ABSTRACT
Edwardsiella piscicida is an Enterobacteriaceae that is abundant in water and causes food and waterborne infections in fish, animals, and humans. The bacterium causes Edwardsiellosis in farmed fish and can lead to severe economic losses in aquaculture worldwide. E. piscicida is an intracellular pathogen that can also cause systemic infection. Type III and type VI secretion systems are the bacterium’s most lethal weapons against host defenses. It also possesses multi-antibiotic resistant genes and is selected and enriched in the environment due to the overuse of antibiotics. Therefore, the bacterium has great potential to contribute to the evolution of the resistome. All these properties have made this bacterium a perfect model to study bacteria virulence mechanisms and the spread of antimicrobial genes in the environment. We summarize recent advance in E. piscicida biology and provide insights into future research in virulence mechanisms, vaccine development and novel therapeutics.
KEYWORDS: Edwardsiella piscicida, T3SS/T6SS and effectors, intracellular and systemic infection, resistome, virulence mechanisms
Introduction
Edwardsiella species are abundant in freshwater and marine environments and one particular species, E. piscicida (old name as E. tarda) is a common and important fish pathogen. E. piscicida causes severe infections in a wide variety of marine and freshwater animals, especially in fish, in the USA, Europe, Asia, and around the world [1,2]. Factors that have contributed to the severity of Edwardsiella infections in fish include intensive fish farming methods, the overuse of antimicrobial chemicals in aquaculture and agriculture, the bacterium’s broad host range and the development of multi-antibiotics resistance. Edwardsiella can move through the food chain to infect humans and other farm animals. This emerging pathogen is a member of the Enterobacteriaceae and is capable of transferring multi-antibiotics resistant genes to other enterics and to the resistome in water and soil microbiomes [3]. Currently, approximately 80% of Edwardsiella infections in humans result in gastroenteritis in patients with other underlying diseases [4]. Among the Edwardsiella species, E. piscicida is the most studied and is therefore a useful model organism to study enterics, intracellular pathogens, systemic infections, and crosstalk between multiple secretion systems. Some strains of Edwardsiella used in research are given in Table 1. Furthermore, understanding the organism’s interactions with the food and human microbiome can further our understanding of the evolution of the resistome in relations to other food and waterborne diseases.
Table 1.
Edwardsiella strains used by researchers in the literature.
| Edwardsiella strain | Isolated from; characteristicsa | Reference |
|---|---|---|
|
E. piscicida ET883T (NCIMB 14824T, CCUG 62,929) |
European eel, Anguilla anguilla; Greaker, Norway | [12] |
| E. piscicida EIB202 (CCTCC M208068) | Turbot, Scophthalmus maximus; Mariculture farm, Yantai, China; Colr Cmr Tcr | [85] |
| E. piscicida PPD130/91 | Ornamental fish, Serpae tetra; PPD, Singapore; Kms Colr Amps | [22,29] |
| E. anguillarum ET080813T | Japanese eel, Anguilla japonica; Fujian, China | [13] |
| E. hoshinae ATCC33379T | Female puffin, Fratercula arctica; France | [5] |
| E. ictaluri ATCC33202T | Channel catfish, Ictalurus punctatus; Georgia, USA | [6] |
| E. ictaluri 93–146 | Channel catfish, Ictalurus punctatus; Baton Rouge, USA | [7] |
| E. tarda ATCC15947T | Human feces; Kentucky, USA | [10] |
| E. tarda TX1* | Japanese flounders, Paralichthys olivaceus; Tcr; Qingdao, China | [8] |
Identification, taxonomy and classification of Edwardsiella bacteria
Edwardsiella was described as a new genus in the mid-1960s in isolates recovered from wounds, blood, urine, and feces of infected humans and animals in the USA, Brazil, Ecuador, Israel and Japan [10]. Some of the first reports of E. tarda infections in aquaculture were reported in channel catfish in Arkansas, USA [11], but the organism is now recognized as a pathogen of farmed and wild fish worldwide [1]. Recently, the genus Edwardsiella was reclassified into five species based on genomic information and phylogenetic analysis. The five species include three fish pathogens (E. piscicida, E. anguillarum, and E. ictaluri) and two non-fish pathogens (E. tarda and E. hoshinae) [2,12–14]. The three fish pathogens infect a wide variety of marine and freshwater fish globally and are major threats to the aquaculture industry worldwide [1,15].
E. piscicida now includes the fish pathogens under the old species name of E. tarda that are isolated from diseased fish and contains one type III and one type VI secretion system (T3SS and T6SS) [12,14]. E. anguillarum contains two T3SSs and three T6SSs and is highly virulent to fish although not much work has been done on this organism [13,15–18]. It is interesting to note that isolates of this species have gone by different names; E. piscicida-like species, atypical E. piscicida, or just E. tarda strains [15,18]. However, E. anguillarum is taxonomically distinct as described by Shao et al. [13] and Buján et al. [15]. E. ictaluri is found in colder climates, harbors one T3SS and one T6SS and is responsible for catfish enteric septicemia (ESC) [14]. E. tarda now describes human or environmental isolates that do not contain any T3SS and T6SS [14,19] whereas E. hoshinae is a pathogen of reptiles and birds [1]. In the Edwardsiella literature prior to 2013, E. tarda described both E. tarda and E. piscicida and this old classification has made it difficult to examine the contribution of E. piscicida to human infections. Likewise, it is not clear whether the new organisms now referred to as E. tarda isolates play any role in fish infections. Finally, work to understand the evolution of virulence genes, passage of these genes to the resistomes or other bacteria and humans, and adaptation to various environments is required in order to understand the pathogenicity of Edwardsiella. Additionally, comparative studies on Edwardsiella and other enterics such as pathogenic Escherichia coli and Salmonella species can shed light on the various virulence mechanisms employed during the infection process.
E. piscicida, a model of the enteric pathogen causing food and waterborne diseases
Isolation of E. tarda/E. piscicida from human feces and from infected fish strongly suggest that these bacteria are important enteric zoonotic pathogens [20]. In fact, Edwardsiella and many other bacteria such as Aeromonas, Salmonella, Vibrio and Yersinia species, have been considered as established zoonotic pathogens that affect both humans and animals [20].
Most studies on virulence mechanisms of bacteria human pathogens (such as enterics) use mammalian tissue cultures and mammalian infection models to gain insights into the mechanisms and principles of bacterial pathogenesis. Although studies of these pathogens in humans are crucial, investigations of bacterial pathogens in non-human hosts can help us gather useful information before extending the applications to humans. Many similarities exist between organisms that cause gastroenteritis, such as E. piscicida, pathogenic E. coli, and Salmonella species. Therefore, E piscicida is increasingly becoming an attractive model organism for studying enteric bacteria in non-human cells and other hosts [2]. E. piscicida infects many fish including blue gourami, turbot, Japanese flounder and zebrafish [1]. Significant differences in LD50 values between virulent strains of E. piscicida and their T3SS and/or T6SS attenuated mutants have been observed, making E. piscicida an attractive model organism for studying food and waterborne pathogens [21,22].
Studying E. piscicida infections in non-mammalian host models can provide vital information for the development of appropriate therapeutics such as live vaccines for the aquaculture industry. These models may also illustrate potential treatments for enteric infections in humans and other animals. E. piscicida is able to infect mammalian infection models such as mice and mammalian tissue culture cells: HeLa, HEK293A, Hep-2, and J774A.1 [23,24]. Using E. piscicida as the model organism to study infection biology, with an emphasis on virulence factors/mechanisms, response to environmental signals, regulation of virulence mechanisms, and interaction between virulence factors and host immune systems, will pave ways to the development of new therapeutics for bacterial diseases in fish. Information gained from such studies has potential for application to the prevention of enteric infections (such as gastroenteritis) in humans as well.
E. piscicida as a model organism for studying intracellular and systemic infections
Eukaryotic cells use endomembrane systems such as secretory and endocytic pathways, for vesicular trafficking to distribute and recycle vital macromolecules and to eliminate harmful pathogens [25,26]. The secretory pathway distributes proteins and lipids produced in the endoplasmic reticulum to the plasma membrane, endosomes and lysosomes. In contrast, the endocytic pathway internalizes extracellular materials such as plasma membrane proteins and lipids. Internalized materials are channeled to the early and late endosomes, to lysosomes for degradation, or to the trans-Golgi for recycling [25].
In the tug of war between pathogens and host cells, some bacteria choose an intracellular lifestyle to avoid the hostile external environments and to access the plentiful nutrients and spacious compartments within host cells [27]. These pathogens often acquire new properties to exploit the endomembrane systems for their survival and replication inside host cells. Intracellular pathogens can reside and replicate within a vacuole after internalization. Some pathogens, such as Salmonella Typhimurium and Legionella pneumophila, choose to modify the vacuole environment and prevent fusion between endosomes and lysosomes to avoid destruction [28]. Others, escape from the vacuoles and replicate inside the host cytoplasm (Listeria monocytogene) or persist within the vacuoles and follow the endocytic trafficking (Helicobacter pylori) [28].
Our previous studies suggested that the actin cloud or actin condensation were important for the internalization of E. piscicida in carp epithelial cells [29]. Sui et al. [30] confirmed the involvement of actin, in addition to microtubules, in E. piscicida internalization using clathrin- and caveolin-mediated endocytosis in mouse macrophage RAW264.7 cells. Upon entering the host, E. piscicida prefers an intracellular lifestyle in Edwardsiella-containing vacuoles (ECVs) in either epithelial [29] or phagocytic cells [31] (Figure 1). The T3SS effector, such as EseJ, empowers the bacteria to thrive intracellularly by disrupting the production of reactive oxygen species (ROS) in J774A.1 cells [32]. The trafficking of Edwardsiella in ECVs followed the pathway from early and late endosomes to lysosomes [30]. Additionally, the acidification of the inside of ECVs was required, similar to that reported for E. ictaluri by Baumgartner et al. [33]. A second T3SS effector, EseG, appears to be translocated and injected into the membrane fraction of host cells and at the vacuole membrane of ECVs after the internalization of E. piscicida in both phagocytic and epithelial cells [34]. EseG may function in microtubule disassembly or remodeling of the endomembrane for the expansion of ECVs [23].
Figure 1.
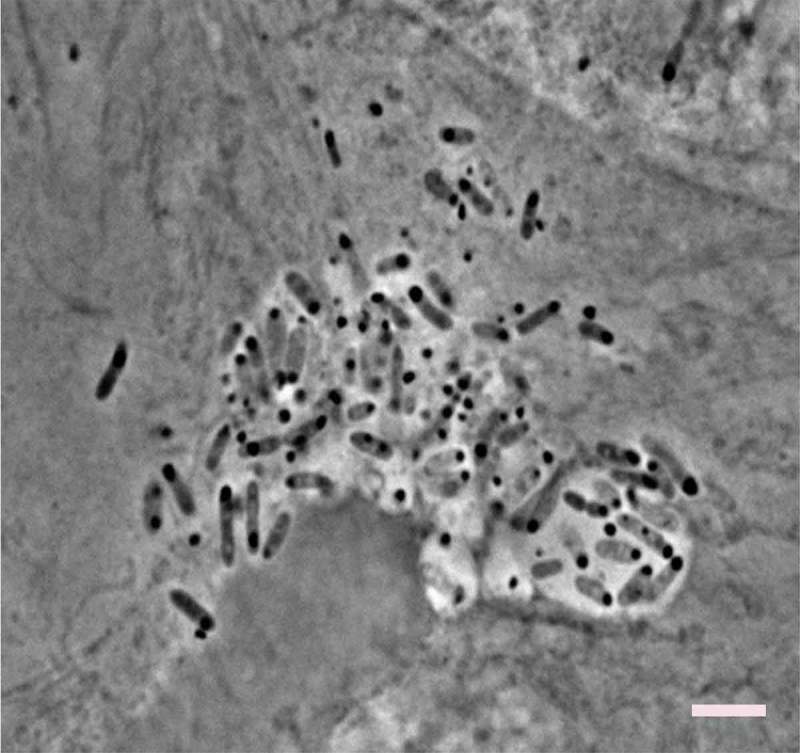
Caco-2 cells showing successful internalization of E. piscicida PPD130/91. Caco-2 cells were infected with wild type PPD130/91 for 6 h at 35°C. Edwardsiella-containing vacuoles (ECVs) with internalized bacteria are clearly visible under phase microscopy. Scale bar = 10 μm.
Studies on the involvement of E. piscicida in systemic infection in animals are limited and the evidence is fragmented. Most evidence comes from histopathological observations of diseased fish, infection kinetics studies in fish, and in situ protein-protein interactions. Clinical signs in diseased fish include poor pigmentation, protrusion and opacity of eyes, lesions on the skin, petechial hemorrhage, liquefaction and necrosis of tissues and organs such as kidney, spleen, and liver [1]. Phagocytes loaded with Edwardsiella were prominent and accompanied watery and bloody ascites [1]. These clinical signs of E. piscicida infection are similar to those observed in fish infected with other fish pathogens such as Aeromonas hydrophila and Vibrio anguillarum [1]; T3SSs and T6SSs are believed to play key roles in this infection pathway. E. piscicida is therefore a good model to study systemic infection in fish and other animals. Novel in vivo and molecular based approaches are needed to fully understand this complicated process. Ling et al [35], used GFP tagged E. piscicida to infect fish by an immersion method to reveal three virulent entry sites; gills, gastrointestinal tract, and skin. The virulent strain but not the avirulent strain, proliferated in various fish tissue and organs and mortalities were reported within three days-post infection. After bacterial entry, intracellular replication is speculated to be the major event (first inside intestinal cells and then inside phagocytic cells) before progressing to systemic infection as bacteria reach a critical mass and spread deeper inside the host’s body [36]. The hosts eventually die due to multiple organ failure and high bacteria load. The surface proteins of Edwardsiella interact with host proteins during local and systemic infection. Protein-protein interactions between outer membrane proteins (OMP) of E. piscicida and proteins in the gills are suggested to be responsible for bacterial entry into the fish [37].
So far, no T3SS or T6SS effectors of E. piscicida appear to be responsible for systemic infections. However, using time series Tn-seq technology and pattern analysis of conditional essentiality (PACE) algorithm to evaluate the fitness costs of each transposon insertion in the E. piscicida genome, the East China University of Science and Technology (ECUST) group in Shanghai was able to systematically assay the virulence factors or determinants in vivo in fish [38]. About 417 genes, including nearly all the previously identified or established virulence factors, were validated to be associated with in vivo colonization and progression of infection in E. piscicida [38]. Advances in these new technologies are unprecedented and show a lot of promise to help us dissect the infection pathway of E. piscicida in a systematic manner. Overall, E. piscicida appears to use its T3SS and T6SS effectors to sustain an intracellular lifestyle and (possibly) systemic infection in the hosts because T3SS and T6SS mutants of E. piscicida have attenuated ability to replicate in phagocytic cells and show reduced bacterial loads during infections [21,39]. One future direction is to elucidate the precise roles played by T3SS and T6SS effectors in the intracellular lifestyle and systemic infection at the molecular level, and to examine separately and collectively their, roles in the infection pathway.
E. piscicida in humans, fish, and environments
E. tarda in humans
E. tarda infections in humans are rare and the reported cases have been associated with Salmonella-like gastroenteritis [40]. Extra-intestinal manifestation includes biliary tract infection, bacteremia, skin and soft tissue infection, liver abscess, peritonitis, intra-abdominal abscess, tubo-ovarian abscess, and mycotic aneurysm [41,42]. Immunocompromised individuals and persons with other underlying diseases such as hepatobiliary diseases, malignancy, and diabetes are most susceptible to infection [41]. Although T3SS and T6SS are the main virulence mechanism in E. piscicida and E. ictulari, these virulent mechanisms are lacking in E. tarda isolates responsible for human infections [13]. It is not clear which virulence factors/mechanisms E. tarda uses to infect human cells. Environmental isolates of E. tarda and E. piscicida may also be active players in the dissemination of multi-antibiotic resistant bacteria resistome in the aquatic microbiome.
E. tarda/E. piscicida in aquaculture
The aquaculture industry has grown rapidly over the past few decades and FAO [43] estimated that the industry contributed about 53% to the total fish consumed in 2016. This rapid growth in the aquaculture industry has raised concerns about the quality and safety of farmed fish [44]. The use of intensive and semi-intensive farming practices greatly increases the risk of bacterial diseases in aquaculture [45]. Although there are many bacteria pathogens of fish, only a few genera are responsible for most of the important economic losses worldwide. Important fish pathogens include species of Edwardsiella, Aeromonas, Vibrio, Flavobacterium, and Streptococcus [45]. E. piscicida causes Edwadsiellosis in many commercially important fish including eels, channel catfish, mullet, chinook salmon, flounder, carp, tilapia, and striped bass [1].
Edwardsiella species and resistome in microbiome
Fish reared in aquaculture are often kept under crowded and stressful conditions and require prophylactic and therapeutic use of antimicrobials to control disease outbreaks [44]. The use of antibiotics in aquaculture introduces a selective pressure on the microbial flora in aquatic environments and often promotes antibiotic resistance [46]. Resistant bacteria can thus multiply after the suppression of sensitive bacteria. Antibiotic resistance and virulence genes are often clustered on pathogenic islands located on the chromosome and or plasmid [47]. Thus, antibiotic resistant bacteria such as E. piscicida can serve as reservoirs of antimicrobial resistance genes in the environment and can facilitate the transfer of these genes to other bacteria resistome [48,49].
Human activity in the form of wastewater discharge, manure disposal, and aquaculture is the main source of antibiotics in the environment [50]. The appearance of antimicrobial resistant zoonotic pathogens in agricultural environments correlates to the use of antimicrobials in animal husbandry [51]. Antimicrobial resistance genes and antimicrobial-resistant bacteria harboring these genes can also pass from industrially grown animals to human beings and vise versa [51]. The role of aquaculture in the transfer antimicrobial resistance genes from bacteria to bacteria or in the spread of antimicrobial resistant bacteria has not received a lot of attention despite the rapid growth of the aquaculture industry [44]. Additionally, aquaculture can facilitate the transfer of antimicrobial resistance genes from aquatic environments to human pathogens and the human resistome [51,52]. Antibiotic resistance mechanisms in bacteria vary and may include inactivation of drugs via hydrolysis or modification, alteration or bypass of the drug target, changes in the permeability of bacterial cell wall, active efflux of the antibiotic from the microbial cell, and biofilm formation to avoid death [53].
The spread of antibiotic resistance and virulence in bacteria is often mediated by mobile genetic elements. These elements include insertion sequence, transposons, integrons, bacteriophage, genomic pathogenicity island, plasmids or combinations of these elements [54,55]. For example, Yersinia ruckerii, the cause of Yersiniosis in fish, shares an antimicrobial resistance plasmid and other antimicrobial resistance genes with Yersinia pestis, which is responsible for the plague in humans [56]. A plasmid encoding tetracycline resistance is shared between different Aeromonas species and E. coli in various environments [57]. The origin of the qnr genes involved in plasmid mediated quinolone resistance in clinical and environmental bacterial species belonging to the Enterobacteriaceae, Aeromonadaceae, Pseudomonadaceae, Xanthomonadaceae, Moraxellaceae, and Shewanellaceae, all have their origin in the waterborne Shewanella species [58].
Edwardsiella species in the environment and aquaculture include human pathogens of E. tarda, non-T3SS and non-T6SS avirulent E. piscicida, and virulent E. piscicida that habour T3SS and T6SS. Edwardsiella species are under constant selection pressure from all the antibiotics used in aquaculture, farms and human medicine. These organisms are part of the multi-antibiotics resistance bacteria (MARB) found in the environment and can exchange antibiotic resistance genes with other bacteria in the microbiome. For example, the E. piscicida plasmid sequence contains six genes known to confer antibiotic resistance to tetracycline (tetA and tetR), streptomycin (strA and strB), sulfonamides (sulII) and chloramphenicol (catA3) [59,60]. Genetic analysis of the E. piscicida plasmid revealed an incomplete set of type IVA secretion (T4AS) genes (VirB2, -B4, -B5, -B6, -B8, -B9, -B10, -B11, -D2, and -D4) [49]. T4AS genes are widespread in nature and promote (conjugative) dissemination of multiple-antibiotic resistance. The presence of plasmid encoded antibiotic resistance genes (tetracycline, streptomycin, sulfonamides, and chloramphenicol) together with T4AS genes, suggests that E. piscicida is a good candidate for the dissemination of antibiotic resistance to other bacteria in the aquatic environment and to the human resistome. Thus, Edwardsiella species may be involved in the dissemination of multi-antibiotic resistant genes to other bacteria. Therefore, Edwardsiella species not only harbour antimicrobial resistance genes but they can also increase the number of MARB in the environment.
Virulence mechanisms and bacterial pathogenesis
Although Edwardsialla has been known as a serious pathogen of aquatic animals for a long time, its pathogenicity mechanisms are yet to be fully elucidated. The pathogenesis of E. piscicida appears to be multifactorial, and include many virulence factors such as the production of exoenzymes (hemolysin) [1], possession of T3SS and T6SS [21,22], ability to adhere, invade, survive and replicate in both epithelial and phagocytic cells [36]. Additionally, the organisms produces virulence regulators such as EsrA-EsrB, EsrC, PhoP-PhoQ, QseB-QseC and PhoB-PhoR [36] (Figure 2). Some virulent genes of E. piscicida are thought to have been acquired by horizontal gene transfer and include the two-component system of EsrA-EsrB that is essential for T3SS and T6SS mediated pathogenesis [61], and the locus enterocyte effacement (LEE) from pathogenic E. coli [16]. Among these factors, the T3SS and T6SS are the leading virulence systems that contribute to the success of E. piscicida pathogenesis and disruptions in T3SS or T6SS resulted in 1–4 logs attenuation in the LD50 values in different fish models [21,22,62].
Figure 2.
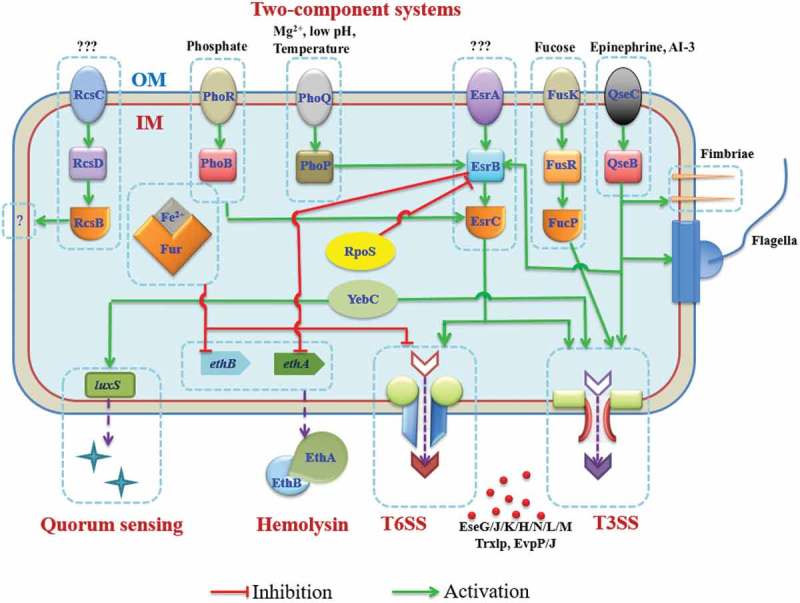
Virulence regulators and crosstalk among the different regulators in E. piscicida. Two-component systems and other global regulators control the delivery of the effectors of T3SS and T6SS, and other virulent factors in response to external environmental stimuli. Green lines indicate activation and red lines indicate inhibition.
T3SS and T6SS as the major players in Edwardsiella pathogenesis
T3SS is a flagellar-like structure and a contact-dependent device consisting of an injectisome, effectors, regulators, and chaperones [63,64]. Cornelis [65] used phylogenetic analyses to propose seven major families of T3SSs in Gram-negative bacteria. These needle-like nano-machines directly inject protein effectors into eukaryotic host cells using a one-step mechanism through an internal conduit of about 25Å in diameter [63]. Effectors have diverse functions and target specific host proteins to enable the pathogens to take control of the host cells [66]. Effector functions include modifications of cytoskeleton components, ubiquitination and phosphorylation of the host proteins [66]. Concerted actions of these effectors disrupt and/or mimic host cellular processes as well as interfere with the host immunity. Some T3SS effectors of plant and animal pathogens have common properties, suggesting that bacteria have evolved similar functions or inherited these genes through horizontal transfers to hijack host cells and disrupt host defense mechanisms [66]. Seventy percent of the T3SS effectors were estimated belonging to 91 families based on BLASTs results [67]. However, the remaining 30% of effectors appear to be species or strain specific, indicating a specific role in host cell/tissue tropism and host specificity [66,67]. Studies on effectors are still in their infancy and we are only now starting to appreciate their complexity and importance in host-pathogen interactions.
Leung’s group was the first to identify a T3SS in E. piscicida and found it to be analogous to the Salmonella pathogenicity island 2 (SPI-2) T3SS family encoded by S. Typhimurium [21]. Using a blue gourami fish model, T3SS deletion mutants were attenuated by 1 log in LD50 values and bacteria replication rates inside fish phagocytes were also reduced [21]. The identified T3SS secreted proteins, EseB, EseC and EseD, are homologous to Salmonella needle and translocon proteins, suggesting that they may form the extracellular conduit to deliver effectors [21]. EscC was characterized as a chaperone for EseB and EseD [68], while EscA and EseE are chaperones for EseC [69,70]. Similar to the corresponding homologs in Salmonella, the EsrA-EsrB two-component system (TCS) regulated the injection apparatus genes of the T3SS [36]. EsrC is an additional regulator (not found in Salmonella species) and used to regulate genes encoding the apparatus and effector proteins of T6SS. Therefore, EsrC enables crosstalk between T3SS and T6SS in E. piscicida [36]. EseG and EseJ were the first two T3SS effectors studied from Leung’s, and researchers at the Institute of Hydrobiology group in Wuhan [23,32]. Using transcriptome analysis, scientists at the ECUST in Shanghai, were able to identify six additional novel T3SS effectors, including EseH, EseK, and Trxl [71,72] (Table 2). Functional studies on T3SS effectors of Edwardsiella is the next frontier to unlock key host-pathogen interactions of this important fish pathogen.
Table 2.
T3SS and T6SS effectors in E. piscicida EIB202.
| # | Protein name or tag # | T3SS or T6SS effector | Function | Location | Reference |
|---|---|---|---|---|---|
| 1 | ETAE_0866, EseG | T3SS | Interact with α-tubulin and disassemble microtubule | Membrane | [23,34,71,72] |
| 2 | ETAE_0888, EseJ | T3SS | Inhibit adhesion and promote intracellular replication, decrease ROS production | [32,71,72] | |
| 3 | ETAE_1586, EseK | T3SS | Inhibit MAPK activation, promote colonization in zebrafish larvae | [24,71,72] | |
| 4 | ETAE_1604 | T3SS | Hypothetical protein | [72] | |
| 5 | ETAE_1757, EseH/EseN* | T3SS | Phosphothreonine lyase, inhibit MAPK signalling pathway | Nucleus | [71,72,84] |
| 6 | ETAE_2186, Trxlp | T3SS | Thioredoxin-like, promote inflammasome NLRC4 activation | [72,73] | |
| 7 | ETAE_2188 | T3SS | Hypothetical protein | [72] | |
| 8 | ETAE_3282 | T3SS | Hypothetical protein | [72] | |
| 9 | ETAE_1303, EseL | T6SS | Major cold shock protein? | [71] | |
| 10 | ETAE_2316, EseM | T6SS | Major cold shock protein? | [71] | |
| 11 | ETAE_2428, EvpP | T6SS | Prevent NLRP3 inflammasome activation, promote colonization in mice | Membrane | [71,77] |
| 12 | ETAE_2438, EvpJ | T6SS | Hypothetical protein | [72] |
T6SSs are also a contact-dependent secretion system reported in about 25% of sequenced genomes of Gram-negative bacteria, especially proteobacteria [63]. A hallmark of T6SSs is the contractile phage-tail-like injection apparatus used to puncture and deliver effectors directly into adjacent host or bacterial cells [74]. The second major component is the membrane complex with some of its inner membrane proteins that have similarities to components of T4SSs [63]. In the tail complex, a spike component called VgrG trimer is responsible for puncturing targeted cells, supported and assisted by the Hcp tube [63,74]. Together, the spike-tube complex or the puncturing device, deliver effectors into target cells.
The majority of T6SS effectors identified so far are related to anti-bacteria action and bacterial fitness or survival in the natural environments. For example, bacteriolytic activity against peptidoglycan in surrounding bacteria allows some bacteria to compete for space in the microbiota [75]. However, there is mounting evidence to suggest other functions such as evading host cell immunity similar to the T3SS anti-host effectors [76]. For example, EvpP of E. piscicida was reported to inhibit the Nod-like receptor P3 (NLRP3) inflammasome by supressing Ca2+-dependent c-Jun N-terminal kinase (Jnk) activation in bone marrow-derived macrophages and J774A.1 cells, and to promote bacterial colonization in vivo [77].
Zheng and Leung [22] first discovered a T6SS gene cluster that encodes 16 E. piscicida virulent proteins (Evp). Mutants with 14 individual evp gene deletions resulted in virulence attenuation of about 2 logs in LD50 values in a fish model. Proteins involved in crosstalk, such as EsrB and EsrC, connected T3SS and T6SS in E. piscicida [36]. Data from Leung’s group provided some of the strongest evidence that the T6SS is important for bacterial pathogenesis in host cells. The ECUST group later identified four novel T6SS effectors, namely EvpP, EseL, EseM, and EvpJ [71,77] (Table 2).
T3SS and T6SS effectors identification and characterization
One of the next frontiers of Edwardsiella research is to characterize all the novel effectors of T3SS and T6SS and to examine their individual and combined functions in bacterial infection and host defence. E. piscicida has one T3SS and one T6SS that together translocate the 12 effectors reported so far (Table 2). Many more effectors are likely to be identified in the future. Some Edwardsiella effectors may share commonalities with effectors from other bacteria. Therefore, knowledge attained through E. piscicida studies is applicable to the biology of bacterial effectors in general. Although T3SS effectors are common in human and plant pathogens, our understanding of effectors in fish pathogens is limited. Studying Edwardsiella effectors will expand our understanding of fish diseases and lead to the development of good control measures. Identified T3SS effectors would form the foundation for the design of vaccines and novel therapeutics. Live E. piscicida vaccines have been developed by constructing mutants with deletions in genes that encoded for the translocon and needle proteins (EseB, EseC and EseD) [78], and regulator proteins (EsrB) [62]. Poly-T3SS effector mutants of E. piscicida such as those involving the disruption of five and nine effectors were used to produce more effective vaccines [71,72]; T3SSs can also serve as vectors for delivering heterologous antigens into the host cells [79].
The majority of the T3SS and T6SS effectors are not in the main pathogenicity gene clusters of the bacteria, but are scattered throughout the genome as hypothetical proteins among the unknown ORFs. For example, E. piscicida strain EIB202 has about 3,700+ genes and 1,000+ (about 30%) of them are hypothetical proteins, some of which may be putative effectors. Novel platform technologies using genome-wide experimental and bioinformatics methods are used to identify potential effectors among the hypothetical proteins before conducting labor intensive confirmation experiments using translocation assays such as TEM-β-lactamase [23] or Cya assay [32]. Several approaches to identify T3SS and T6SS effectors are available. Firstly, a BLAST search on hypothetic proteins in E. piscicida can identify sequence similarities with known T3SS and T6SS effectors of other Gram-negative bacteria proteins in the NCBI database [80]. However, this method may not be able to identify novel Edwardsiella effectors. Secondly, RNA expression in wild type E. piscicida or T3SS/T6SS regulator mutants (such as ΔesrB) can be compared under effector inducing versus non-inducing conditions using RNA sequencing and transcriptome analysis. Using the above methods, two known T3SS effectors and ten novel T3SS and T6SS effectors were identified (Table 2) [71,72]. Interestingly, Zhang et al. [71] also found three effectors that required the outer membrane vesicles (OMV) for translocation but did not require either T3SS or T6SS; the mechanism is unclear or whether these effectors are related to T3SS and/or T6SS.
Future effector prediction approaches include secretome analysis followed by protein identifications using mass spectrometry and machine learning [80]. Machine learning on known Edwardsiella effectors can predict Edwardsiella-specific translocation signals found in T3SS and T6SS effectors. Previous results from Leung’s group suggested that established effector prediction programs such as EffectiveT3 [81] and T3SEpre [82] were not very useful for predicting effectors in E. piscicida strain EIB202 (Leung et al., unpublished data). None of the proteins in the top 30 ranked proteins using the above two algorithms were true effectors and only the needle or translocon proteins were identified (EseC, EseB, and EseD; Leung et al., unpublished data). However, it is possible to create a reliable machine learning method that is species or genus specific. Hobbs et al. [83], used 21 attributes to create a reliable machine learning method called, Genome Search for Effectors Tool (GenSET) to predict T3SS effectors. Known effectors and non-effector sequences from one genome were used to train five machine learning algorithms. An averaging algorithm was then applied to predict known and unknown effectors in a testing set. The GenSET program was species-specific, gave better performance, and successfully predicted effectors in four known genomes including S. Typhimurium and E. coli [83]. A similar approach can be applied to Edwardsiella strains to predict T3SS and T6SS effectors in the future.
After the identification of a putative Edwardsiella effector and confirmation by a translocation assay, the next challenge is to characterize its function(s). In general, the following methods for effector characterization can be used: (a) Assay for a known Edwardsiella key host-alteration phenotype such as its role in intracellular living, decreased rates in adhesion, invasion, or intracellular replication; e.g. EseJ decreases intracellular replication rates in J774A.1 cells [32]. (b) Identify and examine host partner proteins and study their functions in relation to the function of the effector, e.g. EseG interacts with α-tubulin and destabilizes microtubules [23]. (c) Examine known homologs of effectors; e.g. EseH is a phosphothreonine lyase that inhibits the MAPK signalling pathways [84].
E. piscicida as a model for the study of T3SS and T6SS and their crosstalk
E. piscicida is an ideal microbe for studies on T3SS and T6SS because of several reasons: (a) Possesses both T3SS and T6SS and has an intracellular lifestyle, a phenotype believed to be T3SS and T6SS dependent [21,22]. (b) Secretes predominantly T3SS and T6SS proteins that are easy to assay in the supernatants of cultured cells [22]. (3) Its effectors are largely unknown. (4) It is a great candidate for studying the roles of effectors in intracellular lifestyle and systemic infection of hosts. (5) It is an excellent model organism to study the role of T3SS and T6SS in other enterics and fish pathogens. Future studies on effectors of E. piscicida, E. italuri and E. anguillarum will provide a complete picture of effector biology in Edwardsiella and other Gram-negative bacteria.
Virulence regulators and crosstalk regulation of T3SS/T6SS
The precise expression and targeting of virulence factors includes a repertoire of T3SS or T6SS effectors, essential for the pathogenesis of E. piscicida. Many regulators have been implicated, directly or indirectly, in regulating T3SS/T6SS expression. However, exact regulation mechanisms of many of the regulators are presently unknown. E. piscicida strain EIB202 harbors ~33 TCSs as important virulence regulators [85]. These include EsrA-EsrB, PhoQ-PhoP, PhoR-PhoB, QseC-QseB [36], Rcs cascade (RcsC-RcsD-RcsB) [86], and FusK-FusR for fucose signaling [87] (Figure 2). The horizontally acquired EsrA-EsrB controls the expression of ~1,006 genes (27.2% of the genome) through the EsrB binding to the specific 7–4-7 (ATCAGGTgattACCCGAT) motifs in a manner similar to that of SsrB in Salmonella in the activated state [72]. The major virulence traits (i.e. T3SS/T6SS and their effectors), as well as genes related to other virulence factors (EthA and siderophore) and metabolic related genes, are controlled by EsrB [72]. The genes encoding EsrA-EsrB are also under the feedback control of EsrB and from PhoP, PhoB, and QseB in response to the Mg2+, iron, phosphate concentrations, pH, as well as temperatures, epinephrine, or quorum sensing signals such as AI-3. Not much is known about the other 30+ TCSs, although the T3SS defective phenotypes are observed in their respective mutants; but their regulatory mechanisms as well as their distinct signal ligands or substrates are still unclear and remain to be elucidated.
Known as a global regulator for stress adaptation, RpoS, was initially showed to be less related to virulence in zebrafish [88]. However, recent investigations indicated that RpoS is involved in virulence regulation by directly repressing esrB and other genes’ expression by binding to the −6G sites in their respective discriminator sequences in the promoter regions [89]. The regulation of esrB links the stress conditions and virulence expression level during the progression of infection in E. piscicida and other phylogenetically related pathogens such as Salmonella species [89]. Hfq is another global regulator of an mRNA/sRNA chaperon associated with pathogenesis and interacts with ~49 sRNA in E. piscicida; there were ~148 sRNAs including 129 novel sRNAs that were associated with regulation of adversity adaptation and pathogenicity [90]. Although the distinct sRNAs and targeted mRNAs remain unknown, preliminary data in E. pisicicida suggested that Hfq is involved in T3SS/T6SS expression and in vivo pathogenesis (Wang QY et al., unpublished communication). Recently, YebC was shown to regulate quorum sensing and activate T3SS expression by directly binding to the promoter region of the T3SS gene ETAE_0873 involved in bacteria colonization in fish [91]. The ferric uptake regulator (Fur) is a global regulator of iron acquisition, resistance to acids, resistance to oxidative stress, resistance to host serum, hemolysin EthA production, and virulence towards fish [92]. Fur is intertwined with PhoB-PhoR to regulate EsrC expression, and thus to control T3SS/T6SS expression in E. pisicicida [36]. Together, these findings demonstrated that T3SS and T6SS expression is under a complex regulatory network that is triggered by distinct environmental cues and may play different roles in the pathogenicity E. piscicida in fish hosts. This regulatory network and environmental factors may be highly adapted to E. piscicida when compared to the phylogenetically related Salmonella or other pathogens, although the above-mentioned regulators are also present in these pathogens.
Other virulence factors or determinants and mechanisms
Other non-T3SS/T6SS virulence factors of E. piscicida include chondroitinase (that may relate to cartilage degradation and adhesion), hemolysins (EthA and HlyA), adhesins (AIDA), invasin (Inv1), flagellar structures, and other surface structures or extracellular products that may be involved in the initial steps of infection in hosts [1,15,36,93]. Inside the host, a series of factors such as serum resistance, resistance to oxidative stresses, and replication in macrophages are used to evade adverse host defenses [36,92]. E. piscicida circumvents serum attack by preventing, largely, the activation of the complement using the alternative pathway [94]. Sip1, also named as Aur or aureolysin, is a serum-induced zinc metalloprotease implicated in serum resistance and is essential for fish infection [95]. In addition, Sip2 (homologue of HypB, a putative hydrogenase) protects E. piscicida in fish serum and significantly increases cellular and tissue infection by allowing the bacteria to cope with acidic stress [96]. Although the direct regulon is unclear, the cyclic AMP receptor protein (CRP) was shown to be essential for flagellar biosynthesis and motility, hemolytic activity, and in vivo virulence in E. piscicida [97]. Finally, factors such as universal stress proteins [98], TonB [99], E. tarda hemolysin activator (Eha) [100], lysozyme inhibitor (MliC) [101], serine protease autotransporter (Tsh) [102], vibrioferrin siderophore [103], HU proteins [104], and the twin-arginine translocation system (Tat) [105], have all been reported to be closely linked to E. piscicida virulence.
Conclusion
New and cutting-edge technologies in bacterial functional genomics have been used to investigate the pathogenesis of E. piscicida. These include proteomics and genomics studies as well as secretomes, transcriptomes, interactomes, and metabolomics studies. Proteomics and metabolomics studies have enabled researchers to examine protein-protein interactions between E. piscicida cells and host tissues, such as fish gills [37] and livers [106]; in antibiotics resistome variations [107] and stress adaptation [108]. RNA-seq, ChIP-seq and Tn-seq are new and powerful technologies that couple defined transposon mutant library with high-throughput sequencing of transposon insertion sites to comprehensively map genetic determinants of bacterial fitness. Yin et al. [89] used Tn-seq technology to systematically identify the regulators of EsrB, the critical virulence regulator in E. pisicicida. Time resolved Tn-seq analysis termed PACE is a fascinating technology that is used to study the genes dynamically required for in vivo infection [38]. In these studies, PACE facilitated the heuristics identification of targets for live attenuated vaccine development based on fitness curves of the inserted mutants. The above platforms, along with others, will facilitate studies on Edwardsiella-host interactions as well as the development of vaccines and novel disease control.
Increased demand for fish and other healthier proteins in our diet has lead to the rapid growth of the aquaculture industry. Edwardsiella are abundant in marine and freshwater habitats globally. Edwardsiella infection in aquaculture is a major constraint in fish farming and farmers have resorted to using antibiotics and other antimicrobial agents for prophylactic and therapeutic purposes. The overuse or abuse of antibiotics and chemicals in aquaculture is selecting and enriching for these organisms in the environment. Research on E. piscicida has progressed rapidly in the last two decades, from studies on an obscure fish bacterium a few years ago to become an active area of research in China, Asia, and around the world. Edwardsiella is an emerging fish pathogen, and E. piscicida will increasingly become common as a pathogen of both animals and humans. Thus, E. piscicida is a perfect model for elucidating bacteria pathogenesis at the molecular, cellular, and systemic level. Edwardsiella species and E. piciscida in particular is equipped with a deadly arsenal of virulent mechanisms such as a wide range of antibiotic resistance genes, T3SS, and T6SS. Some of these virulent mechanisms are also present in human pathogens and the knowledge generated from Edwardsiella studies will no doubt benefit public health and human medicine. Additionally, Edwardsiella species are capable of acquiring antibiotic resistance genes from other bacteria and can transmit these genes to the microbiomes in soil, water, animals and humans. Hence, Edwardsiella is not only a fish and human pathogen, but it has potential to spread and transmit, using novel methods, antibiotics- or chemical-resistant genes to other bacteria. Therefore, Edwardsiella species may be important players in the resistome and will likely attract a lot of attention in the next decades. New and powerful technologies such as dual RNA-seq, super resolution bio-imaging, all the omics, and structural biology technologies will facilitate studies to elucidate the biology of Edwardsiella. These new technologies will also provide new methods to assess the impact of pathogenic microorganisms on the environment, and lead to better diagnostic and control measures of other bacterial diseases of animals and humans.
Funding Statement
This work was supported by the National Natural Science Foundation of China [#31873048]; Ministry of Agriculture of China [CARS-47]; Science and Technology Commission of Shandong and Shanghai Municipality [2017CXGC0103 and 17391902000]; National Key R&D Program of China [2018YFD0900504].
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant number #31873048) to Dr. Ka Yin Leung; National Key R&D Program of China (2018YFD0900504), Ministry of Agriculture of China (CARS-47), and the Science and Technology Commission of Shandong and Shanghai Municipality (2017CXGC0103 and 17391902000) to Dr. Qiyao Wang. We also acknowledge the micrograph of Figure 1 provided by Dr. Julian A. Guttman and Ms. Priyanka Aggarwal.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Park SB, Aoki T, Jung TS.. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res. 2012;43(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buján N, Toranzo AE, Magariños B. Edwardsiella piscicida: A significant bacterial pathogen of cultured fish. Dis Aquat Organ. 2018;131(1):59–71. [DOI] [PubMed] [Google Scholar]
- [3].Vayssier-Taussat M, Albina E, Citti C, et al. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front Cell Infect Microbiol. 2014;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hirai Y, Asahata-Tago S, Ainoda Y, et al. Edwardsiella tarda bacteremia. A rare but fatal water-and foodborne infection: review of the literature and clinical cases from a single centre. Can J Infect Dis Med Microbiol. 2015;26(6):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grimont PA, Grimont F, Richard C, et al. Edwardsiella hoshinae, a new species of Enterobacteriaceae. Curr Microbiol. 1980;4(6):347–351. [Google Scholar]
- [6].Hawke JP, Mcwhorter AC, Steigerwalt AG, et al. Edwardsiella ictaluri sp. nov., the causative agent of enteric septicemia of catfish. Int J Syst Evol Microbiol. 1981;31(4):396–400. [Google Scholar]
- [7].Williams M, Gillaspy A, Dyer D, et al. Genome sequence of Edwardsiella ictaluri 93-146, a strain associated with a natural channel catfish outbreak of enteric septicemia of catfish. J Bacteriol. 2012;194(3):740–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang M, Jiao XD, Hu YH, et al. Attenuation of Edwardsiella tarda virulence by small peptides that interfere with LuxS/autoinducer type 2 quorum sensing. Appl Environ Microbiol. 2009;75(12):3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang M, Sun K, Sun L. Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain. Microbiology. 2008;154(7):2060–2069. [DOI] [PubMed] [Google Scholar]
- [10].Ewing W, Mcwhorter A, Escobar M, et al. Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E. tarda. Int J Syst Evol Microbiol. 1965;15(1):33–38. [Google Scholar]
- [11].Meyer F, Bullock G. Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl Microbiol. 1973;25(1):155–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abayneh T, Colquhoun D, Sørum H. Edwardsiella piscicida sp. nov., a novel species pathogenic to fish. J Appl Microbiol. 2013;114(3):644–654. [DOI] [PubMed] [Google Scholar]
- [13].Shao S, Lai Q, Liu Q, et al. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813T encoding two distinct T3SS and three T6SS gene clusters: propose a novel species as Edwardsiella anguillarum sp. nov. Syst Appl Microbiol. 2015;38(1):36–47. [DOI] [PubMed] [Google Scholar]
- [14].Yang M, Lv Y, Xiao J, et al. Edwardsiella comparative phylogenomics reveal the new intra/inter-species taxonomic relationships, virulence evolution and niche adaptation mechanisms. PLoS One. 2012;7(5):e36987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buján N, Mohammed H, Balboa S, et al. Genetic studies to re-affiliate Edwardsiella tarda fish isolates to Edwardsiella piscicida and Edwardsiella anguillarum species. Syst Appl Microbiol. 2018;41(1):30–37. [DOI] [PubMed] [Google Scholar]
- [16].Nakamura Y, Takano T, Yasuike M, et al. Comparative genomics reveals that a fish pathogenic bacterium Edwardsiella tarda has acquired the locus of enterocyte effacement (LEE) through horizontal gene transfer. BMC Genomics. 2013;14(1):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Katharios P, Kalatzis PG, Kokkari C, et al. Characterization of a highly virulent Edwardsiella anguillarum strain isolated from Greek aquaculture, and a spontaneously induced prophage therein. Front Microbiol. 2019;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ucko M, Colorni A, Dubytska L, et al. Edwardsiella piscicida-like pathogen in cultured grouper. Dis Aquat Organ. 2016;121(2):141–148. [DOI] [PubMed] [Google Scholar]
- [19].Shao J, Guo Q, Hu R, et al. Comparative genomic insights into the taxonomy of Edwardsiella tarda isolated from different hosts: marine, freshwater and migratory fish. Aquac Res. 2018;49(1):197–204. [Google Scholar]
- [20].Abbott SL, Janda JM. The Genus Edwardsiella. Prokaryotes. 2006;6: 72–89. New York: Springer. [Google Scholar]
- [21].Tan Y, Zheng J, Tung S, et al. Role of type III secretion in Edwardsiella tarda virulence. Microbiology. 2005;151(7):2301–2313. [DOI] [PubMed] [Google Scholar]
- [22].Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66(5):1192–1206. [DOI] [PubMed] [Google Scholar]
- [23].Xie HX, Yu HB, Zheng J, et al. EseG, an effector of the type III secretion system of Edwardsiella tarda, triggers microtubule destabilization. Infect Immun. 2010;78(12):5011–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cao H, Han F, Tan J, et al. Edwardsiella piscicida type III secretion system effector EseK inhibits mitogen-activated protein kinase phosphorylation and promotes bacterial colonization in zebrafish Larvae. Infect Immun. 2018;86(9):e00233–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hilbi H, Haas A. Secretive bacterial pathogens and the secretory pathway. Traffic. 2012;13(9):1187–1197. [DOI] [PubMed] [Google Scholar]
- [26].Raymond B, Young JC, Pallett M, et al. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol. 2013;21(8):430–441. [DOI] [PubMed] [Google Scholar]
- [27].Casadevall A. Evolution of intracellular pathogens. Annu Rev Microbiol. 2008;62:19–33. [DOI] [PubMed] [Google Scholar]
- [28].Smith LM, May RC. Mechanisms of microbial escape from phagocyte killing. Biochem Soc Trans. 2013;41(2):475–490. [DOI] [PubMed] [Google Scholar]
- [29].Ling SHM, Wang X, Xie L, et al Use of green fluorescent protein (GFP) to study the invasion pathways of Edwardsiella tarda in in vivo and in vitro fish models. Microbiology. 2000;146(1):7–19. [DOI] [PubMed] [Google Scholar]
- [30].Sui ZH, Xu HJ, Wang HH, et al. Intracellular trafficking pathways of Edwardsiella tarda: from clathrin-and caveolin-mediated endocytosis to endosome and lysosome. Front Cell Infect Microbiol. 2017;7:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang L, Ni C, Xu W, et al. Intramacrophage infection reinforces the virulence of Edwardsiella tarda. J Bacteriol. 2016;198(10):1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie HX, Lu JF, Zhou Y, et al. Identification and functional characterization of a novel Edwardsiella tarda effector EseJ. Infect Immun. 2015;83(4):1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baumgartner WA, Dubytska L, Rogge ML, et al. Modulation of vacuolar pH is required for replication of Edwardsiella ictaluri in channel catfish (Ictalurus punctatus) macrophages. Infect Immun. 2014;82(6):2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fang S, Zhang L, Lou Y, et al. Intracellular translocation and localization of Edwardsiella tarda type III secretion system effector EseG in host cells. Microb Pathog. 2016;97:166–171. [DOI] [PubMed] [Google Scholar]
- [35].Ling SH, Wang XH, Lim TM, et al Green fluorescent protein-tagged Edwardsiella tarda reveals portal of entry in fish. FEMS Microbiol Lett. 2001;194(2):239–243. [DOI] [PubMed] [Google Scholar]
- [36].Leung KY, Siame BA, Tenkink BJ, et al. Edwardsiella tarda–virulence mechanisms of an emerging gastroenteritis pathogen. Microbes Infect. 2012;14(1):26–34. [DOI] [PubMed] [Google Scholar]
- [37].Liu Y, Zhang H, Liu Y, et al. Determination of the heterogeneous interactome between Edwardsiella tarda and fish gills. J Proteomics. 2012;75(4):1119–1128. [DOI] [PubMed] [Google Scholar]
- [38].Yang G, Billings G, Hubbard TP, et al. Time-resolved transposon insertion sequencing reveals genome-wide fitness dynamics during infection. MBio. 2017;8(5):e01581–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Srinivasa Rao PS, Yamada Y, Tan YP, et al. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol. 2004;53(2):573–586. [DOI] [PubMed] [Google Scholar]
- [40].Schlenker C, Surawicz CM. Emerging infections of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2009;23(1):89–99. [DOI] [PubMed] [Google Scholar]
- [41].Wang IK, Kuo HL, Chen YM, et al. Extraintestinal manifestations of Edwardsiella tarda infection. Int J Clin Pract. 2005;59(8):917–921. [DOI] [PubMed] [Google Scholar]
- [42].Ebisawa KF, Nishimura S, Yamamoto S, et al. Mycotic aneurysm caused by Edwardsiella tarda successfully treated with stenting and suppressive antibiotic therapy: A case report and systematic review. Ann Clin Microbiol Antimicrob. 2018;17(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].FAO In: The State of World Fisheries and Aquaculture 2018 Meeting the sustainable development goals. 2018; Rome: Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- [44].Cabello FC, Godfrey HP, Buschmann AH, et al. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect Dis. 2016;16(7):e127–e133. [DOI] [PubMed] [Google Scholar]
- [45].Xu T, Zhang X-H. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture. 2014;431:129–135. [Google Scholar]
- [46].Miller RA, Harbottle H. Antimicrobial drug resistance in fish pathogens. Microbiol Spectr. 2018;6:1. [DOI] [PubMed] [Google Scholar]
- [47].Groisman EA, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87(5):791–794. [DOI] [PubMed] [Google Scholar]
- [48].Santos L, Ramos F. Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents. 2018;52(2):135–143. [DOI] [PubMed] [Google Scholar]
- [49].Liu Y, Gao Y, Liu X, et al. Transposon insertion sequencing reveals T4SS as the major genetic trait for conjugation transfer of multi-drug resistance pEIB202 from Edwardsiella. BMC Microbiol. 2017;17(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang S, Carlson K. Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Res. 2003;37(19):4645–4656. [DOI] [PubMed] [Google Scholar]
- [51].Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24(4):718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhao JY, Dang HY. Coastal seawater bacteria harbor a large reservoir of plasmid-mediated quinolone resistance determinants in Jiaozhou Bay, China. Microb Ecol. 2012;64(1):187–199. [DOI] [PubMed] [Google Scholar]
- [53].Blair JM, Webber MA, Baylay AJ, et al. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. [DOI] [PubMed] [Google Scholar]
- [54].Sørensen SJ, Bailey M, Hansen LH, et al. Studying plasmid horizontal transfer in situ: A critical review. Nat Rev Microbiol. 2005;3(9):700–710. [DOI] [PubMed] [Google Scholar]
- [55].Juhas M. Horizontal gene transfer in human pathogens. Crit Rev Microbiol. 2015;41(1):101–108. [DOI] [PubMed] [Google Scholar]
- [56].Welch TJ, Fricke WF, McDermott PF, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2(3):e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bruun MS, Schmidt AS, Dalsgaard I, et al. Conjugal transfer of large plasmids conferring oxytetracycline (OTC) resistance: transfer between environmental aeromonads, fish-pathogenic bacteria, and Escherichia coli. J Aquat Anim Health. 2003;15(1):69–79. [Google Scholar]
- [58].Poirel L, Rodriguez-Martinez JM, Mammeri H, et al. Origin of plasmid-mediated quinolone resistance determinant QnrA. Antimicrob Agents Chemother. 2005;49(8):3523–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sun K, Wang HL, Zhang M, et al. Genetic mechanisms of multi-antimicrobial resistance in a pathogenic Edwardsiella tarda strain. Aquaculture. 2009;289(1–2):134–139. [Google Scholar]
- [60].Yu JE, Cho MY, Kim JW, et al. Large antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb Pathog. 2012;52(5):259–266. [DOI] [PubMed] [Google Scholar]
- [61].Lv Y, Xiao J, Liu Q, et al. Systematic mutation analysis of two-component signal transduction systems reveals EsrA-EsrB and PhoP-PhoQ as the major virulence regulators in Edwardsiella tarda. Vet Microbiol. 2012;157(1–2):190–199. [DOI] [PubMed] [Google Scholar]
- [62].Yang W, Wang L, Zhang L, et al. An invasive and low virulent Edwardsiella tarda esrB mutant promising as live attenuated vaccine in aquaculture. Appl Microbiol Biotechnol. 2015;99(4):1765–1777. [DOI] [PubMed] [Google Scholar]
- [63].Costa TR, Felisberto-Rodrigues C, Meir A, et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol. 2015;13(6):343–359. [DOI] [PubMed] [Google Scholar]
- [64].Deng W, Marshall NC, Rowland JL, et al. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol. 2017;15(6):323. [DOI] [PubMed] [Google Scholar]
- [65].Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4(11):811. [DOI] [PubMed] [Google Scholar]
- [66].Dean P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev. 2011;35(6):1100–1125. [DOI] [PubMed] [Google Scholar]
- [67].Hu Y, Huang H, Cheng X, et al. A global survey of bacterial type III secretion systems and their effectors. Environ Microbiol. 2017;19(10):3879–3895. [DOI] [PubMed] [Google Scholar]
- [68].Zheng J, Li N, Tan YP, et al. EscC is a chaperone for the Edwardsiella tarda type III secretion system putative translocon components EseB and EseD. Microbiology. 2007;153(6):1953–1962. [DOI] [PubMed] [Google Scholar]
- [69].Wang B, Mo ZL, Mao YX, et al. Investigation of EscA as a chaperone for the Edwardsiella tarda type III secretion system putative translocon component EseC. Microbiology. 2009;155(4):1260–1271. [DOI] [PubMed] [Google Scholar]
- [70].Yi J, Xiao SB, Zeng ZX, et al. EseE of Edwardsiella tarda augments the secretion of translocon protein EseC and the expression of escC-eseE operon. Infect Immun. 2016;84(8):2336–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang L, Jiang Z, Fang S, et al. Systematic identification of intracellular-translocated candidate effectors in Edwardsiella piscicida. Front Cell Infect Microbiol. 2018;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liu Y, Zhao L, Yang M, et al. Transcriptomic dissection of the horizontally acquired response regulator EsrB reveals its global regulatory roles in the physiological adaptation and activation of T3SS and the cognate effector repertoire in Edwardsiella piscicida during infection toward turbot. Virulence. 2017;8(7):1355–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Xu W, Gu Z, Zhang L, et al. Edwardsiella piscicida virulence effector trxlp promotes the NLRC4 inflammasome activation during infection. Microb Pathog. 2018;123:496–504. [DOI] [PubMed] [Google Scholar]
- [74].Gallique M, Bouteiller M, Merieau A. The type VI secretion system: A dynamic system for bacterial communication? Front Microbiol. 2017;8:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Russell AB, Hood RD, Bui NK, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475(7356):343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hachani A, Wood TE, Filloux A. Type VI secretion and anti-host effectors. Curr Opin Microbiol. 2016;29:81–93. [DOI] [PubMed] [Google Scholar]
- [77].Chen H, Yang D, Han F, et al. The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-Jnk pathway. Cell Host Microbe. 2017;21(1):47–58. [DOI] [PubMed] [Google Scholar]
- [78].Xiao J, Chen T, Liu B, et al. Edwardsiella tarda mutant disrupted in type III secretion system and chorismic acid synthesis and cured of a plasmid as a live attenuated vaccine in turbot. Fish Shellfish Immunol. 2013;35(3):632–641. [DOI] [PubMed] [Google Scholar]
- [79].Figueira R, Watson KG, Holden DW, et al. Identification of Salmonella pathogenicity island-2 type III secretion system effectors involved in intramacrophage replication of S. enterica serovar Typhimurium: implications for rational vaccine design. MBio. 2013;4(2):e00065–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Arnold R, Jehl A, Rattei T. Targeting effectors: the molecular recognition of Type III secreted proteins. Microbes Infect. 2010;12(5):346–358. [DOI] [PubMed] [Google Scholar]
- [81].Arnold R, Brandmaier S, Kleine F, et al. Sequence-based prediction of type III secreted proteins. PLoS Pathog. 2009;5(4):e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang Y, Sun M, Bao H, et al. Effective identification of bacterial type III secretion signals using joint element features. PLoS One. 2013;8(4):e59754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hobbs CK, Porter VL, Stow ML, et al. Computational approach to predict species-specific type III secretion system (T3SS) effectors using single and multiple genomes. BMC Genomics. 2016;17(1):1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hou M, Chen R, Yang D, et al. Identification and functional characterization of EseH, a new effector of the type III secretion system of Edwardsiella piscicida. Cell Microbiol. 2017;19(1):e12638. [DOI] [PubMed] [Google Scholar]
- [85].Wang Q, Yang M, Xiao J, et al. Genome sequence of the versatile fish pathogen Edwardsiella tarda provides insights into its adaptation to broad host ranges and intracellular niches. PLoS One. 2009;4(10):e7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xu Y, Xu T, Wang B, et al. A mutation in rcsB, a gene encoding the core component of the Rcs cascade, enhances the virulence of Edwardsiella tarda. Res Microbiol. 2014;165(3):226–232. [DOI] [PubMed] [Google Scholar]
- [87].Wu J, Liu G, Sun Y, et al. The role of regulator FucP in Edwardsiella tarda pathogenesis and the inflammatory cytokine response in tilapia. Fish Shellfish Immunol. 2018;80:624–630. [DOI] [PubMed] [Google Scholar]
- [88].Xiao J, Wang Q, Liu Q, et al. Characterization of Edwardsiella tarda rpoS: effect on serum resistance, chondroitinase activity, biofilm formation, and autoinducer synthetases expression. Appl Microbiol Biotechnol. 2009;83(1):151–160. [DOI] [PubMed] [Google Scholar]
- [89].Yin K, Guan Y, Ma R, et al. Critical role for a promoter discriminator in RpoS control of virulence in Edwardsiella piscicida. PLoS Pathog. 2018;14(8):e1007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Du HH, Zhou HZ, Tang P, et al. Global discovery of small RNAs in the fish pathogen Edwardsiella piscicida: key regulator of adversity and pathogenicity. Vet Res. 2018;49(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wei L, Wu Y, Qiao H, et al. YebC controls virulence by activating T3SS gene expression in the pathogen Edwardsiella piscicida. FEMS Microbiol Lett. 2018;365:14. [DOI] [PubMed] [Google Scholar]
- [92].Hu YH, Sun L. The global regulatory effect of Edwardsiella tarda Fur on iron acquisition, stress resistance, and host infection: A proteomics-based interpretation. J Proteomics. 2016;140:100–110. [DOI] [PubMed] [Google Scholar]
- [93].Suomalainen LR, Tiirola M, Valtonen E. Chondroitin AC lyase activity is related to virulence of fish pathogenic Flavobacterium columnare. J Fish Dis. 2006;29(12):757–763. [DOI] [PubMed] [Google Scholar]
- [94].Li MF, Sun L, Li J. Edwardsiella tarda evades serum killing by preventing complement activation via the alternative pathway. Fish Shellfish Immunol. 2015;43(2):325–329. [DOI] [PubMed] [Google Scholar]
- [95].Zhou ZJ, Sun BG, Sun L. Edwardsiella tarda Sip1: A serum-induced zinc metalloprotease that is essential to serum resistance and host infection. Vet Microbiol. 2015;177(3–4):332–340. [DOI] [PubMed] [Google Scholar]
- [96].Li M, Sun L. Edwardsiella tarda Sip2: A serum-induced protein that is essential to serum survival, acid resistance, intracellular replication, and host infection. Front Microbiol. 2018;9:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Choe Y, Park J, Yu JE, et al. Edwardsiella piscicida lacking the cyclic AMP receptor protein (Crp) is avirulent and immunogenic in fish. Fish Shellfish Immunol. 2017;68:243–250. [DOI] [PubMed] [Google Scholar]
- [98].Akgul A, Nho SW, Kalindamar S, et al. Universal stress proteins contribute Edwardsiella ictaluri virulence in catfish. Front Microbiol. 2018;9:2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Abdelhamed H, Lawrence ML, Karsi A. The role of TonB gene in Edwardsiella ictaluri virulence. Front Physiol. 2017;8:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gao D, Li Y, Zheng E, et al. Eha, a regulator of Edwardsiella tarda, required for resistance to oxidative stress in macrophages. FEMS Microbiol Lett. 2016;363:20. [DOI] [PubMed] [Google Scholar]
- [101].Li MF, Wang C, Sun L. Edwardsiella tarda MliC, a lysozyme inhibitor that participates in pathogenesis in a manner that parallels Ivy. Infect Immun. 2015;83(2):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hu YH, Zhou HZ, Jin QW, et al. The serine protease autotransporter Tsh contributes to the virulence of Edwardsiella tarda. Vet Microbiol. 2016;189:68–74. [DOI] [PubMed] [Google Scholar]
- [103].Castro N, Osorio C, Buján N, et al. Insights into the virulence‐related genes of Edwardsiella tarda isolated from turbot in Europe: genetic homogeneity and evidence for vibrioferrin production. J Fish Dis. 2016;39(5):565–576. [DOI] [PubMed] [Google Scholar]
- [104].Wang L, Xiao J, Cui S, et al. HU-induced polymorphous filamentation in fish pathogen Edwardsiella tarda leading to reduced invasion and virulence in zebrafish. Vet Microbiol. 2014;171(1–2):165–174. [DOI] [PubMed] [Google Scholar]
- [105].Wang Y, Wang Q, Yang W, et al. Functional characterization of Edwardsiella tarda twin-arginine translocation system and its potential use as biological containment in live attenuated vaccine of marine fish. Appl Microbiol Biotechnol. 2013;97(8):3545–3557. [DOI] [PubMed] [Google Scholar]
- [106].Li H, Zhu QF, Peng XX, et al. Interactome of E. piscicida and grouper liver proteins reveals strategies of bacterial infection and host immune response. Sci Rep. 2017;7:39824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peng B, Su YB, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21(2):249–261. [DOI] [PubMed] [Google Scholar]
- [108].Ma W, Jia J, Huang X, et al. Stable isotope labelling by amino acids in cell culture (SILAC) applied to quantitative proteomics of Edwardsiella tarda ATCC 15947 under prolonged cold stress. Microb Pathog. 2018;125:12–19. [DOI] [PubMed] [Google Scholar]


