Abstract
Background. Evidence of the presence of Plasmodium vivax in the placenta is scarce and inconclusive. This information is relevant to understanding whether P. vivax affects placental function and how it may contribute to poor pregnancy outcomes.
Methods. Histopathologic examination of placental biopsies from 80 Papua New Guinean pregnant women was combined with quantitative polymerase chain reaction (qPCR) to confirm P. vivax infection and rule out coinfection with other Plasmodium species in placental and peripheral blood. Leukocytes and monocytes/macrophages were detected in placental sections by immunohistochemistry.
Results. Monoinfection by P. vivax and Plasmodium falciparum was detected by qPCR in 8 and 10 placentas, respectively. Seven of the 8 women with P. vivax placental monoinfection were negative in peripheral blood. By histology, 3 placentas with P. vivax monoinfection showed parasitized erythrocytes in the intervillous space but no hemozoin in macrophages nor increased intervillous inflammatory cells. In contrast, 7 placentas positive for P. falciparum presented parasites and hemozoin in macrophages or fibrin as well as intervillous inflammatory infiltrates.
Conclusions. Plasmodium vivax can be associated with placental infection. However, placental inflammation is not observed in P. vivax monoinfections, suggesting other causes of poor delivery outcomes associated with P. vivax infection.
Cytoadherence of erythrocytes infected by Plasmodium falciparum has been shown to contribute to microvascular sequestration and to the high case fatality rate of severe falciparum malaria [1]. In contrast, sequestration of Plasmodium vivax within organs has not been confirmed in the very few autopsies and tissue biopsies studied (reviewed in [2]). However, recent reports showing adherence of P. vivax–infected erythrocytes (IEs) to human receptors [2–4] suggest the possibility that this parasite might also be able to sequester in specific organs and tissues.
Histological evidence of P. vivax sequestration from autopsies conducted at the beginning of the 1900s preceded the possibility of molecular confirmation of P. vivax monoinfection [5, 6]. More recently, the postmortem examination of a woman with fatal respiratory distress, and polymerase chain reaction (PCR)–confirmed P. vivax infection showed heavy intravascular monocytic infiltrates provoking inflammatory lesions in the endothelium of the lungs, but without sequestration of IEs in the pulmonary microvasculature [7], suggesting organ damage unrelated to sequestration of P. vivax parasites.
In contrast to the considerable amount of data showing accumulation of P. falciparum IEs in the intervillous spaces of the placenta [8], few reports have described other Plasmodium species in placental smears [9–19]. However, there is a lack of studies combining histology and molecular techniques in placental and peripheral blood to distinguish histological changes by Plasmodium species. Recently, placental histology has demonstrated a deposition of hemozoin associated with antenatal P. vivax infection among Karen women from the Thai-Burmese border, although parasites were rare or absent as antenatal care relied on active detection and prompt treatment and P. vivax monoinfection was not confirmed by PCR [20]. In another study among pregnant women residing in an area of Iquitos, Peru, with low transmission of P. vivax and P. falciparum, hemozoin deposition was observed in 22% of the placentas analyzed [21], including a cord blood infection and placental changes associated only with P. vivax infection. Case reports have revealed the presence of P. vivax in the placenta by PCR, either alone or in combination with P. falciparum, but they lacked histological confirmation [22, 23]. Although P. vivax infection has been associated with adverse pregnancy outcomes [24], the pathophysiological mechanisms underlying these adverse effects remain to be determined.
Detailed histological and molecular studies describing whether P. vivax parasites accumulate in human placentas and the pathological changes associated with the infection are a priority to understand how P. vivax can affect placental function and cause low birth weight [24], as well as to gain insights into the general mechanisms of disease severity associated with P. vivax malaria. The present study was undertaken to determine if P. vivax can be unequivocally associated with placental infection. To address this, we combined histological examination of placental samples with highly specific PCR methods to confirm P. vivax infection and rule out coinfection with other Plasmodium species.
MATERIALS AND METHODS
Study Site and Sample Collection
Pregnant women attending antenatal care at Alexishafen Health Centre in Madang, Papua New Guinea, between September 2005 and October 2007 were recruited in the context of a longitudinal study of malaria in pregnancy, following written informed consent. The study area is characterized by year-round, high level malaria transmission. At the time of the study, the prevalence of malaria in pregnant women in Madang at first antenatal care visit was 29%–41% (with 5%–20% of these infections described as P. vivax by microscopy). At delivery, 7%–18% of women were positive in their peripheral blood, and 16%–24% were placental smear positive, with 4%–20% of these infections due to P. vivax [25]. The study was approved by the Papua New Guinea Medical Research Advisory Council and by the Papua New Guinea Institute of Medical Research Institutional Review Board. All women participating in this study were prescribed chloroquine as a treatment dose followed by weekly prophylaxis in accordance with Papua New Guinea standard treatment guidelines; in the latter part of the study an initial dose of 3 tablets of sulfadoxine-pyrimethamine was coadministered with chloroquine treatment, as recommended by the guidelines. Seventy-five percent of women regularly used bed nets, but <10% of these nets were insecticide-treated. Peripheral blood (6 mL) was collected during labor or shortly after delivery in ethylenediaminetetraacetic acid (EDTA) vacutainers. An incision was made in a macroscopically normal area in the center of the maternal side of the placenta, and blood was collected from the intervillous space with a sterile transfer pipette into an EDTA vacutainer. From the same incision point, one placental biopsy was collected and a full-thickness biopsy about 1 cm thick and 2 cm wide was placed in 10% neutral buffered formalin. Samples collected from 80 pregnant women were included in the study.
Placental Histology
Placental samples (90–168 mm2) were processed for histological examination and stained as previously described [26]. Hematoxylin and eosin–stained slides from all placentas were read independently by 2 pathologists. The following histological findings were recorded in all cases: presence of Plasmodium parasites, presence of hemozoin (identified by light microscopy as a coarse, brown, granular material with birefringency under polarized light) in free macrophages, presence of hemozoin in fibrin (either in macrophages covered by fibrin or free), and intervillous inflammation. Every case with a discrepant result between both readings was subject to a third blinded histological evaluation by one of the pathologists. A 2-out-of-3 consensus evaluation was established after this third evaluation. The percentage of IEs was established after counting 500 intervillous erythrocytes.
Immunohistochemistry
Immunohistochemical studies were performed using the automated immunohistochemical system TechMate 500 TM (Dako) and the EnVision system (Dako) in formalin-fixed, paraffin-embedded tissue. The total number of leukocytes was determined following staining with anti-CD45 (Clone 2B11, Dako) and macrophages were stained with anti-CD68 (Clone KP1, Dako). In brief, paraffin sections were deparaffinized and rehydrated in xylene and graded alcohols. After blocking with peroxidase for 7.5 minutes in ChemMate peroxidase-blocking solution (Dako), the slides were incubated with the primary antibodies (CD45 and CD68) for 30 minutes and washed in ChemMate buffer solution (Dako). After application of the peroxidase-labeled polymer for 30 minutes and washing, the slides were incubated with the diaminobenzidine substrate chromogen solution, washed in water, counterstained with hematoxylin, washed again, dehydrated, and mounted. The number of CD45- and CD68-positive cells per high-power field (HPF = ×400) was calculated after counting 10 HPFs using an Olympus BX41 microscope (total area evaluated = 17.28 mm2).
DNA Template Extraction and Amplification
After peripheral and placental blood centrifugation, DNA was extracted from 200 μL of erythrocyte pellet using the QIAmp DNA blood Mini Kit (Qiagen) and finally resuspended in 200 μL of distilled water according to the supplier's instructions. Three microliters of extracted DNA was used for real-time quantitative PCR (qPCR) following previously published methods [27] with minor modifications. For optimal detection of parasite genomic DNA, 25-μL simplex reactions were performed for P. falciparum and P. vivax. Detection of Plasmodium malariae and Plasmodium ovale was performed using a duplex qPCR assay. Primers and probes corresponded to those previously described with the exception of an improved P. vivax probe, which has been changed to a minor groove binder (MGB) probe (Life Technologies) with a 5′ VIC reporter and a 3′ MGB nonfluorescent quencher to increase specificity of P. vivax detection. Forty-five cycles were performed. Positive and negative controls were included in the PCR experiments. Positive controls consisted of a P. falciparum and P. vivax amplicon cloned into plasmids. A standard curve of 10-fold serial dilutions of these plasmids as well as no template controls were included in each experiment. A sample was considered positive if the cycle threshold (Ct) value was <45.
Samples positive for Plasmodium species detected by qPCR were confirmed by PCR ligase detection reaction fluorescent microsphere assay (PCR-LDR-FMA) using 2.5 μL of DNA extraction as described elsewhere [27].
Definitions and Statistical Analysis
A placental infection was defined as unequivocally attributed to P. vivax if placental blood was positive for P. vivax by qPCR and PCR-LDR-FMA without evidence of coinfection with P. falciparum, P. ovale, or P. malariae either in the placenta or in peripheral blood by qPCR. The Kruskal-Wallis test was used to compare Cts obtained from samples positive for different Plasmodium species and the number of CD45+/CD68+ cells in uninfected placentas with numbers in P. vivax or P. falciparum monoinfected placentas.
RESULTS
The mean age of the 80 pregnant women included into the study was 23.1 years (SD, 7.3). Twenty-seven of the women (34%) were primigravidae. In 8 of the 80 women, qPCR revealed P. vivax monoinfection in the placenta; 7 of these women were negative in the peripheral blood for any Plasmodium species, and 1 woman was positive for P. falciparum. Ten of the women were positive for P. falciparum in the placenta, 6 of whom were also positive for P. falciparum in peripheral blood. Only 1 mixed infection (P. falciparum plus P. vivax) was found in placental blood (Table 1). Plasmodium species–positive samples by qPCR were confirmed by PCR-LDR-FMA. Median Cts obtained by qPCR from placental blood positive for P. falciparum (36.73 [interquartile range {IQR}, 33.49–39.40]) were lower than Cts from P. vivax–positive infections (42.83, [IQR, 41.09–44.53], P = .005). Plasmodium ovale and P. malariae were not detected by qPCR in any of the peripheral or placental blood samples tested.
Table 1.
Placental Histology and Quantitative Polymerase Chain Reaction (qPCR) in Peripheral Blood From Women With qPCR-Confirmed Plasmodium Species Infection in Intervillous Placental Blood Samples
| Placental Infection (qPCR)a | Placental Histology | Peripheral Infection (qPCR)a |
|---|---|---|
| 8 only P. vivax | 3 parasites | Negative |
| 3 hemozoin in fibrin | Negative | |
| 1 parasites + hemozoin in fibrin | P. falciparum | |
| 1 negative | Negative | |
| 10 only P. falciparum | 2 parasites | Negative |
| 7 parasites + hemozoin in free macrophages and fibrin | 6 P. falciparum | |
| 1 negative | Negative | |
| 1 P. falciparum + P. vivax | 1 parasites + hemozoin in free macrophages and fibrin | Negative |
a Plasmodium vivax and Plasmodium falciparum infections detected by qPCR were confirmed by PCR ligase detection reaction fluorescent microsphere assay.
On histological examination, 3 of the 8 placentas positive for P. vivax by qPCR showed malarial parasites in the maternal erythrocytes of the intervillous space, with parasitemias of 1%, 4%, and 25% (Figure 1A and 1B). All IEs showed mature forms of the parasite containing hemozoin, but no hemozoin was observed in free macrophages or in fibrin. One of the 8 placentas had both malaria IEs (2% parasitized erythrocytes), together with mild to moderate deposition of malarial hemozoin in perivillous fibrin (Figure 1C); this woman had a P. falciparum infection in peripheral blood. Among the other 4 placentas not showing any parasite by histology, 3 presented hemozoin in perivillous fibrin. Hemozoin associated with P. vivax infection, either in the cytoplasm of the parasite or in fibrin, showed birefringence under polarized light (Figure 1D). No hemozoin within free macrophages was identified in any of the P. vivax–infected placentas.
Figure 1.
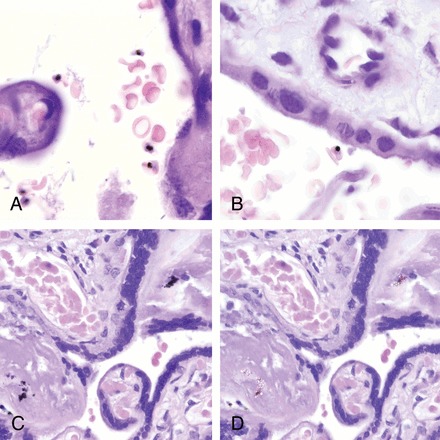
Histological sections of placentas with Plasmodium vivax monoinfections showing parasitized maternal red blood cells. All parasites identified were mature forms containing hemozoin (A and B). C, Malarial hemozoin in perivillous fibrin in a case positive for P. vivax by quantitative polymerase chain reaction. D, Malarial hemozoin showing birefringence under polarized light.
Histology of the 10 placentas that were positive by qPCR only for P. falciparum showed 1 biopsy with no parasites or hemozoin; 2 with only malaria IEs; and 7 with malaria IEs and hemozoin in free macrophages and fibrin. Among those with IEs, placental parasitemias were 1% (4 placentas), 2%, 4%, 15% (1 placenta each), and 25% (2 placentas). Six of the 7 women with placental IEs and hemozoin in free macrophages and fibrin also had a P. falciparum peripheral infection (Table 1).
In the placenta with mixed P. falciparum plus P. vivax infection detected by qPCR, both IEs and hemozoin in macrophages and fibrin were observed by histology. No Plasmodium species were detected in peripheral blood of this woman (Table 1).
Histological examination did not allow discrimination between P. vivax and P. falciparum IEs (Figure 2). None of the placentas with P. vivax monoinfections detected by qPCR showed increased leukocytes (CD45) or macrophages (CD68) (Figure 3A and 3B) compared with uninfected placentas (Figure 4). In contrast, a significant number of P. falciparum–monoinfected placentas showed a moderate to marked increase in the number of total leukocytes (n = 5) and macrophages (n = 3) in the intervillous space (Figure 3C and 3D), compared with uninfected placentas (Figure 4).
Figure 2.
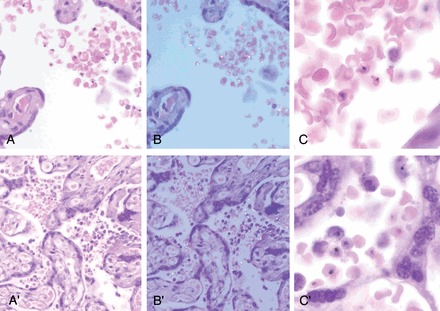
Comparison between Plasmodium vivax (A, B, and C) and Plasmodium falciparum (A′, B′, and C′) parasitized placentas. A and A′ show maternal infected red blood cells in the intervillous space. No inflammatory reaction was observed in P. vivax–infected placenta, whereas an increased number of intervillous macrophages were detected in P. falciparum–infected placenta. B and B′ show the same fields under polarized light showing the birefringence of hemozoin present in the mature forms of the parasites. Birefringent hemozoin in free macrophages was present in the P. falciparum–infected placenta. C and C′, Higher magnification showing the characteristics of the parasites.
Figure 3.
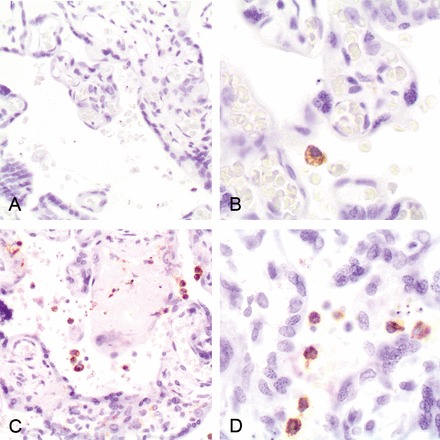
Immunohistochemical staining macrophages (CD68) in Plasmodium vivax–infected (A and B) and Plasmodium falciparum–infected (C and D) placentas.
Figure 4.
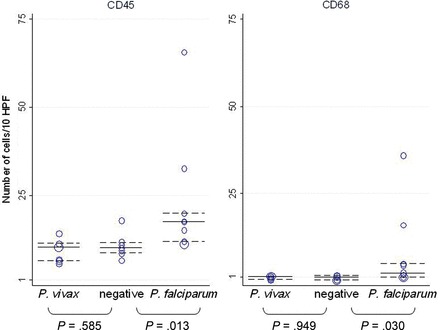
Number of CD45+ (leukocytes) and CD68+ (macrophages) cells after examination of 10 high-power fields (HPF; ×400) in placentas with Plasmodium vivax and Plasmodium falciparum monoinfection and uninfected placentas. Median cell counts and interquartile ranges are indicated by horizontal continuous and dashed lines.
DISCUSSION
The data presented constitute a detailed molecular and histological characterization of P. vivax infection in the placenta. Ten percent of placental blood samples (8 of 80) showed P. vivax monoinfection by qPCR. Histological examination of 3 of these placentas showed malaria IEs in the intervillous space. Plasmodium falciparum monoinfection was detected in 12% (10 of 80) of the placental blood samples, 9 of which were associated with the presence of malaria IEs by histology. Only 1 placental sample had a mixture of both P. falciparum and P. vivax. The 3 P. vivax placental monoinfections confirmed by histology were not associated with a concomitant infection in peripheral blood, in contrast to 6 of the 9 women with P. falciparum placental monoinfections who also presented a qPCR-confirmed P. falciparum infection in peripheral blood. Absence of P. vivax peripheral infection in women with P. vivax placental monoinfection may be explained by low P. vivax peripheral densities below or fluctuating around the detection limit of qPCR. Alternatively, the absence of parasites in peripheral blood may suggest that P. vivax can persist in the placenta even after parasite clearance in the circulation and that P. vivax might be able to selectively sequester in the placenta.
Three of the 8 placentas with qPCR-confirmed P. vivax monoinfections showed hemozoin in fibrin but no parasites in the histological examination. Although the presence of DNA in hemozoin complexes [28] may explain these cases positive by PCR but negative by histology, it is not possible to know with certainty whether the hemozoin corresponds to recent P. vivax infection or a past P. falciparum infection. In this study, parasites and hemozoin deposition in free macrophages and fibrin were detected by histology in 7 of the 9 (78%) women with qPCR-confirmed P. falciparum placental infection, but only in 1 of the 4 women with qPCR-confirmed P. vivax placental infection. However, the fact that this latter woman was also infected by P. falciparum in the peripheral blood means it is not possible to conclude whether the hemozoin resulted from the P. vivax or P. falciparum infection. Although a previous report showed moderate placental deposition of hemozoin in Karen women with antenatal P. vivax infections [20], that study could only confirm by histology the presence of malaria IEs in 1 placenta, probably as a result of the active detection and treatment of women included in that study [20].
The absence of hemozoin deposition and accumulation of inflammatory cells in placentas infected with P. vivax observed in the present study may suggest that placental P. vivax infections occurred during late pregnancy. Alternatively, P. vivax parasites may not remain in the placenta long enough to cause hemozoin deposition and trigger the accumulation of inflammatory cells. Overall, this study suggests that factors other than persistent pathological disturbances of the placenta, such as systemic alterations, may cause the reduction of birth weight associated with P. vivax infection [24].
There are 4 main limitations of the current study. First, the small sample size did not allow statistical comparisons of histological findings between P. vivax– and P. falciparum–infected placentas. Second, it is not possible to exclude the presence of parasites in other sections of the placenta, as only a small section was examined by histology. Third, all the analysis was done with samples at delivery, as blood was not available during pregnancy to assess the presence of Plasmodium infection. And fourth, Plasmodium parasites, particularly P. vivax, which reaches lower densities than P. falciparum, may be present at densities below the detection limit of qPCR, making their detection by histology and even qPCR challenging.
In conclusion, this study shows the presence of P. vivax parasites in the placenta by histology while excluding infection with other Plasmodium species by 2 independent and highly specific molecular methods. This demonstrates for the first time that P. vivax, although at a lower frequency than P. falciparum, can be associated with placental infection. Importantly, histological examination of placentas with P. vivax monoinfections and absence of P. falciparum in peripheral blood detected only parasites but not hemozoin in macrophages, in contrast to the observation of both parasites and hemozoin in P. falciparum monoinfections. Moreover, the presence of P. vivax in the placenta was not associated with an increase of inflammatory cell infiltrates in contrast to P. falciparum placental infections [20]. Even though inflammatory changes were not observed with P. vivax infections, the parasite or its products may have an adverse effect on placental function. Further studies are under way to establish the clinical consequences of P. vivax in pregnant women on pregnancy outcomes.
Notes
Acknowledgments. We express our gratitude to the pregnant women participating in this study; to the field team in Papua New Guinea, particularly to Francesca Baiwog; and to Mireia Piqueras and Laura Puyol, who helped in the management of the study.
Disclaimer. The funding sources did not have any involvement in study design, collection, analysis, and interpretation of data, writing of the report, or in the decision to submit the paper for publication.
Financial support. The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7-2007-HEALTH) under grant agreement n° 201588 and from the Malaria in Pregnancy Consortium. A. M. receives salary support from the Instituto de Salud Carlos III (CP-04/00220) and C. D. from the Ministerio de Ciencia e Innovacion (RYC-2008-02631).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 2.Costa FT, Lopes SC, Ferrer M, et al. On cytoadhesion of Plasmodium vivax: raison d'etre? Mem Inst Oswaldo Cruz. 2011;106(suppl 1):79–84. doi: 10.1590/s0074-02762011000900010. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho BO, Lopes SC, Nogueira PA, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–47. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 4.Chotivanich K, Udomsangpetch R, Suwanarusk R, et al. Plasmodium vivax adherence to placental glycosaminoglycans. PLoS One. 2012;7:e34509. doi: 10.1371/journal.pone.0034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–7. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ewing J. Contribution to the pathological anatomy of malarial fever. J Exp Med. 1902;6:119–80. doi: 10.1084/jem.6.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valecha N, Pinto RG, Turner GD, et al. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg. 2009;81:758–62. doi: 10.4269/ajtmh.2009.09-0348. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ, Romagosa C, Abdelgalil S, et al. The sick placenta—the role of malaria. Placenta. 2004;25:359–78. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Bachschmid I, Soro B, Coulibaly A, et al. Malaria infection during childbirth and in newborns in Becedi (Ivory Coast) Bull Soc Pathol Exot. 1991;84:257–65. [PubMed] [Google Scholar]
- 10.Benet A, Khong TY, Ura A, et al. Placental malaria in women with South-East Asian ovalocytosis. Am J Trop Med Hyg. 2006;75:597–604. [PubMed] [Google Scholar]
- 11.Bruce-Chwatt LJ. Malaria in African infants and children in southern Nigeria. Ann Trop Med Parasitol. 1952;46:173–200. doi: 10.1080/00034983.1952.11685522. [DOI] [PubMed] [Google Scholar]
- 12.Garnham P. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans R Soc Trop Med Hyg. 1938;32:13–34. [Google Scholar]
- 13.Hamer DH, Singh MP, Wylie BJ, et al. Burden of malaria in pregnancy in Jharkhand State, India. Malar J. 2009;8:210. doi: 10.1186/1475-2875-8-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelliffe EF. Low birth-weight and malarial infection of the placenta. Bull World Health Organ. 1968;38:69–78. [PMC free article] [PubMed] [Google Scholar]
- 15.McGready R, Brockman A, Cho T, et al. Haemozoin as a marker of placental parasitization. Trans R Soc Trop Med Hyg. 2002;96:644–6. doi: 10.1016/s0035-9203(02)90339-1. [DOI] [PubMed] [Google Scholar]
- 16.Paksoy N. The incidence of placental malaria in Vanuatu in the South Pacific. Trans R Soc Trop Med Hyg. 1986;80:174–5. doi: 10.1016/0035-9203(86)90237-3. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Saxena A, Awadhia SB, Shrivastava R, Singh MP. Evaluation of a rapid diagnostic test for assessing the burden of malaria at delivery in India. Am J Trop Med Hyg. 2005;73:855–8. [PubMed] [Google Scholar]
- 18.Singh N, Saxena A, Shrivastava R. Placental Plasmodium vivax infection and congenital malaria in central India. Ann Trop Med Parasitol. 2003;97:875–8. doi: 10.1179/000349803225002688. [DOI] [PubMed] [Google Scholar]
- 19.Rijken MJ, Boel ME, Russell B, et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J. 2010;10:113. doi: 10.1186/1475-2875-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGready R, Davison BB, Stepniewska K, et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 21.Parekh FK, Davison BB, Gamboa D, Hernandez J, Branch OH. Placental histopathologic changes associated with subclinical malaria infection and its impact on the fetal environment. Am J Trop Med Hyg. 2010;83:973–80. doi: 10.4269/ajtmh.2010.09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho BO, Matsuda JS, Luz SL, et al. Gestational malaria associated to Plasmodium vivax and Plasmodium falciparum placental mixed-infection followed by foetal loss: a case report from an unstable transmission area in Brazil. Malar J. 2011;10:178. doi: 10.1186/1475-2875-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman RD, Hailemariam A, Jimma D, et al. Burden of malaria during pregnancy in areas of stable and unstable transmission in Ethiopia during a nonepidemic year. J Infect Dis. 2003;187:1765–72. doi: 10.1086/374878. [DOI] [PubMed] [Google Scholar]
- 24.Nosten F, McGready R, Simpson JA, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–9. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 25.Rijken MJ, McGready R, Boel ME, et al. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75–88. doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 26.Ordi J, Ismail MR, Ventura PJ, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Rosanas-Urgell A, Mueller D, Betuela I, et al. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–24. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]


