; Session et al., 1994) or genetic (Czech et al., 2012, 2014; Dewing et al., 2006), or a combination of both. In this study, we developed a dose-response of rotenone-induced neurodegeneration in adult, nonovariectomized female rats to establish a dosing paradigm that closely mirrors well-established pathology in male rats. Non-naïve, intact (retired breeder) adult female rats were selected to model the average time of prodromal onset for women diagnosed with idiopathic PD. A dose of 2.8 mg/kg of rotenone is typically employed in male rats (Cannon et al., 2009; De Miranda et al., 2018; Tapias et al., 2017; Zharikov et al., 2015), but as its toxicity dose-response is nonlinear and very steep (Betarbet et al., 2000), we compared 2.8–3.2 mg/kg/day, and 3.6 mg/kg/day of rotenone exposure in adult female rats until they reached a motor behavioral endpoint, defined as severe bradykinesia. Here, we characterize sex as a variable in rotenone-induced neurodegeneration, and address the experimental details necessary for inclusion of female animals within these studies.
MATERIALS AND METHODS
Chemical reagents and supplies
Rotenone (CAS Number 83-79-4) and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Antibody information is listed in Table 1.
Table 1.
Primary Antibodies Used for Immunohistochemistry
| Antigen | Antibody Catalog Information | Company | Immunohistochemistry Concentration |
|---|---|---|---|
| Tyrosine hydroxylase | AB1542 | EMD Millipore | 1:2000 |
| (Burlington, MA) | |||
| Ser129-α-Synuclein | ab51253 | 1:500 | |
| Lamp1 | ab24170 | Abcam (Cambridge, MA) | 1:500 |
| Iba1 | 019-19741 | Wako Chemicals USA (Irvine, CA) | 1:500 |
| CD-68 (ED1) | MCA341 | BioRad (Hercules, CA) | 1:500 |
| α-Synuclein | 610787 | BD Biosciences | 1:500 |
| (San Jose, CA) | |||
| p62/SQSTM1 (2C11) | H00008878-M01 | Abnova | 1:500 |
| (Tapei, Taiwan) |
Animals and rotenone administration
Adult (10 month) male and female Lewis rats (Envigo) were separated into single-housing 2 weeks prior to the onset of experiments and trained to perform the postural instability test (PIT). Conventional diet and water were available to rats ad libitum. Animals were maintained under standard temperature-controlled conditions with a 12-h light-dark cycle. The average weight of rats at the time of study onset was 528 g (male) and 276 g (female). Rotenone was dissolved in dimethylsulfoxide (DMSO) (2% final concentration) and Miglyol 812 to reach the final concentration of 2.8, 3.2, or 3.6 mg/kg; n = 3. Rotenone handling and disposal was carried out following University of Pittsburgh Environmental Health and Safety procedures.
Rotenone and vehicle (2% DMSO and Miglyol) groups were randomly divided, and each animal was administered a single daily intraperitoneal (i.p.) injection of rotenone until they reached their motor behavioral endpoint. Endpoint in this rotenone study was defined as severe bradykinesia and the inability to perform the PIT (see below), or loss of 25% body mass. This dosing paradigm has been extensively profiled in adult male Lewis rats to occur over a period of 8–15 days of 2.8 mg/kg rotenone treatment (Cannon et al., 2009; De Miranda et al., 2018). Each animal is individually assessed and is euthanized upon reaching endpoint for tissue collection. A separate group of female animals were administered 1.6 mg/kg twice daily in 9-h intervals (BID, 3.2 mg/kg total; n = 3); data reported in Supplementary Figure 2. Animals were euthanized using pentobarbital, followed by transcardial perfusion and 4% paraformaldehyde fixation. All experiments involving animal treatment and euthanasia were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Motor behavior
The PIT (Woodlee et al., 2008) was used as previously described (De Miranda et al., 2018) to measure forelimb motor dysfunction. Briefly, rats were habituated to handling for 2 weeks prior to the onset of the study for habituation and to minimize stress. To perform the PIT, rats were held with 1 forelimb immobilized, and the opposite forelimb placed on a metered surface with texture to prevent slipping. Each animal was slowly moved forward until the forelimb in contact with the surface was forced to take a step (distance to trigger). The centimeter distance was recorded as the PIT (average of three trials per animal, per day).
Rotenone metabolism studies
Adult (9 month) female and male Lewis rats (Envigo) were administered a single i.p. injection of rotenone (2.8 mg/kg) and euthanized 1 h later (n = 6 male, n = 6 female). Brain tissue was rapidly dissected and flash frozen for high-performance liquid chromatography (HPLC)-mass spectrometry analysis. Rotenone analytical standard (≥95%) and 2-isopropyl-8,9-dimethoxy-1,2,12,12a-4h-6ah-chromeno(3,4-b)furo(2,3-h)chromen-6-one, used as internal standard (I.S.), were purchased from Sigma-Aldrich. Solvents used for extractions and mass spectrometric analyses were from Burdick and Jackson (Muskegon, MI).
Sample extraction
Rat brain samples (≈110 mg) were homogenized in a FastPrep-24™5G homogenizer in 0.5 ml phosphate buffer 50 mM pH 7.4. Next, an aliquot of homogenate (50 μl) was spiked with 3.6 pmol I.S. (Caboni et al., 2008), left on ice for 10 min, and 300 μl acetonitrile were added. Samples were vortexed for 30 s, centrifuged at 20 000 × g for 10 min at 4°C, after which the supernatant was analyzed by HPLC-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS).
Analysis of rotenone in rat brain
Rotenone was analyzed by HPLC-ESI-MS/MS using an analytical C18 Luna column (2 × 20 mm, 5 μm, Phenomenex) at a 0.7 ml/min flow rate, with a gradient solvent system consisting of water containing 0.1% acetic acid (solvent A) and acetonitrile containing 0.1% acetic acid (solvent B). Samples were chromatographically resolved using the following gradient program: 30–100% solvent B (0–3 min); 100% solvent B (0.6 min) followed by 1.4 min re-equilibration to initial conditions. Detection of rotenone was performed using a 5000 triple quadrupole mass spectrometer (Applied Biosystems, San Jose, CA) equipped with an ESI in positive mode and the following parameters were used: declustering potential 90 V, entrance potential 10 V, collision cell exit potential 14 V, and a desolvation temperature of 700°C. Multiple reaction monitoring (MRM) transitions were used with the best collision energy (CE): MRM 395.3/367.2, 395.3/213.2, and 395.3/192.2 with CE 30, 32, and 35 eV respectively. The following MRM transitions were used for the analysis of the I.S.: MRM 397.3/369.2, 397.3/215.2, and 397.3/192.2. Quantitation of rotenone in rat brain was performed using calibration curves of rotenone (MRM 395.3/192.2) in the presence of the I.S. (MRM 397.3/192.2; Supplementary Figure 1). Levels of rotenone in rat brain were reported as pmol/mg protein.
Striatal terminal intensity
A series of brain sections (35 µm) encompassing the volume of the rat striatum (one-sixth sampling fraction, approximately 10 sections per animal) were stained for tyrosine hydroxylase (TH) and detected using an infrared secondary antibody (IRDye® 680, LiCor Biosciences). Striatal tissue sections were analyzed using near-infrared imaging for density of dopamine neuron terminals (LiCor Odyssey), and analyzed using LiCor Odyssey software (V3.0; Licor Biosciences, Lincoln, NE). Results are reported as striatal TH intensity in arbitrary fluorescence units.
Stereology
Stereological analysis of dopamine neuron number in the SN was achieved using an adapted protocol from Tapias et al. (2013) as reported in De Miranda et al. (2018) employing an unbiased, automated system. Briefly, nigral tissue sections were stained for TH and counterstained with 4′,6-diamidino-2-phenylindole, nuclear marker (DAPI) and NeuroTrace Dye (640; Life Technologies) and imaged using a Nikon 90i upright fluorescence microscope equipped with high N.A. plan fluor/apochromat objectives, Renishaw linear encoded microscope stage (Prior Electronics) and Q-imaging Retiga cooled CCD camera (Center for Biological Imaging, University of Pittsburgh). Images were processed using Nikon NIS-Elements Advanced Research software (Version 4.5, Nikon, Melville, NY), and quantitative analysis was performed on fluorescent images colocalizing DAPI, TH, and Nissl-positive stains. Results are reported as the number of TH-positive cell bodies (whole neurons) within the SN.
Immunohistochemistry and immunopathology
Brain sections (35 µm) were maintained at −20°C in cryoprotectant, stained while free-floating, and mounted to glass slides for imaging, using a “primary antibody delete” (secondary antibody only) stained section to subtract background fluorescence. Fluorescent immunohistochemical images were collected using an Olympus BX61 confocal microscope and Fluoview 1000 software (Melville, NY). Quantitative fluorescence measurements were thoroughly monitored using standard operating imaging parameters to ensure that images contained no saturated pixels. For quantitative comparisons, all imaging parameters (eg laser power, exposure, and pinhole) were held constant across specimens. Confocal images were analyzed using Nikon NIS-Elements Advanced Research software (Version 4.5, Nikon, Melville, NY). A minimum of 6 images per tissue slice were analyzed per animal, averaging 9–15 neurons per ×60–×100 image (approximately 180 cells per animal, per histological stain). Twenty times magnification was used to generate montage imaging of the ventral midbrain, for which the entire SN was analyzed per image using anatomical region of interest boundaries. Results are reported as a measure of puncta within TH-positive cells, either number of objects (# of objects) or area (μm).
Statistical analysis
All data were expressed as mean values ± standard error of the mean. Statistical significance between male and female rats was evaluated for normally distributed means by two-way analysis of variance (two-way ANOVA) with a Sidak multiple comparisons test to correct for mean comparison between multiple groups, where source of variation was defined as “sex” or “rotenone” groups. P value and F-statistic is reported for the interaction comparison between “sex” and “rotenone” variables as F(DFn, DFd) = x within each figure legend. Prior to study onset, an apriori power analysis was conducted using G*power software (Heinrich-Heine-University Düsseldorf) to determine the sample size required for a 20–40% difference between mean, with a 95% power at α = .05. Statistical significance between treatment groups is represented in each figure as *p < .05, **p < .01, ***p < .001, ****p < .0001, unless otherwise specified on graph. Animal survival was analyzed using the log-rank Mantel-Cox comparison of survival curve (**p = .0011), and postural instability was evaluated using one-way ANOVA with the Dunnett multiple comparisons test (α = .05). Statistical outliers from each data set were determined using the extreme studentized deviate (Grubbs’ test, α = .05). Statistical analyses were carried out using GraphPad Prism software (V. 5.01).
Results
Adult Female Rats Have Decreased Sensitivity to Rotenone-Induced Neurodegeneration
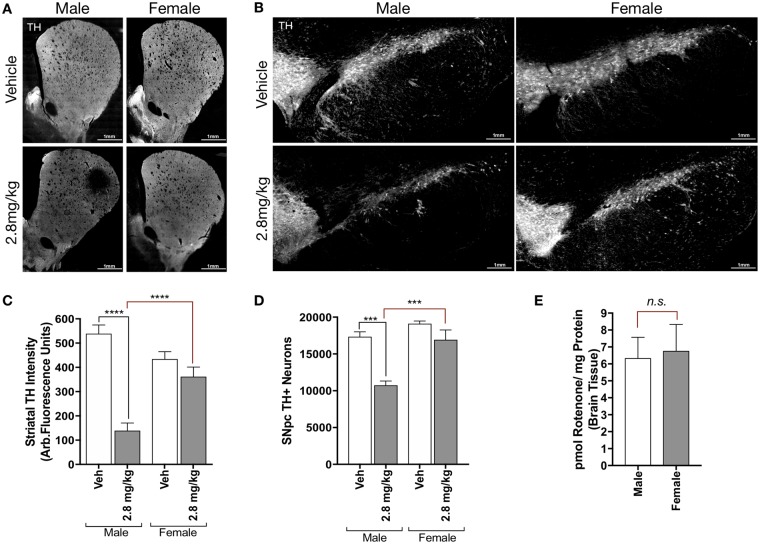
Age-matched adult male and female Lewis rats (9 months old) were obtained from the same commercial source (Envigo) and housed under identical conditions prior to the onset of rotenone exposure. Male and female rats were dosed with 2.8 mg/kg/day rotenone (i.p.) or vehicle (2% DMSO, 98% miglyol) until they reached a phenotypic motor “endpoint,” defined by severe bradykinesia, a result of substantial nigrostriatal dopaminergic neuron degeneration (Cannon et al., 2009). Unexpectedly, female animals dosed with 2.8 mg/kg rotenone did not display any of the predefined motor symptoms of endpoint rotenone treatment, and exhibited similar behavior to vehicle treated male and female animals. Histopathological analysis of dopaminergic neurons within the nigrostriatal tract (TH) revealed that female animals did not show equivalent loss of TH-positive terminals within the ST as male animals (Figs. 1a and 1c). Similarly, stereological analysis of the SN showed that female animals did not lose a significant number of TH-positive neurons (p = .5141), while male animals exhibited approximately 35% loss of dopaminergic neurons from the SN (p < .0001; Figs. 1b and 1d).
Figure 1.
Adult female rats have decreased sensitivity to rotenone-induced neurodegeneration. Representative montage images (×20) of 35 μm brain tissue sections of the striatum (A) and substantia nigra (B) immunostained for tyrosine hydroxylase (TH) from male and female Lewis rats (7–9 months) treated with vehicle or 2.8 mg/kg rotenone (i.p.). C, Average intensity values of quantified TH in striatal terminals (F(1, 126) = 45.74, p < .0001; two-way analysis of variance [ANOVA]). D, Stereological counts of TH-positive neurons within the substantia nigra (F(1, 13) = 18.95, p = .0008; two-way ANOVA). E, Amount of parent rotenone content in male and female Lewis rat brains detected by mass spectrometry 6 h following a single 2.8 mg/kg injection (i.p.) p = .8350, unpaired t test (t = 0.2138, df = 10).
These results prompted the examination of rotenone metabolism in male and female adult Lewis rats, though no sex-related difference in metabolism has been reported. To investigate this, we injected a single i.p. dose of 2.8 mg/kg of rotenone to age-matched male and female Lewis rats (n = 6/group), and assessed the brain concentration of rotenone 6 h later. No difference in rotenone concentration was detected by HPLC-tandem mass spectrometry (HPLC-MS/MS) between male and female brains (p = .8350; Figure 1e), indicating that disparate neurodegeneration between male and female animals is likely not due to differences in rotenone metabolism or concentration in the brain.
Establishing Rotenone-Induced Neurodegeneration Within Female Lewis Rats
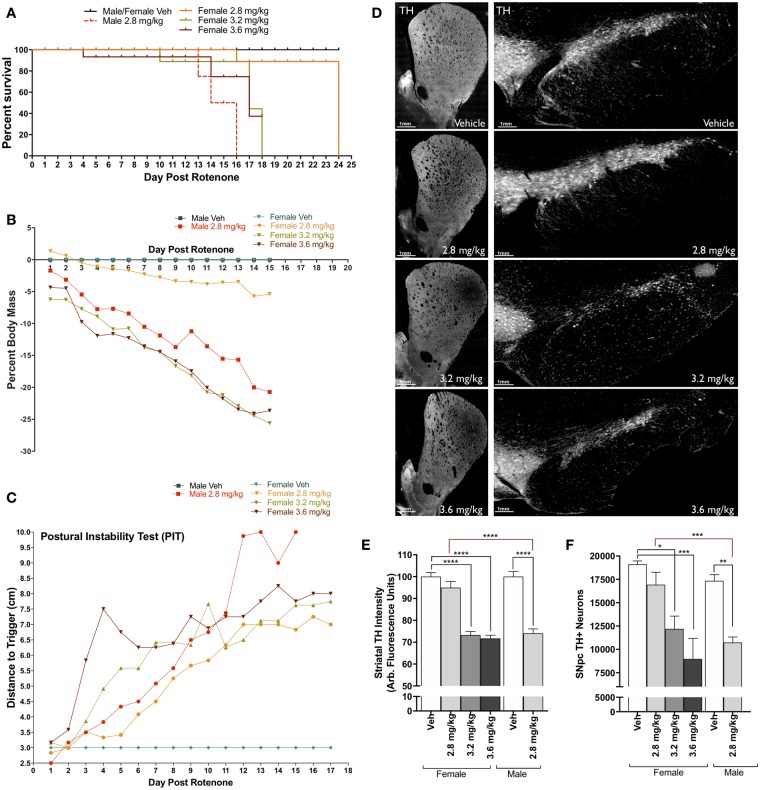
To identify an equivalent rotenone dosage in female animals that reproduces dopaminergic neurodegeneration observed in male animals, we compared daily doses of 2.8 mg/kg, 3.2 mg/kg, and 3.6 mg/kg of rotenone or vehicle (2% DMSO, 98% miglyol) in adult, female Lewis rats. Following the same endpoint dosing paradigm (daily i.p. injection), female animals exposed to higher concentrations of rotenone (3.2 mg/kg, 3.6 mg/kg) exhibited severe bradykinesia, and morbidity over a similar time course as male animals dosed with 2.8 mg/kg rotenone (Figure 2a). Additionally, the 3.2 mg/kg and 3.6 mg/kg female dosing groups lost approximately the same body weight (25%) as male animals receiving 2.8 mg/kg rotenone (Figure 2b). Of note, postural instability testing was not significantly different across any rotenone dose or between sex (Figure 2c).
Figure 2.
Establishing rotenone-induced neurodegeneration within female Lewis rats. A, Survival plot of male and female Lewis rats following daily rotenone injection of 2.8 mg/kg (orange), 3.2 mg/kg (green), 3.6 mg/kg (maroon), or vehicle (black), compared to 2.8 mg/kg in male rats (red). B, Percent loss of body mass of male and female rats over 15 days of rotenone treatment. C, Postural instability test (PIT) motor-behavior scoring following rotenone treatment of male and female rats. D, Representative striatal tyrosine hydroxylase (TH)-positive terminal and substantia nigra (SN) montage images (×20) from female rats dosed with vehicle, 2.8 mg/kg, 3.2 mg/kg, and 3.6 mg/kg rotenone. E, Average intensity values of quantified TH in striatal terminals (F(1, 238) = 90.61, p < .0001; two-way analysis of variance [ANOVA]). F, Stereological counts of TH-positive neurons within the SN (F(1, 24) = 6.946, p = .0145; two-way ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Analysis of dopaminergic neurons and their terminals within the SN and ST respectively, revealed that both 3.2 and 3.6 mg/kg rotenone produced significant neurodegeneration in female Lewis rats; approximately 30% terminal loss (p < .0001) and 33% cell body loss (p = .0425 for 3.2 mg/kg, p = .0008 for 3.6 mg/kg; Figs. 2e and 2f). These are quantitatively indistinguishable from the approximately 30–35% dopaminergic neuron and terminal loss observed in male Lewis rats exposed to 2.8 mg/kg.
A Threshold Dose for Microglial Activation by Rotenone in Male and Female Rats
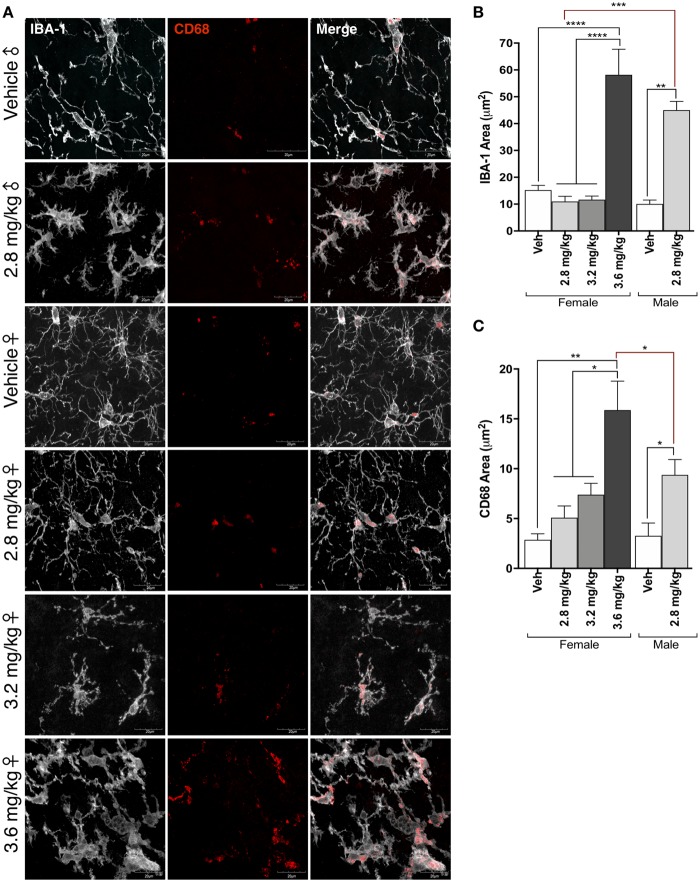
A robust microglial response to rotenone treatment has been previously reported in male Lewis rats following 2.8 mg/kg rotenone (De Miranda et al., 2018; Sherer et al., 2003). Similarly, we observed a marked shift in microglial morphology from resting to activated in the midbrain of male rats receiving 2.8 mg/kg rotenone (Figs. 3a and 3b). In addition, the lysosomal protein CD68 was significantly increased in these cells (p = .0475), indicating a phenotype of reactive microglia (Figs. 3a and 3c). In contrast, no activated phenotype or CD68 increase was observed in the midbrain of female animals receiving 2.8 mg/kg rotenone (p = .9968), however a dose-response of CD68 area to rotenone concentration was observed in the 3.2 mg/kg (p = .5991) and 3.6 mg/kg (p = .0003) dosing groups. Only the highest dose of rotenone in female rats, 3.6 mg/kg, produced a significant morphological shift in microglia (p < .0001) visualized by the microglial marker Iba-1, and this was statistically indistinguishable from microglial activation observed in male rats given 2.8 mg/kg rotenone (p = .0993).
Figure 3.
A threshold dose for microglial activation induced by rotenone in male and female rats. A, Representative confocal microscopy (×100) of microglia within the substantia nigra immunoreactive for IBA-1 (white) and CD68 (red). B, Quantification of IBA-1 area following rotenone injection in male and female rats (F(1, 96) = 12.48, p = .0006; two-way analysis of variance [ANOVA]). C, Quantification of CD68 area following rotenone injection in male and female rats (F(3, 85) = 7.46, p = .0002; two-way ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Sex Differences in α-Synuclein Accumulation Following Rotenone Treatment
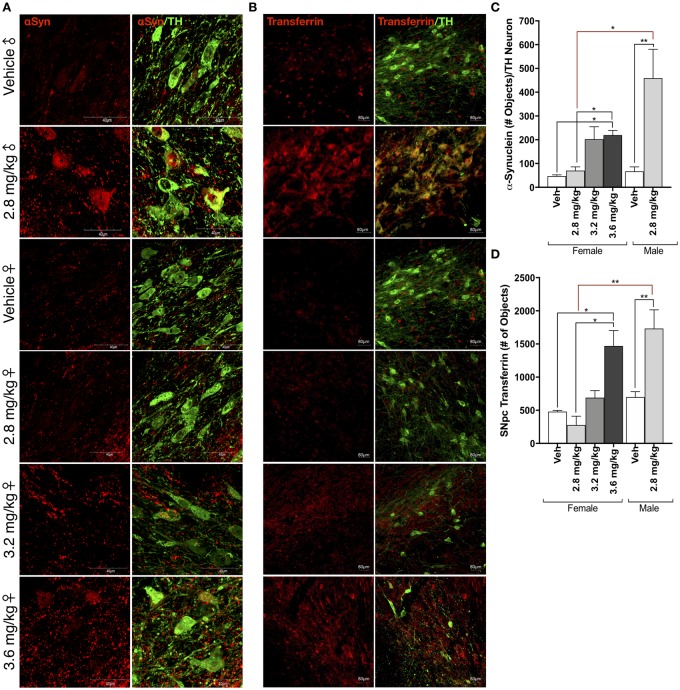
Rotenone treatment causes the accumulation of endogenous α-synuclein within dopaminergic neurons of the SN in male rats (Cannon et al., 2009; Di Maio et al., 2018; Figs. 4a and 4c). In contrast, female rats exposed to 2.8 mg/kg rotenone did not display any measurable increase in α-synuclein protein within dopaminergic neurons or throughout the cytoarchitecture of the ventral midbrain. α-Synuclein accumulation was significantly elevated within TH-positive cells of female animals in the 3.6 mg/kg rotenone dosing group (p = .0396). A direct comparison of α-synuclein accumulation in 2.8 mg/kg rotenone in male animals, and 3.6 mg/kg rotenone in female animals revealed no statistical difference (p = .1884). At the lower dose of 3.2 mg/kg, the increase in α-synuclein with female rat dopaminergic neurons was not statistically significant relative to vehicle (p = .559).
Figure 4.
Sex differences in protein accumulation following rotenone treatment. A, Representative confocal microscopy (×100) of α-synuclein (αSyn, red) within tyrosine hydroxylase (TH)-positive neurons (green) within the substantia nigra (SN) in male and female rats following vehicle or rotenone treatment. B, Representative images (×20 montage) of transferrin (Tf; red) accumulation within tyrosine hydroxylase TH-positive neurons (green) within the SN in male and female rats following vehicle or rotenone treatment. C, Quantification of α-synuclein within TH-immunoreactive cells within the SN (F(1, 15) = 15.12, p = .0015; two-way analysis of variance [ANOVA]). D, Quantification of transferrin within TH-immunoreactive cells within the SN (F(1, 14) = 24.45, p = .0002; two-way ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Transferrin and Iron Metabolism in Male and Female Rats
There is evidence that iron binding and trafficking proteins are sexually dimorphic in the aging human brain (Persson et al., 2015), and excessive iron accumulation within the brain is associated with PD progression and severity (Bartzokis et al., 2007; Persson et al., 2015). Rotenone treatment in male rats and nonhuman primates disrupts iron metabolism and results in the accumulation of iron and iron binding proteins such as transferrin and its receptor (TfR2), within the TH-positive neurons of the SN (Mastroberardino et al., 2009). Similarly, in male rats exposed to 2.8 mg/kg rotenone, there was significant accumulation of transferrin (Tf) within the dopaminergic neurons of the SN (p = .0084; Figs. 4b and 4d). In contrast, only the highest rotenone dose (3.6 mg/kg) produced a significant amount of Tf accumulation in female rat dopaminergic neurons (p = .0226).
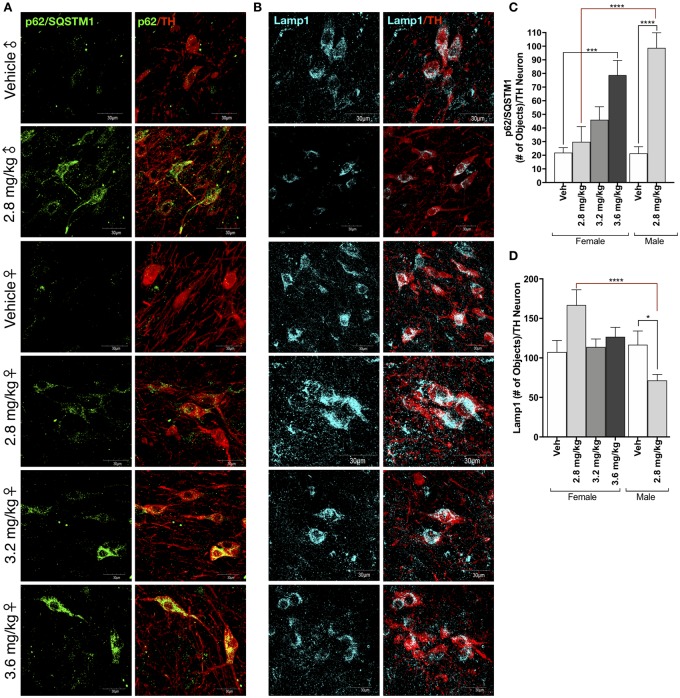
Sex-Dependent Differences in Lysosomal Dysfunction Following Rotenone Treatment
An acute rotenone dosing paradigm (1-dose per day, for 5 days) in male rats using 2.8 mg/kg produces no measurable nigrostriatal neurodegeneration. It does however, result in significant loss of the lysosomal-associated membrane protein (Lamp1) indicative that lysosomal defects precede dopaminergic neurodegeneration (Di Maio et al., 2018). Male rats in this study receiving 2.8 mg/kg/day rotenone displayed significant decreases in Lamp1 protein expression within the surviving dopaminergic neurons of the SN, and this was correlated with a significant increase in p62/SQSTM1 within the same cells (p < .0001; Figs. 5a–d). Interestingly, Lamp1 protein levels were not significantly affected in female rats in any rotenone dosing group, and there was a nonsignificant trend to be mildly increased in animals receiving 2.8 mg/kg rotenone (p = .1769; Fig. 5b and 5d). p62/SQSTM1 accumulated within dopaminergic neurons of female rats in a rotenone dose-dependent manner (Figs. 5a and 5c).
Figure 5.
Sex-dependent differences in lysosomal dysfunction following rotenone treatment. A, Representative confocal microscopy (×100) of the ubiquitin-like protein p62/SQSTM1 (green) within tyrosine hydroxylase (TH)-positive neurons (red) within the substantia nigra (SN) of male and female rats treated with vehicle or rotenone. B, Representative confocal microscopy (×100) of the lysosomal-associated membrane protein (Lamp1) (cyan) within TH-positive neurons (red) within the SN of male and female rats treated with vehicle or rotenone. C, Quantification of the number of p62/SQSTM1-positive puncta within TH-immunoreactive neurons within the SN (F(1, 37) = 26.1, p < .0001; two-way analysis of variance [ANOVA]). D, Quantification of the number of Lamp1-positive puncta within TH-immunoreactive neurons within the SN (F(1, 148) = 25.25, p < .0001; two-way ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
DISCUSSION
Comparative studies identifying sex-dependent variables in human clinical data, in vivo animal studies, or ex vivo cellular assays are complex and often difficult to perform. In addition, the inclusion of sex-specific, experimental animal groups may significantly increase the cost of basic research and preclinical animal models, and decrease statistical power. Despite these challenges, including sex as a variable is essential for comprehensive disease-focused research. This is particularly crucial for PD, which affects 50% more men than women and, once present, affects men and women differentially (Baldereschi et al., 2000; Lubomski et al., 2014; Swerdlow et al., 2001; Van Den Eeden et al., 2003; Wooten et al., 2004). Traditional rodent models of parkinsonism often use one sex of animal (approximately 80% male-only rodent studies), and rarely separate or compare the pathological findings between male and female animals. While the rotenone model of dopaminergic neurodegeneration has been well-characterized in Lewis rats and widely employed for over 15 years (Betarbet et al., 2000; Cannon et al., 2009), there are no data that directly compare male and female neurodegeneration. Because the rotenone model of parkinsonism offers the potential for therapeutic development and preclinical testing (Di Maio et al., 2018; Drolet et al., 2009), it is necessary to fully characterize its effect on female rodents. Here, we investigated rotenone-induced dopaminergic neurodegeneration within the Lewis strain of adult male and female rats, to facilitate the integration of female animals into immediate and future in vivo studies.
Male rats injected with 2.8 mg/kg/day rotenone (i.p.), display an “endpoint” phenotype within 2 weeks of the onset of the study, where endpoint is defined by severe bradykinesia, loss of body mass, and inability to perform motor behavioral testing. Neuropathological analyses from these animals reveal a significant loss of dopaminergic neurons from the SN pars compacta, and their terminal projections to the dorsolateral ST (Betarbet et al., 2000; Cannon et al., 2009; De Miranda et al., 2018; Figure 1). Unexpectedly, age-matched female rats given the same dose display almost no motor phenotype, minimal decline in body mass, and no measurable loss of dopaminergic neurons within the SN or projections to the ST. Although we initially postulated this contrast in pathology may be the result of sex-dependent differences in rotenone metabolism, HPLC-mass spectrometry analysis revealed that rotenone content within brain tissue was equivalent in male and female animals (Figure 1). Importantly, previous work has shown that after bolus administration, specific rotenone binding in brain persists for well over 6 h but is minimal by 24 h (Talpade et al., 2000). Thus, the 6-h time point was chosen as an integrated measure of rotenone distribution, binding, metabolism, and washout.
Rotenone is predominately metabolized by P450 isoenzymes 3A4, 219C, and 2B6 (Caboni et al., 2004). Sex-dependent expression and activity of P450 enzymes is highly variable between species and target compound metabolism (Islam et al., 2017; Pasleau et al., 1980; Waxman and Holloway, 2009), however, the identical content of rotenone parent compound within male and female brains suggests similar brain distribution of the toxin for each sex in this model. To further investigate, we scheduled one group of female animals with bis in die (BID) dosing using a 9-h interval (Udeani et al., 2001) equivalent to 3.2 mg/kg (1.6 mg/kg BID; Supplementary Figure 2). These animals were pathologically indistinguishable from the 3.2 mg/kg dosing group, displaying similar survival time, changes in body mass, and nigrostriatal dopaminergic neurodegeneration, indicating that metabolism in female animals is unlikely to account for sex-dependent variability in rotenone toxicity.
Rotenone treatment in male rats (2.8 mg/kg) produces a robust activation in microglia within the SNpc and surrounding ventral midbrain (De Miranda et al., 2018; Sherer et al., 2003). There is also evidence that rotenone directly activates microglia via proinflammatory signaling pathways, similar in potency to the bacterial endotoxin lipopolysaccharide (Gao et al., 2013; Yuan et al., 2013). The lack of an overt activated microglial phenotype in female rats treated with 2.8 mg/kg rotenone is a strong contrast to male animals at this dose. In part, this may be due to the sensitivity of detecting morphological changes using immunolabeling within fixed tissue, however, the lysosomal protein CD68, increases dose-dependently in female rats treated with rotenone (Figure 3). Together, these data indicate that rotenone does not elicit an equivalent neuroinflammatory response in the female rat brain, even in conditions that produce neurodegeneration (3.2 mg/kg).
Estrogens are likely involved in both neuroinflammation and neurodegeneration (Chakrabarti et al., 2014; Hughes et al., 2009; Sárvári et al., 2011; Siani et al., 2017; Vegeto et al., 2003; Villa et al., 2016), for review see Bjorling and Wang (2001) and Jurado-Coronel et al. (2018), and it is unclear whether endogenous estrogens in female Lewis rats contribute to the observed resistance to rotenone-induced neuroinflammation. Several lines of evidence suggest that estrogen is a contributing factor towards overall risk for idiopathic PD (Gillies and McArthur, 2010; Gillies et al., 2004; Green and Simpkins, 2000; Sárvári et al., 2011; Villa et al., 2016), and should be considered in animal modeling of the disease. In this study, we utilized middle-aged, nonovariectomized retired breeders, roughly equivalent in age to perimenopausal women who might experience early prodromal nonmotor symptoms of PD (de Lau and Breteler, 2006). Furthermore, we note that the goal of this study was to compare and describe sex differences in rotenone toxicity and not to elucidate the mechanisms thereof. Such future studies are undoubtedly important, and the current study represents a first step toward that goal.
A key pathological feature of the rotenone model of parkinsonism is the accumulation of endogenous α-synuclein within the surviving dopaminergic neurons of the SN (Cannon et al., 2009; De Miranda et al., 2018; Di Maio et al., 2018), and we found a requirement for higher doses of rotenone to produce accumulation of α-synuclein in female rat dopaminergic neurons. We have previously shown that α-synuclein accumulation within the dopaminergic neurons of the SN is related to lysosomal deficits, where α-synuclein levels inversely correlate with the Lamp1 (Di Maio et al., 2018). Additionally, in the current study, the autophagy receptor protein, p62/SQSTM1, is increased in male rat dopaminergic neurons following rotenone treatment, indicating that protein degradation and mishandling occurs prior to neuron death. Together, the loss of Lamp1 and accumulation of p62/SQSTM1 suggests a defect in autophagic flux, which might contribute to increased levels of α-synuclein. Interestingly, female rats did not display a decrease in Lamp1—and “ineffective” rotenone doses (2.8 mg/kg) may actually upregulate the lysosomal protein, although this did not reach statistical significance within these study parameters (Figure 5). We also observed a dose-dependent increase in p62/SQSTM1 levels following rotenone treatment in female animals (Figure 5). The increase in p62/SQSTM1 in presence of preserved (or possibly increased) Lamp1, may indicate upregulation of autophagic flux, and this may be partly responsible for the reduced accumulation of α-synuclein observed in female rats. In this context, these data reflect other reports of sex-related differences in autophagy machinery in heart (Campesi et al., 2013) and cerebral cortex (Demarest et al., 2016).
Iron levels within aging human brain tissue are associated with both PD risk and progression (Bartzokis et al., 2010; Matak et al., 2016; Rhodes et al., 2014; Wieler et al., 2015). Additionally, brain iron content appears to be sex-dependent, such that midbrain ferritin and iron levels are higher in men in an aged population of healthy individuals (Bartzokis et al., 2007). Rotenone treatment recapitulates iron accumulation within the SN, measured by significant increases in Tf and its receptor, TfR-2 (Mastroberardino et al., 2009). In the current study, we replicated this result in the male animals treated with 2.8 mg/kg rotenone (Figure 4), and showed increased Tf within the SN. Female rats did not show significant Tf protein accumulation following rotenone except at the highest dose (3.6 mg/kg). The presence of Tf protein within the SN following rotenone exposure correlates with a pathological increase in iron (Kotze et al., 2009) and it may also suggest a defect in endosomal trafficking. Taken together, male rats appear to have a lower threshold for iron-induced toxicity following rotenone exposure, and female rats require a higher toxin dose to produce equivalent pathology.
Parkinson’s disease is more common in men than women, but disease risk is influenced by genetics, lifestyle, and environmental exposures. Current animal models do not allow the incorporation of all variables affecting human PD risk; however, including sex as a variable should be considered standard in future mechanistic and translational studies. Doing so in the current study revealed that (i) females are relatively resistant to equivalent concentrations of rotenone, (ii) show less inflammation and (iii) less accumulation of α-synuclein and Tf, possibly as a result of (iv) preserved autophagy. Whether such sex differences will translate into differences in responses to mechanism-driven therapeutic interventions remains to be determined.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
National Institute of Neurological Disorders and Stroke (R01NS095387, R01NS100744 to J.T.G.); National Institute of Environmental Health Sciences (K99ES029986 to B.R.D. and R21ES027470 to J.T.G.); Parkinson’s Foundation (Postdoctoral Fellowship Transition Award PF-PBS-1892 to B.R.D.); the American Parkinson Disease Association, the Commonwealth of Pennsylvania DHS (4100080420 [project # 601457] to J.T.G.); and the friends and family of Sean Logan.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Meghan Bucher for her technical assistance in these studies.
REFERENCES
- Aksoy D., Solmaz V., Çavuşoğlu T., Meral A., Ateş U., Erbaş O. (2017). Neuroprotective effects of exenatide in a rotenone-induced rat model of Parkinson's disease. Am. J. Med. Sci. 354, 319–324. [DOI] [PubMed] [Google Scholar]
- Anusha C., Sumathi T., Joseph L. D. (2017). Protective role of apigenin on rotenone induced rat model of Parkinson's disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 269, 67–79. [DOI] [PubMed] [Google Scholar]
- Baldereschi M., Di Carlo A., Rocca W. A., Vanni P., Maggi S., Perissinotto E., Grigoletto F., Amaducci L., Inzitari D. (2000). Parkinson's disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 55, 1358–1363. [DOI] [PubMed] [Google Scholar]
- Bartzokis G., Lu P. H., Tishler T. A., Peters D. G., Kosenko A., Barrall K. A., Finn J. P., Villablanca P., Laub G., Altshuler L. L. (2010). Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. J. Alzheimers Dis. 20, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Tishler T. A., Lu P. H., Villablanca P., Altshuler L. L., Carter M., Huang D., Edwards N., Mintz J. (2007). Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging 28, 414–423. [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Bjorling D. E., Wang Z. Y. (2001). Estrogen and neuroinflammation. Urology 57, 40–46. [DOI] [PubMed] [Google Scholar]
- Brann D. W., Dhandapani K., Wakade C., Mahesh V. B., Khan M. M. (2007). Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 72, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboni P., Sarais G., Vargiu S., Luca M. A., Garau V. L., Ibba A., Cabras P. (2008). LC–MS–MS determination of rotenone, deguelin, and rotenolone in human serum. Chroma 68, 739–745. [Google Scholar]
- Caboni P., Sherer T. B., Zhang N., Taylor G., Na H. M., Greenamyre J. T., Casida J. E. (2004). Rotenone, deguelin, their metabolites, and the rat model of Parkinson's disease. Chem. Res. Toxicol. 17, 1540–1548. [DOI] [PubMed] [Google Scholar]
- Campesi I., Straface E., Occhioni S., Montella A., Franconi F. (2013). Protein oxidation seems to be linked to constitutive autophagy: A sex study. Life Sci. 93, 145–152. [DOI] [PubMed] [Google Scholar]
- Cannon J. R., Tapias V., Na H.-M., Honick A. S., Drolet R. E., Greenamyre J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 34, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capello E., Butcher G. Q. (2014). Assessment of the male sex bias in Parkinson's research on non-human subjects: A meta-analysis. IMPULSE: The Premier Undergraduate Neuroscience Journal, 1–6. [Google Scholar]
- Chakrabarti M., Haque A., Banik N. L., Nagarkatti P., Nagarkatti M., Ray S. K. (2014). Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 109, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech D. P., Lee J., Correia J., Loke H., Möller E. K., Harley V. R. (2014). Transient neuroprotection by SRY upregulation in dopamine cells following injury in males. Endocrinology 155, 2602–2612. [DOI] [PubMed] [Google Scholar]
- Czech D. P., Lee J., Sim H., Parish C. L., Vilain E., Harley V. R. (2012). The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J. Neurochem. 122, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau L. M., Breteler M. M. (2006). Epidemiology of Parkinson's disease. Lancet Neurol. 5, 525–535. [DOI] [PubMed] [Google Scholar]
- De Miranda B. R., Rocha E. M., Bai Q., Ayadi El, A., Hinkle D., Burton E. A., Timothy Greenamyre J. (2018). Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson's disease. Neurobiol. Dis. 115, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest T. G., Waite E. L., Kristian T., Puche A. C., Waddell J., McKenna M. C., Fiskum G. (2016). Sex-dependent mitophagy and neuronal death following rat neonatal hypoxia-ischemia. Neuroscience 335, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P., Chiang C. W. K., Sinchak K., Sim H., Fernagut P.-O., Kelly S., Chesselet M.-F., Micevych P. E., Albrecht K. H., Harley V. R., et al. (2006). Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 16, 415–420. [DOI] [PubMed] [Google Scholar]
- Di Maio R., Hoffman E. K., Rocha E. M., Keeney M. T., Sanders L. H., De Miranda B. R., Zharikov A., Van Laar A., Stepan A. F., Lanz T. A., et al. (2018). LRRK2 activation in idiopathic Parkinson's disease. Sci. Transl. Med. 10, eaar5429.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet R. E., Cannon J. R., Montero L., Greenamyre J. T. (2009). Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol. Dis. 36, 96–102. [DOI] [PubMed] [Google Scholar]
- Gao F., Chen D., Hu Q., Wang G. (2013). Rotenone directly induces BV2 cell activation via the p38 MAPK pathway. PLoS One 8, e72046.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., McArthur S. (2010). Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson's disease: A contribution to sex-specific neuroprotection by estrogens. Horm. Behav. 57, 23–34. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Murray H. E., Dexter D., McArthur S. (2004). Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson's disease. Pharmacol. Biochem. Behav. 78, 513–522. [DOI] [PubMed] [Google Scholar]
- Green P. S., Simpkins J. W. (2000). Neuroprotective effects of estrogens: Potential mechanisms of action. Int. J. Dev. Neurosci. 18, 347–358. [DOI] [PubMed] [Google Scholar]
- Hughes Z. A., Liu F., Marquis K., Muniz L., Pangalos M. N., Ring R. H., Whiteside G. T., Brandon N. J. (2009). Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr. Mol. Pharmacol. 2, 215–236. [DOI] [PubMed] [Google Scholar]
- Islam M. M., Iqbal U., Walther B. A., Nguyen P.-A., Li Y.-C. J., Dubey N. K., Poly T. N., Masud J. H. B., Atique S., Syed-Abdul S. (2017). Gender-based personalized pharmacotherapy: A systematic review. Arch. Gynecol. Obstet. 295, 1305–1317. [DOI] [PubMed] [Google Scholar]
- Javed H., Azimullah S., A. Khair S. B., Ojha S., Haque M. E. (2016). Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 17, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado-Coronel J. C., Cabezas R., Ávila Rodríguez M. F., Echeverria V., García-Segura L. M., Barreto G. E. (2018). Sex differences in Parkinson's disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics. Front. Neuroendocrinol. 50, 18–30. [DOI] [PubMed] [Google Scholar]
- Khadrawy Y. A., Salem A. M., El-Shamy K. A., Ahmed E. K., Fadl N. N., Hosny E. N. (2017). Neuroprotective and therapeutic effect of caffeine on the rat model of Parkinson's disease induced by rotenone. J. Diet Suppl. 14, 553–572. [DOI] [PubMed] [Google Scholar]
- Kotze M. J., v. Velden D. P., van Rensburg S. J., Erasmus R. (2009). Pathogenic mechanisms underlying iron deficiency and iron overload: New insights for clinical application. EJIFCC 20, 108–123. [PMC free article] [PubMed] [Google Scholar]
- Lubomski M., Louise Rushworth R., Lee W., Bertram K. L., Williams D. R. (2014). Sex differences in Parkinson's disease. J. Clin. Neurosci. 21, 1503–1506. [DOI] [PubMed] [Google Scholar]
- Mastroberardino P. G., Hoffman E. K., Horowitz M. P., Betarbet R., Taylor G., Cheng D., Na H.-M., Gutekunst C.-A., Gearing M., Trojanowski J. Q., et al. (2009). A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol. Dis. 34, 417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matak P., Matak A., Moustafa S., Aryal D. K., Benner E. J., Wetsel W., Andrews N. C. (2016). Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc. Natl. Acad. Sci. U.S.A. 113, 3428–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasleau F., Kremers P., Gielen J. E. (1980). Sex differences in the activity of different cytochrome P450 dependent steroid 16 alpha-hydroxylases in rat liver. J. Steroid Biochem. 13, 733–736. [DOI] [PubMed] [Google Scholar]
- Persson N., Wu J., Zhang Q., Liu T., Shen J., Bao R., Ni M., Liu T., Wang Y., Spincemaille P. (2015). Age and sex related differences in subcortical brain iron concentrations among healthy adults. NeuroImage 122, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M., Nicoletti A., Fetoni V., Garavaglia B., Barone P., Pellecchia M. T. (2017). The relevance of gender in Parkinson's disease: A review. J. Neurol. 264, 1583–1607. [DOI] [PubMed] [Google Scholar]
- Rhodes S. L., Buchanan D. D., Ahmed I., Taylor K. D., Loriot M.-A., Sinsheimer J. S., Bronstein J. M., Elbaz A., Mellick G. D., Rotter J. I., et al. (2014). Pooled analysis of iron-related genes in Parkinson's disease: Association with transferrin. Neurobiol. Dis. 62, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárvári M., Hrabovszky E., Kalló I., Solymosi N., Tóth K., Likó I., Széles J., Mahó S., Molnár B., Liposits Z. (2011). Estrogens regulate neuroinflammatory genes via estrogen receptors α and β in the frontal cortex of middle-aged female rats. J. Neuroinflammation 8, 82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Session D. R., Pearlstone M. M., Jewelewicz R., Kelly A. C. (1994). Estrogens and Parkinson's disease. Med. Hypotheses 42, 280–282. [DOI] [PubMed] [Google Scholar]
- Sherer T. B., Betarbet R., Kim J.-H., Greenamyre J. T. (2003). Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci. Lett. 341, 87–90. [DOI] [PubMed] [Google Scholar]
- Siani F., Greco R., Levandis G., Ghezzi C., Daviddi F., Demartini C., Vegeto E., Fuzzati-Armentero M.-T., Blandini F. (2017). Influence of estrogen modulation on glia activation in a murine model of Parkinson's disease. Front. Neurosci. 11, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H., Parker W. D., Currie L. J., Bennett J. P., Harrison M. B., Trugman J. M., Wooten G. F. (2001). Gender ratio differences between Parkinson's disease patients and their affected relatives. Parkinsonism Relat. Disord. 7, 129–133. [DOI] [PubMed] [Google Scholar]
- Talpade D. J., Greene J. G., Higgins D. S., Greenamyre J. T. (2000). In vivo labeling of mitochondrial complex I (NADH: ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J. Neurochem. 75, 2611–2621. [DOI] [PubMed] [Google Scholar]
- Tapias V., Cannon J. R., Greenamyre J. T. (2017). Melatonin treatment potentiates neurodegeneration in a rat rotenone Parkinson's disease model. J. Neurosci. Res. 88, 420–427. [DOI] [PubMed] [Google Scholar]
- Tapias, V., Greenamyre, J. T. and Watkins, S. C. (2013). Automated imaging system for fast quantitation of neurons, cell morphology and neurite morphometry in vivo and in vitro. Neurobiology of Disease54, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeani G. O., Zhao G. M., Shin Y. G., Kosmeder J. W., Beecher C. W., Kinghorn A. D., Moriarty R. M., Moon R. C., Pezzuto J. M. (2001). Pharmacokinetics of deguelin, a cancer chemopreventive agent in rats. Cancer Chemother. Pharmacol. 47, 263–268. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden S. K., Tanner C. M., Bernstein A. L., Fross R. D., Leimpeter A., Bloch D. A., Nelson L. M. (2003). Incidence of Parkinson's disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 157, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Belcredito S., Etteri S., Ghisletti S., Brusadelli A., Meda C., Krust A., Dupont S., Ciana P., Chambon P., et al. (2003). Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. U.S.A. 100, 9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Vegeto E., Poletti A., Maggi A. (2016). Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev. 37, 372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Holloway M. G. (2009). Sex differences in the expression of hepatic drug metabolizing enzymes. Mol. Pharmacol. 76, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieler M., Gee M., Martin W. R. W. (2015). Longitudinal midbrain changes in early Parkinson's disease: Iron content estimated from R2*/MRI. Parkinsonism Relat. Disord. 21, 179–183. [DOI] [PubMed] [Google Scholar]
- Woodlee M. T., Kane J. R., Chang J., Cormack L. K., Schallert T. (2008). Enhanced function in the good forelimb of hemi-parkinson rats: Compensatory adaptation for contralateral postural instability? Exp. Neurol. 211, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten G. F., Currie L. J., Bovbjerg V. E., Lee J. K., Patrie J. (2004). Are men at greater risk for Parkinson's disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.-H., Sun J.-D., Wu M.-M., Hu J.-F., Peng S.-Y., Chen N.-H. (2013). Rotenone could activate microglia through NFκB associated pathway. Neurochem. Res. 38, 1553–1560. [DOI] [PubMed] [Google Scholar]
- Zharikov A. D., Cannon J. R., Tapias V., Bai Q., Horowitz M. P., Shah V., Ayadi El, A., Hastings T. G., Greenamyre J. T., et al. (2015). shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J. Clin. Invest. 125, 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.