ABSTRACT
An accumulating body of evidence has shown that capsaicin induces apoptosis in various tumor cells as a mechanism of its anti-tumor activity. However, the effects of capsaicin on osteosarcoma have not been studied extensively. In the current study, we explore the molecular mechanism of capsaicin-mediated tumor suppressive function in osteosarcoma. We found that capsaicin-induced apoptosis and the activation of transient receptor potential receptor vanilloid 1 (TRPV1) in a dose- and time-dependent manner in human osteosarcoma MG63 cells in vitro. Blocking TRPV1 using capsazepine attenuated the capsaicin-induced cytotoxicity, mitochondrial dysfunction, overproduction of reactive oxygen species (ROS) and decrease in superoxide dismutase (SOD) activity. In addition, the results demonstrated that capsaicin induced the activation of adenosine 5ʹ-monophosphate-activated protein kinase (AMPK), p53 and C-jun N-terminal kinase (JNK). In addition, Compound C (antagonist of AMPK) attenuated the activation of p53, which appeared to be TRPV1 independent. Taken together, the present study suggests that capsaicin effectively causes cell death in human osteosarcoma MG63 cells via the activation of TRPV1-dependent (mitochondrial dysfunction, and overproduction of ROS and JNK) and TRPV1-independent (AMPK-p53) pathways. Thus, capsaicin may be a potential anti-osteosarcoma agent.
KEYWORDS: Osteosarcoma, capsaicin, mitochondria, TRPV1, apoptosis
Introduction
Osteosarcoma, which occurs most frequently in those in their twenties or those aged >60 years, is the most common form of primary malignant bone tumor [1]. With a high tendency of local invasion and early lung metastasis, the 5-year overall survival rate of those diagnosed with metastatic osteosarcoma is 20–30% [2]. In the past two decades, multidisciplinary therapeutic strategies, including wide tumor excision, adjuvant chemotherapy, and radiotherapy, have significantly improved patient prognosis, and the survival rate has risen rises to 60–70% in patients with localized disease [1,3]. However, many problems associated with chemotherapy have subsequently emerged. Many patients show no improvement in their condition due to resistance to the treatment and some present with severe side effects in other organs of the body [4,5]. Accordingly, the search for novel effective anti-osteosarcoma agents is urgently required.
Capsaicin (8-methyl-N-vanillyl-6-noneamide), one of the main components of chili peppers with pungency and pharmacological properties, is widely and frequently consumed throughout the world and can be extracted from the plant genus Capsicum [6]. Capsaicin is a natural agonist of transient receptor potential receptor vanilloid 1 (TRPV1), which has been shown to have anti-inflammatory, antifungal and analgesic properties [7,8]. Recently, studies have also revealed that capsaicin restricts tumor cell growth and progression as well as its induction of apoptosis, whereas capsaicin fails to induce cytotoxicity in normal healthy cells [9,10]. Due to its pro-apoptotic activity and lack of genotoxic function, capsaicin has been proposed to be a novel candidate for cancer therapy [11,12].
TRPV1 is a non-selective cation channel, which is frequently overexpressed in highly malignant cancers [13,14]. There is a growing body of evidence that suggests that capsaicin may be able to induce cell death in urothelial cancer and glioma cells via TRPV1-dependent stimulation of excessive calcium (Ca2+) influx [15], which can be inhibited via the activation of cannabinoid receptors [16]. TRPV receptors alter membrane structure and function, depolarize the mitochondria of intact cells, and mediate apoptosis [17].
Recently, several independent studies have demonstrated that capsaicin can disrupt the mitochondrial membrane potential (MMP), trigger rapid reactive oxygen species (ROS) overproduction, and induce mitochondria-mediated apoptosis in cancer cells, which make mitochondria as a target for cancer prevention and treatment [18,19]. Therefore, the underlying molecular mechanisms and targets of capsaicin involved in its antitumor activity are multifaceted and dependent on specific cell types. For example, capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action [20]. Previous studies have revealed that capsaicin inhibited cell growth and induced apoptosis in osteosarcoma cells [21–25]. For example, capsaicin inducedapoptosis via adenosine 5ʹ-monophosphate-activated protein kinase (AMPK)-dependent and -independent signaling pathways in human osteosarcoma cells [21,25]. Another study reported that capsaicin induced apoptosis through the caspase cascade and the antioxidant enzyme system in osteosarcoma cells [23]. However, the effect of capsaicin on human osteosarcoma cells and whether it produces these effects via TRPV1 have not been fully elucidated. In the present study, human osteosarcoma MG63 cells were treated with capsaicin and the effects of capsazepine, an antagonist of TRPV1, on cell viability, apoptosis, mitochondrial function, and ROS production were investigated as well as several potential underlying mechanisms of capsaicin’s anti-osteosarcoma effects.
Results
Capsaicin induces cell viability loss and apoptosis
The results of the CCK-8 assay and Annexin V-FITC/PI staining revealed that MG63 cell viability was reduced in a time- and dose-dependent manner following capsaicin treatment. Compared with the vehicle (0.1% DMSO) controls, following cell treatment with 5, 10, 20, or 40 µM of capsaicin for 48 h, the number of MG 63 cells was reduced to 79.3 ± 8.7, 45.1 ± 10.5 and 35.3 ± 7.2%, respectively (Figure 1(a)). These effects of capsaicin on cell growth were also time-dependent (Figure 1(b)). Cell apoptosis was observed following capsaicin administration to MG63 cells for 48 h (Figure 1(c,d)). Specifically, 20 µM capsaicin induced late apoptotic forms in 15% of the control group and 22% in MG63 cells (Figure 1(c)). Notably, capsaicin mainly induced late cell apoptosis in MG63 cells (Figure 1(c,d), and Figure S1). Since 20 μM was an effective capsaicin concentration in reducing cell death and inducing apoptosis, the following experiments were conducted using this concentration. In addition, a decrease in the level of B-cell lymphoma 2 (Bcl-2), and an increase in the levels of Cytochrome C, cleaved-caspase-3 and cleaved polyadenosine diphosphate-ribose polymerase (PARP) in a time-dependent manner following capsaicin treatment in MG63 cells (Figure 1(e,f)).
Figure 1.
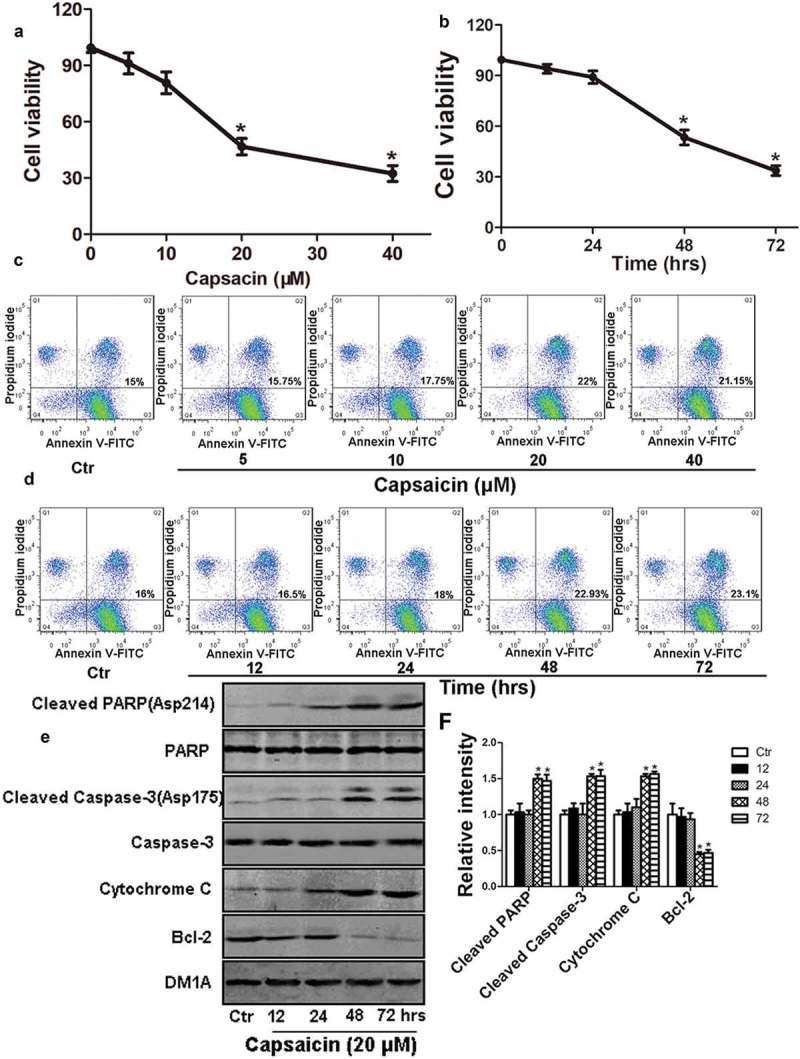
Capsaicin reduces cell viability and induces apoptosis in human osteosarcoma MG63 cells. (a) Capsaicin-induced dose-dependent decreases in cell viability following 48 h of drug treatment. Significant effects were observed with 20 or 40 μM of capsaicin. (b) Time course of capsaicin-induced cell death following treatment with 20 μM of capsaicin. Significant effects were observed following after 48 or 72 h of capsaicin treatment. (c) Capsaicin-induced dose-dependent apoptosis following 48 h of treatment. Significant effects were observed with 20 or 40 μM of capsaicin. (d) Time course of capsaicin-induced apoptosis. Significant effects were observed following 48 or 72 h of capsaicin treatment. (e) Changes in apoptosis-associated proteins including cleaved PARP, PARP, cleaved caspase-3, caspase-3, Cytochrome C and Bcl-2 were detected by western blotting following MG63 cells treatment with 20 μM capsaicin for various lengths of time. (f) Quantification of the phosphorylation levels of cleaved-PARP, cleaved caspase-3, Cytochrome C and Bcl-2. Data are expressed as the mean ± SD. *, p < 0.05, compared with control.
TRPV1 activation serves a pivotal role in capsaicin-induced cytotoxicity
As shown in Figure 2(a,b), TRPV1 was activated in a time-dependent manner by capsaicin. In addition, capsazepine attenuated the capsaicin-induced activation of TRPV1 (Figure 2(c)), apoptosis (Figure 2(d) and Figure S2) cell viability loss (Figure 2(e)), and expression of apoptosis-associated proteins (Figure 2(f,g)), in MG63 cells. These results indicated that TRPV1 activation serves a pivotal role in capsaicin-induced cytotoxicity in MG63 cells.
Figure 2.

TRPV1 activation is required for capsaicin-induced mitochondrial dysfunction in human osteosarcoma MG63 cells. (a) Time course of the capsaicin-induced expression of TRPV1 mRNA expression. Capsaicin inducedan increase in TRPV1 mRNA expression at 4–12 h following the capsaicin (20 μM) treatment. (b) Capsaicin inducedsimilar increases in TRPV1 protein expression, although a slightly delayed time course was observed; TRPV1 protein increased 8–12 h following the treatment. (c-e) The Capsaicin-induced effects were TRPV1-dependent: (c) TRPV1 protein expression induced by Ctr (vehicle), 20 μM of capsaicin, 20 μM of capsazepine (Cpz, TRPV1 antagonist), or capsaicin + Cpz; (d) cell viability induced by the same treatments; and (e), apoptosis levels induced by the same treatments. (f-g) Capsaicin induced changes in apoptosis-associated proteins were also TRPV1-dependent: (f) Representative Western blotting presenting the levels of cleaved PARP, PARP, cleaved caspase-3, caspase-3, Cytochrome C and Bcl-2 as well as DM1A (α-tubulin) from the same experiment; (g) Quantification of the protein levels detected in (f). The data in (f) and (g) are representatives of the results from three separate experiments. Data are expressed as the mean ± SD. *, p < 0.05, compared with control; #, p < 0.05, compared with capsaicin group.
TRPV1 activation is required for capsaicin-induced mitochondrial dysfunction
Capsaicin-induced tumor cell apoptosis by disrupting MMP (25). In the present study, MMP (Figure 3(a)), Complex I activity (Figure 3(b)) and ATP content (Figure 3(c)) decreased in a time-dependent manner following the capsaicin treatment. Blocking TRPV1 by using capsazepine attenuated the capsaicin-induced decrease of MMP (Figure 3(d)), Complex I activity (Figure 3(e)) and ATP content in MG63 cells (Figure 3(f)). These results suggest that TRPV1 activation is required for capsaicin-induced mitochondrial dysfunction in MG63 cells.
Figure 3.
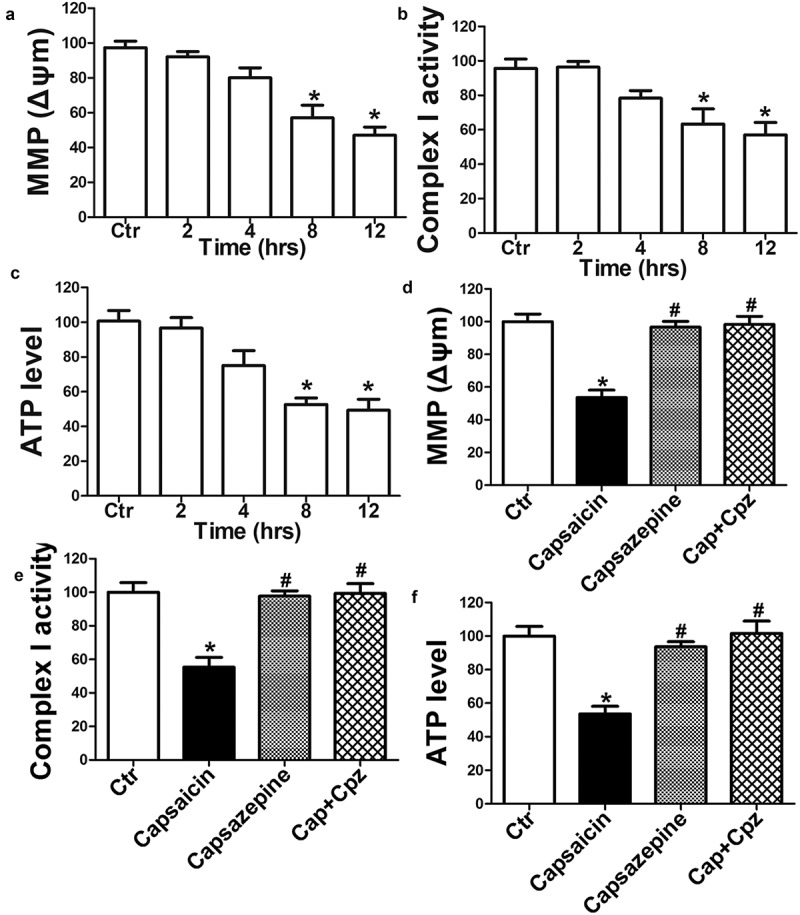
TRPV1 activation is required for capsaicin-induced mitochondrial dysfunction in human osteosarcoma MG63 cells. (a-c) Time course of capsaicin-induced reductions in MMP (a), complex I (b), and ATP levels (c). Significant effects were observed between 8 and 12 h following the capsaicin treatment. (d-f) Effects of capsazepine (Cpz) on capsaicin-induced decrease of MMP (d), complex I (e) and ATP (f), respectively. Blocking TRPV1 using Cpz attenuated the capsaicin-induced decrease in MMP, complex I activity and ATP content in MG63 cells. The values are expressed as the mean ± SD. *, p < 0.05, compared with control, #, p < 0.05, compared with capsaicin group.
TRPV1 activation is required for capsaicin-induced Cytochrome C release into the cytoplasm from the mitochondria and the decrease in Bcl-2 in the mitochondria
Next, the present study sought to determine whether capsaicin-induced the mitochondrial-apoptosis pathway through TRPV1 in MG63 cells. Cytoplasmic and mitochondrial proteins were harvested from each treatment group. The results revealed capsaicin caused increased the levels of Cytochrome C in the cytoplasm, which was accompanied with a decrease in Bcl-2 expression in the mitochondria in a time-dependent manner in MG63 cells (Figure 4(a,c)). Blocking TRPV1 via capsazepine treatment attenuated Cytochrome C release into the cytoplasm from the mitochondria as well as the increase in Bcl-2 protein in the mitochondria induced by capsaicin in MG63 cells (Figure 4(b,d)). These results suggest that TRPV1 activation mediates capsaicin-induced Cytochrome C release into the cytoplasm from mitochondria and the decreased Bcl-2 expression in the mitochondria of MG63 cells.
Figure 4.

TRPV1 activation is required for capsaicin-induced Cytochrome C release into the cytoplasm from the mitochondria and the decrease in Bcl-2 in the mitochondria of human osteosarcoma MG63 cells. (a) Time course of capsaicin-induced Cytochrome C release into the cytoplasm; significant effects were observed at 48 and 72 h following treatment. (b) Increased levels of Cytochrome C in the cytoplasm induced by capsaicin were blocked by the TRPV1 antagonist capsazepine. (c) Time course of the capsaicin-induced reduction of mitochondrial Bcl-2; significant effects were observed at 48 and 72 h following the capsaicin treatment. (d) The capsaicin-induced decrease in the mitochondrial Bcl-2 was blocked by capsazepine. Cytochrome C levels in the cytoplasm were normalized to DM1A, and Bcl-2 in the mitochondria was normalized to COX IV. The values are expressed as the mean ± SD. *, p < 0.05, compared with control; #, p < 0.05, compared with capsaicin group.
TRPV1 activation is required for the capsaicin-induced overproduction of ROS and decrease in SOD activity
Activation of TRPV1 promoted the generation of ROS, which is harmful to cells if generated in excessive amounts [26,27]. When compared with the vehicle control, ROS was overproduced and SOD activity decreased in a time-dependent manner following capsaicin treatment in MG63 cells (Figure 5(a,b)), suggesting that decreasing the capacity to clear ROS via SOD could be a key factor contributing to the overproduction of ROS induced by capsaicin. Blocking TRPV1 using capsazepine attenuated the effects induced by capsaicin in MG63 cells (Figure 5(c,d)). These results suggest that TRPV1 activation mediated the capsaicin-induced overproduction of ROS and decrease in SOD activity in MG63 cells.
Figure 5.
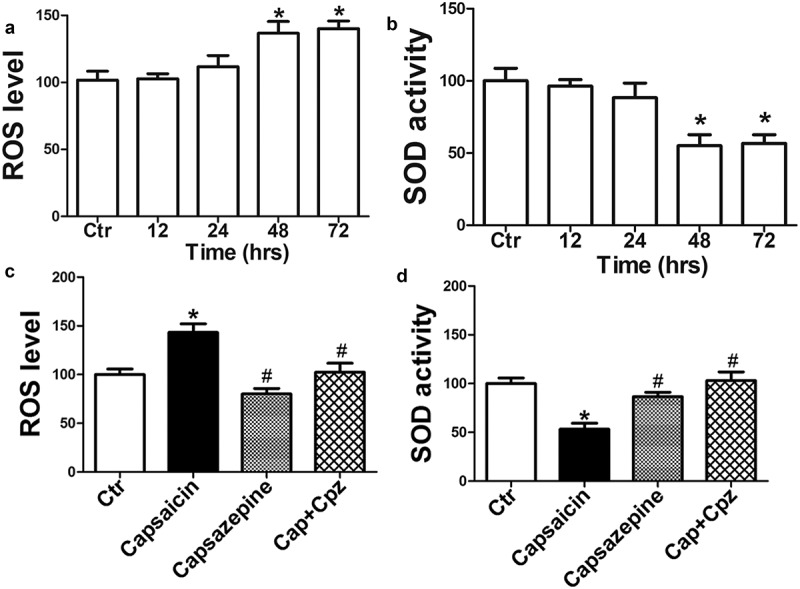
TRPV1 activation is required for the capsaicin-induced overproduction of ROS and the decrease in SOD activity in human osteosarcoma MG63 cells. (a) Time course of capsaicin-induced ROS generation. Capsaicin (20 μM) caused significant ROS production 48 and 72 h following the capsaicin treatment. (b) Time course of capsaicin-induced decrease in SOD activity. Significant effects were observed at 48 and 72 h following the capsaicin treatment. (c) Effects of capsazepine (20 μM) on the capsaicin-induced increase of ROS generation. (d) Effects of capsazepine on the capsaicin-induced decrease in SOD activity. The values are expressed as the mean ± SD. *, p < 0.05, compared with control, #, p < 0.05, compared with capsaicin group.
Capsaicin activates AMPK, p53 and c-Jun N-terminal Kinase (JNK)
An Increasing body of evidence has shown that capsaicin can suppress the migration of HuCCT1 cholangiocarcinoma cells and induce apoptosis via the activation of AMPK, p53 or JNK in different types of tumor cells [28–31]. In the present study, capsaicin induced significant AMPK (Figure 6(a)), p53 (Figure 6(b)) and JNK (Figure 6(c)) activation in a time-dependent manner. The activation of AMPK, p53, and JNK were indicated by the increased levels of phosphorylated-AMPKα (Thr172), p-p53 (Ser15) and p-JNK (Thr83/Tyr185) with unchanged total levels of AMPK, p53, and JNK in MG63 cells.
Figure 6.
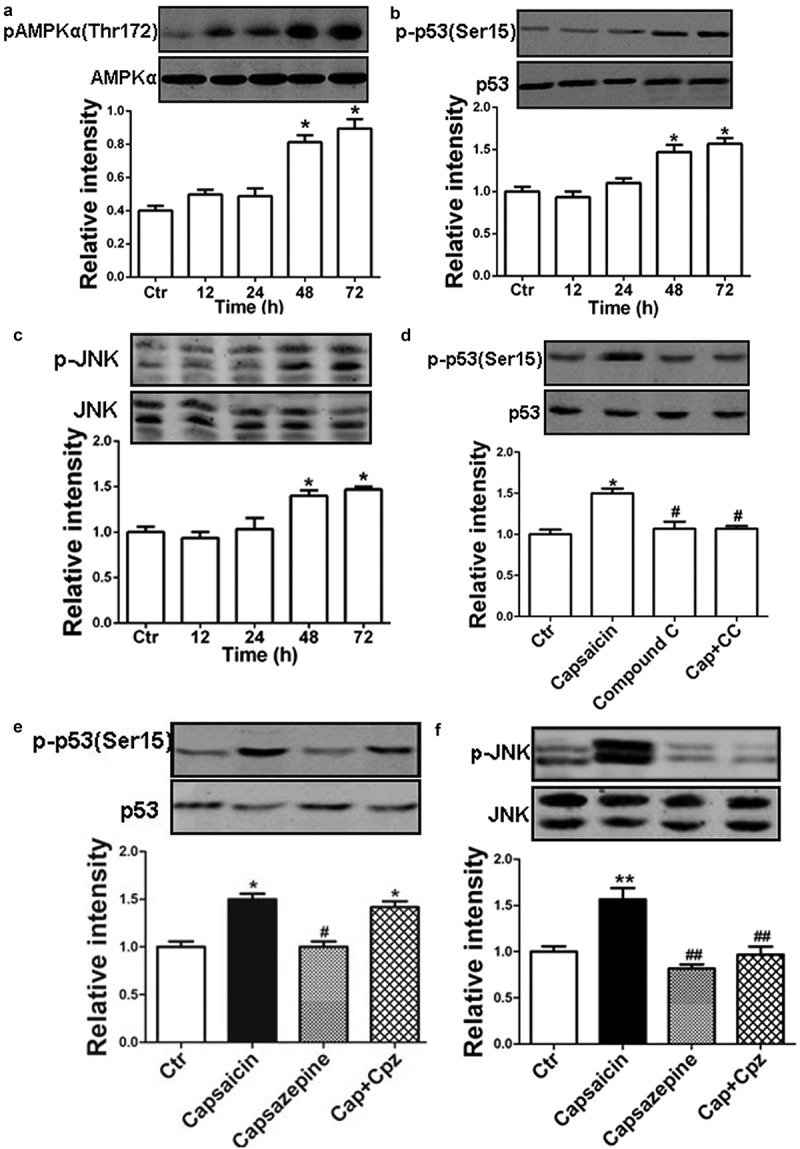
Roles of AMPK in the capsaicin-induced effects. (a-c) Time courses of capsaicin-induced phosphorylation of AMPKα (a), p53 (b), and JNK (c) detected by western blotting analysis. The phosphorylated AMPKα, p53, and JNK were normalized, respectively, to the total level of each corresponding protein. (d) MG63 cells were treated with capsaicin (20 μM), Compound C (20 μM), capsaicin + Compound C (1 h prior to administration of capsaicin) for 48 h, respectively. Treatment with the AMPK antagonist compound C (CC) blocked the capsaicin-induced activation of p53. (e) MG63 cells were treated with capsaicin (20 μM), capsazepine (20 μM), and capsaicin plus capsazepine (1 h prior to addition of capsaicin) for 48 h, respectively. (f) Treatment with capsazepine blocked capsaicin-induced activation of JNK. The values are expressed as the mean ± SD. *, p < 0.05, compared with control; #, p < 0.05, compared with the capsaicin group. **, p<0.01, compared wuth control; ##, p<0.01, compared with the capsaicin group.
Blocking AMPK using Compound C (20 μM) attenuated the capsaicin-induced activation of p53 in MG63 cells (Figure 6(d)), without affecting the level of p-JNK, mitochondrial function, ROS production or SOD activity (data not shown). Blocking TRPV1 was not associated with the activation of p53 (Figure 6(e)), but it did attenuate the level of p-JNK (Figure 6(f)) in MG63 cells. These results suggest that capsaicin induced the activation of AMPK and p53 in a TRPV1-independent manner, which may also be involved in the cell death of MG63 cells.
Discussion
TRPV1 is a member of the TRP channel superfamily, a large and unusually diverse family of nonselective cation channels that primarily exist in sensory neurons and respond to a wide range of environmental and endogenous noxious stimuli of a chemical and/or physical nature [32,33]. Capsaicin is a natural agonist of TRPV1 [7], in addition to capsaicin, the channel can also be activated by heat, chemical mediators, such as acidic pH and lipid mediators, including anandamide and eicosanoids [17]. Once the channel is activated, it can enable the rapid increase of intracellular calcium (Ca2+) levels and initiate cell death [34]. Recently, studies have demonstrated that capsaicin-induced apoptosis and suppression of tumorigenesis via TRPV1-dependent stimulation of excessive calcium (Ca2+) influx [13,16]. In line with these reports, we found that capsaicin can induce cell viability loss, apoptosis and activate TRPV1 in a dose- and time-dependent manner in MG63 cells. Blocking TRPV1 using capsazepine ameliorated these capsaicin-induced changes, suggesting that activation of TRPV1 may be involved in capsaicin-induced cell death in MG63 cells.
TRPV receptors alter membrane structure and function, depolarize the mitochondria of intact cells, and mediate apoptosis [17]. Mitochondria are the cell’s source of energy as they can turn nutrients into energy [35]. Capsaicin and resiniferatoxin are structurally similar to the cyclic portion of coenzyme Q of the mitochondria respiratory chains [36]. In cancer cells, mitochondrial metabolism is often altered from reliance on oxidative phosphorylation (OXPHOS) in normal cells to glycolytic intermediates, which is called the Warburg effect [37,38]. Recently, several independent studies have demonstrated that capsaicin disrupted MMP (Δψm) and induced tumor cell apoptosis [10,18,19]. The loss of mitochondrial membrane depolarization (decreased levels of Δψm) triggers the opening of a mitochondrial mega channel pores with the concomitant ATP hydrolysis and mitochondrial complex I inactivation [39]. Consistent with previous reports [20,40], the present study demonstrated that capsaicin induced significant mitochondrial dysfunction (represented by the decreases in Δψm, ATP levels and complex I activity in the mitochondria) in MG63 cells. Blocking of TRPV1 by capsazepine treatment ameliorated mitochondrial dysfunction, suggesting the activation of TRPV1 via capsaicin may be the underlying mechanism of capsaicin-induced cell death in MG63 cells.
It has been known that decreased levels of Δψm triggered Cytochrome C release from the mitochondria to the cytoplasm, and the subsequent process is prevented by the anti-apoptotic Bcl-2 protein family. In the cytoplasm, Cytochrome C initiates mitochondrial apoptotic signaling, during which caspase-3 is cut and modified to cleaved caspase-3 and PARP to cleaved PARP, the activated forms of caspase-3 and PARP [41]. In accordance with previous research, the present study revealed that capsaicin induced the activation of caspase-3 and PARP, increased the level of Cytochrome C in the cytoplasm and decreased Bcl-2 expression in the mitochondria in MG63 cells. Blocking TRPV1 ameliorated the capsaicin-induced increases in the expression of mitochondrial apoptosis-associated proteins and the decrease in Bcl-2 expression, suggesting that TRPV1 is crucial in the capsaicin-induced mitochondrial apoptotic pathway.
Previous studies have demonstrated that activation of TRPV1 promoted the generation of ROS, which are involved in regulating many physiological processes, but are harmful to cells if generated in excessive amounts [26,27]. Exposure of cells to capsaicin leads to the overproduction of ROS, which can interact with many cell components and cause damage to cell structures [42–44], with subsequent apoptosis induction in solid tumors [45], inhibition of ROS accumulation prevents capsaicin from inducing cell death [19]. Mitochondrial dysfunction may cause abnormal electron transfer, which in turn may result in oxidative stress and ROS-mediated cell death [46], therefore, ROS may be beneficial in chemotherapy and cancer apoptosis [47,48]. Consistent with previous research [46], the present results also demonstrated that capsaicin induced the overproduction of ROS and reduced SOD activity; blocking TRPV1 ameliorated these changes in MG63 cells. SOD, an antioxidant enzyme, is capable of clearing intracellular ROS [47], suggesting that the decrease in SOD activity induced by capsaicin via the TRPV1 channel may be a crucial factor in ROS overproduction in MG63 cells.
AMPK (5ʹAMP-activated protein kinase) serves vital roles in sensing and regulating mitochondrial function via acute and long-term modulation of mitochondrial activity and controlling substrate selection for mitochondrial fuel (36, 58). Recently, studies have demonstrated that capsaicin could induce cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action [20], induce apoptosis via the activation of caspase cascades and the antioxidant enzyme system, or the AMPK-dependent signaling pathway in human osteosarcoma cells [21,23]. In accordance with a recent study that established an association with AMPK-p53 in MG63 cells [21], the present results also demonstrated that capsaicin-induced activation of AMPK was combined with p53 and JNK activation. Blocking of AMPK ameliorated the capsaicin-induced increase in p-p53 at serine 15 without affecting p-JNK. Blocking of TRPV1 also ameliorated the capsaicin-induced increase in p-JNK at sites, threonine 83 and tyrosine 185, but did not affect the levels of p-p53 protein. One possible explanation of this phenomenon might be the activation of p53 induced by capsaicin through AMPK, and JNK through the TRPV1/ROS signaling pathway [31]. However, the detailed mechanisms underlying the activation of AMPK and JNK by capsaicin in MG63 cells required further investigations.
Taken together, in the present study demonstrated that capsaicin has strong anti-tumor activity in human osteosarcoma MG63 cells. Molecular mechanism studies revealed that capsaicin activated TRPV1-dependent (mitochondrial dysfunction, overproduction of ROS and activation of JNK) and TRPV1-independent (AMPK-p53) signaling pathways to promote cell death. The results suggest that capsaicin may be an effective anti-osteosarcoma agent. A recent study reported that capsaicin in combination with cisplatin had synergistic inhibitory effects on human osteosarcoma cells [49]. Therefore, capsaicin could be effective in the treatment of osteosarcoma with chemotherapeutic drugs.
Materials and methods
Cell culture and capsaicin treatment
Human osteosarcoma MG63 cells, purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences (Shanghai, China), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Sigma Aldrich Merck KGaA, Darmstadt, Germany). Subcultures were prepared using 0.05% trypsin solution (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) and seeded in 6- or 96-well tissue culture plates. To study capsaicin-induced cytotoxicity, MG63 cells were treated with capsaicin (Sigma-Aldrich) at various concentrations for different time. To evaluate the underlying molecular mechanisms, MG63 cells were treated with capsaicin (20 μM), capsazepine (20 μM), capsaicin + capsazepine (1 h prior to the addition of capsaicin), Compound C (20 μM, an antagonist of AMPK) or with capsaicin + Compound C (1 h prior to the administration of capsaicin), respectively, for 48 h. Capsaicin, capsazepine and Compound C were all dissolved in Dimethyl Sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA).
Real-time quantitative PCR
The total RNA of MG63 cells in each group was isolated with Trizol (Invitrogen, Carlsbad) and reverse-transcribed to cDNA with Reverse Transcription System [50]. Quantitative real-time PCR was performed on an ABI 7900 System with SYBR green (Takara, China) Primers of TRPV1 were as follows: CTGATGGCAAGGACGACT (forward, 5ʹ-3ʹ) and TCTGCCTGAAACTCTGCTT (reverse, 5ʹ-3ʹ). Transcripts were quantified with GAPDH (glyceraldehyde 3-phosphate dehydrogenase) as an internal standard. GAPDH, forward primer 5′- ACC CAG AAG ACT GTG GAT GG −3′ and reverse primer 5′- CAG TGA GCT TCC CGT TCA G- 3′.
Cell viability assay
The viability of MG63 cells was assessed using a Cell Counting Kit-8 (CCK8, Dojindo Molecular Technologies, InC., Kumamoto, Japan) [51]. Briefly, MG63 cells in the logarithmic growth phase were inoculated in 96-well plates at 100 μl/well with a density of 2 × 104/ml. Following 24 h of incubation, the cells were randomly divided into the control and capsaicin groups (5, 10, 20 or 40 µΜ) with six duplicates per group. Following 12, 24, 48 or 72 h of incubation, 10 µl CCK-8 solution was added to each well. To study the role of TRPV1, the cells were treated for 48 h with the following groups: Control vehicle only, capsaicin only, capsazepine only, and capsaicin + capsazepine. Then, 10 μl CCK-8 solution was added to each well, following 2 h of incubation, the absorption value was measured at a wavelength of 450 nm using a Quant spectrophotometer (Bio-Rad laboratories, InC., Hercules, CA, USA). Cell viability was calculated using the following formula: Cell viability = (A Capsaicin− A Blank)/(A Control− A Blank) ×100%.
Measurement of apoptotic cells
Apoptotic cell death was detected using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining kit (BestBio, Shanghai, China) [22]. Briefly, following MG63 cells inclubation with the different treatment groups, the cells were harvested, washed with phosphate-buffered saline (PBS) and resuspended in the binding buffer provided in the kit. The cells were then labeled with AnnexinV- FITC/PI and the percentage of apoptotic cells was quantified using Multicycle AV software (FACSAria; BD Biosciences, San Jose, CA, USA).
Western blotting and data quantification
Western blotting was performed according to previously established methods [51,52]. Briefly, MG63 cells in the logarithmic growth phase were rinsed twice with ice-cold PBS (pH 7.4), lysed in a cooled buffer and sonicated for 5 sec on ice. Then, the lysates were boiled in a water bath for 10 min and centrifuged at 12,000 x g for 10 min at 4°C. The protein concentrations in the supernatant were measured using a Bicinchoninic Acid kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Amersham; GE Healthcare, Chicago, IL, Germany). To prevent non-specific binding, the membranes were blocked with 5% non-fat milk in PBS plus 0.5% Tween-20 for 1 h and incubated with primary antibodies for 24 h at 4°C. The blots were incubated with secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature and visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences.). The intensity of phosphorylated protein was normalized to the total counterpart or the DM1A (α-tublin) or COX IV (in mitochondrial protein) internal control band.
Mitochondrial membrane potential (MMP)
To determine the release of MMP (Δψm), the mitochondria were isolated using a cell mitochondria isolation kit and Δψm was measured using a 5,5ʹ,6,6ʹ-tetrachloro-1,1,3,3ʹtetraethylbenzim- idazolylcarbocyanineiodide (JC-1) kit (Beyotime Institute of Biotechnology, Haimen, China) [51,53]. Briefly, MG63 cells were harvested using pre-cooled mitochondrial buffer. The lysate was centrifuged at 1,500 x g for 5 min and then at 16,000 x g for 30 min at 4°C. Following centrifugation, the supernatant was comprised of the cytoplasm and the sediment was the mitochondria. Isolated mitochondria were immediately incubated with the JC-1 staining kit, then the fluorescence intensity of the JC-1 aggregates (excitation 550 nm, emission 600 nm) and monomers (excitation 490 nm, emission 535 nm) were detected using the Synergy 2 multi-mode microplate reader (BioTek Instruments, InC., Winooski, VT, USA). The Δψm of the mitochondria in each group was calculated as the fluorescence ratio of JC-1 aggregates to monomers.
Mitochondrial complex I activity
The complex I enzyme activity microplate assay kit (Abcam, Cambridge, UK) was used to determine the mitochondrial complex I activity as previously described [51]. Briefly, 50 μg mitochondrial protein samples (each sample at a final concentration 5.5 mg/ml) were used for assaying the activity. Complex I activity was determined as the change in absorbance per min per quantity of protein sample in the assay kit; the experiment was run in triplicates.
Adenosine triphosphate (ATP) content assays
Intracellular ATP levels were measured using an ATP assay kit (Beyotime Institute of Biotechnology) [54]. MG63 cells were harvested with lysate buffer and then centrifuged at 1,000 x g for 2 min at 4°C. A total of 50 μl ATP extract was then mixed with 50 μl of the luciferase reagent included in the kit. The fluorescence intensity at 562 nm was measured using a luminometer Synergy 2 multi-mode microplate reader. A standard curve for a series of defined ATP concentrations was prepared, and ATP content was calculated using the following formula: ATP content = ATP concentration/protein concentration.
ROS measurement
The level of ROS in MG63 cells in each group was assessed using a ROS assay kit (Abcam, UK) [20]. Briefly, 2,7-dichlorofluorescein diacetate (DCFH-DA) was converted to the highly fluorescent dichlorofluorescein in the presence of a proper oxidant, then MG63 cells were homogenized with 0.01 M PBS. All of the samples were centrifuged at 1,000 x g for 10 min at 4°C, the supernatants were collected and incubated with 1 mM DCFH-DA in PBS, protected from light for 30 min at 37°C. Following the incubation, the fluorescence intensity of the mixture was quantified at an excitation of 485 nm and an emission of 525 nm using the Synergy 2 multi-mode microplate reader. The results were expressed as units of enzymatic activity per mg protein contained in each sample (U/mg protein).
Superoxide dismutase (SOD) activity assay
SOD activity was determined using a SOD assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (30). Briefly, MG63 cells in each group were harvested with 0.9% normal saline and centrifuged at 3,000 x g for 10 min at 4°C; the Supernatants were then collected. The SOD activity kit, which utilized tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine, was used to detect the SOD activity at 450 nm using Synergy 2 multi-mode microplate reader. One unit of SOD was defined as the amount of enzyme required to produce 50% dismutation of the superoxide radical. The final SOD activity was defined as units of enzymatic activity per mg protein contained in the samples (U/mg protein).
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Multiple comparisons between groups were performed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Differences between two groups were evaluated using Student’s t-test. P < 0.05 was considered to indicate a statistically significant difference. Statistical tests were performed using GraphPad Prism version 5 for Windows (GraphPad Software Inc., La Jolla, CA, USA).
Funding Statement
This work was supported by the Nature Science Major and Key Program of College and University of Anhui Province (No. KJ2018ZD024 and KJ2016A460), Key Projects of Support Program for Outstanding Young Talents in Colleges and Universities of Anhui Province (No. gxyq2017032), and the Nature Science Foundation of Bengbu Medical University (No.BYKF1771 and No. BYKF1708ZD).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18:39–50. [DOI] [PubMed] [Google Scholar]
- [2].Hayden JB, Hoang BH.. Osteosarcoma: basic science and clinical implications. Orthop Clin North Am. 2006;37:1–7. [DOI] [PubMed] [Google Scholar]
- [3].Saraf AJ, Fenger JM, Roberts RD.. Osteosarcoma: accelerating progress makes for a hopeful future. Front Oncol. 2018;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Yang J, Zhao N, et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16:6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016;82:690–698. [PubMed] [Google Scholar]
- [6].Patowary P, Pathak MP, Zaman K, et al. Research progress of capsaicin responses to various pharmacological challenges. Biomed Pharmacother. 2017;96:1501–1512. [DOI] [PubMed] [Google Scholar]
- [7].Chatterjee S, Asakura M, Chowdhury N, et al. Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Microbiol Lett. 2010;306:54–60. [DOI] [PubMed] [Google Scholar]
- [8].Cho SC, Lee H, Choi BY. An updated review on molecular mechanisms underlying the anticancer effects of capsaicin. Food Sci Biotechnol. 2017;26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mori A, Lehmann S, O’Kelly J, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–3229. [DOI] [PubMed] [Google Scholar]
- [10].Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PloS One. 2011;6:e20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chakraborty S, Adhikary A, Mazumdar M, et al. Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis. PloS One. 2014;9:e99743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Georgescu SR, Sarbu MI, Matei C, et al. Capsaicin: friend or foe in skin cancer and other related malignancies? Nutrients. 2017 Dec 16;9(12). pii: E1365. doi: 10.3390/nu9121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Jong PR, Takahashi N, Harris AR, et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J Clin Invest. 2014;124:3793–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mistretta F, Buffi NM, Lughezzani G, et al. Bladder cancer and urothelial impairment: the role of TRPV1 as potential drug target. Biomed Res Int. 2014;2014:987149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amantini C, Mosca M, Nabissi M, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. [DOI] [PubMed] [Google Scholar]
- [16].Amantini C, Mosca M, Lucciarini R, et al. Distinct thymocyte subsets express the vanilloid receptor VR1 that mediates capsaicin-induced apoptotic cell death. Cell Death Differ. 2004;11:1342–1356. [DOI] [PubMed] [Google Scholar]
- [17].Gunthorpe MJ, Benham CD, Randall A, et al. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. [DOI] [PubMed] [Google Scholar]
- [18].Ip SW, Lan SH, Huang AC, et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria-dependent and -independent pathways. Environ Toxicol. 2012;27:332–341. [DOI] [PubMed] [Google Scholar]
- [19].Zhang R, Humphreys I, Sahu RP, et al. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13:1465–1478. [DOI] [PubMed] [Google Scholar]
- [20].Skrzypski M, Sassek M, Abdelmessih S, et al. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell Signal. 2014;26:41–48. [DOI] [PubMed] [Google Scholar]
- [21].Ying H, Wang Z, Zhang Y, et al. Capsaicin induces apoptosis in human osteosarcoma cells through AMPK-dependent and AMPK-independent signaling pathways. Mol Cell Biochem. 2013;384:229–237. [DOI] [PubMed] [Google Scholar]
- [22].Chien CS, Ma KH, Lee HS, et al. Dual effect of capsaicin on cell death in human osteosarcoma G292 cells. Eur J Pharmacol. 2013;718:350–360. [DOI] [PubMed] [Google Scholar]
- [23].Cho WH, Lee HJ, Choi YJ, et al. Capsaicin induces apoptosis in MG63 human osteosarcoma cells via the caspase cascade and the antioxidant enzyme system. Mol Med Rep. 2013;8:1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin T, Wu H, Wang Y, et al. Capsaicin induces immunogenic cell death in human osteosarcoma cells. Exp Ther Med. 2016;12:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Deng X, Lei T, et al. Capsaicin inhibits proliferation and induces apoptosis in osteosarcoma cell lines via the mitogenactivated protein kinase pathway. Oncol Rep. 2017;38:2685–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schulz TJ, Zarse K, Voigt A, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. [DOI] [PubMed] [Google Scholar]
- [27].Song IS, Kim HK, Jeong SH, et al. Mitochondrial peroxiredoxin III is a potential target for cancer therapy. Int J Mol Sci. 2011;12:7163–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ito K, Nakazato T, Murakami A, et al. Induction of apoptosis in human myeloid leukemic cells by 1ʹ-acetoxychavicol acetate through a mitochondrial- and Fas-mediated dual mechanism. Clin Cancer Res off J Am Assoc Cancer Res. 2004;10:2120–2130. [DOI] [PubMed] [Google Scholar]
- [29].Kim YM, Hwang JT, Kwak DW, et al. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci. 2007;1095:496–503. [DOI] [PubMed] [Google Scholar]
- [30].Lee GR, Jang SH, Kim CJ, et al. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK-NF-kappaB signaling pathway. Clin Exp Metastasis. 2014;31:897–907. [DOI] [PubMed] [Google Scholar]
- [31].Sanchez AM, Malagarie-Cazenave S, Olea N, et al. Apoptosis induced by capsaicin in prostate PC-3 cells involves ceramide accumulation, neutral sphingomyelinase, and JNK activation. Apoptosis. 2007;12:2013–2024. [DOI] [PubMed] [Google Scholar]
- [32].Caprodossi S, Amantini C, Nabissi M, et al. Capsaicin promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells. Carcinogenesis. 2011;32:686–694. [DOI] [PubMed] [Google Scholar]
- [33].Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. [DOI] [PubMed] [Google Scholar]
- [34].Amantini C, Ballarini P, Caprodossi S, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30:1320–1329. [DOI] [PubMed] [Google Scholar]
- [35].Niu NK, Wang ZL, Pan ST, et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des Devel Ther. 2015;9:1555–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hartel M, Di Mola FF, Selvaggi F, et al. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Esteves P, Pecqueur C, Ransy C, et al. Mitochondrial retrograde signaling mediated by UCP2 inhibits cancer cell proliferation and tumorigenesis. Cancer Res. 2014;74:3971–3982. [DOI] [PubMed] [Google Scholar]
- [38].Song IS, Jeong JY, Jeong SH, et al. Mitochondria as therapeutic targets for cancer stem cells. World J Stem Cells. 2015;7:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–102. [DOI] [PubMed] [Google Scholar]
- [40].Huang SP, Chen JC, Wu CC, et al. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009;29:165–174. [PubMed] [Google Scholar]
- [41].Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. [DOI] [PubMed] [Google Scholar]
- [42].Bechtel W, Bauer G. Catalase protects tumor cells from apoptosis induction by intercellular ROS signaling. Anticancer Res. 2009;29:4541–4557. [PubMed] [Google Scholar]
- [43].Gao F, Yi J, Yuan JQ, et al. The cell cycle related apoptotic susceptibility to arsenic trioxide is associated with the level of reactive oxygen species. Cell Res. 2004;14:81–85. [DOI] [PubMed] [Google Scholar]
- [44].Yang ZH, Wang XH, Wang HP, et al. Capsaicin mediates cell death in bladder cancer T24 cells through reactive oxygen species production and mitochondrial depolarization. Urology. 2010;75:735–741. [DOI] [PubMed] [Google Scholar]
- [45].Bley K, Boorman G, Mohammad B, et al. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol. 2012;40:847–873. [DOI] [PubMed] [Google Scholar]
- [46].Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci. 2000;7:2–15. [DOI] [PubMed] [Google Scholar]
- [47].Battisti V, Maders LD, Bagatini MD, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41:511–518. [DOI] [PubMed] [Google Scholar]
- [48].McClay EF. Epidemiology of bone and soft tissue sarcomas. Semin Oncol. 1989;16:264–272. [PubMed] [Google Scholar]
- [49].Wang Y, Deng X, Yu C, et al. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J Exp Clin Cancer Res. 2018;37:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tao YS, Ma XY, Chai DM, et al. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie. 2012;35:651–656. [DOI] [PubMed] [Google Scholar]
- [51].Du LL, Chai DM, Zhao LN, et al. AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimers Dis. 2015;43:775–784. [DOI] [PubMed] [Google Scholar]
- [52].Cheng XS, Zhao KP, Jiang X, et al. Nmnat2 attenuates Tau phosphorylation through activation of PP2A. J Alzheimers Dis. 2013;36:185–195. [DOI] [PubMed] [Google Scholar]
- [53].Parone PA, James DI, Da Cruz S, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Egawa T, Hamada T, Kameda N, et al. Caffeine acutely activates 5ʹadenosine monophosphate-activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metabolism. 2009;58:1609–1617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


