OBESITY
Obesity is a pandemic that has increased exponentially during the past decades due in large part to recent changes in lifestyle and food delivery systems. Yet, there is still a great variability in the burden of obesity across ancestral populations. For example, in the USA prevalence estimates of adult obesity vary from 13 to 48% in Asian Americans and African Americans.1 Such variable observations within the same obesogenic environment have motivated a wide array of inquiry into the genetic, epigenetic and social determinants of obesity, and their complex interactions with modern lifestyles and food systems.
EVOLUTIONARY PERSPECTIVES
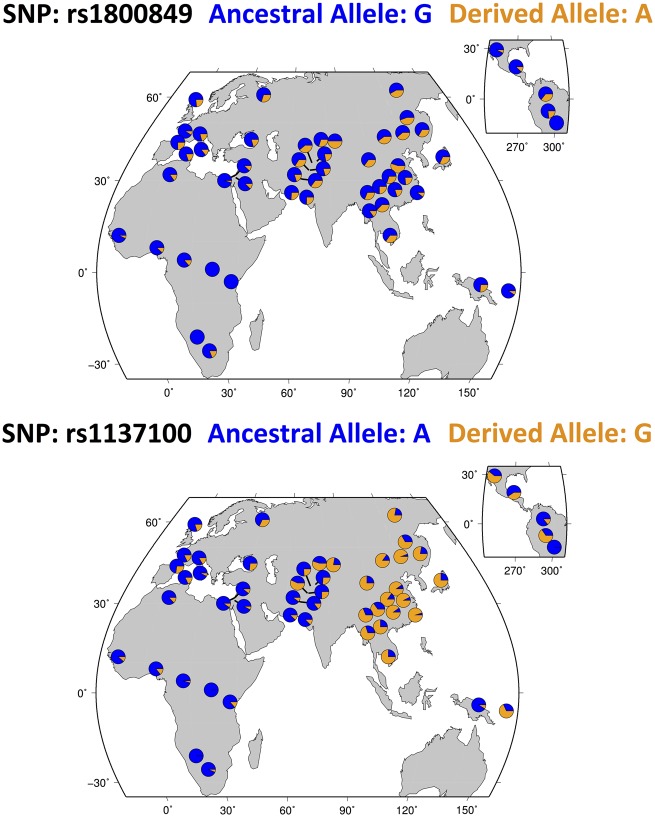
Variation in obesity burden across ancestral populations may be partially explained by human history. After migrating out of Africa, humans were exposed to a broader range of environmental conditions. For example, exposure to colder climates may have shaped human metabolism by positively selecting for a higher resting metabolic rate (RMR), at the expense of building lower adipose stores for times of food insecurity.2 This evolutionary trade-off has been supported by the fact that RMR varies between populations, being highest in indigenous-arctic populations, intermediate for European ancestry and lowest in African Americans.3,4 This could partially explain why certain ancestral groups within the same country, e.g. European Americans or East Asian Americans, have a lower obesity burden.5 In fact, there is evidence of selection by climate across the genome,6 and specifically in genes related to the non-shivering thermogenesis of brown adipose tissue (BAT) like those encoding the uncoupling proteins (i.e. UCP1-3) or the leptin receptor (LEPR).4,7 As shown in Fig. 1 for rs1800849 at UCP3 and rs1137100 at LEPR, adaptation to the colder environmental conditions of high latitudes may have selected for a higher RMR in these populations, as compared with populations with a higher frequency of the ancestral alleles that protect against overheating and promote greater storage of excess energy.
Figure 1.
Geographical distribution of rs1800849 at UCP3 and rs1137100 at LEPR single nucleotide polymorphisms (SNPs; adapted from the selection browser of the Human Genome Diversity Panel8)
FUTURE IMPLICATIONS
The complex genetic architecture of obesity has been extensively studied by genome-wide association studies. Yet, additional insights on how the frequency of obesity-related variants was shaped by selective pressures and further modified by possibly heritable epigenetic processes are needed to form a more integrated view of the complex origins of obesity. For example, DNA methylation sites are enriched in regions of the genome with signals of natural selection.9 Both genetic variation and epigenetic processes have been implicated in UCP1 expression in BAT in response to cold exposure.10 Prediction models of thermogenesis-dependent expression and protein–protein interactions, along with the search for selection signatures could identify strong obesity candidate genes for future study. This could improve our knowledge of the genetic basis of differential obesity susceptibility and move clinical care closer to the goal of precision medicine. Evolutionary principles may inform the prevention and treatment of obesity via the development of tailored lifestyle guidelines. For example, caloric intake recommendations may want to take differences in RMR across ancestral populations into account.5 Thus, taking an evolutionary perspective to the study of obesity may shed light on the role of evolutionary trade-offs and mismatches in both clinical care and global health.
REFERENCES
- 1. Flegal KM, Kruszon-Moran D, Carroll MD. et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sellayah D, Cagampang FR, Cox RD.. On the evolutionary origins of obesity: a new hypothesis. Endocrinology 2014;155:1573–88. [DOI] [PubMed] [Google Scholar]
- 3. Leonard WR, Sorensen MV, Galloway VA. et al. Climatic influences on basal metabolic rates among circumpolar populations. Am J Hum Biol 2002;14:609–20. [DOI] [PubMed] [Google Scholar]
- 4. Hancock AM, Clark VJ, Qian Y. et al. Population genetic analysis of the uncoupling proteins supports a role for UCP3 in human cold resistance. Mol Biol Evol 2011;28:601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sellayah D. The Impact of early human migration on brown adipose tissue evolution and its relevance to the modern obesity pandemic. J Endocr Soc 2019;3:372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hancock AM, Witonsky DB, Alkorta-Aranburu G. et al. Adaptations to climate-mediated selective pressures in humans. PLoS Genet 2011;7:e1001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sazzini M, Schiavo G, De Fanti S. et al. Searching for signatures of cold adaptations in modern and archaic humans: hints from the brown adipose tissue genes. Heredity 2014;113:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pickrell JK, Coop G, Novembre J. et al. Signals of recent positive selection in a worldwide sample of human populations. Genome Res 2009;19:826–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quinana-Murci L. Genetic and epigenetic variation of human populations: an adaptive tale. C R Biol 2016;339:278–83. [DOI] [PubMed] [Google Scholar]
- 10. Villarroya F, Peyrou M, Giralt M.. Transcriptional regulation of the uncoupling protein-1 gene. Biochimie 2017;134:86–92. [DOI] [PubMed] [Google Scholar]