Abstract
Since the first outbreak of avian influenza A(H7N9) virus in China in early 2013, several interventions to control the transmission of H7N9 virus from poultry to humans have been implemented. Temporarily closing live poultry markets reduced the risk of human infection to an extent, but it did not prevent the spread of the H7N9 virus among poultry, and this spread eventually led to more human cases. Nevertheless, the mass vaccination of poultry after September 2017 has been highly effective in preventing the H7N9 virus infection in both poultry and humans. In light of the emergence of highly pathogenic H7N9 and H7N2 viruses in unimmunized ducks, vaccination among poultry, especially for ducks, should be accompanied with continued surveillance of H7N9 variants and other avian influenza A viruses that could signal a heightened pandemic risk.
Keywords: avian influenza viruses, H7N9 viruses, interventions, vaccination
During the past 5 years, novel strains of avian influenza A(H7N9) virus, with pandemic potential, have emerged and spread rapidly among poultry across mainland China. Human H7N9 infections have subsequently been reported in most of China’s provinces, with occasional infections occurring among travelers to Hong Kong, Malaysia, and Taiwan. As of January 18, 2019, 1564 laboratory-confirmed human cases, including at least 614 deaths, had been documented, with a case fatality among clinical H7N9 infections of 39% [1].
Genetic and biological characteri-zations of H7N9 virus have revealed that this virus is already better adapted to human nasal passages and the human tracheobronchial tree than any other avian viruses—a feature that is thought to be required for a virus to be transmissible from human-to-human [2]. Also remarkable, highly pathogenic avian influenza (HPAI) H7N9 strains have emerged and infected both poultry and humans during the fifth Wave (2016–2017) [3] and were found to be more pathogenic in mice and ferrets than previous H7N9 strains [4]. Hence, the H7N9 virus has earned the highest risk score among zoonotic influenza A virus threats as evaluated by the US Centers for Disease Control and Prevention using the Influenza Risk Assessment Tool [5].
In this study, we (1) review the numerous interventions China has taken to reduce the risk of human H7N9 infections, (2) report new data documenting the success of the new poultry vaccine, and (3) suggest additional measures to prevent the further spread of novel influenza viruses among poultry and humans.
CLOSURE OF LIVE POULTRY MARKETS
Considering previously published research, live poultry markets (LPMs) are an ongoing source of H7N9 virus exposure for poultry and humans and represent a contact point target where control of H7N9 could be implemented [6]. Hence, closure of LPMs has been a central intervention strategy in China’s H7N9 control efforts. Although there was wide variation in the closure strategies across different cities, LPM closure seems to have been at least initially effective in reducing the incidence of H7N9 virus infections in people. Yu et al [7] estimated that closure of LPMs reduced the mean daily number of human infections from 97% to 99% in several major Chinese cities. However, such gains are now viewed as transient. For instance, after multiple interventions including LPM closure were implemented in Shanghai during 2013, H7N9 transmission to humans was thought be controlled and LPMs were reopened in early 2014. Soon thereafter, 8 new human H7N9 infections were detected and LPMs were again closed on January 31, 2014 [8]. Similar short-term benefits to LPM closure were seen in other cities [9, 10]. It is now understood that LPM closure has reduced the risk of human infection to an extent, but these interventions were imperfect, leading to multiple complex social and economic changes [11], such as likely encouraging the emergence of hidden LPMs, which have increased the dispersion of H7N9 virus among poultry across China.
MARKET REST DAYS
Likewise, market rest days have been found to reduce the prevalence of low pathogenic avian influenza viruses in retail markets [12, 13]. Although complete cleaning and disinfecting in LPMs after rest day seems effective in eliminating avian influenza viruses, the viruses often become prevalent again once the markets are reopened [14]. In Guangdong Province, China, all LPMs employed a once-a-month market closure day beginning in March 2015. Although the H7N9 virus detection prevalence significantly declined, the prevalence was again found to increase 2 days after closure day [15–18]. Market rest days are meant to interrupt the persistence of introduced virus within LPMs, but the duration of this effect is now understood to be relatively short-lived because other avian viruses are often reintroduced, and thus the virus shedding among poultry returned to its elevated baseline rate. A key factor for such transmission is now recognized to be the continual introduction of immunologically naive poultry, which results in virus amplification and persistence in these markets [19].
BAN ON KEEPING LIVE POULTRY OVERNIGHT
Prohibiting keeping poultry overnight in the markets have also been studied and seems to have been effective [13]. For instance, during January 2014 in Guangzhou city of Guangdong Province, the prevalence of H7N9 virus was 91.7% in stalls where poultry were kept overnight and only 33% where poultry were not kept overnight [14]. We speculate that banning live poultry holding overnight is effective in reducing virus circulation and the infection of incoming chickens during the next day [20]. However, cleaning and disinfecting markets while live poultry remain in the market is ineffective because live poultry continue to shed virus infecting other poultry and again contaminating the environment [21]. Because avian influenza viruses can sometimes survive for weeks in the environment [22], it seems crucial that fecal matter, poultry drinking water, and poultry feed should be removed and replenished each day to reduce avian influenza viruses transmission within the markets.
VACCINATION IN POULTRY
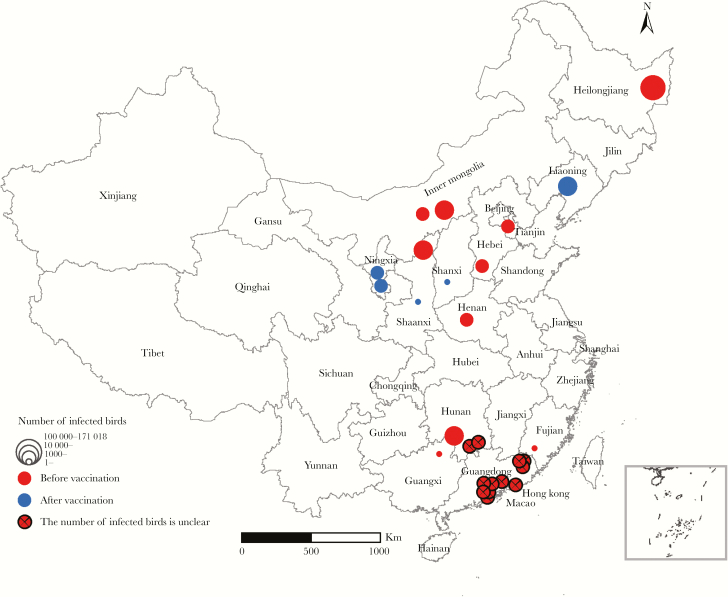
The fifth wave of the H7N9 virus was the most severe with the greatest spread and highest number of human cases. In addition, HPAI H7N9 strains emerged during this wave and caused high mortality among poultry and humans. In an attempt to limit the spread of both low pathogenic and HPAI H7N9 strains, in July 2017 the Chinese government conducted a pilot program in Guangdong and Guangxi Provinces using a bivalent poultry vaccine that offered protective immunity against recent H7N9 strains and clade 2.3.4.4 H5 viruses [23]. The pilot program was followed by the launch of a nationwide compulsory vaccination program in early September 2017. Our unpublished surveillance work conducted in several provinces of China showed a marked increase in H7N9 prevalence among LPM poultry moving from an average of 0.27% during waves 1 through 4 to an average of 2.2% during wave 5 (Table 1). After the H7N9 vaccination, specimens collected in 53 LPMs in April and May of 2018 yielded no H7N9 virus detections. Correspondingly, from 2013 to 2018, our surveillance in poultry farms yielded no H7N9 virus detections during wave 5. Although our surveillance finding might have other explanations, our observations of H7N9 control are supported by a recent independent study documenting a significant decrease of H7N9 prevalence among poultry before (approximately 1% isolation rate) and after (approximately 0.07% isolation rate) the mass poultry immunization program. Likewise, although many human cases were reported in waves 1 through 5, only 3 human infections have occurred after the vaccination program (wave 6, 2017–2018). In addition, the vaccination program also seems to have been successful in controlling outbreaks of HPAI H7N9 in poultry. Twenty-two outbreaks were reported before the immunization program (January to June 2017), but only 5 outbreaks have been reported afterwards (February to May 2018) (Figure 1) [24]. From these results, it is evident that the vaccination program has been very successful in reducing the prevalence of H7N9 viruses in both LPMs and poultry farms in China.
Table 1.
Detections of Avian Influenza A(H7N9) Virus in Poultry At Live Poultry Markets and Poultry Farms in China, 2013–2018
| LPMs | PFs | ||||||
|---|---|---|---|---|---|---|---|
| Year of Sampling | Sampling Provincesa | No. of LPMs | No. of Samples | No. of H7N9 Positive (%) | No. of PFs | No. of Samples | No. of H7N9 Positive (%) |
| 2013 | |||||||
| March–June | LN, SD, SX, HN, NX, JS, AH, HB, HN, ZJ, FJ, GD, GX, JX, GZ, CQ, SC, YN, HAN | 62 | 2480 | 24 (0.97) | 152 | 4551 | 0 (0) |
| October–December | 79 | 3448 | 8 (0.23) | 24 | 698 | 0 (0) | |
| 2014 | |||||||
| April–May | HLJ, NX, JS, AH, ZJ, SH, FJ, GD, GX, GZ, CQ, SC | 58 | 5801 | 75 (1.29) | 45 | 1425 | 0 (0) |
| October–December | 54 | 5921 | 35 (0.59) | 40 | 1515 | 0 (0) | |
| 2015 | |||||||
| April–May | BJ, SX, JL, SH, JS, AH, FJ, JX, GD, GX, HAN, CQ, QH, NX, HB | 51 | 5756 | 119 (2.07) | 46 | 1758 | 0 (0) |
| October–December | 68 | 4824 | 3 (0.06) | 53 | 1452 | 0 (0) | |
| 2016 | |||||||
| March–June | JS, SH, FJ, SX, HB, GD, GZ, QH, JX, NX, YN, GX, JL | 53 | 4063 | 4 (0.10) | 3 | 169 | 0 (0) |
| October–December | 56 | 4092 | 39 (0.95) | 36 | 1040 | 0 (0) | |
| 2017 | |||||||
| February | SH, FJ, HN, AH | 69 | 1959 | 77 (3.93) | 43 | 1445 | 11 (0.76) |
| March–June | JS, SH, GD, ZJ, HN, GZ, QH, JX, HB, NX, GX, HLJ, XJ, TB, YN | 83 | 4176 | 55 (1.32) | 175 | 5431 | 2 (0.04) |
| October–December | JS, SH, GD, GZ, QH, JX, HB, NX, GX, HLJ, TB, YN | 69 | 4095 | 11 (0.27) | 17 | 500 | 0 (0) |
| 2018 | |||||||
| April–May | GD, GX, GZ, HLJ, HB, JS, JX, NX, SH, TB, YN | 53 | 4032 | 0 (0) | 10 | 450 | 0 (0) |
Abbreviations: AH, Anhui; BJ, Beijing; CQ, Chongqing; FJ, Fujian; GD, Guangdong; GX, Guangxi; GZ, Guizhou; HAN, Hainan; HB, Hebei; HLJ, Heilongjiang; HN, Henan; JL, Jilin; JS, Jiangsu; JX, Jiangxi; LN, Liaoning; LPMs, live poultry markets; NX, Ningxia; PFs, poultry farms; QH, Qinghai; SC, Sichuan; SD, Shandong; SH, Shanghai; SX, Shanxi; TB, Tibet; XJ, Xinjiang; YN, Yunnan; ZJ, Zhejiang.
aThe H7N9 virus detection was conducted according to the protocol recommended by the World Health Organization (available at http://www;who;int/influenza/gisrs_laboratory/a_h7n9/en/).
Figure 1.
The geographic distribution of highly pathogenic avian influenza A(H7N9) virus outbreaks in Chinese poultry farms, 2017–2018.
REMAINING GAPS
Although modern integrated poultry production systems in China now provide approximately 50% of poultry through chilled and frozen meat, which is thought to reduce the H7N9 transmission risk to humans, these modern production systems may actually enhance long distance H7N9 transmission. This occurs because some production farms must move their poultry long distances for processing. During such long transport, avian influenza viruses spread among both transported poultry and poultry on farms adjacent to road ways, as well as increase the contamination of poultry transport vehicles. Taken together, these factors may likely increase the geographical spread of H7N9 among poultry in China. This is especially true if poultry are taken to wholesale poultry sales centers and redistributed to smaller retail markets or multiple local processing centers. Hence, we posit that China will need to conduct continued H7N9 virus surveillance not only in LPMs but also throughout modern poultry production systems. China will also need to strongly enforce the careful cleaning and treatment of poultry transportation vehicles as China switches from LPMs to modern poultry processing methods.
Although in the aggregate, the above-mentioned multiple interventions are now considered effective in controlling the current H7N9 threat, newly emergent avian influenza strains may entirely undermine current control programs. For instance, independent of H7N9 control measures, a novel emerged reassortment H7N2 virus was recently isolated from an unimmunized duck and found to be 1000-fold more lethal in mice than other HPAI H7N9 strains [25]. Such emerging viruses have potential to undermine the bivalent vaccination program control efforts. Hence, the long-term effectiveness of the current avian influenza control measures is very vulnerable to emerging virus threats. For sustainable effectiveness, avian influenza control programs must be coupled with comprehensive surveillance of novel influenza viruses. When novel viruses emerge, aggressive culling programs must be used. It also seems prudent to now include ducks in the current vaccination programs.
Conclusions
China’s mass poultry vaccination program has demonstrated considerable initial effectiveness in containing the spread of H7N9 among poultry and people, but its long-term effectiveness remains to be evaluated. To curb the circulation of H7N9 and to avert the threat of other emerging avian influenza viruses with pandemic potential, mass poultry vaccination will need to be coupled with comprehensive viral surveillance in the multiple venues for emergence of novel avian influenza viruses.
Acknowledgments
Financial support. This study was funded by the National Natural Science Foundation of China (Grant numbers 81773494 and 81402730; to M.-J. M.); China Mega-Project on Infectious Disease Prevention (Grant number 2017ZX10303401-006; to M.-J. M.); Beijing Scientific and Technology Nova Program (Grant number Z171100001117088; to M.-J. M.); the State Key Laboratory of Pathogen and Biosecurity (Grant number SKLPBS1819); and National Institutes of Health/National Institute of Allergy and Infectious Diseases (Grant number R01AI108993; to G. C. G.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Chinese National Influenza Center. Chinese Influenza Weekly Report Available at: http://www.chinaivdc.cn/cnic/zyzx/lgzb/201806/P020180624806846812091.pdf. Accessed 17 June 2018.
- 2. Chan MC, Chan RW, Chan LL, et al. Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med 2013; 1:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Agriculture Organization of the United Nations. H7N9 situation update Available at: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/situation_update.html. Accessed 5 June 2019.
- 4. Ma MJ, Yang Y, Fang LQ. Highly pathogenic avian H7N9 influenza viruses: recent challenges. Trends Microbiol 2019; 27:93–5. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Influenza risk assessment tool (IRAT) Available at: https://www.cdc.gov/flu/pandemic-resources/monitoring/irat-virus-summaries.htm. Accessed 27 July 2018.
- 6. Jernigan DB, Cox NJ. H7N9: preparing for the unexpected in influenza. Annu Rev Med 2015; 66:361–71. [DOI] [PubMed] [Google Scholar]
- 7. Shi J, Deng G, Kong H, et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res 2017; 27:1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He Y, Liu P, Tang S, et al. Live poultry market closure and control of avian influenza A(H7N9), Shanghai, China. Emerg Infect Dis 2014; 20:1565–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu P, Jiang H, Wu JT, et al. Poultry market closures and human infection with influenza A(H7N9) virus, China, 2013–14. Emerg Infect Dis 2014; 20:1891–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu H, Wu JT, Cowling BJ, et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet 2014; 383:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Wang Y, Shen C, et al. Closure of live bird markets leads to the spread of H7N9 influenza in China. PLoS One 2018; 13:e0208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lau EH, Leung YH, Zhang LJ, et al. Effect of interventions on influenza A (H9N2) isolation in Hong Kong’s live poultry markets, 1999–2005. Emerg Infect Dis 2007; 13:1340–7. [DOI] [PubMed] [Google Scholar]
- 13. Leung YH, Lau EH, Zhang LJ, et al. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerg Infect Dis 2012; 18:1339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu H, Chen Z, Xiao X, et al. Effects of resting days on live poultry markets in controlling the avian influenza pollution. Zhonghua Liu Xing Bing Xue Za Zhi 2014; 35:832–6. [PubMed] [Google Scholar]
- 15. Peng C, Peng NX, Duan XD, et al. The cohort study on effect of monthly closing day in live poultry markets in Guangzhou city. China Animal Helath Inspect 2016; 33:3–7. [Google Scholar]
- 16. Yuan J, Lau EH, Li K, et al. Effect of live poultry market closure on avian influenza A(H7N9) virus activity in Guangzhou, China, 2014. Emerg Infect Dis 2015; 21:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Liu S, Mao H, et al. Surveillance of avian H7N9 virus in various environments of Zhejiang Province, China before and after live poultry markets were closed in 2013–2014. PLoS One 2015; 10:e0135718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang M, He J, Song T, et al. Environmental sampling for avian influenza A(H7N9) in live-poultry markets in Guangdong, China. PLoS One 2015; 10:e0126335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fournié G, Guitian FJ, Mangtani P, Ghani AC. Impact of the implementation of rest days in live bird markets on the dynamics of H5N1 highly pathogenic avian influenza. J R Soc Interface 2011; 8:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepin KM, Lloyd-Smith JO, Webb CT, et al. Minimizing the threat of pandemic emergence from avian influenza in poultry systems. BMC Infect Dis 2013; 13:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan J, Tang X, Yang Z, et al. Enhanced disinfection and regular closure of wet markets reduced the risk of avian influenza A virus transmission. Clin Infect Dis 2014; 58:1037–8. [DOI] [PubMed] [Google Scholar]
- 22. Wood JP, Choi YW, Chappie DJ, et al. Environmental persistence of a highly pathogenic avian influenza (H5N1) virus. Environ Sci Technol 2010; 44:7515–20. [DOI] [PubMed] [Google Scholar]
- 23. Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Ministry of Agriculture News Available at: http://www.moa.gov.cn/xw/qg/201708/t20170807_5770956.htm. Accessed 7 August 2017.
- 24. OIE. OIE Situation Report for Avian Influenza Available at: http://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/OIE_AI_situation_report/OIE_SituationReport_AI__6_8May2017.pdf. Accessed 9 May 2017.
- 25. Shi J, Deng G, Ma S, et al. Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host Microbe 2018; 24:558–568.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]