Abstract
Interleukin-10 (IL-10) mRNA levels are increased within intestinal mucosa after Eimeria infection. IL-10 apical receptor presence on enterocytes suggests IL-10 is secreted into the intestinal lumen. Increased IL-10 has been shown to be central to the pathogenesis of numerous intracellular pathogens; we hypothesize luminal secretion of IL-10 enables Eimeria spp. infection in chickens. This study examines intestine luminal IL-10 levels and performance in broilers challenged with Eimeria when fed an anti-IL-10 antibody. Chicks were fed a diet (1 to 21 d) with control or anti-IL-10 antibody (0.34 g egg yolk antibody powder/Kg diet) with a saline or 10× dose of Advent coccidiosis vaccine on d 3. One chick per pen was euthanized on days 2, 4, 7, 10, 13, 16, and 19 post-challenge, bled, and intestines were collected for luminal fluid IL-10 concentrations. Body weight and feed intake were measured on d 21, and oocyst shedding was assessed on d 7 post-challenge. A significant Eimeria × antibody interaction on d 21 body weight (P < 0.05) showed chicks fed control antibody, but not anti-IL-10, had significant reductions in body weight when challenged with Eimeria spp. Oocyst shedding was increased with Eimeria challenge, but dietary antibody had no effect. Plasma carotenoid levels were reduced in Eimeria challenged chicks 4, 7, 10, and 16 days post-challenge compared to unchallenged chicks. Lack of an Eimeria × antibody interaction showed anti-IL-10 was not protective against Eimeria-induced decreases in plasma carotenoids. Eimeria challenge increased intestine luminal IL-10 on days 4 and 7 post-challenge in the cecum and jejunum, respectively, compared to unchallenged. Dietary anti-IL-10 decreased luminal IL-10 in the ileum on day 2 post-challenge when compared to control antibody fed chicks. No interaction between Eimeria challenge and antibody was observed on intestine luminal contents of IL-10, suggesting anti-IL-10 was ineffective at preventing increased Eimeria-induced luminal IL-10. In conclusion, Eimeria challenge increased intestinal luminal IL-10 and anti-IL-10 was effective at preventing Eimeria-induced decreased body weight, however the mechanism anti-IL-10 antibody protects body weight during Eimeria challenge remains unknown.
Keywords: Interleukin-10, Eimeria, coccidiosis, egg antibody, anti-IL-10
INTRODUCTION
Interleukin-10 (IL-10) is an anti-inflammatory cytokine important in balancing inflammatory responses to pathogens, and is secreted by macrophages, dendritic cells, T cells, B cells, neutrophils, eosinophils, and mast cells (Moore et al., 2001). The role of IL-10 as a key regulator of the inflammatory process has been extensively reviewed (Couper et al., 2008; Cyktor and Turner, 2011). Interleukin-10 activity diminishes the capacity of innate immune cells to respond to pathogens by down regulating major histocompatibility complex class II proteins, costimulatory molecules, and the production of reactive oxygen intermediates in active macrophages. IL-10 also reduces the adaptive immune response by decreasing the generation of antigen specific T-cells. Typically after a pro-inflammatory immune response, IL-10 prevents further inflammation by the aforementioned mechanisms and by inhibiting pro-inflammatory cytokine production (gamma interferon (IFN-γ), tumor necrosis factor alpha, interleukin 1 beta, interleukin 2, and granulocyte-monocyte colony-stimulating factor). IL-10s anti-inflammatory properties are typically necessary to prevent further inflammation that may be detrimental to host tissue.
However, anti-inflammatory properties of IL-10 also provide a pathway for intracellular pathogens to evade host immune responses. A compilation of literature indicates that IL-10 regulation may be exploited by bacterial, fungal, and parasitic infections through a variety of pathways involving toll-like receptor 2 and 4 (TLR-2 and TLR-4) signaling, (Cyktor and Turner, 2011). For example, macrophages stimulated with Yersinia enterocolitica (a pathogenic intracellular bacteria) LcrV antigens increase IL-10 secretion through transmembrane signaling via lipopolysaccharide binding protein/CD14/TLR-2 complexes. A pathogen-induced increase in IL-10 leads to a hypo-responsive state by decreasing nuclear factor kappa-light-chain-enhancer of activated B cells signal transduction, and tumor necrosis factor alpha production (and other cytokines associated with cell apoptosis and inflammation during infection), which favors intracellular pathogen survival (Sing et al., 2002). Furthermore, animal models that have reduced or eliminated IL-10 levels (e.g., knockout or anti-IL-10 receptor monoclonal antibody) have exhibited a greater capacity than their wild-type or control antibody counterparts to clear some pathogenic infections (Cyktor and Turner, 2011). Similar to other intracellular pathogens, Eimeria acervulina has also been found to increase IL-10 mRNA production in the duodenum and cecum 5 days post-infection (Hong et al., 2006). E. tenella-induced increases in IL-10 suggests that Eimeria species may be utilizing a similar tactic to elude host immune responses, allowing the parasite to complete its life cycle within intestinal epithelial cells.
Eimeria spp. are intracellular protozoan parasites that cause gastrointestinal dysfunction, decreased growth and feed efficiency, and increased mortality in floor-raised chickens (Williams, 1999). Currently Eimeria infection is managed by vaccination and anti-coccidials (i.e., ionophores and chemicals). Due to inconsistent coverage, vaccines can reduce overall flock weight gain or offer limited protection against subsequent exposure, while anti-coccidials create Eimeria resistance (Lillehoj and Lillehoj, 2000; Vermeulen et al., 2001).
Evidence of uncontrolled inflammation in IL-10 knockout models and animals treated systemically with antibodies to IL-10 (Cyktor and Turner, 2011) suggested that alternative means of controlling Eimeria-induced release of IL-10 were needed. A recent finding of apical IL-10 receptors on intestinal epithelia (Kominsky et al., 2014) led Sand et al. (2016) to explore targeting IL-10 secretion into the intestinal lumen. Preliminary findings of luminal IL-10 supported an alternative method for controlling Eimeria infection without inducing systemic inflammation, the feeding of anti-IL-10 antibodies (Sand et al., 2016).
Recently, successful targeting of host peptides in the intestinal lumen using egg antibodies was demonstrated (Cook and Trott, 2010; Bobeck et al., 2015). The recent findings of Sand et al. (2016) that oral antibodies to chicken IL-10 prevented Eimeria-induced decreases in growth rate of broilers lead us to develop a quantitative assay for intestine luminal IL-10 and to better understand IL-10s regulation in Eimeria challenged chickens. In this study we induce a subclinical Eimeria challenge by orally gavaging chicks with a 10× dose of low virulent coccidiosis vaccine. This model has been shown to reduce performance without an increase in chick mortality (Pederson et al., 2008). The effects of Eimeria challenge and oral anti-IL-10 on intestine luminal IL-10 levels as well as chick performance were measured.
MATERIALS AND METHODS
All experimental procedures involving chickens were approved by the College of Agricultural and Life Sciences Animal Care Committee at the University of Wisconsin-Madison.
Antibody preparation and specificity
Antibody to avian IL-10 was prepared using a procedure similar to those described in Bobeck et al. (2015) and was recently described in more detail in Sand et al. (2016). Briefly, an 8 amino acid peptide val-leu-pro-arg-ala-met-gln-thr (vlpramqt) synthesized by GeneScript (Piscatawy, NJ), was conjugated to bovine gamma globulin (BGG, Sigma, St. Louis, MO) using the previously described glutaraldehyde procedure (Bobeck et al., 2012). The control vaccine consisted of glutaraldehyde treated BGG and Freund's Complete and Incomplete adjuvants (Difco Laboratories, Detroit, MI), the same adjuvants used for making anti-IL-10 antibody. Hens were injected as previously described and eggs containing the antibody were collected beginning 21 days after the first injection, yolks separated, and dried by lyophilization (Cook and Trott, 2010). Presence of the antibody was determined using ELISA, where the coating peptide (vlpramqt) was attached to ovalbumin (Bobeck et al., 2015).
Antibody neutralization ability was determined using the procedures described by Hillyer and Woodward (2003) with minor modifications. In this assay, the ability of anti-IL-10 antibody to prevent IL-10-induced inhibition of IFN-γ production in concanavalin A (ConA) stimulated chick splenocytes was determined. Briefly, chick splenocytes were harvested (Ren et al., 2015) and re-suspended at 4 × 106 cells/mL RPMI-1640 medium (without phenol red) supplemented with 10% fetal calf serum, 100U/mL penicillin, and 100μg/mL streptomycin (Sigma). Cells were treated with 20 μg/mL ConA alone, ConA (Sigma) plus 0.2 ng/mL IL-10 (Kingfisher Biotech, Inc., St. Paul, MN), or ConA, plus IL-10 plus 0.85 μg/mL of affinity purified egg yolk anti-IL-10 antibody. Cultures were incubated at 41.5°C in a humidified 5% CO2 chamber for 24 h. Culture fluid was removed from each well and centrifuged at 1,000 g for 20 minutes. A commercial ELISA kit was used to quantify IFN-γ in supernatants (Invitrogen, Frederick, MD).
Chick Experiment
Day-old broiler pullets from Welp Hatchery, Bancroft, Iowa, (400 in total) were divided into 40 pens (10 chicks per pen) and housed in a battery brooder with raised wire floors. Ten pens of chicks were assigned to each of 4 treatments in a 2 (Antibody) × 2 (Eimeria) factorial arrangement in a complete randomized design. The four treatments were: dietary control antibody and saline challenge, dietary control antibody and Eimeria challenge, dietary anti-IL-10 antibody and saline challenge, and dietary anti-IL-10 antibody and Eimeria challenge. Diets consisted of a standard broiler starter diet supplemented with either control dried egg yolk antibody (from hens injected with BGG carrier in adjuvant) or an anti-IL-10 dried egg yolk antibody (0.341 g/Kg diet). Since anti-IL-10 antibody replaced control antibody containing the exact same nutrient profiles (nutrient profiles of dried egg yolk powder), the nutrient content of all diets were identical and came from the same lot of feed. The dietary level of anti-IL-10 antibody was based on the level used by Sand et al. (2016), and was a level that prevented Eimeria-induced growth depression in chicks. Chicks assigned each diet treatment were either orally gavaged with a saline solution or an Advent Coccidiosis vaccine (10× vaccine dose consisting of a proprietary blend of live Eimeria acervulina, Eimeria maxima, and Eimeria tenella oocysts) at 3 d. Since chicks were euthanized throughout the study for blood and tissue sampling, only the body weights of remaining live chicks were determined at d 21 (n = 3 chicks per pen). Feed consumption was assessed by feed consumed/bird/day since birds were sacrificed mid-trial. Feed conversion was calculated by dividing feed consumption by body weight over the 21-day period. Oocysts were quantified using McMaster technique on day 4, 6, 8, and 10 days post infection (dpi) (Haug et al., 2006). On 2, 4, 7, 10, 13, 16, and 19 dpi, 1 bird per pen was randomly selected, bled into heparinized tubes, and blood was centrifuged and plasma was stored at −80°C for eventual determination of plasma carotenoids and corticosterone levels. At the same collection times, the luminal contents of the duodenum, jejunum, ileum, and ceca were collected by gentle squeezing and contents were flash frozen in liquid nitrogen. At a later date, luminal contents were thawed, diluted 1:5 in PBS, centrifuged (10,000 rpm, 15 minutes) to remove fecal matter, and supernatants were frozen at −80°C for eventual IL-10 determination. Preliminary data analyzing IL-10 levels in an intestinal luminal content sample compared to an intestinal mucosal scraping indicated on average that the lumen had 2 times the amount of IL-10 compared to the mucosal scraping. These findings suggested that mucosal IL-10 levels would have minimal effects on intestinal luminal content IL-10 levels if cells were damaged from the gentle squeeze during collection.
Measurement of Luminal IL-10 Using a Capture ELISA
Protein levels of thawed luminal supernatants (day 2, 4, and 7 post infection) were determined using Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL)). A capture ELISA was developed to quantify the amount of IL-10 in luminal contents. Rabbit anti-IL-10 (Bioss, Woburn, MA, diluted 1:1,000) served as the capture antibody and was bound to a Costar EIA/RIA flat bottom polystyrene 96 well plate (Corning Inc., Corning, NY) using a sodium carbonate buffer (pH 9.6), overnight at 4°C. The plate was washed 3× with PBS Tween 20 (Thermo Fisher Scientific, Rockford, IL) and then blocked for 1 hour at room temperature with 200 μL/well Pierce protein-free (TBS) blocking buffer (Thermo Scientific, Rockford, IL). Recombinant chicken IL-10 was used to develop a standard curve of optical density versus commercial recombinant IL-10 concentrations (16 ng to 1000 ng/mL in serial 2-fold dilutions, where concentrations were expressed as Log2 concentrations for linear curve fit, R2 = 0.98). The standard or luminal supernates (at 2.5 mg protein/mL) were then added to each of the wells and allowed to shake overnight at room temperature, then washed. Anti-IL-10 egg antibody to peptide vlpramqt (affinity purified, 0.85 mg/mL) was diluted at 1:1,000 in 1% nonfat dry milk powder was then added to the plate, incubated for 4 hours then washed. Rabbit anti-Chicken IgY-horseradish peroxidase (Bethyl, Montgomery, TX) was diluted in blocking buffer 1:10,000, added to each well, and shaken for 30 min at room temperature. After washing 6 times, the plate was developed by adding 1-Step Ultra TMB-ELISA (Thermo Scientific). Development was stopped by the addition of 100 μL/well 0.5 M H2SO4. The plate was read on an EL800 plate reader (BioTek, Winooski, VT) at 450 nm, and data were expressed in μg IL-10/mg protein.
Plasma Carotenoid Concentration
The carotenoid content of the plasma, a known biomarker for malabsorption, was determined using a modification method of Wilson and Wakabayashi (1956). The plasma was diluted 1:10 in acetone (0.1 mL of plasma to 0.9 mL of acetone) to precipitate the protein. This was followed by centrifugation for 10 minutes at 1,000 g. The optical density of the supernatant fluid was measured in a Beckman Coulter DU 800 spectrophotometer (Beckman Coulter, Brea, CA) at 445 nm (Wilson and Wakabayashi, 1956; Ruff and Fuller, 1975; Pettersson et al., 1995). Lutein (Indofine Chemical, Hillsborough, NJ) was used to establish a standard curve and was diluted 2-fold starting at 31.25 ng/mL of lutein in acetone. The R2 of the standard curve was 0.993.
Statistical Analysis
The collected data were analyzed using ANOVAs PROC MIXED of SAS 9.4 (SAS Institute Inc., Cary, NC). The LSD test was used for multiple treatment comparisons using the LSMEANS statement of SAS 9.4 with letter grouping obtained using the SAS pdmix800 macro. For the different statistical tests, significance was declared at a P-value of ≤0.05. Post hoc analyses for treatment differences were conducted if interactions were significant.
RESULTS
IL-10 Bioactivity Assay
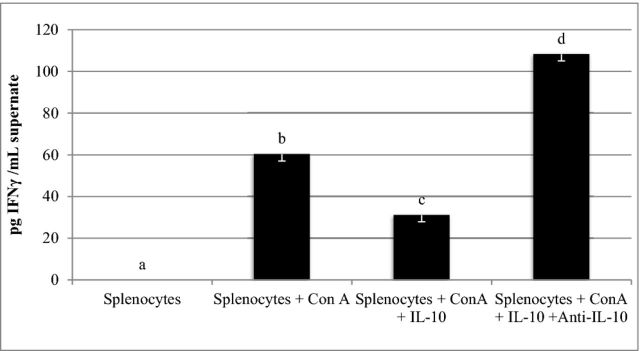
ConA-stimulated splenocytes cultured with IL-10 had significantly lower IFN-γ/mL than ConA splenocytes cultured without IL-10 (Figure 1, P < 0.05). Affinity purified egg yolk anti-IL-10 prevented IL-10s reduction of IFN-γ, and actually increased IFN-γ levels in the supernatant to 108 pg IFN-γ/mL, a level higher than in Con-A stimulated cells (Figure 1, P < 0.0001).
Figure 1.
The neutralizing effects of affinity purified egg yolk anti-IL-10 antibody were determined by measuring the ability of recombinant chicken IL-10 to inhibit IFN-γ production in Concanavalin A (Con A) stimulated chicken splenocytes. Splenocytes were untreated, stimulated with 20 μg Con A, stimulated with Con A and 0.2 ng/mL, recombinant chicken IL-10, or stimulated with Con A, IL-10, and 0.85 μg/mL affinity purified egg anti-IL-10 specific antibody. Culture media was analyzed for IFN-γ 24 hours after treatment. Anti-IL-10 was effective at neutralizing IL-10s inhibition of IFN-γ release (overall ANOVA P < 0.0001, standard error of the mean bars n = 3). a–dTreatment means with different letter superscripts are statistically different (P < 0.05).
Broiler Performance and Oocyst Shedding
Broiler chicks fed control antibody and challenged with Eimeria had a 20% reduction in body weight at 21 days of age when compared to chicks fed the control antibody and unchallenged (Table 1). Challenged chicks fed anti-IL-10 had similar 21 d body weight to unchallenged chicks fed anti-IL-10, and only a 13% reduction in 21 d body weight compared to those fed the control diet and unchallenged (significant Eimeria × antibody interaction). Feed conversion was unaffected by Eimeria challenge or dietary antibody. Oocyst shedding was increased due to Eimeria challenge. Antibody had no effect on oocyst shedding (Table 1).
Table 1.
Effect of Eimeria challenge and anti-IL-10 on chick performance and oocyst shedding.
| Treatment Groups | Average Weight (g)1 | Feed Conversion2 | Oocysts/g Excreta2 | |||
|---|---|---|---|---|---|---|
| Eimeria Infection | ||||||
| Antibody | Unchallenged | Challenged | Unchallenged | Infected | Unchallenged | Challenged |
| Control | 809a | 645c | 1.11 | 1.27 | 0 | 1,159,180 |
| Anti-IL-10 | 753a,b | 705b,c | 1.19 | 1.14 | 0 | 819,010 |
| SEM | 24 | 18 | 115,624 | |||
| P-values | ||||||
| Eimeria | 0.01 | 0.21 | 0.01 | |||
| Antibody | 0.93 | 0.57 | 0.15 | |||
| Eimeria X Antibody | 0.02 | 0.07 | 0.15 | |||
a–cMeans with different superscripts within a column were significantly different (P < 0.05).
1Average weight (n = 10) and feed consumption (n = 10) were measured in grams. SEM = Standard error of the mean.
2Feed conversion is calculated by dividing feed consumption by average pen body weight.
Anti-IL-10 Capture ELISA and Luminal IL-10 Levels
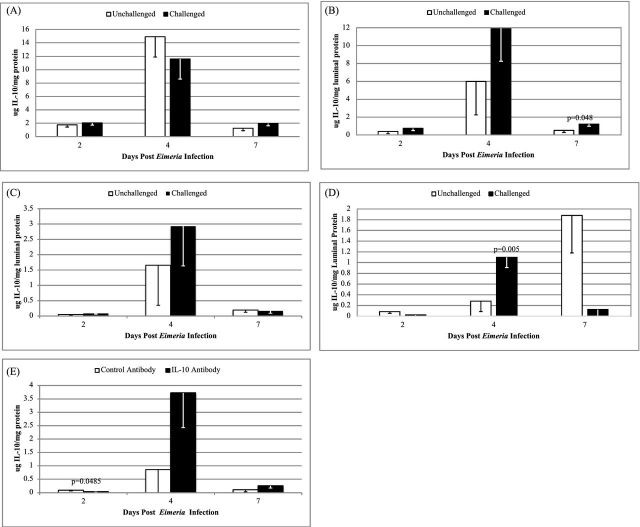
The sensitivity and linearity of the capture ELISA for detecting recombinant chicken IL-10 was demonstrated. Recombinant chicken IL-10 could be detected at concentrations as low as 16 ng/mL and the standard curve was linear (Log2) to 1,000 ng/mL (R2 = 0.98). Addition of luminal fluid at 0.25 mg protein/mL was adequate for detecting IL-10 within the range of the standard curve. When luminal contents were analyzed for the concentration of IL-10, Eimeria challenge (Eimeria main effect) increased luminal IL-10 levels on 4 dpi in the cecum (Figure 2D; P = 0.005), and on 7 dpi in the jejunum (Figure 2B, P < 0.05), but not in the duodenum or ileum at time points measured (Figure 2A and C). Feeding anti-IL-10 reduced the luminal IL-10 levels (Figure 2E; P < 0.05), but had no effect on lumen fluid levels in other tissues and at other time points (data not shown). No significant interactions for Eimeria × antibody were present in luminal contents of different regions of the intestine. Luminal IL-10 levels were notably increased on 4 dpi compared to 2 and 7 dpi in all sections of the intestine (effect of day; P < 0.05). Luminal IL-10 levels were again reduced on 7 dpi (except the cecum).
Figure 2.
Intestinal luminal IL-10 of Eimeria challenged chicks. Only the main effect of Eimeria challenge is shown for duodenum (2A), jejunum (2B), ileum (2C), cecum (2D) at each time point sampled. Main effect of antibody is shown for the ileum (2E). Error bars denote standard error of the mean (n = 20/treatment).
Plasma Carotenoid Levels
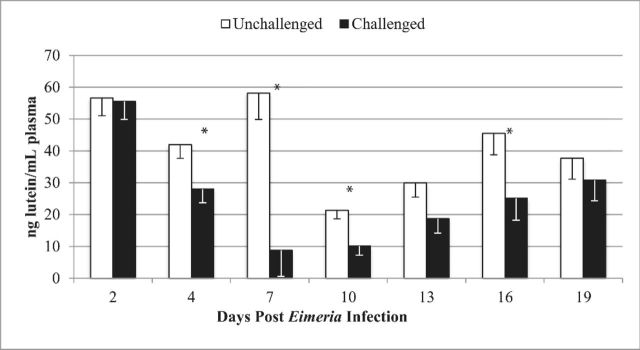
Infected chicks had reduced plasma carotenoid levels on 4, 7, 10, and 16 dpi (main effect of infection, Figure 3; P < 0.05). Coccidia caused the largest reduction (−85%) in plasma carotenoid levels on 7 dpi. No significant Eimeria × antibody interaction or antibody main effect was observed.
Figure 3.
Plasma carotenoid levels of Eimeria challenged chicks. Within each day post challenge *denote significant difference between unchallenged and challenged treatment groups (P < 0.05). Error bars represent the standard error of the mean (n = 20/treatment).
DISCUSSION
Sand et al. (2016) reported that feeding Eimeria-challenged chicks an anti-IL-10 egg yolk powder (antibody to IL-10 peptide vlpramqt) prevented reduced body weight compared to challenged chicks fed control antibody. The anti-IL-10 antibody did not improve body weight over control antibody when fed to unchallenged chicks, hence the anti-IL-10 benefit was the result of protecting against decreased body weights caused by Eimeria challenge. In a similar manner, anti-IL-10, in the experiment described here, protected against reduced body weight associated with Eimeria challenge. Unlike Sand et al. (2016) anti-IL-10 was not completely effective at preventing reduced body weights associated with Eimeria challenge, since the anti-IL-10 fed and challenged chick weighed less than the control antibody fed and unchallenged chicks. The experiment described in this paper was not designed as a growth trial, since every 2 to 3 days, chicks were removed from the experiment for tissue sampling. By d 21, only 3 chicks per pen (with 10 pens/experimental treatment) remained for body weight determination. Even with the shortcoming described, the results presented in this paper largely confirm the findings or Sand et al. (2016) with regards to the protective effects of anti-IL-10 against the reduced body weight due to Eimeria challenge.
One possible mechanism by which anti-IL-10 could be protective against reduced body weights due to Eimeria challenge was that anti-IL-10 served as a protectant against intestinal mucosa dysfunction and decreased nutrient absorption during Eimeria challenge (Ruff and Wilkins, 1984). Intestinal lesion scores are commonly used to estimate mucosal damage and the severity of Eimeria challenge since there is a negative correlation between lesion score and final body weight or weight gain (Mathis et al., 1984). However, the challenge used in this study (10× dose of a Advent coccidiosis vaccine) resulted in few lesions upon necropsy (lesion scores not collected). Low lesion scores were likely due to housing conditions. In this study, chicks were in pens with raised wire floors, and the excreta-oral cycling of oocysts was interrupted, limiting the severity of infection relative to birds raised in floor pens (Chapman et al., 2002). Plasma carotenoid levels are a known biomarker for malabsorption, and have been shown to decrease during coccidiosis (Ruff et al., 1974; Ruff and Fuller, 1975). In the study reported here, plasma carotenoids, independent of challenge, showed a bi-phasic curve, where plasma levels declined from day 5 to 13 of age (d 2 to d 12 post challenge), then increased from d 15 to d 18. This finding is consistent with the finding of Benito et al. (2011) who showed in post-hatch tern chicks, plasma levels of carotenoids decline post hatch, probably from the decay of carotenoids originating from the egg yolk. Dietary levels of carotenoids increase as dietary (corn) carotenoids were consumed. Our finding of an apparent accelerated loss of plasma carotenoids between d 2 to d 7 in Eimeria challenged chicks versus unchallenged chicks has been previously reported (Ruff and Fuller, 1975), and the mechanism may be related to the pro-oxidative state of an inflammatory process in challenged chicks (Shanmugasundaram and Selvaraj, 2011). Eimeria acervulina challenge, but not E. tenella, has been shown to interfere with carotenoid absorption and reduced plasma carotenoid levels in Eimeria challenged compared to unchallenged chicks (Ruff and Fuller, 1975). Our finding of reduced plasma carotenoids beginning on day 10 post challenge was probably the result of Eimeria's (possibly E. acervulina, one of the species used in challenging chicks) direct effect on carotenoid absorption. In this study anti-IL-10 antibody was ineffective in preventing Eimeria-induced decreased plasma carotenoids. The finding that anti-IL-10 protected body weights during Eimeria challenge on the one hand, but did not protect against a measure of nutrient absorption on the other, seems contradictory. Carotenoids (i.e., lutein) are lipophilic and are not required for broiler or turkey growth (Shanmugasundaram and Selvaraj, 2011; Moraes et al., 2015). In addition, E. acervulinia infectious dosage that affects carotenoid absorption had no effect on broiler growth (Hernandez-Velasco et al., 2014). Anti-IL-10 may be protective against the absorption of nutrients essential for growth or protective against Eimeria species known to impact chick growth.
The accepted method to measure the bioactivity of chicken IL-10 is by measuring its ability to reduce IFN-γ production in Con A stimulated splenocytes (Hillyer and Woodward, 2003). In the experiment provided, commercial recombinant IL-10 was effective at inhibiting Con A-induced IFN-γ production. Using this cell culture assay, we were then able to investigate if the anti-IL-10 produced against IL-10 peptide vlpramqt was useful in neutralizing the bioactivity of chicken IL-10. Evidence that the anti-IL-10 antibody neutralized the bioactivity of IL-10 (particularly with regards to IFN-γ regulation) provides support that IL-10 neutralization in feeding trials and sandwich ELISA were linked to IL-10s.
Inhibition of IL-10 systemically is well known to cause a pro-inflammatory condition in animals (Couper et al., 2008). We previously passively transferred IL-10 peptide antibodies to chicks by maternal vaccination. Passively transferred systemic antibodies decreased chick growth and increased intestinal inflammation; hence this research strategy was abandoned (unpublished). Orally administered antibodies, except in newborn calves and pigs during the “open gut” period, are not absorbed in a form that retains binding activity (Carlander et al., 2000; Cook and Trott, 2010); hence for an oral antibody to have biological activity, its ligand must be present in the intestinal lumen or accessible to the ligand. A recent finding of an apical IL-10 receptor on enterocytes suggested that functional IL-10 might be secreted into the intestine lumen (Kominsky et al., 2014). In preliminary studies, we were able to measure luminal IL-10 in two animal models, chicks challenged with Eimeria spp. (Sand et al., 2016), and mice with collagen-induced arthritis (unpublished). The data reported here confirms the presence of luminal IL-10 in both unchallenged and challenged chicks.
Striking in the luminal IL-10 data is the increased levels at 7 days of age (corresponding to 7 dpi) independent of Eimeria challenge (duodenum, jejunum, and ileum). In all tissue lumen samples, except the cecum, IL-10 levels decreased by 10 days of age (7 dpi). Studies on the developmental patterns of cytokine expression in the spleen and gut associated lymphoid tissue (GALT) show a similar peak of IL-10 and other cytokine production on day 7 of age (Bar-Shira et al., 2003; Abdul-Careem et al., 2007). The general peak in luminal IL-10 production on d7 may coincide with the maturation of GALT.
Increased luminal IL-10 (jejunum and cecum) in Eimeria challenged chicks was consistent with previous demonstrations of increased mucosal IL-10 mRNA in coccidia infected chicks (Hong et al., 2006; Haritova and Stanilova, 2012). The use of three Eimeria species in the infection model (E. acervulina, E. maxima, and E. tenella) in the study reported here, allowed for the examination of multiple intestinal regions for increased IL-10 release. The inability to detect consistent changes in IL-10 following challenge could be due to sampling time and sampling method. In this study we used three Eimeria species during the challenge. Each species infects at a different location of the intestine and has a life cycle that is independent of the other species. Focusing on one Eimeria species may have provided more consistent data. In Hong et al. (2006), during an E. maxima challenge, IL-10 mRNA peaks on day 4 and is back to baseline on day 5 post infection. The 2-day sampling interval for each tissue may be too broad an interval to accurately measure lumen IL-10 levels in response to Eimeria challenge. Regardless of the limitations outlined, the data presented demonstrates that IL-10 is secreted into the intestinal lumen and the levels are increased as a result of Eimeria challenge.
In all tissues sampled and at all sampling time points, there was no effect of anti-IL-10 (antibody or interaction with Eimeria) on luminal IL-10 levels (with the exception of day 2 post challenge in the ileum). If the oral anti-IL-10 antibodies were binding and neutralizing luminal IL-10, one would expect that detectable luminal IL-10 would decrease with anti-IL-10 feeding as observed in the ileum IL-10 levels on day 2 post challenge. One possible explanation is that the dietary level of anti-IL-10 fed was not sufficient to neutralize the luminal IL-10. This explanation does not appear reasonable, since the level used was effective at preventing Eimeria-induced decreased body weight. However, Sand et al. (2016) provided estimates of binding capacity of antibody fed. As mentioned previously, sampling time may be have interfered with our ability to adequately detect the feeding of anti-IL-10 on luminal IL-10. The capture ELISA was developed to detect IL-10 with an available bioactive region. In reality, this sandwich ELISA will also detect IL-10 bound by the dietary anti-IL-10 with equal efficacy as IL-10 that is not bound; hence the inability to detect a difference in luminal IL-10 levels between those fed control antibody and anti-IL-10 could be an artifact of the ELISA. Discrepancies between IL-10 levels detected by sandwich ELISA and bioassays in the plasma has been previously reported (Hillyer and Woodward, 2003) and presents a problem when attempting to describe meaning to plasma cytokine levels. While outside of the current scope of this study, several changes in experimental protocol or assay conditions could help determine if feeding anti-IL-10 is affecting luminal levels of IL-10. First, a different anti-IL-10 antibody to the peptide of interest, other than chicken IL-10, could be used as a feed ingredient. This would permit the current ELISA to specifically detect luminal chicken IL-10 where the peptide of interest in not bound. Such an experiment would be difficult since a large quantity of antibody, currently produced in high productive laying hens, is not readily available. Second, the IL-10 bioactivity of luminal fluids could be determined directly using the bioactivity assay described. Problems with this approach would be the presence of contaminating antigens, such as lipopolysaccharide, which could interfere with the cell culture bioassay. A third approach would be the development of a mouse monoclonal antibody to the chicken IL-10 vlpramqt peptide. An indirect method of assessing an effect of the anti-IL-10 antibody would be to quantify luminal IFN-γ. If feeding anti-IL-10 effectively inhibited biologically relevant luminal IL-10, IFN-γ in the intestinal lumen or surrounding mucosa may be increased. At this time, literature does not support the presence of luminal IFN-γ (i.e., no apical receptor on the enterocytes has been reported). Hong et al. (2006) showed that both IL-10 and IFN-γ mRNA in intraepithelial lymphocytes peaked at 4 to 6 days post E. acervulina infection, implying the IFN-γ levels may not be a good indicator of IL-10 regulation in the complex intestinal milieu, hence a IFN-γ surrogate for estimating effects of dietary IL-10 on IL-10 activity is not supported by the literature. Regardless of our ability to clearly demonstrate that anti-IL-10 directly affects luminal IL-10 levels, the findings reported here and by Sand et al. (2016), that oral anti-IL-10 protects chicks against Eimeria spp.-induced reduction in body weight, clearly demonstrates that neutralizing IL-10 through anti-IL-10 antibody feeding is protective in challenged chicks.
A pattern has emerged in the scientific literature; disruption of IL-10, its receptor or signaling pathway often confers resistance to a number of pathogens. Equally clear in the literature is that many pathogens exploit IL-10s down regulation of inflammation by directly inducing its release (Cyktor and Turner, 2011). In the case of Eimeria infection, intraepithelial lymphocytes levels of IL-10 mRNA increased post Eimeria spp. infection (Hong et al., 2006), resistance to Eimeria infection was linked to chicken lines that do not up-regulate intestinal IL-10 mRNA during Emieria infection (Rothwell et al., 2004), and cytokines considered important to immune dense against Eimeria infection (interleukin 2 and IFN-γ) are down-regulated by IL-10 (Choi et al., 1999; Li et al., 2002). Eimeria macrophage migratory inhibitory factor (eMIF) may be a means by which Eimeria spp. up-regulate host IL-10. While a direct link between eMIF and chicken IL-10 secretion has not been demonstrated in Eimeria spp. infected chickens directly, Paark et al. (2012) showed that MIF like peptide of the helminthes, Anisakis simplex, increased IL-10 production in human peripheral blood mononuclear cells. Protection against the Eimeria-induced decrease in body weight, as observed here and in Sand et al. (2016) has recently been demonstrated by direct targeting eMIF (Jang et al., 2011). Jang et al. (2011) showed that when embryos were vaccinated in ovo with a recombinant Eimeria MIF antigen, vaccinated chicks were protected against Eimeria tenella-induced decreased weight gain, and vaccinated chicks had reduced oocyst shedding (reduced approximately 25%) relative to sham vaccinated infected control chicks. Therefore, whether eMIF or host IL-10 is targeted, the outcome appears to be the same; protection against Eimeria-induced reduction in body weights.
Use of oral anti-IL-10 antibodies to protect against coccidia-induced growth depression represents a novel approach to controlling Eimeria infections. Efficacy of using oral antibodies to various host intestinal proteins has been well documented (Hatta et al., 1993; Cook and Trott, 2010; Rahman et al., 2013; Bobeck et al., 2015). The ability of egg antibody to transit the gastrointestinal tract and reach their target has been shown in multiple species (Cook and Trott, 2010). Consequently the ability for anti-IL-10 egg antibody to inhibit luminal IL-10 activity was predictable if luminal IL-10 played an important role in the pathogenesis of Eimeria spp (as documented here). Since oral antibodies are not absorbed (Cook and Trott, 2010), oral anti-IL-10 approaches to treat coccidiosis may avoid the inflammatory outcome of systemic inhibition of IL-10 (Gazzinelli et al., 1996).
In conclusion, oral anti-IL-10 antibody was effective at preventing Eimeria-induced growth suppression independent of an effect on oocyst shedding. The finding of increased IL-10 in the lumen of the intestine of Eimeria challenged chicks, and recent studies suggesting that bacteria, protozoans, and helminthes may induce IL-10 production as a part of its pathogenic strategy, supports a hypothesis that oral anti-IL-10 antibody acts as an anti-coccidial by allowing normal immune reactivity. Based on emerging scientific literature, it is reasonable to hypothesize that oral anti-IL-10 antibody may have broad protective effects in microbes that use up-regulation of IL-10 as a pathogenesis strategy.
Acknowledgments
The authors would like to thank Maria Jose, Jason Ren, Jake Olson, Zachary Simons, Christina Stevenson, Dylan Easley, Megan Mezera, and Christopher Nguyen for aiding in sample collection and analysis, as well as the UW Animal Care Staff (Dawn Irish, and John Kemper) for animal care. Laying hens used for making antibodies in these studies were kindly donated by S&R Farms, Whitewater, WI.
FUNDING
Research was supported in part by Wisconsin Alumni Research Foundation (WARF) Accelerator Grant, royalties received from WARF and University of Wisconsin- School of Veterinary Medicine- 12 Month Mentored Research Program, and the College of Agricultural and Life Sciences.
DISCLOSURES
MEC and JMS have an ownership interest in AbE Discovery, LLC, which has licensed technology reported in this publication. All of the remaining authors declare no financial conflict of interest.
AUTHOR CONTRIBUTIONS
MK Arendt- Concept, experimental design, experimentation, manuscript preparation.
JM Sand- Concept, IL-10 ELISA.
TM Marcone- Carotenoid assay.
ME Cook- Concept, experimental design, manuscript preparation.
REFERENCES
- Abdul-Careem M. F., Hunter D. B., Lambourne M. D., Barta J., Sharif S. Ontogeny of cytokine gene expression in the chicken spleen. Poultry Sci. 2007;86:1351–355. doi: 10.1093/ps/86.7.1351. [DOI] [PubMed] [Google Scholar]
- Bar-Shira E., Sklan D., Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003;27:147–157. doi: 10.1016/s0145-305x(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Benito M. M., Gonzalez-Solis J., Becker P. H. Carotenoid supplementation and sex-specific trade-offs between colouration and condition in common tern chicks. J. Comp. Physiol. B. 2011;181:539–549. doi: 10.1007/s00360-010-0537-z. [DOI] [PubMed] [Google Scholar]
- Bobeck E. A., Hellestad E. M., Sand J. M., Piccione M. L., Bishop J. W., Helvig C., Petkovich M., Cook M. E. Oral peptide specific egg antibody to intestinal sodium-dependent phosphate co-transporter-2b is effective at altering phosphate transport in vitro and in vivo. Poultry Sci. 2015;94:1128–1137. doi: 10.3382/ps/pev085. [DOI] [PubMed] [Google Scholar]
- Bobeck E. A., Burgess K., Jamres T., Piccione M. L., Cook M. E. Maternally-derived antibody to fibroblast growth factor-23 reduced dietary phosphate requirements in growing chicks. Biochem Biophys Res. Commun. 2012;420:686–670. doi: 10.1016/j.bbrc.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Carlander D., Kollberg H., Wejåker R., Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol. Res. 2000;21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. D., Cherry T. E., Danforth H. D., Richards G., Shirley M. W., Williams R. B. Sustainable coccidosis control in poultry production: the role of live vaccines. Intl. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Choi K. D., Lillehoj H. S., Zalenga D. S. Changes in local IFN-γ and TGF-β4 mRNA expression and intraepithelial lymphocytes following Eimeria acervulina infection. Vet. Immunol. Immunopathol. 1999;71:263–275. doi: 10.1016/s0165-2427(99)00103-8. [DOI] [PubMed] [Google Scholar]
- Cook M. E., Trott D. L. IgY- Immune component of eggs as a source of pasive immunity for animals and humans. World's Poultry Sci. J. 2010;66:215–226. [Google Scholar]
- Couper K. N., Blount D. G., Riley E. M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Cyktor J. C., Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect. Imm. 2011;79:2964–973. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kühn R., Müller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4 T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Haritova A. M., Stanilova S. A. Enhanced expression of IL-10 in contrast to IL-12B mRNA in poultry with experimental coccidiosis. Expl. Parasit. 2012;132:378–382. doi: 10.1016/j.exppara.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Akachi S., Kim M., Yamamota T., Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosci. Biotechnol. Biochem. 1993;57:1077–1081. doi: 10.1271/bbb.57.1077. [DOI] [PubMed] [Google Scholar]
- Hernández-Velasco X., Chapman H. D., Owens C. M., Kuttappan V. A., Fuente-Martinez B., Menconi A., Latorre J. D., Kallapura G., Bielke L. R., Rathinam T., Harrgis B. M., Tellez G. Absorption and deposition of xanthophylls in broilers challenged with three dosages of Eimeria acervulina oocysts. Brit. Poultry Sci. 2014;55:167–173. doi: 10.1080/00071668.2013.879095. [DOI] [PubMed] [Google Scholar]
- Haug A., Williams R. B., Larsen S. Counting coccidial oocysts in chicken faeces: A comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 2006;136:233–242. doi: 10.1016/j.vetpar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Hillyer L. M., Woodward B. Interleukin-10 concentration determined by sandwich enzyme linked immunosorbent assay is unrepresentative of bioactivity in murine blood. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1514–R1519. doi: 10.1152/ajpregu.00378.2003. [DOI] [PubMed] [Google Scholar]
- Hong Y. H., Lillehoj H. S., Lee S. H., Dalloul R. A., Lillehoj E. P. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jang S. I., Lillehoj H. S., Lee S. H., Kim D. K., Pagés M., Hong Y. H., Min W., Lillehoj E. P. Distinct immunorgulatory properties of macrophage migration inhibitory factors encoded by Eimeria parasites and their chicken host. Vaccine. 2011;29:8998–9004. doi: 10.1016/j.vaccine.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Kominsky D. J., Campbell E. L., Ehrentraut S. F., Wilson K. E., Kelly C. J., Glover L. E., Collins C. B., Bayless A. J., Saeedi B., Dobrinskikh E., Bowers B. E., MacManus C. F., Muller W., Colgan S. P., Bruder D. IFN-gamma-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 2014;192:1267–1276. doi: 10.4049/jimmunol.1301757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Lillehoj E. P., Lillehoj H. S. Interleukin-2 production in SC and TK chickens infected with Eimeria tenella. Avian Dis. 2002;46:2. doi: 10.1637/0005-2086(2002)046[0002:IPISAT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Lillehoj E. P. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. 2000;44:408–425. [PubMed] [Google Scholar]
- Mathis G. F., Washburn K. W., McDougald L. R. Genetic variability of resistance to Eimeria acervulina and E. tenella. Theor. Appl. Genet. 1984;68:385–389. doi: 10.1007/BF00254803. [DOI] [PubMed] [Google Scholar]
- Moraes M. L., Ribeiro A. M. L., Santin E., Klasing K. C. Effects of conjugated linoleic acid and letein on the growth performance and immune response of broiler chickens. Poultry Sci. 2015 doi: 10.3382/ps/pev325. dx.doi.org/10.3382/ps/pev325. [DOI] [PubMed] [Google Scholar]
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Paark H. K., Cho M. K., Park H. Y., Kim K. U., Kim Y. S., Lee M. K., Park S. K., Kim D. H., Yu H. S. Macrophage Migration Inhibitory Factor Isolated from a Parasite Inhibited Th2 Cytokine Production in PBMCs of Atopic Asthma Patients. Journal of Asthma. 2012;49:10–15. doi: 10.3109/02770903.2011.637593. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Bjerrum L., Heuer O. E., Wong D., Nauerby B. Reproducible infection model for Clostridium perfringens in broiler chickens. Avian Dis. 2008;52:34–39. doi: 10.1637/7955-022307-Reg. [DOI] [PubMed] [Google Scholar]
- Pettersson T., Ernstrom U., Griffiths W., Sjovall J., Bergman T., Jornvall H. Lutein associated with a transthyretin indicates carotenoid derivation and novel multiplicity of transthyretin ligands. FEBS. Letters. 1995;365:23–26. doi: 10.1016/0014-5793(95)00389-q. [DOI] [PubMed] [Google Scholar]
- Rahman S., Van Nguyen S., Icatlo F. C., Jr., Umeda K., Kodama Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 2013;9:1039–1048. doi: 10.4161/hv.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Wang Y., Deng H., Deng Y., Deng J., Zuo Z., Wang Y., Peng X., Cui H., Shen L., Ma X., Fang J. Deoxynivalenol-induced cytokines and related genes in concanavalin A-stimulated primary chicken splenic lymphocytes. Toxicol. In Vitro. 2015;29:558–563. doi: 10.1016/j.tiv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Rothwell L., Young J. R., Zoorob R., Whittaker C. A., Hesketh P., Archer A., Smith A. L., Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Ruff M. D., Reid W. M., Johnson J. K. Lowered blood carotenoid levels in chickens infected with coccidia. Poultry Science. 1974;53:1801–1809. doi: 10.3382/ps.0531801. [DOI] [PubMed] [Google Scholar]
- Ruff M. D., Fuller H. L. Some mechanisms of reduction of carotenoid levels in chickens infected with Eimeria acervulinia or E. tenella. J. Nutr. 1975;105:1447–1456. doi: 10.1093/jn/105.11.1447. [DOI] [PubMed] [Google Scholar]
- Ruff M. D., Wilkins G. C. Intestinal absorption following challenge of immune chicks with Eimeria acervulina. Avian Pathol. 1984;13:25–35. doi: 10.1080/03079458408418505. [DOI] [PubMed] [Google Scholar]
- Sand J. M., Arendt M. K., Repasy Alec, Deniz Gűlay, Cook M. E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poultry Sci. 2016 doi: 10.3382/ps/pev352. xxx–xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundaran R., Selvaraj R. K. Lutein supplementation alters inflammatory cytokine production and antioxidant status in F-line Turkeys. Poultry Sci. 2011;90:971–976. doi: 10.3382/ps.2010-01150. [DOI] [PubMed] [Google Scholar]
- Sing A., Rost D., Tvardovskaia N., Roggenkamp A., Wiedemann A., Kirschning C. J., Aepfelbacher M., Heesemann J. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 2002;196:1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., Schaap D. C., Schettlers T. P. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001;100:13–20. doi: 10.1016/s0304-4017(01)00479-4. [DOI] [PubMed] [Google Scholar]
- Williams R. B. A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int. J. Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Wilson W. O., Wakabayashi G. Identifying Non-Laying Chicken Hens. Poultry Science. 1956;35:226–27. [Google Scholar]