Abstract
3, 4-Methylenedioxymethamphetamine (MDMA) is a hallucinogenic amphetamine derivative. The acute effects of MDMA are hyperthermia, hyperactivity, and behavioral changes, followed by long-term serotonergic neurotoxicity in rats and primates. However, the underlying mechanisms of MDMA neurotoxicity remain elusive. We reported that pretreatment of rats with Ro 4-1284, a reversible inhibitor of the vesicular monoamine transporter 2 (VMAT2), reduced MDMA-induced hyperactivity in rats, abolished the hyperthermic response, and the long-term neurotoxicity. Current studies focused on the effects of co- and/or postinhibition of VMAT2 on the acute and chronic effects of MDMA and on the dose-response relationship between MDMA-induced elevations in body temperature and subsequent reductions in indolamine concentrations. Sprague Dawley rats were treated with MDMA (20, 25, or 27.5 mg/kg sc), and either co- and/or posttreatment with the VMAT2 inhibitor (10 mg/kg ip). Rats simultaneously treated with Ro 4-1284 and MDMA exhibited a more rapid increase in body temperature compared to just MDMA. However, the duration of the elevated body temperature was significantly shortened (approximately 3 h vs approximately 8 h, respectively). A similar body temperature response was observed in rats posttreated (7 h after MDMA) with Ro 4-1284. Despite decreases in the area under the curve (Δtemp X time) of body temperature caused by Ro 4-1284, there were no significant differences in the degree of indolamine depletion between any of the MDMA-treated groups. The results suggest that the neuroprotective effects of VMAT2 inhibition is likely due to the indirect monoamine depleting effects of the Ro 4-1284 pretreatment, rather than by the direct inhibition of VMAT2 function.
Keywords: MDMA, VMAT2, Ro 4-1284, hyperthermia, neurotoxicity
3, 4-(±)-Methylenedioxymethamphetamine (MDMA) is a psychostimulant amphetamine derivative. MDMA has biphasic neuropharmacological effects. Initially, MDMA increases synaptic monoamine levels, mainly serotonin (5-HT), leading to euphoria, increased empathy, hyperesthesia, hyperactivity, mild hallucinations, and physiological changes, mainly hyperthermia. Due to its short-term effects, MDMA is abused at “rave parties,” along with other drugs, rendering abusers susceptible to a higher degree of injury. The major long-term effects of MDMA exposure in rats and in primates is serotonergic neurotoxicity, manifest as a sustained depletion in 5-HT, reductions in 5-HT axonal density, and increased inflammation (Capela et al., 2009; Hatzidimitriou et al., 1999; Moratalla et al., 2017). In the initial phase; 5-HT levels decline within 3 h of MDMA administration, but return to normal levels by 24 h. The second phase of MDMA toxicity begins 1 day after administration, at which time 5-HT levels begin to gradually decrease, declining to 50% of control levels by 1 week after exposure (Schmidt, 1987). MDMA-induced inflammation is also biphasic, where acute induction of microglia subsides after 24 h followed by gradual increase of long-term inflammation (Herndon et al. 2014). 5-HT uptake sites also decline after MDMA administration and remain 75% of control levels at 6 months, returning to control levels 1 year after MDMA administration (Battaglia et al., 1988; Stone et al., 1986). However, the underlying multifactorial mechanisms of MDMA neurotoxicity remain unclear.
MDMA’s ability to increase synaptic 5-HT, dopamine (DA), and norepinephrine (NE) is attributed to reversal of the 5-HT, DA, and NE reuptake transporters (SERT, DAT, and NET, respectively) (Berger et al., 1992; Crespi et al., 1997; Gudelsky and Nash, 2002; Ramamoorthy and Blakely, 1999). MDMA also disrupts vesicular monoamine transporter 2 (VMAT2) function (Partilla et al., 2006), the main amine transporter on storage vesicles. Furthermore, MDMA partially inhibits monoamine oxidase (MAO)-A, and to a lesser extend MAO-B (Leonardi and Azmitia, 1994; Steuer et al., 2016), enzymes involved in 5-HT, DA, and NE metabolism, located on mitochondrial membranes and in glial cells. Amphetamines and related compounds, including MDMA, also disrupt the pH gradient across vesicle membranes, which are important for appropriate VMAT2 function (Fleckenstein and Hanson, 2003). In summary, MDMA increases cytoplasmic neurotransmitter levels by inhibiting their storage and degradation and causes carrier-mediated neurotransmitter release leading to increases in neurotransmitter levels in the synaptic cleft.
The mechanism(s) by which MDMA induces long-term neurotoxicity is unlikely due to a single factor, but multifactorial. MDMA causes acute hyperthermia, inhibition of which is protective against MDMA neurotoxicity (Capela et al., 2009; Moratalla et al., 2017). However, repeated administration of MDMA (4 mg/kg, twice per day, 4 days) to rats produces long-term neurotoxicity in the absence of marked hyperthermia (O’Shea et al., 1998). The long-term neurotoxicity of MDMA may even occur under hypothermic conditions (O’Shea et al., 2006). Although compounds that inhibit MDMA neurotoxicity also tend to inhibit MDMA-induced acute hyperthermia (Farfel and Seiden, 1995; Malberg et al., 1996; Soleimani Asl et al., 2013), such compounds (ketanserine, MK801, N-nitro-L-arginine) can offer protection in a temperature-independent manner (Capela et al., 2006, 2007). For example, inhibition of MAO-B provides neuroprotection against MDMA in the presence of acute hyperthermia (Alves et al., 2007; Sprague and Nichols, 1995). Finally, we have shown that acivicin-mediated potentiation of MDMA neurotoxicity occurs in the context of acivicin-mediated decreases in rectal body temperature (Jones et al., 2005). Thus, hyperthermia may be neither sufficient nor necessary for MDMA-induced long-term neurotoxicity.
Our own studies, and those of others, have demonstrated the importance of SERT and VMAT2 function in the short- and long-term effects of MDMA. Pharmacological inhibition of SERT by selective 5-HT reuptake inhibitors, or SERT knockout, results in protection against MDMA-induced long-term neurotoxicity without reversal of hyperthermia (Li et al., 2010; Lizarraga et al., 2014; Orio et al., 2004; Sanchez et al., 2001). Furthermore, pretreatment of rats with a reversible VMAT2 inhibitor, Ro 4-1284, 1 h prior to MDMA administration, attenuates hyperactivity, hyperthermia, and long-term serotonergic neurotoxicity (Lizarraga et al., 2015). However, these studies could not distinguish whether the protective effects of Ro 4-1284 were due to inhibition of VMAT2 function or due to depletion of monoamines. We suspect that the protective effects of Ro 4-1284 are not a direct function of VMAT2 inhibition, but rather are due to its ability to deplete catecholamines. In the present study, we therefore investigated the mechanism by which Ro 4-1284 affords protection against MDMA-induced neurotoxicity by examining the effects of co- or posttreatment with Ro 4-1284 on MDMA-mediated hyperthermia and indolamine concentrations. We also examined the dose-response relationship between MDMA-mediated effects on body temperature and subsequent (7-day posttreatment) effects on indolamine concentrations.
MATERIALS AND METHODS
Drugs and chemicals
(±)MDMA-HCl was obtained from the NIDA Drug Supply Program (Bethesda, Maryland). The VMAT2 inhibitor, Ro 4-1284 (2-hydroxy-2-ethyl-3-isobutyl-9, 10-dimethoxy-1, 2, 3, 4, 5, 6, 7-hexahydrobenzo[a]chinolizine), was purchased from Sigma-Aldrich.
Animals
Male Sprague Dawley rats, weighing approximately 200 g (approximately 50 days old), were obtained from Harlan Laboratories (Indianapolis, Indiana) and housed in groups of 3–4 per cage on a 12:12 h light/dark cycle (lights on at 07:00 h). Food and water were provided ad libitum. Animals were acclimated for 1 week prior to initiating experiments. Procedures were carried out in accordance with the University of Arizona Institutional Animal Care and Use Committee.
Dosing
Pharmacological inhibition of VMAT2 was achieved by co- and/or posttreatment with Ro 4-1284 (10 mg/kg, ip) dissolved in DMSO (0.667 ml/kg). During the experiments animals were housed in groups of 3–4 per cage. Lizarraga et al. (2015) demonstrated that Ro 4-1284 (10 mg/kg, ip) treatment alone does not affect acute animal behavior or hyperthermia in response to MDMA, nor does it have any effect on long-term neurotransmitter levels. Consequently, the Ro 4-1284 alone treatment group was not replicated in accordance with the ethics of avoiding unnecessary animal use. Animals dosed with MDMA (20, 25, and/or 27.5 mg/kg, sc) received a single injection of Ro 4-1284 immediately after the MDMA dose, or 7 h after the MDMA dose. MDMA hyperthermia subsides after 7 h, and therefore this timepoint was chosen specifically for posttreatment with Ro 4-1284 so that the inhibitor did not interfere with MDMA-induced hyperthermia. Principles of interspecies scaling predict a dose of 1.28 mg/kg (96 mg/75 kg) in humans to be equivalent to a neurotoxic dose of MDMA of 20 mg/kg in rats (Ricaurte et al., 2000). Recreational doses of MDMA have been found to range from 75 to 125 mg of (±)-MDMA for a single dose, which are within the neurotoxic threshold dictated by interspecies scaling (Ricaurte et al., 2000).
Core body temperature
Mini subcue data-loggers from SubCue (Calgary, Canada) were implanted into the peritoneal cavity. Animals were anesthetized with isoflurane (5% in air) and kept under anesthesia with 2%–3% isoflurane in air for the duration of the surgery. A small incision (approximately 1.5 cm) was made along the top of the right leg, where the temperature data-logger was inserted. Rats were permitted a 4-day recovery period. Body temperature was monitored for 24 h prior to any treatment to establish a baseline, and 48 h posttreatment, at intervals of 5 min. Ambient temperature was maintained at approximately 22°C prior to and during the entire treatment period.
Neurotransmitter isolation
Animals were euthanized 7 days after MDMA administration via CO2 asphyxiation, followed by decapitation. Brains were excised and the frontal cortex and striatum microdissected for subsequent neurotransmitter concentration analysis. Tissue was weighed and suspended in 10 volumes of ice-cold 0.1 M perchloric acid (170 μM EDTA and 263 μM octane-sulfonic acid sodium salt). The tissue was then sonicated for 15 s followed by centrifugation at 16 000 × g (4°C) for 20 min. The resulting supernatant was filtered at 0.45 μm and used for monoamine detection via high performance liquid chromatography coupled to an electrochemical detector (HPLC-ECD).
HPLC-ECD
Neurotransmitter analysis was achieved using a Shimadzu 10ADvp system equipped with an EICOMPAK SC-3 ODS, 3 μm, 3 × 100 mm column (San Diego, California) coupled to an EICOM ECD 700 system with a Power Chrome EPC 500 data processor (San Diego, California). The mobile phase consisted of 42 mM citric acid monohydrate, 3.78 M sodium acetate, 786 μM octane-sulfonic acid sodium salt, 17 μM EDTA, and 20% (vol/vol) methanol, pH 3.5, at a flow rate of 0.345 ml/min. Potentials were set at + 750 mV. Aliquots of tissue supernatant (30 μl) were used per injection and peak areas were compared to a standard curve of DA, 3, 4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-Hydroxyindoleacetic acid (5-HIAA) to achieve quantitation.
Statistics
Results are expressed as the mean ± SE (n = 3–10). Effects of MDMA on core body temperature were analyzed by two-way ANOVA (treatment group × time interval) with time as a repeated measure followed by Bonferroni’s post hoc tests. Peak body temperature, temperature area under the curve (AUC) (Δtemp X time), and neurotransmitter concentrations were analyzed with one-way ANOVA followed by Bonferroni’s post hoc tests. All analyses were performed using GraphPad Prism software (Version 6.07, La Jolla, California).
RESULTS
Co- and Posttreatment With a VMAT2 Antagonist Alter MDMA-induced Hyperthermia
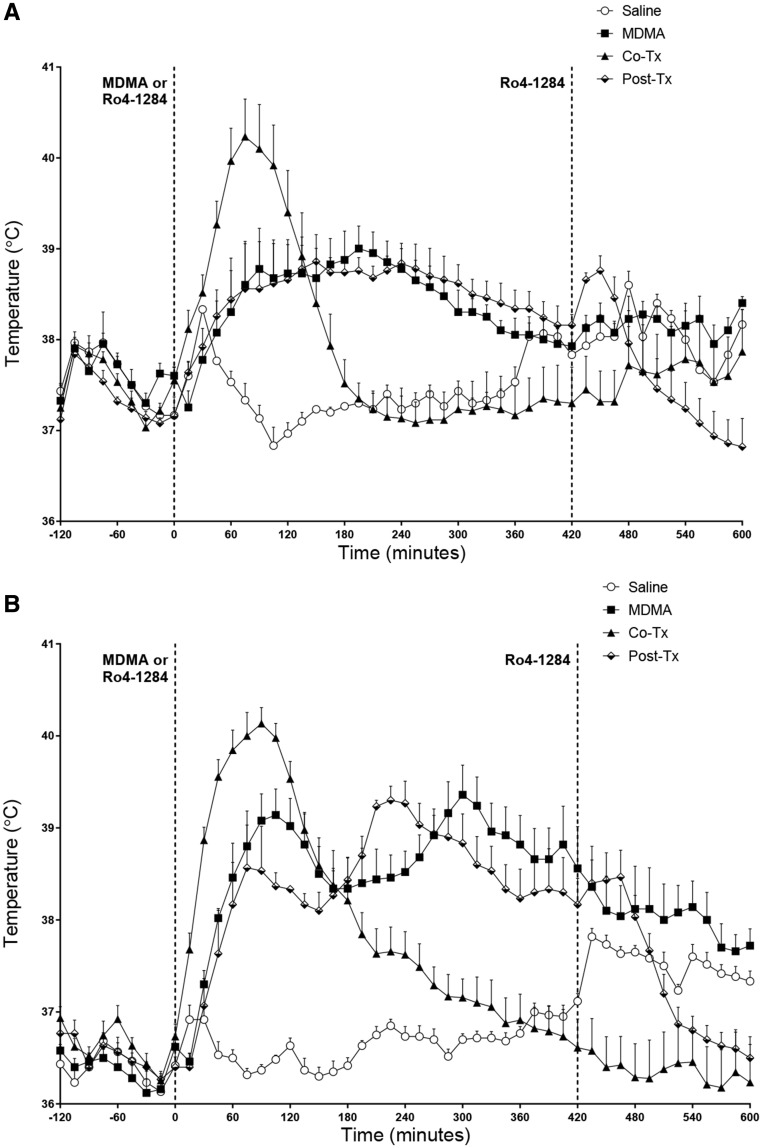
Cotreatment of animals with Ro 4-1284 and 20 mg/kg MDMA resulted in a sharp increase in body temperature relative to animals treated with MDMA alone, or with MDMA followed by posttreatment (+ 7 h) with Ro 4-1284 (Figure 1A). Similarly, animals treated with 27.5 mg/kg MDMA and cotreated with 10mg/kg of Ro 4-1284 experienced a more rapid rise in body temperature relative to animals treated with MDMA alone or with MDMA followed by posttreatment with Ro 4-1284 (Figure 1B). However, the duration of the hyperthermia was curtailed in the animals cotreated with Ro 4-1284 relative to the animals treated with MDMA alone, or with MDMA and posttreated with Ro 4-1284, with body temperature returning to those of controls after approximately 3–6 h (Figs. 1A and 1B). Although two-way repeated measures ANOVA of body temperature for the 20 mg/kg MDMA groups (Figure 1A) did not reveal significant effects between treatment groups, significant effects of time interval (F[432, 2016] = 5.688, p < .0001), and treatment × time interaction (F[144, 2016] = 6.683, p < .0001) were observed. Bonferroni’s post hoc tests confirmed a significant hyperthermic effect in animals cotreated with 20 mg/kg MDMA and Ro 4-1284 when compared with their saline counterparts, between 50 and 135 min after treatment. The 20 mg/kg MDMA/Ro 4-1284 cotreatment group also exhibited significant intermittent increases (approximately 1 h) and decreases (approximately 4 h) in body temperature compared to the groups receiving either 20 mg/kg MDMA alone or posttreated with Ro 4-1284.
Figure 1.
Ro 4-1284 co- and/or posttreatment does not affect 3, 4-methylenedioxymethamphetamine (MDMA)-induced hyperthermia. Body temperature was recorded using temperature data-loggers implanted in the intraperitoneal cavity. A, 20 mg/kg MDMA dose. B, 27.5 mg/kg MDMA dose. Results are expressed as means ± SE (n = 3–9) and represent temperature measurements collected from all 4 treatment groups.
Selection of the time at which posttreatment with Ro 4-1284 was based on prior studies showing that the body temperature in MDMA-treated animals returned to those of saline-treated counterparts after approximately 7 h (Lizarraga et al., 2015), and these findings were replicated in the present study (Figure 1). Two-way repeated measures ANOVA of body temperature for animals treated with 25 mg/kg MDMA (Supplementary Figure 1) revealed a significant effect of treatment group (F[1, 10] = 47.57, p < .0001), time interval (F[167, 1670] = 6.999, p < .0001), and treatment × time interaction (F[167, 1670] = 8.410, p < .0001). Bonferroni’s post hoc tests for the 27.5 mg/kg MDMA group (Figure 1B) showed a significant increase in hyperthermia relative to their saline counterparts between 0.75 and 7 h. Two-way repeated measures ANOVA of body temperature for 27.5 mg/kg MDMA group (Figure 1B) showed significant effects of treatment group (F[3, 19] = 11.21, p < .001), significant effects of time interval (F[144, 2736] = 27.42, p < .0001), and a significant treatment × time interaction (F[432, 2736] = 14.38, p < .0001).
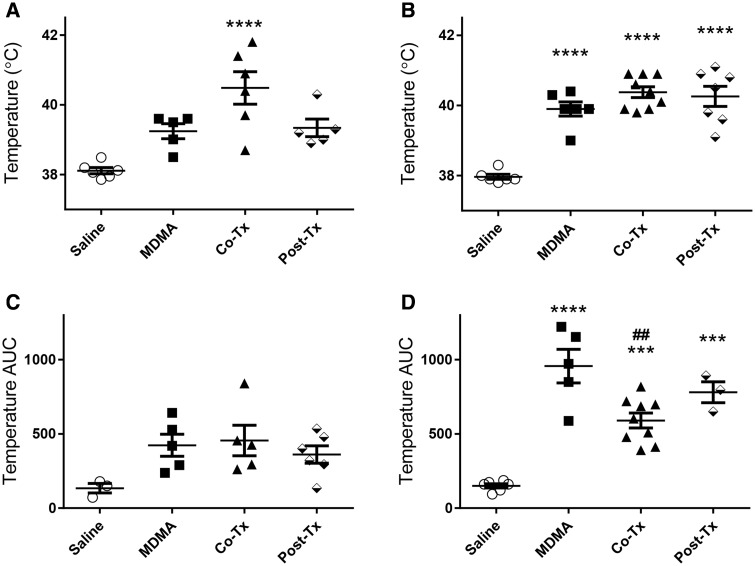
Bonferroni’s post hoc tests confirmed a significant increase in body temperature in animals receiving 27.5 mg/kg MDMA (Figure 1B) and then posttreated with Ro 4-1284 compared to their saline counterparts (0.75–7 h). As previously mentioned, the 27.5 mg/kg MDMA-cotreatment group showed significant increases in body temperature from 30 min to 3 h 20 min compared to controls. Similarly, the 27.5 mg/kg MDMA-cotreatment group also caused a significant increase (approximately 1 h) with a subsequent significant decrease (approximately 4 h) in body temperature compared to 27.5 mg/kg MDMA alone and 27.5 mg/kg MDMA-posttreatment groups (Figure 1B). It is interesting to note that both MDMA-posttreatment groups (20 and 27.5 mg/kg) exhibited a dramatic decrease in body temperature when Ro 4-1284 was administered 7 h post MDMA treatment, with a significant decrease in the 27.5 mg/kg MDMA-posttreatment group compared to 27.5 mg/kg MDMA alone (Figure 1B). One-way ANOVA analysis (F[3, 18] = 11.29, p < .001) with Bonferroni’s post hoc tests of peak body temperature (Figs. 2A and 2B) showed a significant increase in the 20 mg/kg MDMA-cotreatment group (Figure 2A) compared to controls. However, we did not observe significant hyperthermic changes in either the 20 mg/kg MDMA alone or 20 mg/kg MDMA-posttreatment group. Two-tailed t test of 25 mg/kg MDMA group (Supplementary Figure 2) showed the MDMA-treated group had significantly higher peak body temperature compared to controls. One-way ANOVA analysis of the 27.5 mg/kg group’s peak body temperature (F[3, 24] = 29.91, p < .0001) with Bonferroni’s multiple comparisons test showed a significant increase in all treatment groups. For temperature AUC analysis (Figs. 2C and 2D), the average of each control group over the duration of the experiment was considered as baseline (20 mg/kg = 37.61°C, 25 mg/kg = 36.89°C, and 27.5 mg/kg = 36.83°C). One-way ANOVA analysis with Bonferroni’s post hoc tests of temperature AUC showed no significant difference in any of the 20 mg/kg MDMA-treated groups (Figure 2D). However, all other groups revealed a significant increase in body temperature AUC compared to controls (Figure 2D, Supplementary Figure 2). One-way ANOVA analysis of 27.5 mg/kg group’s temperature AUC (F[3, 19] = 25.95, p < .0001) with Bonferroni’s multiple comparisons test showed a significant increase in all treatment groups. Noteworthy is the significant decrease in temperature AUC (2-fold) of the 27.5 mg/kg MDMA-cotreatment group compared to the 27.5 mg/kg MDMA alone group (Figure 2D). In summary, although co- and/or posttreatment of Ro 4-1284 with MDMA does not fully abolish hyperthermia, the cotreatment significantly reduces the period of time during which animals experience hyperthermia. However, because the extent of hyperthermia is much higher in cotreatment groups AUC data should be interpreted with caution.
Figure 2.
Ro 4-1284 treatment does not significantly affect peak body temperature but partially decreases body temperature AUC. Peak body temperature (A-B) and body temperature AUC (C-D). Graphs represent peak body temperature of (A) 20 mg/kg MDMA dose and (B) 27.5 mg/kg MDMA dose. Graphs represent body temperature AUC of (C) 20 mg/kg MDMA dose and (D) 27.5 mg/kg MDMA dose. AUC was calculated by a baseline body temperature set at the average of saline body temperature over the entire experiment. Values are different from the saline group at ***p < .001, ****p < .0001, and from MDMA group at ##p < .01.
Co- and Posttreatment With a VMAT2 Antagonist Does Not Protect Against MDMA-induced Neurotoxicity
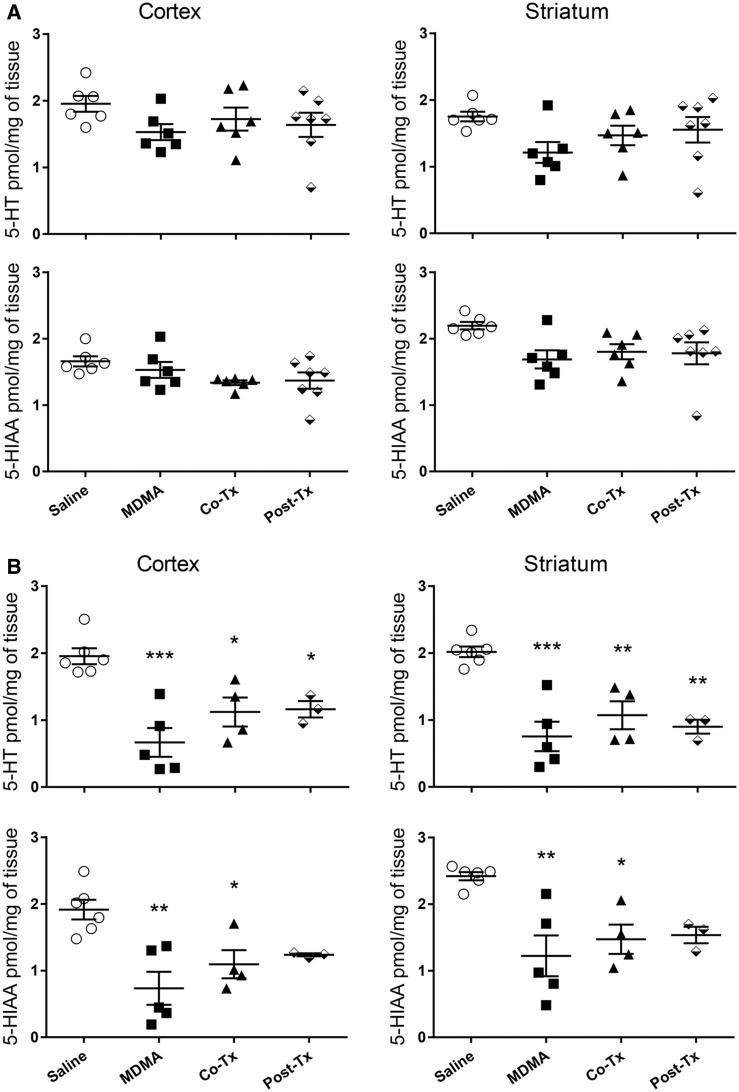
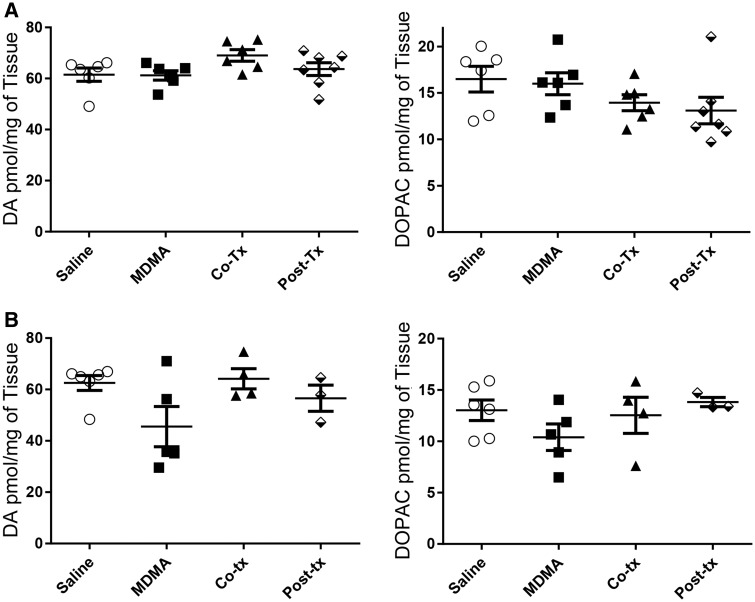
One week after the treatment of the animals with the MDMA/Ro 4-1284 dosing regimen, animals were euthanized and assessed for indolamine depletions, an accepted marker of serotonergic neurotoxicity in rats (Capela et al., 2009). Although there were slight reductions of indolamines in animals treated with the lowest dose of MDMA (20 mg/kg), these changes did not reach statistical significance (Figure 3A). In contrast, significant decreases occurred in 5-HT and 5-HIAA levels in animals treated with either 25 or 27.5 mg/kg MDMA, compared to the saline controls (Figure 3B, 3S) with the highest dose causing a > 60% decrease in 5-HT levels in the striatum and cortex (approximately 2 pmol 5-HT/mg of tissue for control compared to approximately 0.7 pmol 5-HT/mg tissue for MDMA alone). Although both the co- and posttreatment Ro 4-1284 protocols caused a minor attenuation in MDMA-induced depletions in 5-HT and 5-HIAA, these levels were not significantly different from animals treated with MDMA alone. Catecholamine levels were analyzed in the striatum of all the animals to confirm the selective neurotoxicity of MDMA to 5-HT neurons (Figure 4, Supplementary Figure 4). MDMA (20, 25, and/or 27.5 mg/kg) had no significant effect on DA or DOPAC levels in the striatum compared to controls.
Figure 3.
Ro 4-1284 co- and/or posttreatment does not protect against 3, 4-methylenedioxymethamphetamine (MDMA)-induced depletions in serotonin (5-HT) or 5-HIAA. 5-HT and 5-HIAA levels were measured in cortex and striatum of animals 7 days after treatment with Ro 4-1284 (10 mg/kg, ip) and/or MDMA (sc); (A) 20 mg/kg MDMA dose and (B) 27.5 mg/kg MDMA dose. Values are different from the saline group at *p < .01, **p < .001, ***p < .0001.
Figure 4.
Ro 4-1284 co- and posttreatment with 3, 4-methylenedioxymethamphetamine (MDMA) does not affect striatal catecholamine levels. Dopamine (DA) and 3, 4-dihydroxyphenylacetic acid (DOPAC) concentrations were measured from striatum 7 days after MDMA exposure by high performance liquid chromatography coupled to an electrochemical detector (HPLC-ECD). Ro 4-1284 (10 mg/kg, ip) was administered co- and/or 7 h post-MDMA treatment; (A) 20 mg/kg MDMA dose and (B) 27.5 mg/kg MDMA dose. No significance is observed between any of the treatment groups.
DISCUSSION
Although all 3 doses of MDMA (20, 25, and 27.5 mg/kg) caused elevations in body temperature (Figure 1, Supplementary Figure 1), only the 2 highest doses of MDMA caused subsequent reductions in cortical and striatal 5-HT and 5-HIAA concentrations, indices of serotonergic neurotoxicity. Moreover, although cotreatment with Ro 4-1284 with the lowest dose of MDMA (20 mg/kg) resulted in peak body temperatures (Figure 2, Supplementary Figure 2) similar to those that resulted in subsequent neurotoxicity in other groups, such neurotoxicity did not occur in the 20 mg/kg MDMA group. MDMA can induce acute indolamine release even at lower doses (4 mg/kg), but the long-term neurotoxicity is seen with higher doses (single dose 20 mg/kg) or with repeated low dose exposures (O’Shea et al., 1998, 2006). Taken together, the findings support the contention that hyperthermia per se is insufficient to produce MDMA-mediated depletions in cortical and striatal indolamine concentrations.
Pretreatment with Ro 4-1284, a reversible VMAT2 inhibitor, 1 h prior to MDMA administration, protects against the short-term hyperthermic effects of MDMA, and from the subsequent long-term serotonergic neurotoxicity. Monoamine depletion with Ro 4-1284 treatment is rapid. Lizarraga et al. (2015) showed that after 1 h of treatment both DA and 5-HT levels were significantly reduced and returned to baseline levels within 12 h. Due to the rapid loss of indolamines and catecholamines, and that Ro 4-1284 is a short-acting reversible inhibitor of VMAT2, we could not differentiate between a direct VMAT2 inhibiting effect or monoamine depletion (Lizarraga et al., 2015). Furthermore, compounds with similar mechanisms of action as Ro 4-1284 (ie, lobeline or reserpine) also protect against hyperthermia and neurotoxicity induced by various amphetamines, including MDMA (Eyerman and Yamamoto, 2004; Yuan et al., 2002). Interestingly, lobeline-mediated neuroprotection may be both ambient temperature-dependent and -independent, depending on context (Eyerman and Yamamoto, 2004). Coadministration of Ro 4-1284 (10 mg/kg ip) with MDMA (20 or 27.5 mg/kg sc) accelerates the hyperthermic response, with a concomitant reduction in the duration of hyperthermia (Figure 1). This latter effect results in an overall reduction in the exposure to elevated temperatures (AUC) in animals cotreated with Ro 4-1284 and the highest dose of MDMA (27.5 mg/kg) (Figure 2D). However, despite the reduced exposure to elevated body temperature, cotreatment with Ro 4-1284 does not protect against MDMA (27.5 mg/kg) induced long-term neurotoxicity, as assessed by reductions in cortical and striatal 5-HT and 5-HIAA levels (Figure 3). Thus, it is likely that the protective effects of VMAT2 inhibition prior to MDMA administration (Lizarraga et al., 2015) are ostensibly the result of indolamine and catecholamine depletion prior to MDMA exposure which prevents the neurotoxic cascade initiated by MDMA, including the hyperthermic response.
As noted above, hyperthermia per se is insufficient to produce MDMA-mediated serotonergic neurotoxicity, and thus other factors must play an important role in neurotoxicity. Oxidative stress may be one such factor that contributes to MDMA-induced neurotoxicity. Indeed, hydroxyl radicals (Gorska et al., 2014; Shankaran et al., 1999) and lipid peroxidation (Alves et al., 2009a; Cadet et al., 2001) are detected in the brain after administration of MDMA. A potential source of oxidative stress after MDMA administration may be due to (1) the oxidation of metabolites of MDMA, (2) via the redox cycling of DA, (3) increased nitric oxide production by nitric oxide synthase, (4) excitotoxicity, and/or (5) disruptions in calcium homeostasis (Capela et al., 2009; Moratalla et al., 2017; Sarkar and Schmued, 2010). Metabolism of MDMA metabolites, indolamines, and catecholamines by MAO-B produces hydrogen peroxide, which, in the presence of iron can yield the hydroxyl radical, with subsequent damage to mitochondria, including mitochondrial DNA. Inhibition of MAO-B is protective against MDMA-induced neurotoxicity, without attenuating hyperthermia (Capela et al., 2009). Similarly, trapping of hydroxyl radicals and antioxidants are also protective against MDMA-induced neurotoxicity (Alves et al., 2009a; Sprague and Nichols, 1995). Interestingly, oxidative stress can be modulated by temperature, with hyperthermia being favorable to the induction of oxidative stress, and hypothermia having the opposite effect (Colado et al., 1999).
DA has been directly implicated as a major player in MDMA-induced neurotoxicity. In addition to hydroxyl radical formation, DA metabolism by MAO enzymes generates 3, 4-dihydroxyphenylacetaldehyde (DOPAL), a well-known aldehyde neurotoxicant (Segura-Aguilar et al., 2014). Moreover, SERT can transport DA into the cytoplasm without the stimulation of MDMA (Larsen et al., 2011). Vesicular DA is protected from oxidation by the low pH within vesicles (pH approximately 5.5), but it autoxidizes in the cytosol under physiological conditions (pH approximately 7.4), leading to the generation of electrophilic quinones which can react with free thiols and thiols on proteins (Segura-Aguilar et al., 2014). Because MDMA is known to disrupt vesicular storage, uptake of DA by serotonergic cells would lead to generation of toxic quinones and hydroxyl radicals in the cytoplasm, leading to oxidative stress, protein adduction, decreased neuronal thiol content and eventually axonal degeneration, and long-term-depletions of indolamines. In this context, thioether metabolites of MDMA stimulate extracellular DA uptake in hSERT transfected SK-N-MC cells (Jones et al., 2004).
Depletion of DA or lesioning of dopaminergic cells with 6-hydroxydopamine prior to MDMA administration is neuroprotective (Schmidt et al., 1990; Stone et al., 1988). Moreover, increased levels of VMAT2 is protective against methamphetamine (Meth) induced toxicity, which is most likely due to increased vesicularization of DA (Lohr et al., 2015), whereas decreased levels of VMAT2 exacerbate Meth induced neurodegeneration (Guillot et al., 2008). However the increased levels of VMAT2 do not change the basal body temperature of animals or the hyperthermic response to Meth (Lohr et al., 2015). Yet, low VMAT2 levels seem to increase basal body temperature, without significantly affecting Meth induced hyperthermia (Guillot et al., 2008). Consistent with these findings, Lizarraga et al. (2015) showed a protective effect with the monoamine depleting agent Ro 4-1284. Conversely, administration of DA precursors, tyrosine or L-DOPA, with MDMA potentiates MDMA-induced neurotoxicity (Goni-Allo et al., 2008; Schmidt et al., 1991), As noted, inhibition of MAO-B is protective against MDMA neurotoxicity. In contrast, inhibition of MAO-A prior to MDMA treatment promotes hyperactivity, hyperthermia, and high mortality in experimental animals (Alves et al., 2009b). MAO-A, the main enzyme in dopaminergic cells, has a higher affinity for DA than MAO-B, the main enzyme in 5-HT cells (Segura-Aguilar et al., 2014). Moreover, in dopaminergic cells the DA synthesizing enzymes, tyrosine hydroxylase and aromatic amino acid decarboxylase, form a complex with VMAT2, coupling DA synthesis to the vesicular storage of DA, preventing the accumulation of free DA in the cytosol (Cartier et al., 2010). A similar interaction exists between the dopamine transporter (DAT), synaptogyrin-3 (vesicular protein), and VMAT2 in dopaminergic cells (Egana et al., 2009), facilitating uptake of DA from the extracellular space directly into vesicles, simultaneously reducing cytosolic DA.
Dopaminergic cells express high levels of aldehyde and alcohol dehydrogenase enzymes, which should protect them from DOPAL neurotoxicity. A role for these dehydrogenases is recognized in Parkinson’s disease and other dopaminergic related disorders, with DOPAL postulated as the main culprit (Marchitti et al., 2008). In this context, serotonergic cells exposed to MDMA would facilitate uptake of DA from the extracellular space by SERT, accumulating DA in the cytosol, promoting hydroxyl radical, and toxic aldehyde accumulation, leading to severe oxidative stress and DA-induced toxicity. Consequently, pretreatment with Ro 4-1284 would likely deplete indolamines and catecholamines prior to MDMA administration, resulting in protection against MDMA-induced long-term neurotoxicity, as demonstrated by Lizarraga et al. (2015). In contrast, co- or posttreatment with Ro 4-1284 would not be sufficient to prevent the MDMA-mediated release of these neurotransmitters. Consistent with this view, cotreatment with Ro 4-1284 and MDMA caused severe short-lived hyperthermia (approximately 3 h) (Figure 1), most likely due to the combined effects of inhibition of VMAT2 by Ro 4-1284 and release of neurotransmitters by MDMA. Interestingly, posttreatment (7 h after MDMA) did not cause hyperthermia (Figure 1), yet 4 of 7 (approximately 57%) animals in the posttreatment group (27.5 mg/kg MDMA) perished. In contrast, 6 of 9 (56%) animals in the cotreatment group (27.5 mg/kg MDMA), and only 1 of 6 (17%) in the MDMA alone group (27.5 mg/kg) did not survive. It is plausible that inhibition of VMAT2 after MDMA treatment prevents the appropriate storage of neurotransmitters, resulting in severe toxicity and death, whereas cotreatment exacerbates the acute effects of MDMA. Further studies are required to support this view.
Depletion of catecholamines prior to exposure to MDMA might also prevent MDMA-induced hyperthermia, because antagonism of D1 receptors protects against MDMA-induced hyperthermia, and the long-term neurotoxicity (Granado et al., 2014; Mechan et al., 2002). However, although cotreatment of animals with Ro 4-1284 and MDMA reduced the duration of the elevated body temperatures by approximately 50% (Figure 2, Supplementary Figure 2), the reduction in the hyperthermic response did not significantly affect long-term 5-HT depletions (Figure 3, Supplementary Figure 3).
In summary, the protective effects of pretreatment with Ro 4-1284 (Lizarraga et al., 2015) are most likely mediated by the ability of Ro 4-1284 to deplete indolamines and catecholamines (specifically DA) prior to exposure to MDMA, which subsequently prevents the ability of MDMA to induce hyperthermia. Co- or posttreatment with the VMAT2 inhibitor is insufficient to prevent the MDMA-mediated release of neurotransmitters and the events that subsequently lead to neurotoxicity.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank the NIDA Drug Supply Program for kindly providing (±) MDMA.
FUNDING
This work was supported by a grant from the National Institute on Drug Abuse at the National Institutes of Health (DA023525 to T.J.M.) and the National Institute of Environmental Health Sciences Graduate Training Fellowship from the Environmental Toxicology of Complex Diseases Training grant at the National Institutes of Health (5T32ES007091 to A.B.C.). The authors also acknowledge the support of the Southwest Environmental Health Sciences Center and the Integrative Health Sciences Facility Core (P30 ES006694) at the University of Arizona.
REFERENCES
- Alves E., Binienda Z., Carvalho F., Alves C. J., Fernandes E., de Lourdes Bastos M., Tavares M. A., Summavielle T. (2009a). Acetyl-L-carnitine provides effective in vivo neuroprotection over 3, 4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience 158, 514–523. [DOI] [PubMed] [Google Scholar]
- Alves E., Summavielle T., Alves C. J., Gomes-da-Silva J., Barata J. C., Fernandes E., Bastos Mde L., Tavares M. A., Carvalho F. (2007). Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria. J. Neurosci. 27, 10203–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves E., Summavielle T., Alves C. J., Custodio J. B., Fernandes E., de Lourdes Bastos M., Tavares M. A., Carvalho F. (2009b). Ecstasy-induced oxidative stress to adolescent rat brain mitochondria in vivo: Influence of monoamine oxidase type A. Addict. Biol. 14, 185–193. [DOI] [PubMed] [Google Scholar]
- Battaglia G., Yeh S. Y., De Souza E. B. (1988). MDMA-induced neurotoxicity: Parameters of degeneration and recovery of brain serotonin neurons. Pharmacol. Biochem. Behav. 29, 269–274. [DOI] [PubMed] [Google Scholar]
- Berger U. V., Gu X. F., Azmitia E. C. (1992). The substituted amphetamines 3, 4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur. J. Pharmacol. 215, 153–160. [DOI] [PubMed] [Google Scholar]
- Cadet J. L., Thiriet N., Jayanthi S. (2001). Involvement of free radicals in MDMA-induced neurotoxicity in mice. Ann. Med. Interne (Paris) 152(Suppl. 3), IS57–IS59. [PubMed] [Google Scholar]
- Capela J. P., Carmo H., Remiao F., Bastos M. L., Meisel A., Carvalho F. (2009). Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: An overview. Mol. Neurobiol. 39, 210–271. [DOI] [PubMed] [Google Scholar]
- Capela J. P., Fernandes E., Remiao F., Bastos M. L., Meisel A., Carvalho F. (2007). Ecstasy induces apoptosis via 5-HT(2A)-receptor stimulation in cortical neurons. Neurotoxicology 28, 868–875. [DOI] [PubMed] [Google Scholar]
- Capela J. P., Ruscher K., Lautenschlager M., Freyer D., Dirnagl U., Gaio A. R., Bastos M. L., Meisel A., Carvalho F. (2006). Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience 139, 1069–1081. [DOI] [PubMed] [Google Scholar]
- Cartier E. A., Parra L. A., Baust T. B., Quiroz M., Salazar G., Faundez V., Egana L., Torres G. E. (2010). A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J. Biol. Chem. 285, 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado M. I., O’Shea E., Esteban B., Granados R., Green A. R. (1999). In vivo evidence against clomethiazole being neuroprotective against MDMA (‘ecstasy’)-induced degeneration of rat brain 5-HT nerve terminals by a free radical scavenging mechanism. Neuropharmacology 38, 307–314. [DOI] [PubMed] [Google Scholar]
- Crespi D., Mennini T., Gobbi M. (1997). Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3, 4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br. J. Pharmacol. 121, 1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egana L. A., Cuevas R. A., Baust T. B., Parra L. A., Leak R. K., Hochendoner S., Pena K., Quiroz M., Hong W. C., Dorostkar M. M., et al. (2009). Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci. 29, 4592–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerman D. J., Yamamoto B. K. (2004). Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J. Pharmacol. Exp. Ther. 312, 160–169. [DOI] [PubMed] [Google Scholar]
- Farfel G. M., Seiden L. S. (1995). Role of hypothermia in the mechanism of protection against serotonergic toxicity. I. Experiments using 3, 4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J. Pharmacol. Exp. Ther. 272, 860–867. [PubMed] [Google Scholar]
- Fleckenstein A. E., Hanson G. R. (2003). Impact of psychostimulants on vesicular monoamine transporter function. Eur. J. Pharmacol. 479, 283–289. [DOI] [PubMed] [Google Scholar]
- Goni-Allo B., Puerta E., Mathuna B. O., Hervias I., Lasheras B., de la Torre R., Aguirre N. (2008). On the role of tyrosine and peripheral metabolism in 3, 4-methylenedioxymethamphetamine-induced serotonin neurotoxicity in rats. Neuropharmacology 54, 885–900. [DOI] [PubMed] [Google Scholar]
- Gorska A. M., Noworyta-Sokolowska K., Golembiowska K. (2014). The effect of caffeine on MDMA-induced hydroxyl radical production in the mouse striatum. Pharmacol. Rep. 66, 718–721. [DOI] [PubMed] [Google Scholar]
- Granado N., Ares-Santos S., Moratalla R. (2014). D1 but not D4 dopamine receptors are critical for MDMA-induced neurotoxicity in mice. Neurotox. Res. 25, 100–109. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. A., Nash J. F. (2002). Carrier-mediated release of serotonin by 3, 4-methylenedioxymethamphetamine: Implications for serotonin-dopamine interactions. J. Neurochem. 66, 243–249. [DOI] [PubMed] [Google Scholar]
- Guillot T. S., Shepherd K. R., Richardson J. R., Wang M. Z., Li Y., Emson P. C., Miller G. W. (2008). Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J. Neurochem. 106, 2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzidimitriou G., McCann U. D., Ricaurte G. A. (1999). Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/-)3, 4-methylenedioxymethamphetamine seven years previously: Factors influencing abnormal recovery. J. Neurosci. 19, 5096–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J. M., Cholanians A. B., Lau S. S., Monks T. J. (2014). Glial cell response to 3, 4-(+/-)-methylenedioxymethamphetamine and its metabolites. Toxicol. Sci. 138, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. C., Duvauchelle C., Ikegami A., Olsen C. M., Lau S. S., de la Torre R., Monks T. J. (2005). Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J. Pharmacol. Exp. Ther. 313, 422–431. [DOI] [PubMed] [Google Scholar]
- Jones D. C., Lau S. S., Monks T. J. (2004). Thioether metabolites of 3, 4-methylenedioxyamphetamine and 3, 4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. J. Pharmacol. Exp. Ther. 311, 298–306. [DOI] [PubMed] [Google Scholar]
- Larsen M. B., Sonders M. S., Mortensen O. V., Larson G. A., Zahniser N. R., Amara S. G. (2011). Dopamine transport by the serotonin transporter: A mechanistically distinct mode of substrate translocation. J. Neurosci. 31, 6605–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi E. T., Azmitia E. C. (1994). MDMA (ecstasy) inhibition of MAO type A and type B: Comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology 10, 231–238. [DOI] [PubMed] [Google Scholar]
- Li I. H., Huang W. S., Shiue C. Y., Huang Y. Y., Liu R. S., Chyueh S. C., Hu S. H., Liao M. H., Shen L. H., Liu J. C., et al. (2010). Study on the neuroprotective effect of fluoxetine against MDMA-induced neurotoxicity on the serotonin transporter in rat brain using micro-PET. Neuroimage 49, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Lizarraga L. E., Cholanians A. B., Phan A. V., Herndon J. M., Lau S. S., Monks T. J. (2015). Vesicular monoamine transporter 2 and the acute and long-term response to 3, 4-(+/-)-methylenedioxymethamphetamine. Toxicol. Sci. 143, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga L. E., Phan A. V., Cholanians A. B., Herndon J. M., Lau S. S., Monks T. J. (2014). Serotonin reuptake transporter deficiency modulates the acute thermoregulatory and locomotor activity response to 3, 4-(+/-)-methylenedioxymeth-amphetamine, and attenuates depletions in serotonin levels in SERT-KO rats. Toxicol. Sci. 139, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Stout K. A., Dunn A. R., Wang M., Salahpour A., Guillot T. S., Miller G. W. (2015). Increased vesicular monoamine transporter 2 (VMAT2; Slc18a2) protects against methamphetamine toxicity. ACS Chem. Neurosci. 20, 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg J. E., Sabol K. E., Seiden L. S. (1996). Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J. Pharmacol. Exp. Ther. 278, 258–267. [PubMed] [Google Scholar]
- Marchitti S. A., Brocker C., Stagos D., Vasiliou V. (2008). Non-P450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 4, 697–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan A. O., Esteban B., O’Shea E., Elliott J. M., Colado M. I., Green A. R. (2002). The pharmacology of the acute hyperthermic response that follows administration of 3, 4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br. J. Pharmacol. 135, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R., Khairnar A., Simola N., Granado N., García-Montes J. R., Porceddu P. F., Tizabi Y., Costa G., Morelli M. (2017). Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog. Neurobiol. 155, 149–170. [DOI] [PubMed] [Google Scholar]
- Orio L., O’Shea E., Sanchez V., Pradillo J. M., Escobedo I., Camarero J., Moro M. A., Green A. R., Colado M. I. (2004). 3, 4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: Studies on the relationship with acute hyperthermia and 5-HT depletion. J. Neurochem. 89, 1445–1453. [DOI] [PubMed] [Google Scholar]
- O’Shea E., Granados R., Esteban B., Colado M. I., Green A. R. (1998). The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 37, 919–926. [DOI] [PubMed] [Google Scholar]
- O’Shea E., Orio L., Escobedo I., Sanchez V., Camarero J., Green A. R., Colado M. I. (2006). MDMA-induced neurotoxicity: Long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br. J. Pharmacol. 148, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partilla J. S., Dempsey A. G., Nagpal A. S., Blough B. E., Baumann M. H., Rothman R. B. (2006). Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 319, 237–246. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Blakely R. D. (1999). Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 285, 763–766. [DOI] [PubMed] [Google Scholar]
- Ricaurte G. A., Yuan, J., McCann, U. D. (2000). (±)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology42, 5–10. [DOI] [PubMed] [Google Scholar]
- Sanchez V., Camarero J., Esteban B., Peter M. J., Green A. R., Colado M. I. (2001). The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (‘ecstasy’)-induced degeneration of 5-HT nerve endings in rat brain. Br. J. Pharmacol. 134, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Schmued L. (2010). Neurotoxicity of ecstasy (MDMA): An overview. Curr. Pharm. Biotechnol. 11, 460–469. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J. (1987). Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 240, 1–7. [PubMed] [Google Scholar]
- Schmidt C. J., Black C. K., Taylor V. L. (1990). Antagonism of the neurotoxicity due to a single administration of methylenedioxymethamphetamine. Eur. J. Pharmacol. 181, 59–70. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Black C. K., Taylor V. L. (1991). L-DOPA potentiation of the serotonergic deficits due to a single administration of 3, 4-methylenedioxymethamphetamine, p-chloroamphetamine or methamphetamine to rats. Eur. J. Pharmacol. 203, 41–49. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Paris I., Munoz P., Ferrari E., Zecca L., Zucca F. A. (2014). Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 129, 898–915. [DOI] [PubMed] [Google Scholar]
- Shankaran M., Yamamoto B. K., Gudelsky G. A. (1999). Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3, 4-methylenedioxymethamphetamine. Eur. J. Pharmacol. 385, 103–110. [DOI] [PubMed] [Google Scholar]
- Soleimani Asl S., Mousavizedeh K., Pourheydar B., Soleimani M., Rahbar E., Mehdizadeh M. (2013). Protective effects of N-acetylcysteine on 3, 4-methylenedioxymethamphetamine-induced neurotoxicity in male Sprague-Dawley rats. Metab. Brain Dis. 28, 677–686. [DOI] [PubMed] [Google Scholar]
- Sprague J. E., Nichols D. E. (1995). The monoamine oxidase-B inhibitor L-deprenyl protects against 3, 4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J. Pharmacol. Exp. Ther. 273, 667–673. [PubMed] [Google Scholar]
- Steuer A. E., Boxler M. I., Stock L., Kraemer T. (2016). Inhibition potential of 3, 4-methylenedioxymethamphetamine (MDMA) and its metabolites on the in vitro monoamine oxidase (MAO)-catalyzed deamination of the neurotransmitters serotonin and dopamine. Toxicol. Lett. 243, 48–55. [DOI] [PubMed] [Google Scholar]
- Stone D. M., Johnson M., Hanson G. R., Gibb J. W. (1988). Role of endogenous dopamine in the central serotonergic deficits induced by 3, 4-methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 247, 79–87. [PubMed] [Google Scholar]
- Stone D. M., Stahl D. C., Hanson G. R., Gibb J. W. (1986). The effects of 3, 4-methylenedioxymethamphetamine (MDMA) and 3, 4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 128, 41–48. [DOI] [PubMed] [Google Scholar]
- Yuan J., Cord B. J., McCann U. D., Callahan B. T., Ricaurte G. A. (2002). Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J. Neurochem. 80, 960–969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.