Abstract
Background:
Canine brucellosis, caused by the bacterium Brucella canis, is a zoonotic and largely reproductive disease of dogs. The disease is a recognized problem in canine breeding populations, and the risk to individuals assisting with birthing is well described. Prior to 2015, all cases of canine brucellosis reported to the Minnesota Board of Animal Health were in dogs used for breeding. In 2015, canine brucellosis was identified in eight Minnesota rescue dogs, all originating from specific geographic areas in South Dakota. Our objective was to measure the seroprevalence of B. canis in stray and previously owned dogs entering a large Minnesota animal rescue organization to determine if our observations represented a localized or generalized disease issue among rescue dogs.
Methods:
A stratified random sample of stray and previously owned dogs entering the largest Minnesota animal rescue organization between November 1, 2016 and November 7, 2017, was tested for B. canis antibodies by the 2-Mercaptoethanol Rapid Slide Agglutination Test (2ME-RSAT) (Zoetis D-TEC® CB kit). Sample sizes for each strata were calculated using previously published seroprevalence estimates. Blood from selected dogs was collected, serum harvested, and transported to the Minnesota Veterinary Diagnostic Laboratory for testing. Positive samples in the 2ME-RSAT were shipped to Cornell University for confirmation by Agarose Gel Immunodiffusion (AGID) testing. Demographics, state and setting of origin, and health status were collected on study-dogs.
Results:
Of the 10,654 dogs accepted by AHS during the study period, 943 (8.9%) were selected for testing. Most study dogs arrived from Oklahoma (28%), Alabama (18%), and Minnesota (12%). The median age of study dogs was 1.5 years; 303 (32%) were intact males and 294 (31%) were intact females. Most study dogs were strays (n=716, 76%). Of the total, 22 (3.1%) stray and eight (3.5%) owner-surrendered dogs were presumptively positive by RSAT; one (0.11%) of the stray dogs was positive by 2ME-RSAT and confirmed by AGID. The positive dog was a healthy-appearing 1 year-old neutered male beagle from Texas.
Conclusions:
The seroprevalence of canine brucellosis in dogs entering Minnesota for adoption from multiple states was low. Never-the-less, care must to be taken to consider all potential risks and outcomes of interstate and international dog trade, including the spread of infectious diseases such as canine brucellosis.
Keywords: canine brucellosis, Brucella canis, seroprevalence, rescue dogs, zoonosis
Introduction
In recent years, more Americans are joining the “adopt, don’t shop” trend in obtaining new pets (American Veteinary Medical Association, 2012; Bershaker, 2017; Humane Society of the United States, 2018). In Minnesota, the size of the stray or unowned dog population fails to meet the demand of Minnesotans who want to add a new dog to their family. Therefore, many animal rescue organizations and humane societies import dogs from other states where dog overpopulation is a larger concern. This approach is mutually beneficial to consumers in Minnesota and animal care and control organizations in overpopulated states, and it decreases the rate of euthanasia of dogs in overpopulated shelters.
Canine brucellosis is a zoonotic disease of dogs caused by Brucella canis, widely considered incurable. It was first described in 1966 in a population of beagle dogs experiencing an outbreak of abortions (Carmichael, 1966). In addition to reproductive complications and genitourinary signs, discospondylitis, colitis, uveitis, and weight loss have also been described in cases of canine brucellosis (Bramlage et al., 2015; National Association of State Public Health Veterinarians, 2012). Because of its reproductive implications, canine brucellosis in breeding settings is well described, and standard control measure recommendations are available (American College of Theriogenologists; Bramlage et al., 2015).
Brucella canis can also infect humans and cause non-specific illnesses, but may also cause serious complications including endocarditis, similar to other Brucella species infections. Infection in people assisting with whelping of an infected dog or disposing of aborted or stillborn fetuses has been characterized (Blankenship and Sanford, 1975; Lucero et al., 2010; Nomura et al., 2010; Rifkin et al., 1978; Rumley and Chapman, 1986; Tosi and Nelson, 1982). There are also case reports of B. canis infection in people with limited or general dog contact and even some cases who reported no contact with dogs (Dentinger et al., 2015; Lawaczeck et al., 2011; Lucero et al., 2005; Lucero et al., 2010; Marzetti et al., 2013; Munford et al., 1975; Piampiano et al., 2000; Polt et al., 1982; Rousseau, 1985; Swenson et al., 1972; Ying et al., 1999). In these situations, where people have household contact with dogs in the community (i.e., not associated with breeding stock), transmission and infection risk are poorly understood.
Canine brucellosis is reportable in Minnesota to the Minnesota Board of Animal Health (BAH), and surveillance responsibilities are shared between BAH and the Minnesota Department of Health (MDH). Between 2009 and 2017, 117 dogs tested positive for canine brucellosis and were determined to be infected with Brucella canis by the BAH. Of 27 dogs for which clinical information was available, 17 (63%) had at least one clinical sign associated with Brucella canis infection; those without clinical signs were tested because of an epidemiologic link to other identified positive dogs or were incidentally identified by routine wellness testing. Of the dogs with clinical signs, a majority (94%) had back pain or lameness with diagnosed or presumed discospondylitis. Most dog owners (80%) elected humane euthanasia of the infected dog. Prior to 2015, all cases of canine brucellosis in dogs reported in Minnesota were related to purposeful dog breeding. In contrast, of the 34 dogs identified with canine brucellosis in Minnesota in 2015 and 2016, 13 were used in purposeful breeding and 21 were rescue dogs not used for breeding. These 21 dogs originated from specific geographic areas in South Dakota (Minnesota Department of Health: unpublished data). Based on the increased number of canine brucellosis reports in rescue dogs, we wanted to explore if canine brucellosis in canine rescue and shelter populations was an emerging public health issue.
Because canine brucellosis is considered an incurable and lifelong infection in dogs, and animals can potentially remain infectious for years, the presence of B. canis antibodies is considered indicative of B. canis infection and potential infectivity (Bramlage et al., 2015; National Association of State Public Health Veterinarians, 2012). Seroprevalence studies for B. canis antibodies have been used to estimate disease burden in dog populations. In the United States, prevalence of canine brucellosis is higher in stray animals, sexually intact animals, and animals originating from the southern United States (National Association of State Public Health Veterinarians, 2012). Much of the work to estimate the prevalence of canine brucellosis in dog populations in the United States and Canada was performed in the late 1970s and early 1980s. Studies used varying sampling and testing methodologies and reported seroprevalences from 3.6% to 9.4% in stray dogs from Florida, Georgia, and Tennessee (Brown et al., 1976; Fredrickson and Barton, 1974; Hoff and Nichols, 1974; Lovejoy et al., 1976; Wooley et al., 1977); 6.8% to 8% in stray dogs from Illinois, Wisconsin, and Michigan (Boebel et al., 1979; Theirmann, 1980); and 0 to 1.9% in owned dogs from Tennessee, Georgia, and Quebec, Canada (Brown et al., 1976; Fredrickson and Barton, 1974; Higgins et al., 1979; Lovejoy et al., 1976).
The study aim was to determine whether the identification of a cluster of canine brucellosis in dogs from South Dakota was a localized issue, or indicative of a more widespread problem among rescue dogs. Therefore, we measured the seroprevalence of B. canis antibodies in stray and owner-surrendered dogs entering a large Minnesota animal rescue organization, which accounts for approximately half of all rescue dogs in Minnesota. We also intended to characterize seropositive dogs and their risk factors.
Material and Methods
We conducted a cross-sectional seroprevalence study, as measured by the seroresponse of randomly selected dogs to Brucella canis antigens.. All dogs accepted by the Animal Humane Society between November 1, 2016 and November 7, 2017 were eligible for inclusion, except for any puppy that was part of a litter in which a littermate had been selected for testing. A stratified random sample of stray and owner-surrendered dogs was selected to estimate the seroprevalence of B. canis seropositive dogs. Sample sizes for each strata were calculated using an expected 8% seroprevalence in the stray population and 2% seroprevalence in the owner-surrendered population, with a 2% allowed error and 95% confidence level. Therefore, we planned to sample 707 stray and 189 owner-surrendered dogs, aiming to sample 60 stray and 16 owner dogs per month. Dogs were randomly selected for testing using a random number generator that was applied to a list identifying each group of incoming dogs. Blood was drawn from selected dogs during routine intake examination; serum was harvested and transported to the Minnesota Veterinary Diagnostic Laboratory (MN VDL) for testing. Serum samples were screened using a commercial rapid slide agglutination test (RSAT) (Zoetis D-TEC CB Canine Brucellosis Antibody Test Kit, Kansas City, MO); RSAT-positive samples were then tested using the 2-mercaptoethanol (2ME) RSAT procedure. Positive samples by RSAT or 2ME-RSAT were batched and shipped to the Cornell University Animal Health Diagnostic Center for agarose gel immunodiffusion (AGID) testing. Only dogs positive by AGID were considered B. canis-positive for the purpose of this study; all other dogs were considered B. canis-negative. A data collection form was completed by animal rescue facility staff during intake examination to gather information on demographics, state and setting of origin, and current health status (as determined by trained veterinary technicians) of study dogs. A condition of AHS’s participation in this study was that data collection and specimen collection along with test results not disrupt the management or movement of dogs within their facility or delay the placement of adoptable dogs. The average length of stay for a dog at AHS is 10 days; any delay could be detrimental to the dog and would impact the AHS’s ability to help other animals. Therefore, test results were provided in aggregate to the Animal Humane Society, were not linked to individual dogs, and had no influence on the outcome or management of the study dogs. A disease reporting exemption was provided to the MN VDL by the BAH for B. canis test results associated with this study.
Data were entered into a Microsoft Access database; apparent seroprevalence and Wilson’s 95% confidence intervals (CI) were calculated for each strata. Percentages were tabulated for characteristics of dogs. Descriptive analyses were performed using statistical software (SAS 9.4, SAS Institute, Cary, NC).
The study protocol was reviewed and approved by the University of Minnesota’s Institutional Animal Care and Use Committee (protocol ID 1604-33665A), and followed the American Kennel Club Canine Health Foundation humane use of animals policy. As the responsible agency for the dogs included in the study, the Animal Humane Society gave informed consent for participation in the study.
Results
A total of 10,654 dogs were accepted by the animal rescue organization from November 1, 2016 through November 7, 2017; The overall population of dogs at AHS during this time period consisted of 59% stray and 41% owner-surrendered dogs. Of the 10,654 total dogs, 943 (9%) were selected for this study including 716 (76%) stray and 227 (24%) owner-surrendered dogs (Figures 1, 2). Study dogs originated from 13 states and one foreign country with most study dogs arriving from Oklahoma (28%), Alabama (18%), and Minnesota (12%) (Table 1). The median age of study dogs was 1.5 years (range, 6.5 weeks to 19 years); 303 (32%) were intact males, and 294 (31%) were intact females. The majority (93%) were apparently healthy (Table 1). Conditions of non-healthy dogs included apparent kennel cough (n=20), lameness (n=8), upper respiratory signs exclusive of apparent kennel cough (n=7), dermatologic conditions (n=6), and diarrhea (n=5). Sixty-seven (7%) study dogs had current or recently treated heartworm infection noted on their data collection form.
Figure 1.
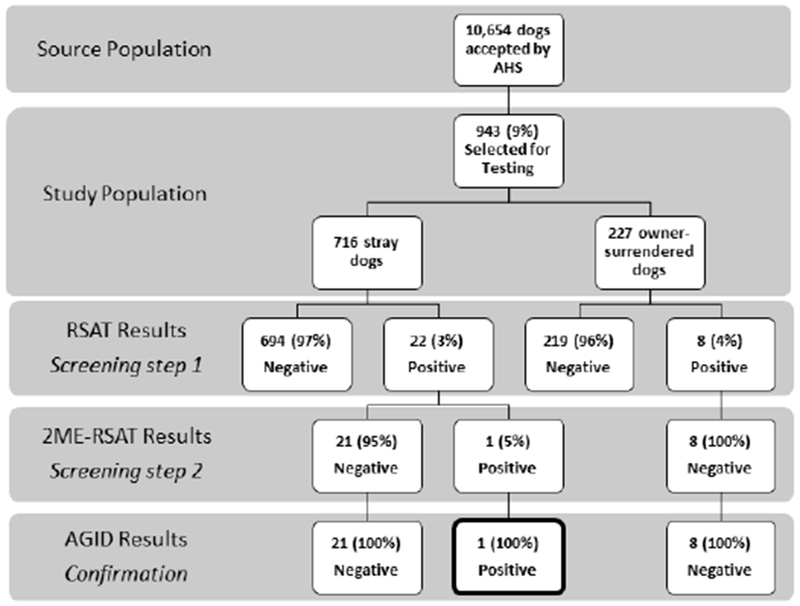
Brucella canis Antibody Test Results, Minnesota, November 2016–2017 (n=943)
Figure 2.
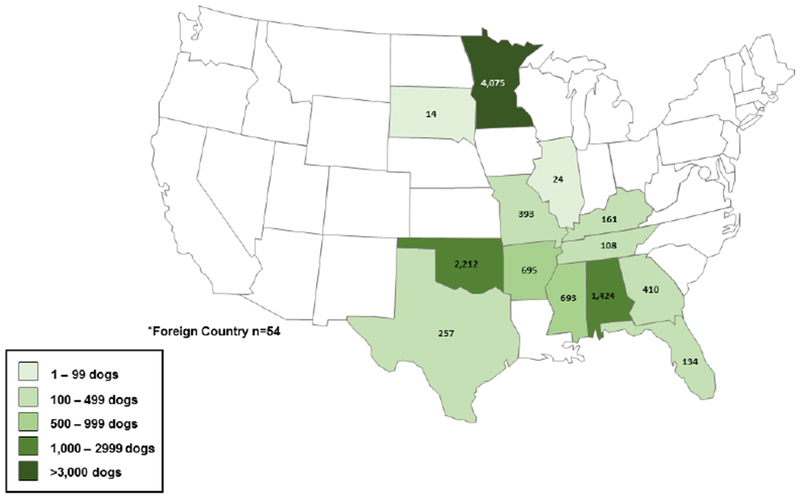
Total Number of Dogs Accepted by the Animal Humane Society by State* of Origin, November 2016–2017 (n=10,654)
Table 1.
Characteristics of dogs tested for canine brucellosis, Minnesota, 2016–2017 n=943
| Stray n=716 n (%) | Owner-Surrendered n=227 n (%) | Total n=943 n (%) | |
|---|---|---|---|
| Age in years, median (IQR) | 1.5 (1.0–2.0) | 2.0 (0.92–3.0) | 1.5 (1.0–2.0) |
| Sex | |||
| Male | 241 (34) | 62 (27) | 303 (32) |
| Neutered male | 119 (17) | 45 (20) | 164 (17) |
| Female | 235 (33) | 59 (26) | 294 (31) |
| Spayed female | 115 (16) | 59 (26) | 174 (18) |
| Female, unknown | 6 (0.84) | 2 (0.88) | 8 (0.85) |
| State of origin | |||
| Alabama | 151 (21) | 19 (8) | 169 (18) |
| Arkansas | 95 (13) | 10 (4) | 105 (11) |
| Florida | 11 (2) | 6 (3) | 17 (2) |
| Georgia | 77 (11) | 16 (7) | 93 (10) |
| Illinois | 4 (0.56) | 0 | 4 (0.42) |
| Kentucky | 13 (2) | 1 (0.44) | 14 (1) |
| Minnesota | 14 (2) | 103 (45) | 117 (12) |
| Missouri | 0 | 2 (0.9) | 2 (0.21) |
| Mississippi | 98 (14) | 22 (10) | 120 (13) |
| Oklahoma | 220 (31) | 44 (19) | 264 (28) |
| South Dakota | 0 | 1 (0.44) | 1 (0.1) |
| Tennessee | 5 (0.70) | 1 (0.44) | 6 (0.6) |
| Texas | 26 (4) | 3 (1) | 29 (3) |
| Foreign country (South Korea) | 2 (0.28) | 0 | 2 (0.21) |
| Setting of origin | |||
| Breeding operation | 0 | 8 (4) | 8 (0.8) |
| Indian Reservation | 1 (0.14) | 5 (2) | 6 (0.64) |
| Urban area*, n=147 | 47 (82) | 46 (51) | 93 (10) |
| Rural area*, n=147 | 10 (18) | 44 (49) | 54 (37) |
| Clinical history | |||
| Healthy | 671 (94) | 210 (93) | 881 (93) |
| History of recent or current antibiotics | 56 (8) | 20 (9) | 76 (8) |
| Reproductive history (female) | |||
| Evidence of prior litter | 76 (22) | 18 (15) | 94 (10) |
| Arrived with litter | 8 (2) | 2 (2) | 10 (1) |
Twenty-two (3.1%; 95% CI, 2.0% to 4.6%) of the 716 stray dogs were presumptively positive by RSAT. Only one stray dog was also positive by 2ME-RSAT and was subsequently confirmed B. canis antibody-positive by AGID. The positive dog was an apparently healthy, 1-year-old neutered-male beagle that arrived from Texas. Eight (3.5%; 95% CI, 1.8% to 6.8%) of the 227 owner-surrendered dogs were presumptively positive by RSAT. However, all eight were negative by 2ME-RSAT and AGID (Figure 1). In total, only one dog was confirmed positive, giving an overall seroprevalence of 0.11% (95% CI, 0.02% to 0.60%).
Due to the low number of canine brucellosis-positive dogs, comparative analyses to determine risk factors were not performed.
Discussion
Each year, hundreds of thousands of dogs are collected from overpopulated states, comingled, and transported across the country to states where the demand for adoptable dogs is high, such as in Minnesota (Bershaker, 2017; Shelter Animals Count, 2016). The participating animal rescue organization in this study, the Animal Humane Society, is the principal humane society in Minnesota and cannot meet the demand for rescued animals; therefore, accepts large numbers of dogs from many areas of the United States. A similar interstate dog trade occurs with breeding dogs, and has been shown to spread brucellosis among states (Brower et al., 2007; Johnson et al., 2018). This means that infected dogs can quickly translocate diseases that were once regionally limited, thereby accelerating spread of infectious diseases.
A recent study conducted in Mississippi estimated the seroprevalence of canine brucellosis in shelter dogs to be 7.4%, consistent with previously published seroprevalence estimates which ranged from 0% to 9% (Boebel et al., 1979; Brown et al., 1976; Fredrickson and Barton, 1974; Higgins et al., 1979; Hoff and Nichols, 1974; Hubbard et al., 2018, Lovejoy et al., 1976; Theirmann, 1980; Wooley et al., 1977). Our study results further support a low seroprevalence of B.canis in canine rescue populations and found an even lower estimated seroprevalence than the previously published estimates (0.11%; 95% CI, 0.02% to 0.60%). This suggests that general screening of individual dogs for canine brucellosis may not be a sensible use of limited animal shelter resources, in agreement with the recent findings of Hubbard et al. (Hubbard et al., 2018).
Extrapolating the seroprevalence found in this study, we can estimate with 95% confidence that if all dogs entering the AHS during the time period were tested, between 2 and 64 dogs would have been identified with canine brucellosis using the study’s test methods. Because we oversampled stray dogs, our data suggest the actual number of undiagnosed dogs would be on the lower end of the estimated range. Even so, each of these dogs presents some zoonotic risk to family members; especially children, the elderly, pregnant women, and those with underlying medical conditions who are at higher risk for serious illness from zoonotic diseases. Although canine brucellosis is considered less severe than other types of brucellosis, there are documented cases of severe complications due to B. canis infection including endocarditis, peripheral aneurysms, osteomyelitis, and epidural abscess (Marzetti et al., 2013; Piampiano et al., 2000; Ying et al., 1999).
The largest limitation of this study was our inability to perform comparative analyses and identify risk factors for seropositivity due to the low number of positive tests. Additionally our methods did not allow for a blood culture or second/paired serum sample to confirm the 2ME-RSAT negative results as suggested in the test kit instructions. Therefore, we could not distinguish between early infection and nonspecific reactions in the RSAT-positive, 2ME RSAT-negative animals. Finally, because our study population consisted predominately of dogs that had been recently transported into Minnesota, our findings may not represent the seroprevelance of all dogs in the rescue population.
The low seroprevalence found in this study supports the notion that the cluster of canine brucellosis in rescue dogs from South Dakota was a localized issue and not indicative of a more widespread problem among rescue dogs or emerging public health issue. Still, care must to be taken to consider all potential risks and outcomes of interstate and international dog trade, including the spread of infectious diseases such as canine brucellosis. Precautions including thorough veterinary examinations and screening for diseases known to be prevalent in a region prior to transfer may help mitigate spread of infectious disease.
This study demonstrates that it is possible for one health collaborators to work together toward solutions to animal and human health issues. We suggest a one health approach to conduct a nationwide prevalence study using direct and indirect testing methodologies to further understand the distribution and burden of this zoonotic disease.
Figure 3.
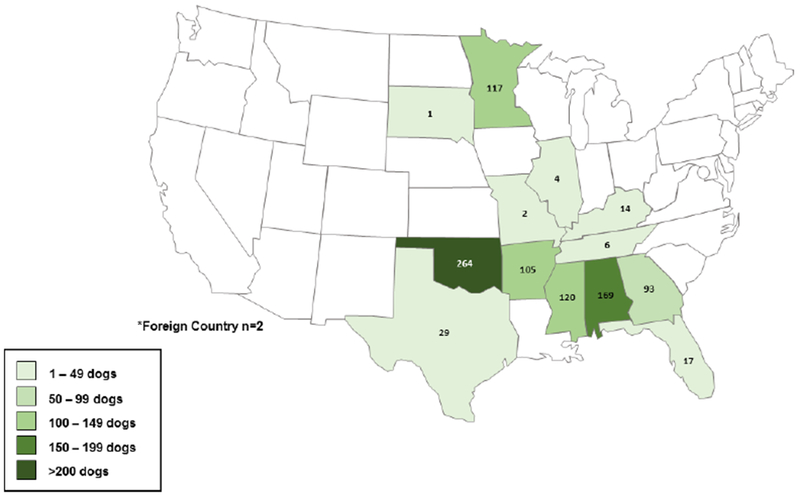
Number of Dogs Selected for Canine Brucellosis Seroprevalence Study by State* of Origin, November 2016–2017 (n=943)
Acknowledgements
The authors would like to acknowledge Dr. Paul Anderson and Kayla Peterson with the Minnesota Board of Animal Health, and Dr. Stephanie Rossow with the Minnesota Veterinary Diagnostic Laboratory for their assistance with beginning this project, and Dr. Jeff Bender with the University of Minnesota for his expertise and careful review of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
This project was supported by the AKC Canine Health Foundation [grant number 02267-A]. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the Foundation.
Study design, interpretation of the data, and writing of this report was partially supported by the National Institutes of Health [grant number NIH T32 OD010993] in the form of salary support for one author (Dr. Larson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Theriogenologists. Position statement: welfare of breeding dogs. Available at: https://www.theriogenology.org/page/PositionStatements#Welfare. Accessed April 27, 2018.
- American Veterinary Medical Association, 2012: American Veterinary Medical Association Household Pet Survey US pet ownership and demographics sourcebook. AVMA, Shaumburg, IL. [Google Scholar]
- Bershaker M The President’s Blog: Are animal shelter outcomes improving? ASPCA Professional; Available at: https://www.aspcapro.org/blog/2017/04/19/presidents-blog-are-animal-shelter-outcomes-improving. Accessed May 10, 2018. [Google Scholar]
- Blankenship RM, Sanford JP. Brucella canis. A cause of undulant fever. AM J Med 1975;59:424–426. [DOI] [PubMed] [Google Scholar]
- Boebel FW, Ehrenford FA, Brown GM, Angus RD, Thoen CO. Agglutinins to Brucella canis in stray dogs from certain counties in Illinois and Wisconsin. J Am Vet Med Assoc 1979;175:276–277. [PubMed] [Google Scholar]
- Bramlage DJ, Fortney W, Kesler RM, et al. Best practices for Brucella canis prevention and control in dog breeding facilities. United States Department of Agriculture Animal and Plant Health Inspection Service, 2015. [Google Scholar]
- Brower A, Okwumabua O, Massengill C, Muenks Q, Vanderloo P, Duster M, Homb K, Kurth K, 2007. Investigation of the spread of Brucella canis via the U.S. interstate dog trade. Int J Infect Dis 11, 454–458. [DOI] [PubMed] [Google Scholar]
- Brown J, Blue JL, Wooley RE, Dreesen DW, Carmichael LE. A serologic survey of a population of Georgia dogs for Brucella canis and an evaluation of the slide agglutination test. J Am Vet Med Assoc 1976;169:1214–1216. [PubMed] [Google Scholar]
- Carmichael LE. Abortion in 200 beagles. J Am Vet Med Assoc 1966;149:1126. [Google Scholar]
- Dentinger CM, Jacob K, Lee LV, Mendez HA, Chotikanatis K, McDonough PL, Chico DM, De BK, Tiller RV, Traxler RM, Campagnolo ER, Schmitt D, Guerra MA, Slavinski SA, 2015. Human Brucella canis Infection and Subsequent Laboratory Exposures Associated with a Puppy, New York City, 2012. Zoonoses Public Health 62, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson LE, Barton CE. A serologic survey for canine brucellosis in a metropolitan area. J Am Vet Med Assoc 1974;165:987–989. [PubMed] [Google Scholar]
- Higgins R, Hoquet F, Bourque R, Gosselin Y. A serological survey for Brucella canis in dogs in the province of Quebec. Can Vet J 1979;20:315–317. [PMC free article] [PubMed] [Google Scholar]
- Hoff GL, Nichols JB. Canine brucellosis in Florida: serologic survey of pound dogs, animal shelter workers, and veterinarians. Am J Epidemiol 1974;100:35–39. [DOI] [PubMed] [Google Scholar]
- Hubbard K, Wang M, Smith DR. Seroprevalence of brucellosis in Mississippi shelter dogs. Prev Vet Med 2018;159:82–86. [DOI] [PubMed] [Google Scholar]
- Humane Society of the United States. Pets by the numbers. Animal sheltering online by the Humane Society of the United States. Available at https://www.animalsheltering.org/page/pets-by-the-numbers. Accessed May 10, 2018.
- Johnson CA, Carter TD, Dunn JR, Baer SR, Schalow MM, Bellay YM, Guerra MA, Frank NA. Investigation and characterization of Brucella canis infections in pet-quality dogs and associated human exposures during a 2007-2016 outbreak in Michigan. J Am Vet Med Assoc 2018;253:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keid LB, Soares RM, Vasconcellos SA, Megid J, Salgado VR, Richtzenhain LJ. Comparison of agar gel immunodiffusion test, rapid slide agglutination test, microbiological culture and PCR for the diagnosis of canine brucellosis. Res Vet Sci 2009;86:22–26. [DOI] [PubMed] [Google Scholar]
- Lawaczeck E, Toporek J, Cwikla J, Mathison BA. Brucella canis in a HIV-infected patient. Zoonoses Public Health 2011;58:150–152. [DOI] [PubMed] [Google Scholar]
- Lovejoy GS, Carver HD, Moseley IK, Hicks M. Serosurvey of dogs for Brucella canis infection in Memphis, Tennessee. Am J Public Health 1976;66:175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero NE, Jacob NO, Ayala SM, Escobar GI, Tuccillo P, Jacques I. Unusual clinical presentation of brucellosis caused by Brucella canis. J Med Microbiol 2005;54:505–508. [DOI] [PubMed] [Google Scholar]
- Lucero NE, Corazza R, Almuzara MN, Reynes E, Escobar GI, Boeri E, Ayala SM Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect 2010;138:280–285. [DOI] [PubMed] [Google Scholar]
- Marzetti S, Carranza C, Roncallo M, Escobar GI, Lucero NE, 2013. Recent trends in human Brucella canis infection. Comp Immunol Microbiol Infect Dis 2013;36, 55–61. [DOI] [PubMed] [Google Scholar]
- Munford RS, Weaver RE, Patton C, Feeley JC, Feldman RA. Human disease caused by Brucella canis a clinical and epidemiologic study of two cases. J Am Med Assoc 1975;231:1267–1269. [PubMed] [Google Scholar]
- National Association of State Public Health Veterinarians. Public health implications of Brucella canis infections in humans. March, 2012. Available at: http://www.nasphv.org/Documents/BrucellaCanisInHumans.pdf.
- Nomura A, Imaoka K, Imanishi H, Shimizu H, Nagura F, Maeda K, Tomino T, Fujita Y, Kimura M, Stein GH. Human Brucella canis infections diagnosed by blood culture. Emerg Infect Dis 2010;16:1183–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piampiano P, McLeary M, Young LW, Janner D. Brucellosis: unusual presentations in two adolescent boys. Pediatr Radiol 2000;30:355–357. [DOI] [PubMed] [Google Scholar]
- Polt SS, Dismukes WE, Flint A, Schaefer J. Human brucellosis caused by Brucella canis: clinical features and immune response. Ann Intern Med 1982;97:717–719. [DOI] [PubMed] [Google Scholar]
- Rifkin GD, Supena RB, Axelson JA. Case report. Brucella canis bacteremia: a case with negative B canis agglutinins. AM J Med Sci 1978;276:113–115. [PubMed] [Google Scholar]
- Rousseau P Brucella canis infection in a woman with fever of unknown origin. Postgrad Med J 1985;78:249. [DOI] [PubMed] [Google Scholar]
- Rumley RL, Chapman SW. Brucella canis: an infectious casue of prolonged fever of undetermined origin. South Med J 1986;79:626–628. [DOI] [PubMed] [Google Scholar]
- Shelter Animals Count. 2016. Animal Sheltering Statistics. Available at:https://shelteranimalscount.org/data/data-reports/2016-animal-sheltering-statistics. Accessed May 10, 2018.
- Swenson RM, Carmichael LE, Cundy KR. Human infection with Brucella canis. Ann Intern Med 1972;76:435–438. [DOI] [PubMed] [Google Scholar]
- Theirmann AB. Brucellosis in stray dogs in Detroit. J Am Vet Med Assoc 1980;177:1216–1217. [PubMed] [Google Scholar]
- Tosi MF, Nelson TJ. Brucella canis infection in a 17-month-old child successfully treated with moxalactam. J Pediatr 1982;101:725–727. [DOI] [PubMed] [Google Scholar]
- Wooley RE, Brown J, Shotts EB, Blue JL, Dreesen DW. Serosurvey of Brucella canis antibodies in urban and rural stray dogs in Georgia. Vet Med Small Anim Clin 1977;72:1584–1584. [PubMed] [Google Scholar]
- Ying W, Nguyen MQ, Jahre JA. Brucella canis Endocarditis: Case Report. Clin Infect Dis 1999;29:1593–1594. [DOI] [PubMed] [Google Scholar]


